Characterizing Kinetics and Avidity of SARS-CoV-2 Antibody Responses in COVID-19 Greek Patients
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Serological Assays
2.3. Statistical Analyses
3. Results
3.1. Characteristics of the Study Population
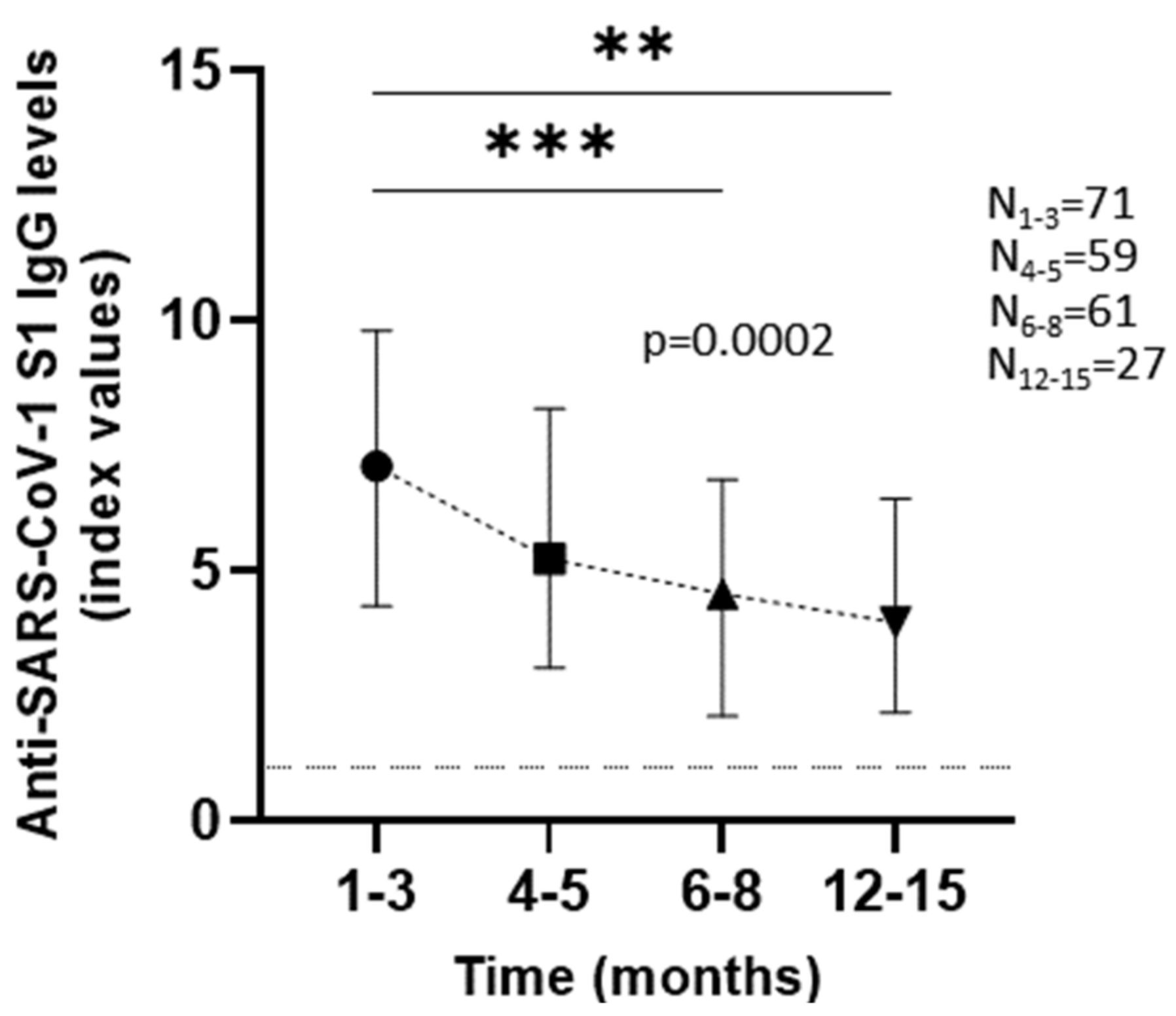
3.2. Anti-SARS-CoV-2 S1 Antibody Kinetics
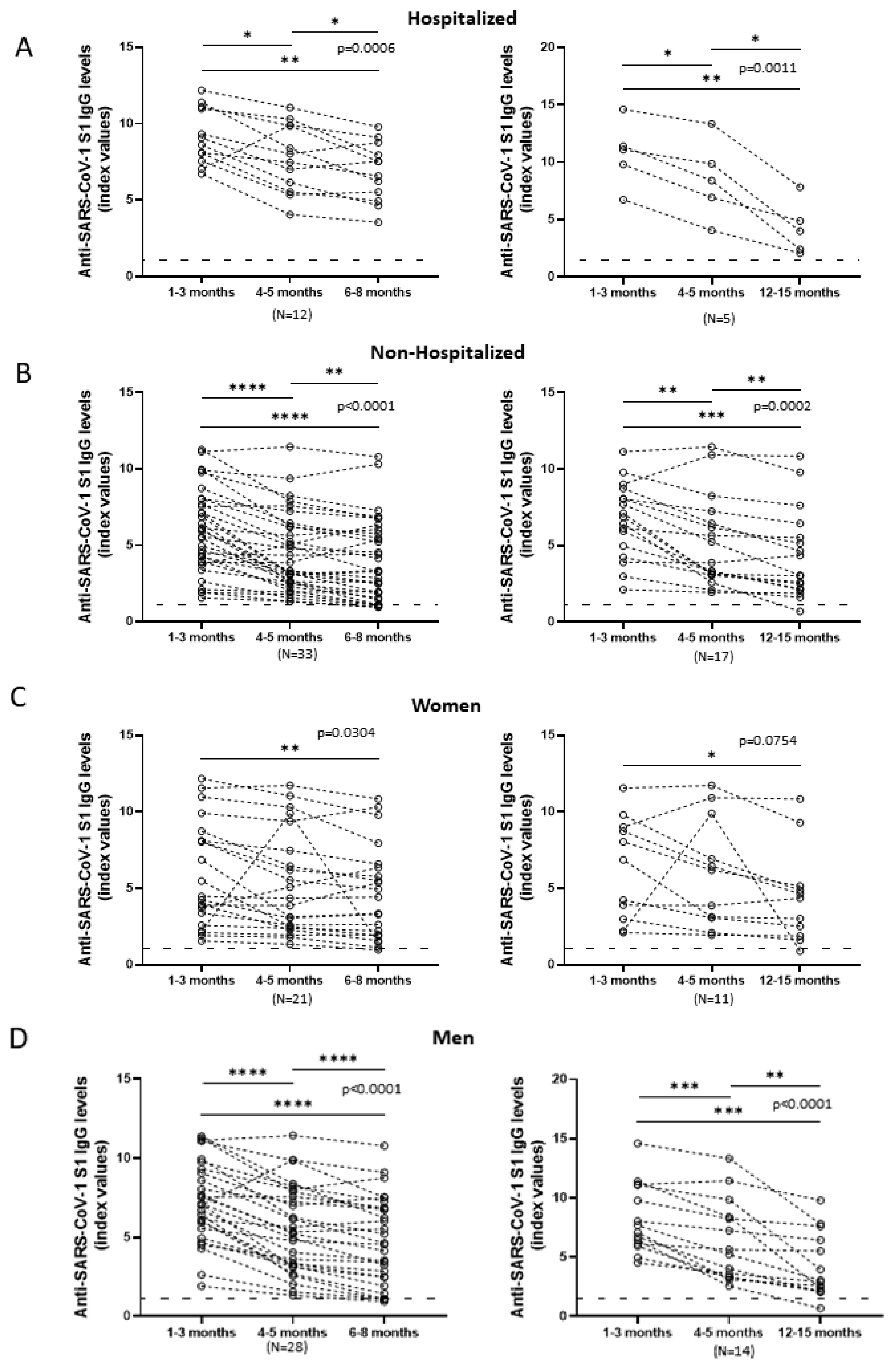
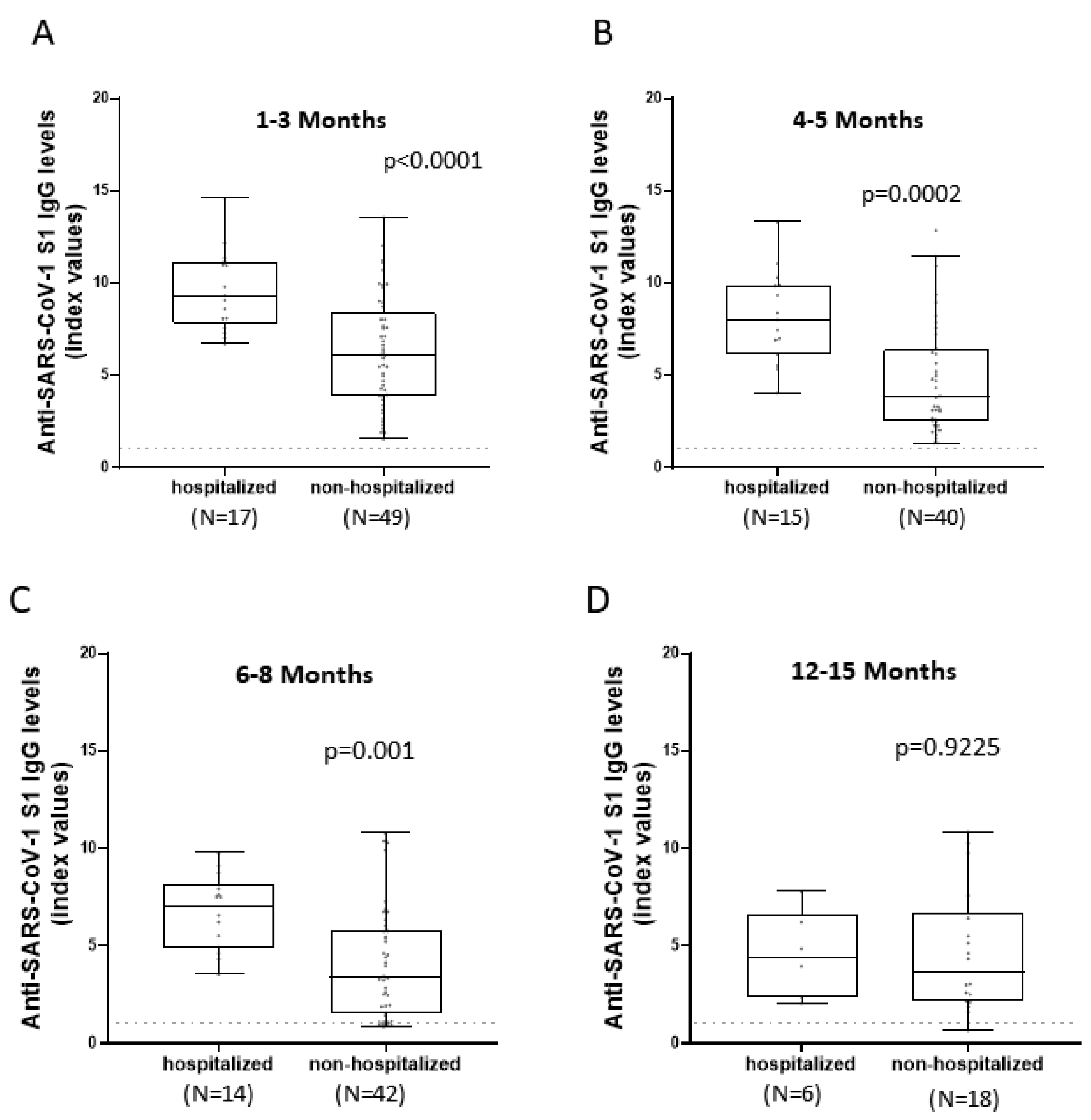
3.3. Comparison of Anti-SARS-CoV-2 S1 Antibody Kinetics among Hospitalized and Non-Hospitalized Patients
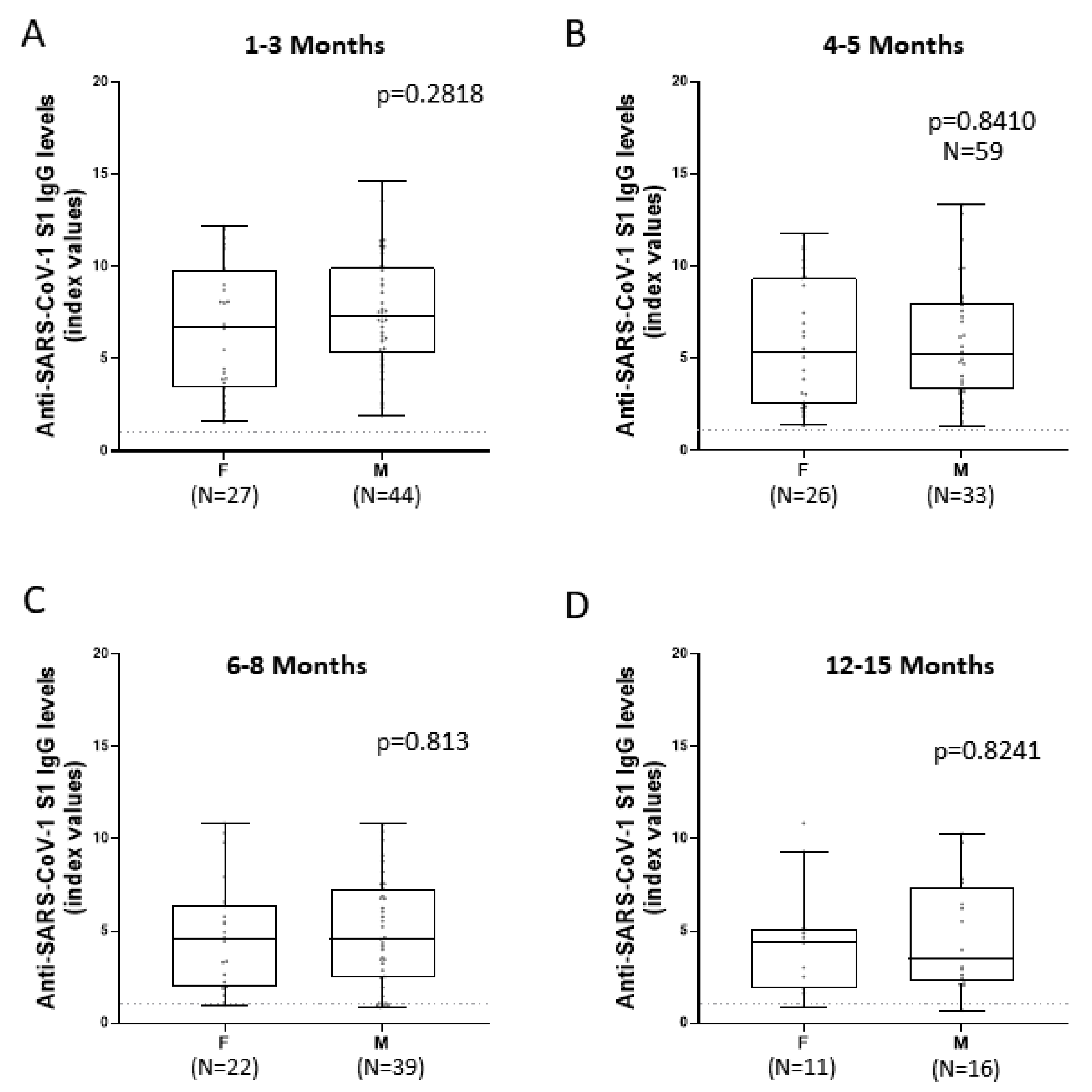
3.4. Comparison of Anti-SARS-CoV-2 S1 Antibody Kinetics among Female and Male Patients
3.5. Comparison of Anti-SARS-CoV-2 S1 Antibody Kinetics among Hospitalized and Non-Hospitalized and Female and Male Patients in Relation to Time
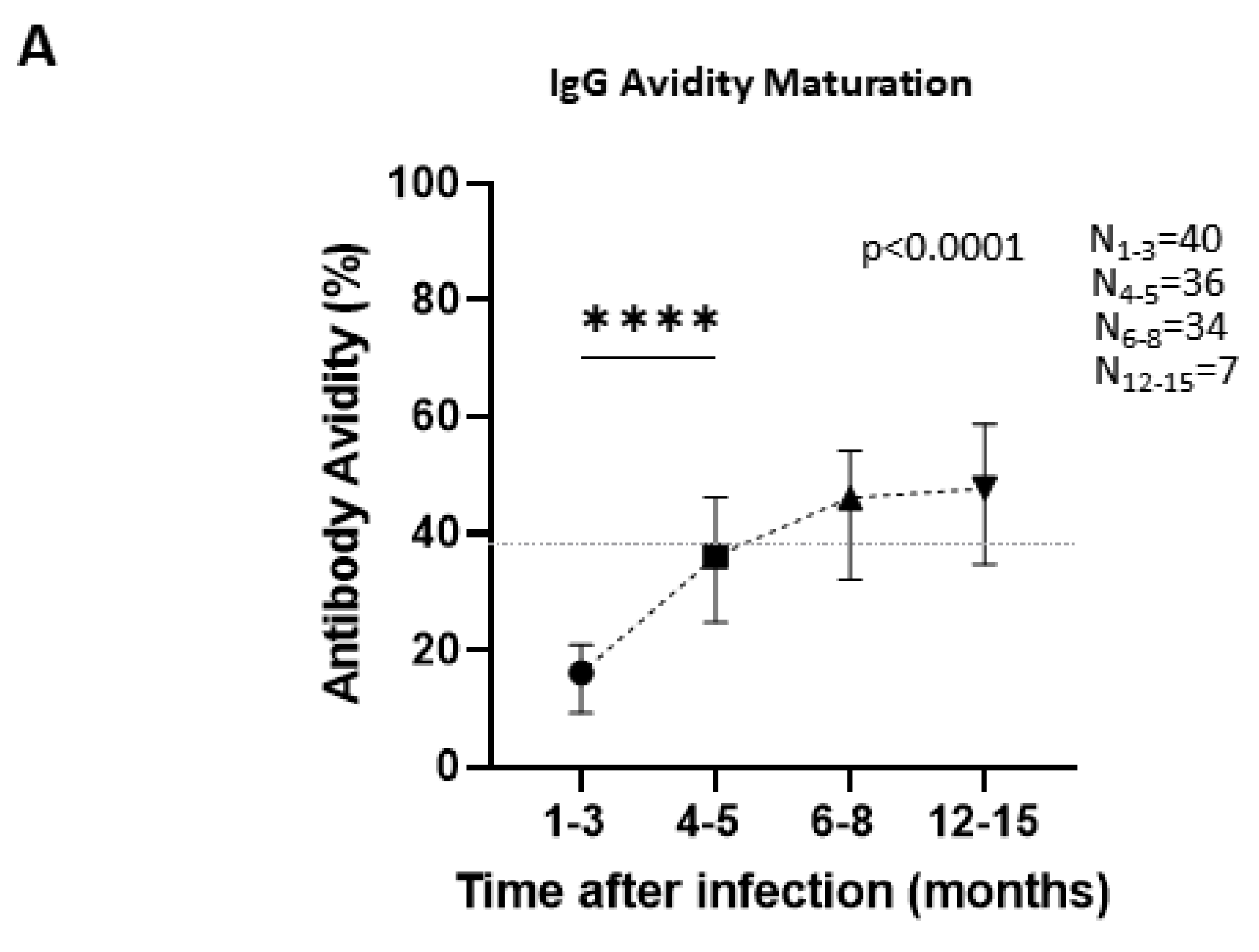
3.6. Antibody Avidity Maturation over Time Is an Important Tool in Determining Stage of the Infection
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Nicola, M.; Alsafi, Z.; Sohrabi, C.; Kerwan, A.; Al-Jabir, A.; Iosifidis, C.; Agha, M.; Agha, R. The socio-economic implications of the coronavirus pandemic (COVID-19): A review. Int. J. Surg. 2020, 78, 185–193. [Google Scholar] [CrossRef]
- WHO Coronavirus (COVID-19) Dashboard. Available online: https://Covid19.who.int/ (accessed on 3 March 2022).
- Coronavirus Disease (COVID-19). Available online: https://www.who.int/emergencies/diseases/novel-coronavirus-2019/question-and-answers-hub/q-a-detail/coronavirus-disease-covid-19 (accessed on 2 March 2022).
- Mpekoulis, G.; Frakolaki, E.; Taka, S.; Ioannidis, A.; Vassiliou, A.G.; Kalliampakou, K.I.; Patas, K.; Karakasiliotis, I.; Aidinis, V.; Chatzipanagiotou, S.; et al. Alteration of L-Dopa decarboxylase expression in SARS-CoV-2 infection and its association with the interferon-inducible ACE2 isoform. PLoS ONE 2021, 16, e0253458. [Google Scholar] [CrossRef]
- Chen, S.; Han, Y.; Yang, L.; Kim, T.; Nair, M.; Harschnitz, O.; Wang, P.; Zhu, J.; Koo, S.Y.; Tang, X.; et al. SARS-CoV-2 Infection Causes Dopaminergic Neuron Senescence. Res. Sq. 2021, rs-3. [Google Scholar] [CrossRef]
- Nikitopoulou, I.; Fanidis, D.; Ntatsoulis, K.; Moulos, P.; Mpekoulis, G.; Evangelidou, M.; Vassiliou, A.G.; Dimakopoulou, V.; Jahaj, E.; Tsipilis, S.; et al. Increased Autotaxin Levels in Severe COVID-19, Correlating with IL-6 Levels, Endothelial Dysfunction Biomarkers, and Impaired Functions of Dendritic Cells. Int. J. Mol. Sci. 2021, 22, 6. [Google Scholar] [CrossRef]
- Wong, R.S.Y. Inflammation in COVID-19: From pathogenesis to treatment. Int. J. Clin. Exp. Pathol. 2021, 14, 831–844. [Google Scholar]
- Bauer, G. The variability of the serological response to SARS-corona virus-2: Potential resolution of ambiguity through determination of avidity (functional affinity). J. Med. Virol. 2021, 93, 311–322. [Google Scholar] [CrossRef]
- Chaimayo, C.; Kaewnaphan, B.; Tanlieng, N.; Athipanyasilp, N.; Sirijatuphat, R.; Chayakulkeeree, M.; Angkasekwinai, N.; Sutthent, R.; Puangpunngam, N.; Tharmviboonsri, T.; et al. Rapid SARS-CoV-2 antigen detection assay in comparison with real-time RT-PCR assay for laboratory diagnosis of COVID-19 in Thailand. Virol. J. 2020, 17, 177. [Google Scholar] [CrossRef]
- Pelleau, S.; Woudenberg, T.; Rosado, J.; Donnadieu, F.; Garcia, L.; Obadia, T.; Gardais, S.; Elgharbawy, Y.; Velay, A.; Gonzalez, M.; et al. Kinetics of the SARS-CoV-2 antibody response and serological estimation of time since infection. J. Infect. Dis. 2021, 224, 1489–1499. [Google Scholar] [CrossRef]
- Lynch, K.L.; Whitman, J.D.; Lacanienta, N.P.; Beckerdite, E.W.; Kastner, S.A.; Shy, B.R.; Goldgof, G.M.; Levine, A.G.; Bapat, S.P.; Stramer, S.L.; et al. Magnitude and Kinetics of Anti-Severe Acute Respiratory Syndrome Coronavirus 2 Antibody Responses and Their Relationship to Disease Severity. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 2021, 72, 301–308. [Google Scholar] [CrossRef]
- Legros, V.; Denolly, S.; Vogrig, M.; Boson, B.; Siret, E.; Rigaill, J.; Pillet, S.; Grattard, F.; Gonzalo, S.; Verhoeven, P.; et al. A longitudinal study of SARS-CoV-2-infected patients reveals a high correlation between neutralizing antibodies and COVID-19 severity. Cell. Mol. Immunol. 2021, 18, 318–327. [Google Scholar] [CrossRef]
- Dan, J.M.; Mateus, J.; Kato, Y.; Hastie, K.M.; Yu, E.D.; Faliti, C.E.; Grifoni, A.; Ramirez, S.I.; Haupt, S.; Frazier, A.; et al. Immunological memory to SARS-CoV-2 assessed for up to 8 months after infection. Science 2021, 371, eabf4063. [Google Scholar] [CrossRef]
- Terpos, E.; Mentis, A.; Dimopoulos, M.A. Loss of Anti-SARS-CoV-2 Antibodies in Mild Covid-19. N. Engl. J. Med. 2020, 383, 1695. [Google Scholar] [CrossRef]
- Huang, A.T.; Garcia-Carreras, B.; Hitchings, M.D.T.; Yang, B.; Katzelnick, L.C.; Rattigan, S.M.; Borgert, B.A.; Moreno, C.A.; Solomon, B.D.; Trimmer-Smith, L.; et al. A systematic review of antibody mediated immunity to coronaviruses: Kinetics, correlates of protection, and association with severity. Nat. Commun. 2020, 11, 4704. [Google Scholar] [CrossRef]
- Dobano, C.; Ramirez-Morros, A.; Alonso, S.; Vidal-Alaball, J.; Ruiz-Olalla, G.; Vidal, M.; Rubio, R.; Cascant, E.; Parras, D.; Rodrigo Melero, N.; et al. Persistence and baseline determinants of seropositivity and reinfection rates in health care workers up to 12.5 months after COVID-19. BMC Med. 2021, 19, 155. [Google Scholar] [CrossRef]
- Rosati, M.; Terpos, E.; Ntanasis-Stathopoulos, I.; Agarwal, M.; Bear, J.; Burns, R.; Hu, X.; Korompoki, E.; Donohue, D.; Venzon, D.J.; et al. Sequential Analysis of Binding and Neutralizing Antibody in COVID-19 Convalescent Patients at 14 Months After SARS-CoV-2 Infection. Front. Immunol. 2021, 12, 793953. [Google Scholar] [CrossRef]
- Iyer, A.S.; Jones, F.K.; Nodoushani, A.; Kelly, M.; Becker, M.; Slater, D.; Mills, R.; Teng, E.; Kamruzzaman, M.; Garcia-Beltran, W.F.; et al. Persistence and decay of human antibody responses to the receptor binding domain of SARS-CoV-2 spike protein in COVID-19 patients. Sci. Immunol. 2020, 5, eabe0367. [Google Scholar] [CrossRef]
- Iyer, A.S.; Jones, F.K.; Nodoushani, A.; Kelly, M.; Becker, M.; Slater, D.; Mills, R.; Teng, E.; Kamruzzaman, M.; Garcia-Beltran, W.F.; et al. Dynamics and significance of the antibody response to SARS-CoV-2 infection. Medrxiv Serv. Health Sci. 2020. preprint. [Google Scholar] [CrossRef]
- Cameron, A.; Porterfield, C.A.; Byron, L.D.; Wang, J.; Pearson, Z.; Bohrhunter, J.L.; Cardillo, A.B.; Ryan-Muntz, L.; Sorensen, R.A.; Caserta, M.T.; et al. A Multiplex Microsphere IgG Assay for SARS-CoV-2 Using ACE2-Mediated Inhibition as a Surrogate for Neutralization. J. Clin. Microbiol. 2021, 59, e02489-20. [Google Scholar] [CrossRef]
- Graham, N.R.; Whitaker, A.N.; Strother, C.A.; Miles, A.K.; Grier, D.; McElvany, B.D.; Bruce, E.A.; Poynter, M.E.; Pierce, K.K.; Kirkpatrick, B.D.; et al. Kinetics and isotype assessment of antibodies targeting the spike protein receptor-binding domain of severe acute respiratory syndrome-coronavirus-2 in COVID-19 patients as a function of age, biological sex and disease severity. Clin. Transl. Immunol. 2020, 9, e1189. [Google Scholar] [CrossRef]
- Zeng, F.; Dai, C.; Cai, P.; Wang, J.; Xu, L.; Li, J.; Hu, G.; Wang, Z.; Zheng, F.; Wang, L. A comparison study of SARS-CoV-2 IgG antibody between male and female COVID-19 patients: A possible reason underlying different outcome between sex. J. Med. Virol. 2020, 92, 2050–2054. [Google Scholar] [CrossRef]
- Luo, H.; Camilleri, D.; Garitaonandia, I.; Djumanov, D.; Chen, T.; Lorch, U.; Täubel, J.; Wang, D. Kinetics of anti-SARS-CoV-2 IgG antibody levels and potential influential factors in subjects with COVID-19: A 11-month follow-up study. Diagn. Microbiol. Infect. Dis. 2021, 101, 115537. [Google Scholar] [CrossRef] [PubMed]
- Terpos, E.; Trougakos, I.P.; Apostolakou, F.; Charitaki, I.; Sklirou, A.D.; Mavrianou, N.; Papanagnou, E.D.; Liacos, C.I.; Gumeni, S.; Rentziou, G.; et al. Age-dependent and gender-dependent antibody responses against SARS-CoV-2 in health workers and octogenarians after vaccination with the BNT162b2 mRNA vaccine. Am. J. Hematol. 2021, 96, E257–E259. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Hu, B.; Hu, C.; Zhu, F.; Liu, X.; Zhang, J.; Wang, B.; Xiang, H.; Cheng, Z.; Xiong, Y.; et al. Clinical Characteristics of 138 Hospitalized Patients With 2019 Novel Coronavirus-Infected Pneumonia in Wuhan, China. JAMA 2020, 323, 1061–1069. [Google Scholar] [CrossRef] [PubMed]
- Ojeda, D.S.; Gonzalez Lopez Ledesma, M.M.; Pallares, H.M.; Costa Navarro, G.S.; Sanchez, L.; Perazzi, B.; Villordo, S.M.; Alvarez, D.E.; BioBanco Working, G.; Echavarria, M.; et al. Emergency response for evaluating SARS-CoV-2 immune status, seroprevalence and convalescent plasma in Argentina. PLoS Pathog. 2021, 17, e1009161. [Google Scholar] [CrossRef]
- Wu, F.; Liu, M.; Wang, A.; Lu, L.; Wang, Q.; Gu, C.; Chen, J.; Wu, Y.; Xia, S.; Ling, Y.; et al. Evaluating the Association of Clinical Characteristics With Neutralizing Antibody Levels in Patients Who Have Recovered From Mild COVID-19 in Shanghai, China. JAMA Intern. Med. 2020, 180, 1356–1362. [Google Scholar] [CrossRef]
- Terpos, E.; Politou, M.; Sergentanis, T.N.; Mentis, A.; Rosati, M.; Stellas, D.; Bear, J.; Hu, X.; Felber, B.K.; Pappa, V.; et al. Anti-SARS-CoV-2 Antibody Responses in Convalescent Plasma Donors Are Increased in Hospitalized Patients; Subanalyses of a Phase 2 Clinical Study. Microorganisms 2020, 8, 1885. [Google Scholar] [CrossRef]
- Bauer, G.; Struck, F.; Schreiner, P.; Staschik, E.; Soutschek, E.; Motz, M. The serological response to SARS corona virus-2 is characterized by frequent incomplete maturation of functional affinity (avidity). Eur. EMC 2020. preprint. [Google Scholar] [CrossRef]
- Benner, S.E.; Patel, E.U.; Laeyendecker, O.; Pekosz, A.; Littlefield, K.; Eby, Y.; Fernandez, R.E.; Miller, J.; Kirby, C.S.; Keruly, M.; et al. SARS-CoV-2 Antibody Avidity Responses in COVID-19 Patients and Convalescent Plasma Donors. J. Infect. Dis. 2020, 222, 1974–1984. [Google Scholar] [CrossRef]
- Lofstrom, E.; Eringfalt, A.; Kotz, A.; Wickbom, F.; Tham, J.; Lingman, M.; Nygren, J.M.; Unden, J. Dynamics of IgG-avidity and antibody levels after Covid-19. J. Clin. Virol. Off. Publ. Pan Am. Soc. Clin. Virol. 2021, 144, 104986. [Google Scholar] [CrossRef]
- Liu, T.; Hsiung, J.; Zhao, S.; Kost, J.; Sreedhar, D.; Hanson, C.V.; Olson, K.; Keare, D.; Chang, S.T.; Bliden, K.P.; et al. Quantification of antibody avidities and accurate detection of SARS-CoV-2 antibodies in serum and saliva on plasmonic substrates. Nat. Biomed. Eng. 2020, 4, 1188–1196. [Google Scholar] [CrossRef]
- Chan, K.H.; Ng, M.H.; Seto, W.H.; Peiris, J.S. Epstein-Barr virus (EBV) DNA in sera of patients with primary EBV infection. J. Clin. Microbiol. 2001, 39, 4152–4154. [Google Scholar] [CrossRef] [Green Version]
- Chawla, A.; Murphy, G.; Donnelly, C.; Booth, C.L.; Johnson, M.; Parry, J.V.; Phillips, A.; Geretti, A.M. Human immunodeficiency virus (HIV) antibody avidity testing to identify recent infection in newly diagnosed HIV type 1 (HIV-1)-seropositive persons infected with diverse HIV-1 subtypes. J. Clin. Microbiol. 2007, 45, 415–420. [Google Scholar] [CrossRef] [Green Version]
- Levett, P.N.; Sonnenberg, K.; Sidaway, F.; Shead, S.; Niedrig, M.; Steinhagen, K.; Horsman, G.B.; Drebot, M.A. Use of immunoglobulin G avidity assays for differentiation of primary from previous infections with West Nile virus. J. Clin. Microbiol. 2005, 43, 5873–5875. [Google Scholar] [CrossRef] [Green Version]
- Chan, K.H.; Sonnenberg, K.; Niedrig, M.; Lam, S.Y.; Pang, C.M.; Chan, K.M.; Ma, S.K.; Seto, W.H.; Peiris, J.S. Use of antibody avidity assays for diagnosis of severe acute respiratory syndrome coronavirus infection. Clin. Vaccine Immunol. CVI 2007, 14, 1433–1436. [Google Scholar] [CrossRef] [Green Version]
- Bauer, G.; Struck, F.; Schreiner, P.; Staschik, E.; Soutschek, E.; Motz, M. The challenge of avidity determination in SARS-CoV-2 serology. J. Med. Virol. 2021, 93, 3092–3104. [Google Scholar] [CrossRef]
- Scott, E.; Maxwell, H.D.D.; Kelley, K. Designing Experiments and Analyzing Data: A Model Comparison Perspective, 2nd ed.; Routledge: New York, NY, USA, 2005; Volume 168. [Google Scholar]
- De Greef, J.; Scohy, A.; Zech, F.; Aboubakar, F.; Pilette, C.; Gerard, L.; Pothen, L.; Yildiz, H.; Belkhir, L.; Yombi, J.C. Determinants of IgG antibodies kinetics after severe and critical COVID-19. J. Med. Virol. 2021, 93, 5416–5424. [Google Scholar] [CrossRef]
- Violán, C.; Torán, P.; Quirant, B.; Lamonja-Vicente, N.; Carrasco-Ribelles, L.A.; Chacón, C.; Manresa-Dominguez, J.M.; Ramos-Roure, F.; Roso-Llorach, A.; Pujol, A.; et al. Antibody kinetics to SARS-CoV-2 at 13.5 months, by disease severity. medRxiv 2021. preprint. [Google Scholar] [CrossRef]
- Moura, A.D.; da Costa, H.H.M.; Correa, V.A.; de, S.L.A.K.; Lindoso, J.A.L.; De Gaspari, E.; Hong, M.A.; Cunha-Junior, J.P.; Prudencio, C.R. Assessment of avidity related to IgG subclasses in SARS-CoV-2 Brazilian infected patients. Sci. Rep. 2021, 11, 17642. [Google Scholar] [CrossRef]
- Luo, Y.R.; Chakraborty, I.; Yun, C.; Wu, A.H.B.; Lynch, K.L. Kinetics of Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) Antibody Avidity Maturation and Association with Disease Severity. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 2021, 73, e3095–e3097. [Google Scholar] [CrossRef]
- Pichler, D.; Baumgartner, M.; Kimpel, J.; Rossler, A.; Riepler, L.; Bates, K.; Fleischer, V.; von Laer, D.; Borena, W.; Wurzner, R. Marked Increase in Avidity of SARS-CoV-2 Antibodies 7-8 Months After Infection Is Not Diminished in Old Age. J. Infect. Dis. 2021, 224, 764–770. [Google Scholar] [CrossRef]
- Scepanovic, P.; Alanio, C.; Hammer, C.; Hodel, F.; Bergstedt, J.; Patin, E.; Thorball, C.W.; Chaturvedi, N.; Charbit, B.; Abel, L.; et al. Human genetic variants and age are the strongest predictors of humoral immune responses to common pathogens and vaccines. Genome Med. 2018, 10, 59. [Google Scholar] [CrossRef] [Green Version]
- Vassilaki, N.; Gargalionis, A.N.; Bletsa, A.; Papamichalopoulos, N.; Kontou, E.; Gkika, M.; Patas, K.; Theodoridis, D.; Manolis, I.; Ioannidis, A.; et al. Impact of Age and Sex on Antibody Response Following the Second Dose of COVID-19 BNT162b2 mRNA Vaccine in Greek Healthcare Workers. Microorganisms 2021, 9, 1725. [Google Scholar] [CrossRef]
- Schlickeiser, S.; Schwarz, T.; Steiner, S.; Wittke, K.; Al Besher, N.; Meyer, O.; Kalus, U.; Pruss, A.; Kurth, F.; Zoller, T.; et al. Disease Severity, Fever, Age, and Sex Correlate With SARS-CoV-2 Neutralizing Antibody Responses. Front. Immunol. 2020, 11, 628971. [Google Scholar] [CrossRef]
- Vashisht, R.; Patel, A.; Crews, B.O.; Garner, O.B.; Dahm, L.; Wilson, C.; Butte, A.J. Age- and Sex-Associated Variations in the Sensitivity of Serological Tests Among Individuals Infected With SARS-CoV-2. JAMA Netw. Open 2021, 4, e210337. [Google Scholar] [CrossRef]
- Cervia, C.; Nilsson, J.; Zurbuchen, Y.; Valaperti, A.; Schreiner, J.; Wolfensberger, A.; Raeber, M.E.; Adamo, S.; Weigang, S.; Emmenegger, M.; et al. Systemic and mucosal antibody responses specific to SARS-CoV-2 during mild versus severe COVID-19. J. Allergy Clin. Immunol. 2021, 147, 545–557.e549. [Google Scholar] [CrossRef]
- Yu, H.Q.; Sun, B.Q.; Fang, Z.F.; Zhao, J.C.; Liu, X.Y.; Li, Y.M.; Sun, X.Z.; Liang, H.F.; Zhong, B.; Huang, Z.F.; et al. Distinct features of SARS-CoV-2-specific IgA response in COVID-19 patients. Eur. Respir. J. 2020, 56, 2001526. [Google Scholar] [CrossRef]
- Zhang, B.; Zhou, X.; Zhu, C.; Song, Y.; Feng, F.; Qiu, Y.; Feng, J.; Jia, Q.; Song, Q.; Zhu, B.; et al. Immune Phenotyping Based on the Neutrophil-to-Lymphocyte Ratio and IgG Level Predicts Disease Severity and Outcome for Patients With COVID-19. Front. Mol. Biosci. 2020, 7, 157. [Google Scholar] [CrossRef]
- Kowitdamrong, E.; Puthanakit, T.; Jantarabenjakul, W.; Prompetchara, E.; Suchartlikitwong, P.; Putcharoen, O.; Hirankarn, N. Antibody responses to SARS-CoV-2 in patients with differing severities of coronavirus disease 2019. PLoS ONE 2020, 15, e0240502. [Google Scholar] [CrossRef]
- Huang, C.; Wang, Y.; Li, X.; Ren, L.; Zhao, J.; Hu, Y.; Zhang, L.; Fan, G.; Xu, J.; Gu, X.; et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020, 395, 497–506. [Google Scholar] [CrossRef] [Green Version]
- Guan, W.J.; Ni, Z.Y.; Hu, Y.; Liang, W.H.; Ou, C.Q.; He, J.X.; Liu, L.; Shan, H.; Lei, C.L.; Hui, D.S.C.; et al. Clinical Characteristics of Coronavirus Disease 2019 in China. N. Engl. J. Med. 2020, 382, 1708–1720. [Google Scholar] [CrossRef]
- Zhou, F.; Yu, T.; Du, R.; Fan, G.; Liu, Y.; Liu, Z.; Xiang, J.; Wang, Y.; Song, B.; Gu, X.; et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: A retrospective cohort study. Lancet 2020, 395, 1054–1062. [Google Scholar] [CrossRef]
- Wandinger, K.P.; Saschenbrecker, S.; Steinhagen, K.; Scheper, T.; Meyer, W.; Bartelt, U.; Enders, G. Diagnosis of recent primary rubella virus infections: Significance of glycoprotein-based IgM serology, IgG avidity and immunoblot analysis. J. Virol. Methods 2011, 174, 85–93. [Google Scholar] [CrossRef]
- Valdivia, A.; Torres, I.; Huntley, D.; Alcaraz, M.J.; Albert, E.; Colomina, J.; Ferrer, J.; Carratala, A.; Navarro, D. Qualitative assessment of SARS-CoV-2-specific antibody avidity by lateral flow immunochromatographic IgG/IgM antibody assay. J. Med. Virol. 2021, 93, 1141–1144. [Google Scholar] [CrossRef]
- Bassi, J.; Giannini, O.; Silacci-Fregni, C.; Pertusini, L.; Hitz, P.; Terrot, T.; Franzosi, Y.; Muoio, F.; Saliba, C.; Meury, M.; et al. Poor neutralization and rapid decay of antibodies to SARS-CoV-2 variants in vaccinated dialysis patients. PLoS ONE 2022, 17, e0263328. [Google Scholar] [CrossRef]
- Wratil, P.R.; Stern, M.; Priller, A.; Willmann, A.; Almanzar, G.; Vogel, E.; Feuerherd, M.; Cheng, C.C.; Yazici, S.; Christa, C.; et al. Three exposures to the spike protein of SARS-CoV-2 by either infection or vaccination elicit superior neutralizing immunity to all variants of concern. Nat. Med. 2022, 28, 496–503. [Google Scholar] [CrossRef]


| 71 (Total) | IgG Levels (Median; IQR) | ||||
| 1–3 Months (N = 71) | 4–5 Months (N = 59) | 6–8 Months (N = 61) | 12–15 Months (N = 27) | ||
| Patients | 7.1 (4.3–9.8) | 5.24 (3.06–8.24) | 4.55 (2.09–6.83) | 3.97 (2.17–6.44) | |
| 40 | Antibody Avidity % (Median; IQR) | ||||
| 1–3 Months (N = 40) | 4–5 Months (N = 36) | 6–8 Months (N = 34) | 12–15 Months (N = 7) | ||
| 16.1 (9.32–20.9) | 36.1 (24.8–46.23) | 46.1 (32.13–54.13) | 47.7 (34.7–58.8) | ||
| IgG levels (Median; IQR) | |||||
| 1–3 Months | 4–5 Months | 6–8 Months | 12–15 Months | ||
| Sex | |||||
| Male | 44 | 7.21 (5.2–9.92) | 5.24 (3.24–7.96) | 4.55 (2.48–7.28) | 3.51 (2.22–7.32) |
| Female | 27 | 6.65 (3.38–9.8) | 5.3 (2.47–9.35) | 4.53 (1.94–6.41) | 4.35 (1.88–5.15) |
| p value > 0.999 a | |||||
| Clinical features | |||||
| Hospitalized | 17 | 9.32 (7.82–11.15) | 8.03 (6.16–9.9) | 7.06 (4.86–8.15) | 4.42 (2.31–6.62) |
| Non-Hospitalized | 49 | 6.07 (3.885–8.385) | 3.84 (2.52–6.4) | 3.4 (1.54–5.84) | 3.7 (2.16–6.74) |
| NA | 5 | ||||
| p value > 0.999 b | |||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Labropoulou, S.; Vassilaki, N.; Milona, R.S.; Terpos, E.; Politou, M.; Pappa, V.; Pagoni, M.; Grouzi, E.; Dimopoulos, M.A.; Mentis, A.; et al. Characterizing Kinetics and Avidity of SARS-CoV-2 Antibody Responses in COVID-19 Greek Patients. Viruses 2022, 14, 758. https://doi.org/10.3390/v14040758
Labropoulou S, Vassilaki N, Milona RS, Terpos E, Politou M, Pappa V, Pagoni M, Grouzi E, Dimopoulos MA, Mentis A, et al. Characterizing Kinetics and Avidity of SARS-CoV-2 Antibody Responses in COVID-19 Greek Patients. Viruses. 2022; 14(4):758. https://doi.org/10.3390/v14040758
Chicago/Turabian StyleLabropoulou, Stavroula, Niki Vassilaki, Raphaela S. Milona, Evangelos Terpos, Marianna Politou, Vasiliki Pappa, Maria Pagoni, Elisavet Grouzi, Meletios A. Dimopoulos, Andreas Mentis, and et al. 2022. "Characterizing Kinetics and Avidity of SARS-CoV-2 Antibody Responses in COVID-19 Greek Patients" Viruses 14, no. 4: 758. https://doi.org/10.3390/v14040758
APA StyleLabropoulou, S., Vassilaki, N., Milona, R. S., Terpos, E., Politou, M., Pappa, V., Pagoni, M., Grouzi, E., Dimopoulos, M. A., Mentis, A., Emmanouil, M., & Angelakis, E. (2022). Characterizing Kinetics and Avidity of SARS-CoV-2 Antibody Responses in COVID-19 Greek Patients. Viruses, 14(4), 758. https://doi.org/10.3390/v14040758








