West Nile Virus Neuroinfection in Humans: Peripheral Biomarkers of Neuroinflammation and Neuronal Damage
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cells and Virus Strains
2.2. Mouse Experiments
2.3. Patients and Sample Collection
2.4. Multiplex Assay
2.5. In Vitro Human Blood-Brain Barrier Model
2.6. Statistical Analyses
3. Results
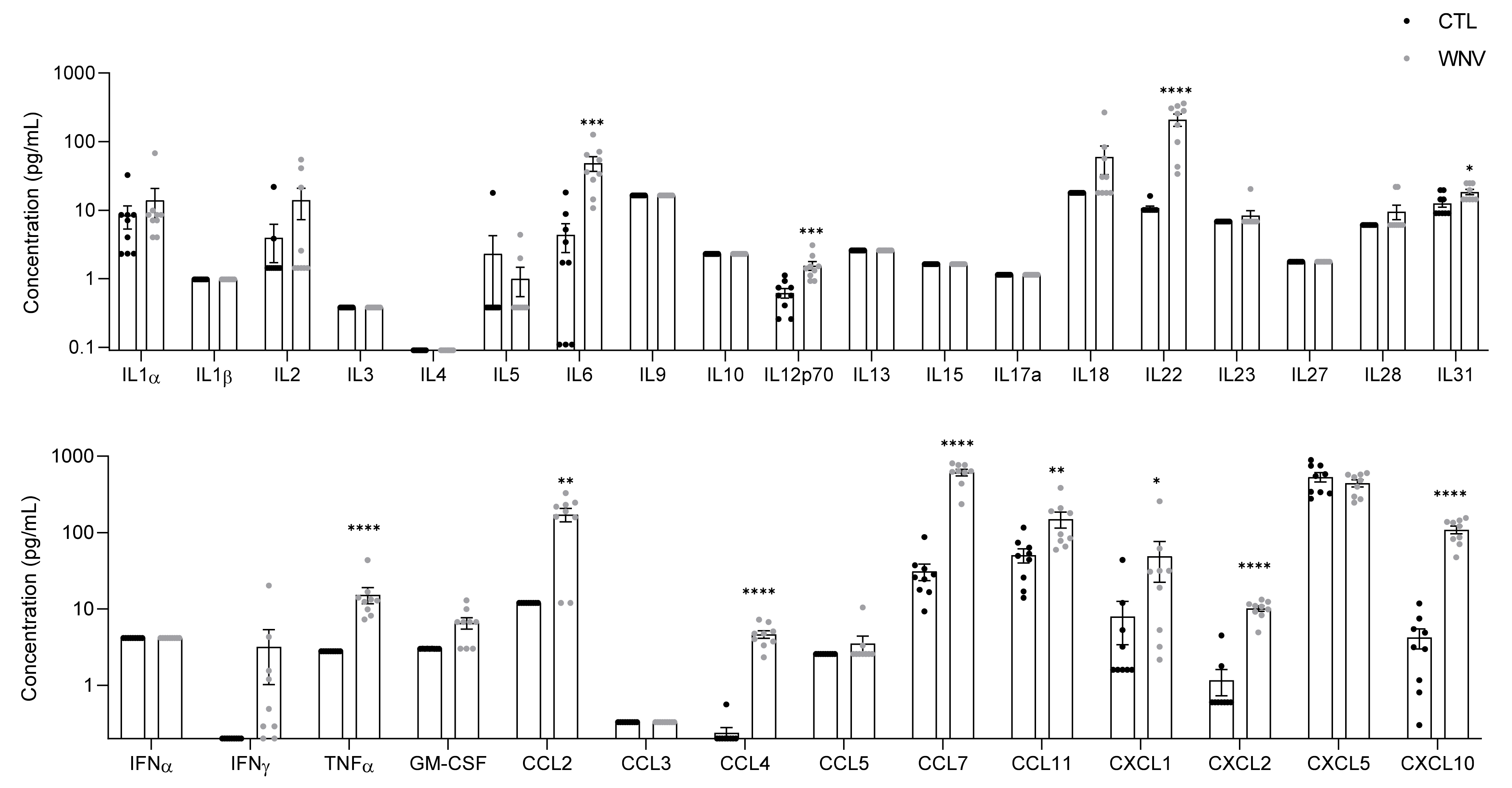
3.1. Characterization of Systemic Inflammation in Immunocompetent Mice
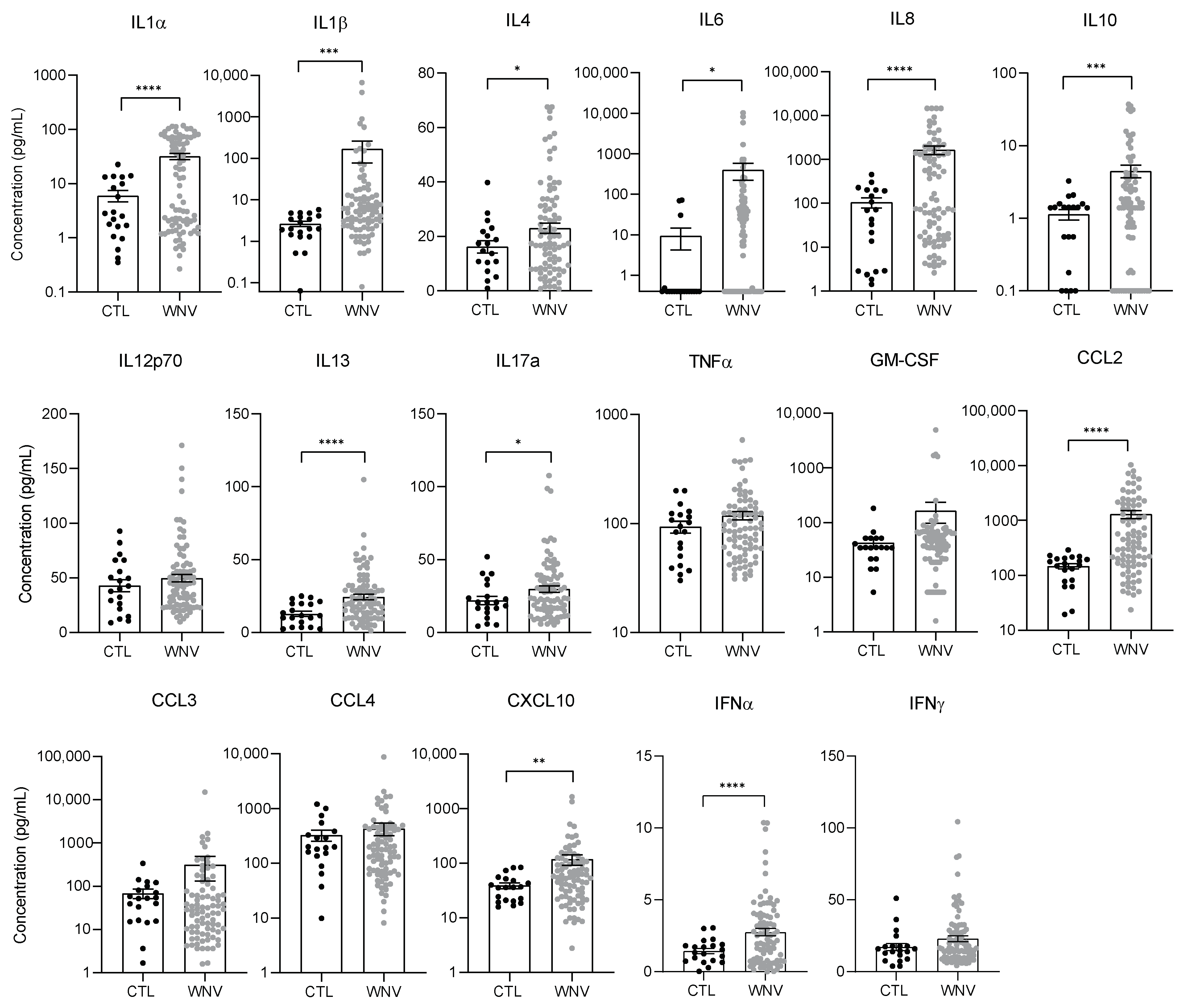
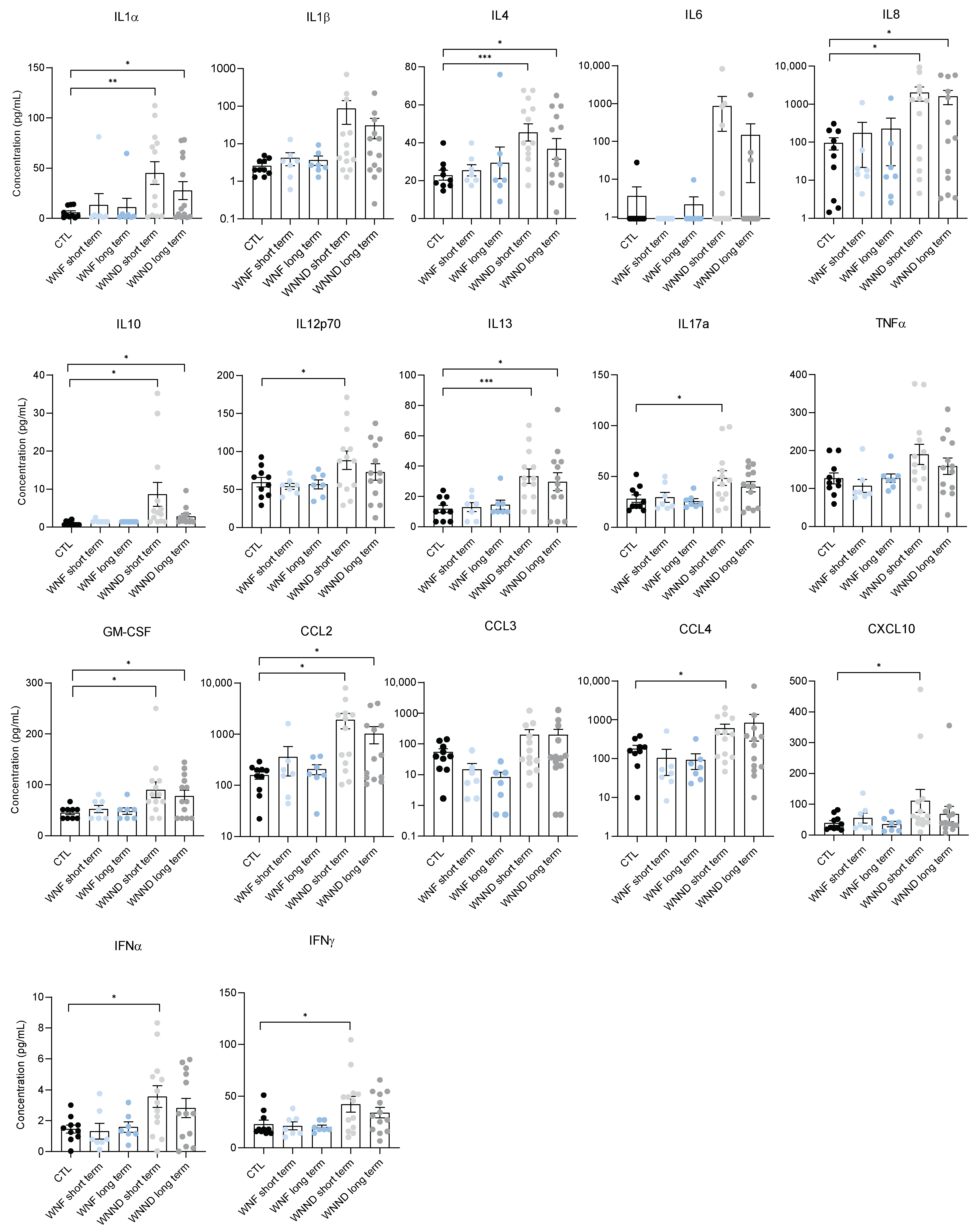
3.2. WNV Infection Leads to Systemic Inflammation, Particularly in Patients with Neuroinvasive Disease
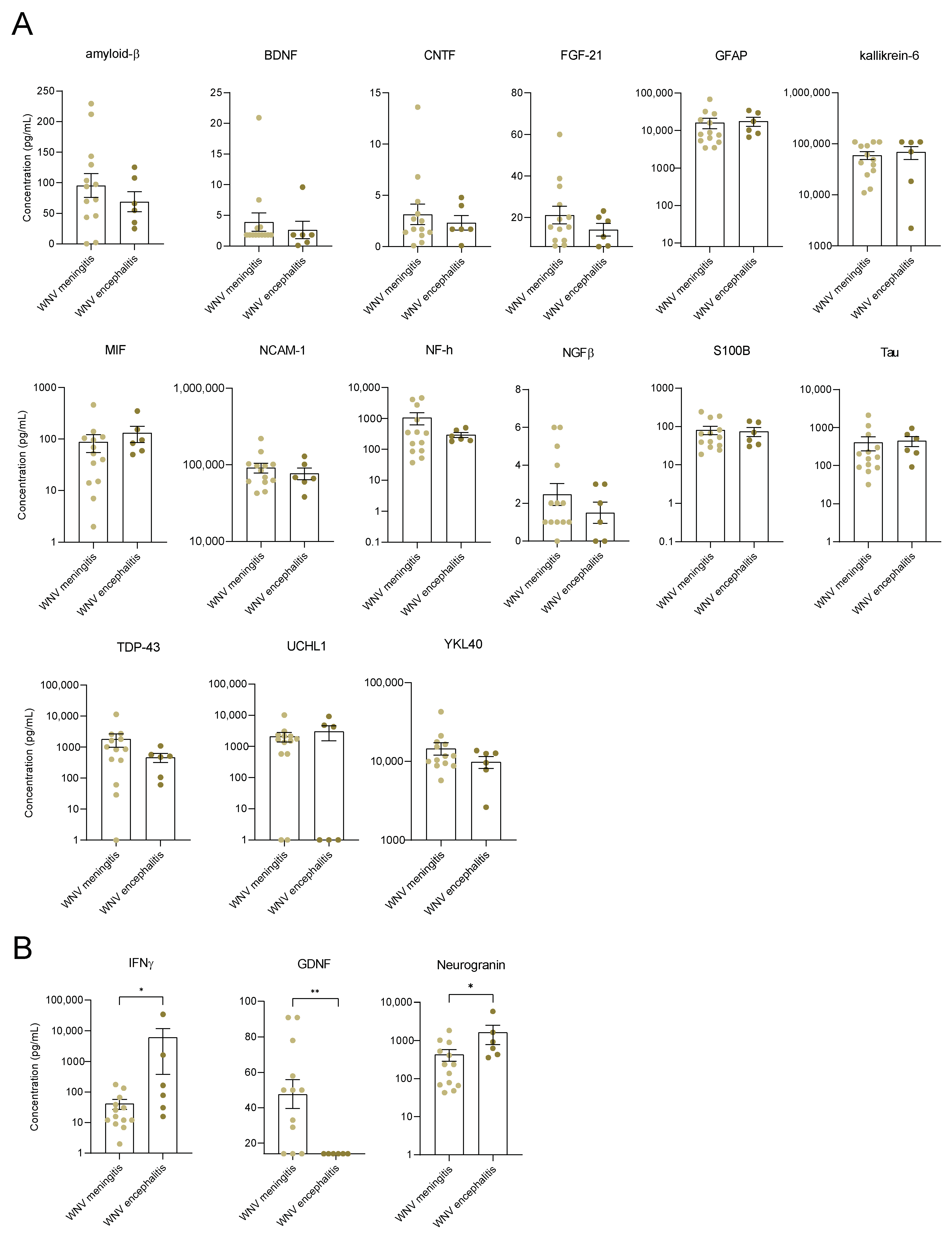
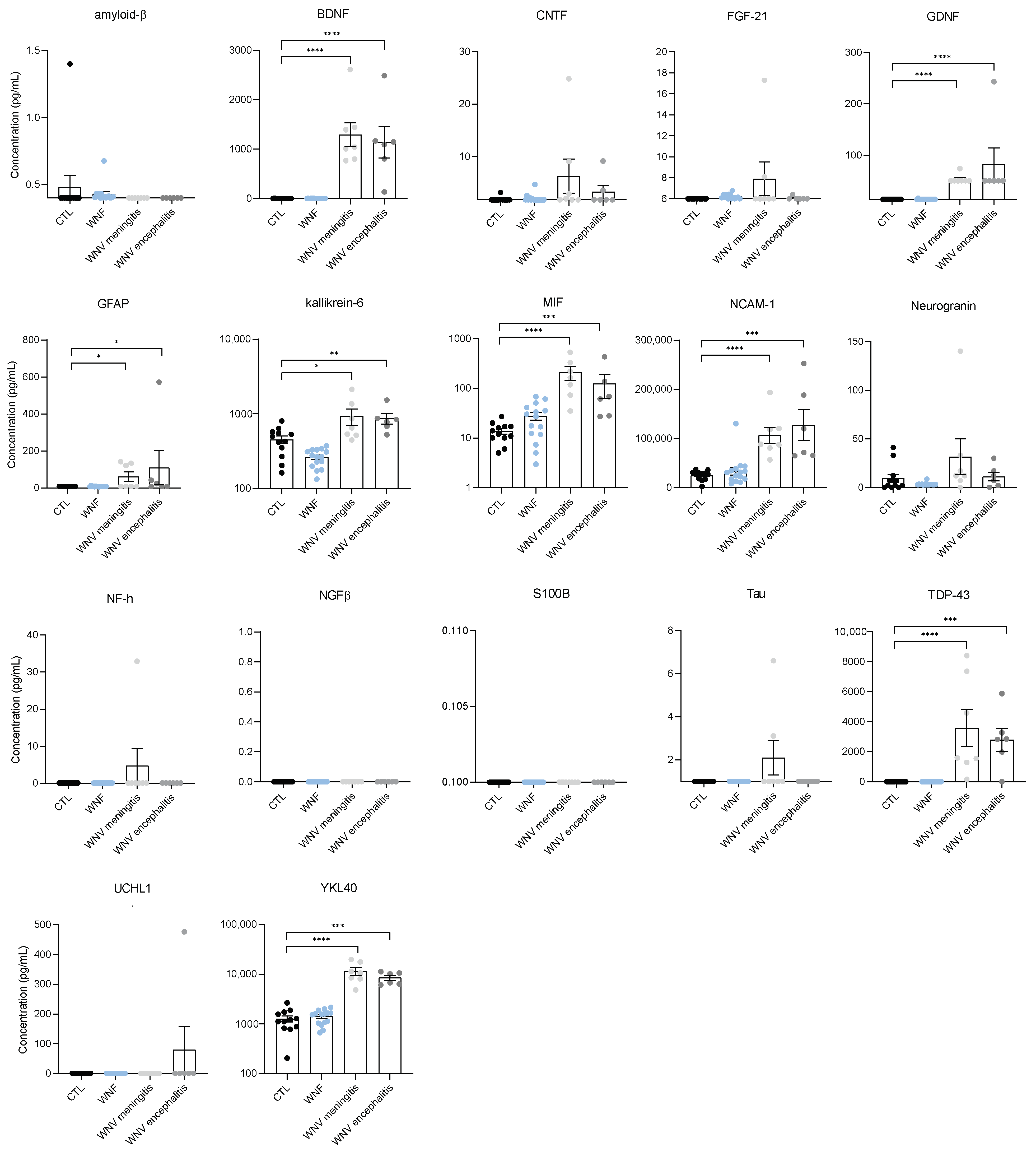
3.3. Identification of Neuroinflammation and Neuronal Damage Factors in WNND
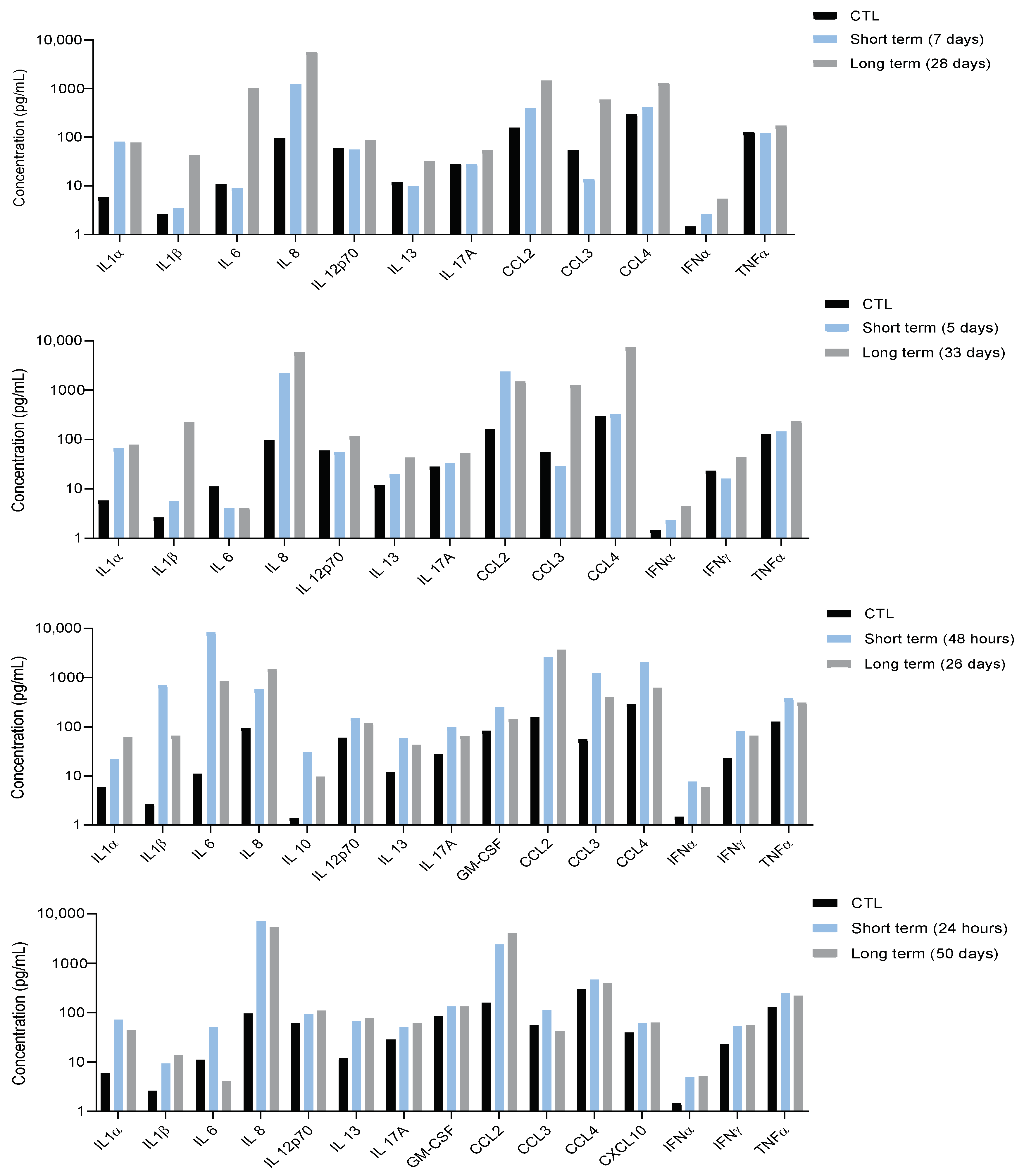
3.4. Systemic Inflammation Is Persistent
3.5. Serum from Patients with WNND Could Affect BBB Integrity
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
Appendix A

References
- Kramer, L.D.; Li, J.; Shi, P.Y. West Nile virus. Lancet Neurol. 2007, 6, 171–181. [Google Scholar] [CrossRef]
- Campbell, G.L.; Marfin, A.A.; Lanciotti, R.S.; Gubler, D.J. West Nile virus. Lancet Infect. Dis. 2002, 2, 519–529. [Google Scholar] [CrossRef]
- Sejvar, J.J.; Marfin, A.A. Manifestations of West Nile neuroinvasive disease. Rev. Med. Virol. 2006, 16, 209–224. [Google Scholar] [CrossRef] [PubMed]
- Hayes, E.B.; Gubler, D.J. West Nile virus: Epidemiology and clinical features of an emerging epidemic in the United States. Annu. Rev. Med. 2006, 57, 181–194. [Google Scholar] [CrossRef] [PubMed]
- Calisher, C.H.; Gould, E.A. Taxonomy of the virus family Flaviviridae. Adv. Virus Res. 2003, 59, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Petersen, L.R.; Brault, A.C.; Nasci, R.S. West Nile Virus: Review of the Literature. JAMA 2013, 310, 308. [Google Scholar] [CrossRef] [PubMed]
- Lecollinet, S.; Pronost, S.; Coulpier, M.; Beck, C.; Gonzalez, G.; Leblond, A.; Tritz, P. Viral Equine Encephalitis, a Growing Threat to the Horse Population in Europe? Viruses 2019, 12, 23. [Google Scholar] [CrossRef] [Green Version]
- ECDC. West Nile Virus Infection Key Facts; ECDC: Stockholm, Sweden, 2019. Available online: https://www.ecdc.europa.eu/en/west-nile-virus-infection (accessed on 1 January 2019).
- Brinton, M.A. Replication cycle and molecular biology of the West Nile virus. Viruses 2013, 6, 13–53. [Google Scholar] [CrossRef] [Green Version]
- Tobler, L.H.; Cameron, M.J.; Lanteri, M.C.; Prince, H.E.; Danesh, A.; Persad, D.; Lanciotti, R.S.; Norris, P.J.; Kelvin, D.J.; Busch, M.P. Interferon and interferon-induced chemokine expression is associated with control of acute viremia in west nile virus-infected blood donors. J. Infect. Dis. 2008, 198, 979–983. [Google Scholar] [CrossRef] [Green Version]
- Martin, M.-F.; Nisole, S. West Nile Virus Restriction in Mosquito and Human Cells: A Virus under Confinement. Vaccines 2020, 8, 256. [Google Scholar] [CrossRef]
- Klein, R.S.; Garber, C.; Funk, K.E.; Salimi, H.; Soung, A.; Kanmogne, M.; Manivasagam, S.; Agner, S.; Cain, M. Neuroinflammation during RNA Viral Infections. Annu. Rev. Immunol. 2019, 37, 73–95. [Google Scholar] [CrossRef]
- Morganti-Kossmann, M.C.; Kossmann, T.; Wahl, S.M. Cytokines and neuropathology. Trends Pharmacol. Sci. 1992, 13, 286–291. [Google Scholar] [CrossRef]
- Samuel, M.A.; Wang, H.; Siddharthan, V.; Morrey, J.D.; Diamond, M.S. Axonal transport mediates West Nile virus entry into the central nervous system and induces acute flaccid paralysis. Proc. Natl. Acad. Sci. USA 2007, 104, 17140–17145. [Google Scholar] [CrossRef] [Green Version]
- Roe, K.; Orillo, B.; Verma, S. West nile virus-induced cell adhesion molecules on human brain microvascular endothelial cells regulate leukocyte adhesion and modulate permeability of the in vitro blood-brain barrier model. PLoS ONE 2014, 9, e102598. [Google Scholar] [CrossRef] [Green Version]
- Bai, F.; Kong, K.F.; Dai, J.; Qian, F.; Zhang, L.; Brown, C.R.; Fikrig, E.; Montgomery, R.R. A paradoxical role for neutrophils in the pathogenesis of West Nile virus. J. Infect. Dis. 2010, 202, 1804–1812. [Google Scholar] [CrossRef] [Green Version]
- Verma, S.; Lo, Y.; Chapagain, M.; Lum, S.; Kumar, M.; Gurjav, U.; Luo, H.; Nakatsuka, A.; Nerurkar, V.R. West Nile virus infection modulates human brain microvascular endothelial cells tight junction proteins and cell adhesion molecules: Transmigration across the in vitro blood-brain barrier. Virology 2009, 385, 425–433. [Google Scholar] [CrossRef] [Green Version]
- Wang, P.; Dai, J.; Bai, F.; Kong, K.-F.; Wong, S.J.; Montgomery, R.R.; Madri, J.A.; Fikrig, E. Matrix Metalloproteinase 9 Facilitates West Nile Virus Entry into the Brain. J. Virol. 2008, 82, 8978–8985. [Google Scholar] [CrossRef] [Green Version]
- Bai, F.; Ashley Thompson, E.; Vig, P.J.S.; Arturo Leis, A. Current understanding of west nile virus clinical manifestations, immune responses, neuroinvasion, and immunotherapeutic implications. Pathogens 2019, 8, 193. [Google Scholar] [CrossRef] [Green Version]
- Winkelmann, E.R.; Luo, H.; Wang, T. West Nile Virus Infection in the Central Nervous System. F1000Research 2016, 5, 105. [Google Scholar] [CrossRef] [Green Version]
- Fares-Gusmao, R.; Rocha, B.C.; Sippert, E.; Lanteri, M.C.; Áñez, G.; Rios, M. Differential Pattern of Soluble Immune Markers in Asymptomatic Dengue, West Nile and Zika Virus Infections. Sci. Rep. 2019, 9, 17172. [Google Scholar] [CrossRef]
- Hoffman, K.W.; Sachs, D.; Bardina, S.V.; Michlmayr, D.; Rodriguez, C.A.; Sum, J.; Foster, G.A.; Krysztof, D.; Stramer, S.L.; Lim, J.K. Differences in early cytokine production are associated with development of a greater number of symptoms following west nile virus infection. J. Infect. Dis. 2016, 214, 634–643. [Google Scholar] [CrossRef] [PubMed]
- Garcia, M.N.; Hause, A.M.; Walker, C.M.; Orange, J.S.; Hasbun, R.; Murray, K.O. Evaluation of prolonged fatigue post-west nile virus infection and association of fatigue with elevated antiviral and proinflammatory cytokines. Viral Immunol. 2014, 27, 327–333. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hasbun, R.; Garcia, M.N.; Kellaway, J.; Baker, L.; Salazar, L.; Woods, S.P.; Murray, K.O. West nile virus retinopathy and associations with long term neurological and neurocognitive sequelae. PLoS ONE 2016, 11, e0148898. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leis, A.A.; Stokic, D.S. Neuromuscular Manifestations of West Nile Virus Infection. Front. Neurol. 2012, 3, 37. [Google Scholar] [CrossRef] [Green Version]
- Schafernak, K.T.; Bigio, E.H. West Nile virus encephalomyelitis with polio-like paralysis & nigral degeneration. Can. J. Neurol. Sci. 2006, 33, 407–410. [Google Scholar]
- Danics, K.; Forrest, S.L.; Kapas, I.; Erber, I.; Schmid, S.; Törő, K.; Majtenyi, K.; Kovacs, G.G. Neurodegenerative proteinopathies associated with neuroinfections. J. Neural Transm. 2021, 128, 1551–1566. [Google Scholar] [CrossRef]
- Kärber, G. Beitrag zur kollektiven Behandlung pharmakologischer Reihenversuche. Naunyn. Schmiedebergs. Arch. Exp. Pathol. Pharmakol. 1931, 162, 480–483. [Google Scholar] [CrossRef]
- Clé, M.; Constant, O.; Barthelemy, J.; Desmetz, C.; Martin, M.F.; Lapeyre, L.; Cadar, D.; Savini, G.; Teodori, L.; Monaco, F.; et al. Differential neurovirulence of Usutu virus lineages in mice and neuronal cells. J. Neuroinflamm. 2021, 18, 11. [Google Scholar] [CrossRef]
- Cecchelli, R.; Aday, S.; Sevin, E.; Almeida, C.; Culot, M.; Dehouck, L.; Coisne, C.; Engelhardt, B.; Dehouck, M.P.; Ferreira, L. A stable and reproducible human blood-brain barrier model derived from hematopoietic stem cells. PLoS ONE 2014, 9, e99733. [Google Scholar] [CrossRef] [Green Version]
- Heymans, M.; Figueiredo, R.; Dehouck, L.; Francisco, D.; Sano, Y.; Shimizu, F.; Kanda, T.; Bruggmann, R.; Engelhardt, B.; Winter, P.; et al. Contribution of brain pericytes in blood-brain barrier formation and maintenance: A transcriptomic study of cocultured human endothelial cells derived from hematopoietic stem cells. Fluids Barriers CNS 2020, 17, 48. [Google Scholar] [CrossRef]
- Candela, P.; Saint-Pol, J.; Kuntz, M.; Boucau, M.C.; Lamartiniere, Y.; Gosselet, F.; Fenart, L. In vitro discrimination of the role of LRP1 at the BBB cellular level: Focus on brain capillary endothelial cells and brain pericytes. Brain Res. 2015, 1594, 15–26. [Google Scholar] [CrossRef]
- Errett, J.S.; Suthar, M.S.; McMillan, A.; Diamond, M.S.; Gale, M. The essential, nonredundant roles of RIG-I and MDA5 in detecting and controlling West Nile virus infection. J. Virol. 2013, 87, 11416–11425. [Google Scholar] [CrossRef] [Green Version]
- Klein, R.S.; Lin, E.; Zhang, B.; Luster, A.D.; Tollett, J.; Samuel, M.A.; Engle, M.; Diamond, M.S. Neuronal CXCL10 directs CD8+ T-cell recruitment and control of West Nile virus encephalitis. J. Virol. 2005, 79, 11457–11466. [Google Scholar] [CrossRef] [Green Version]
- Kumar, M.; Belcaid, M.; Nerurkar, V.R. Identification of host genes leading to West Nile virus encephalitis in mice brain using RNA-seq analysis. Sci. Rep. 2016, 6, 26350. [Google Scholar] [CrossRef]
- Wang, T.; Town, T.; Alexopoulou, L.; Anderson, J.F.; Fikrig, E.; Flavell, R.A. Toll-like receptor 3 mediates West Nile virus entry into the brain causing lethal encephalitis. Nat. Med. 2004, 10, 1366–1373. [Google Scholar] [CrossRef]
- Garcia-Tapia, D.; Hassett, D.E.; Mitchell, W.J.; Johnson, G.C.; Kleiboeker, S.B. West Nile virus encephalitis: Sequential histopathological and immunological events in a murine model of infection. J. Neurovirol. 2007, 13, 130–138. [Google Scholar] [CrossRef]
- Peña, J.; Plante, J.A.; Carillo, A.C.; Roberts, K.K.; Smith, J.K.; Juelich, T.L.; Beasley, D.W.C.; Freiberg, A.N.; Labute, M.X.; Naraghi-Arani, P. Multiplexed digital mRNA profiling of the inflammatory response in the West Nile Swiss Webster mouse model. PLoS Negl. Trop. Dis. 2014, 8, e3216. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- King, N.J.C.; Getts, D.R.; Getts, M.T.; Rana, S.; Shrestha, B.; Kesson, A.M. Immunopathology of flavivirus infections. Immunol. Cell Biol. 2007, 85, 33–42. [Google Scholar] [CrossRef]
- Puerta-Guardo, H.; Glasner, D.R.; Espinosa, D.A.; Biering, S.B.; Patana, M.; Ratnasiri, K.; Wang, C.; Beatty, P.R.; Harris, E. Flavivirus NS1 Triggers Tissue-Specific Vascular Endothelial Dysfunction Reflecting Disease Tropism. Cell Rep. 2019, 26, 1598–1613.e8. [Google Scholar] [CrossRef] [Green Version]
- Teuwen, L.A.; Geldhof, V.; Pasut, A.; Carmeliet, P. COVID-19: The vasculature unleashed. Nat. Rev. Immunol. 2020, 20, 389–391. [Google Scholar] [CrossRef]
- Wimmer, I.; Tietz, S.; Nishihara, H.; Deutsch, U.; Sallusto, F.; Gosselet, F.; Lyck, R.; Muller, W.A.; Lassmann, H.; Engelhardt, B. PECAM-1 stabilizes blood-brain barrier integrity and favors paracellular T-cell diapedesis across the blood-brain barrier during neuroinflammation. Front. Immunol. 2019, 10, 711. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Constant, O.; Barthelemy, J.; Bolloré, K.; Tuaillon, E.; Gosselet, F.; Chable-Bessia, C.; Merida, P.; Muriaux, D.; Van de Perre, P.; Salinas, S.; et al. SARS-CoV-2 Poorly Replicates in Cells of the Human Blood-Brain Barrier without Associated Deleterious Effects. Front. Immunol. 2021, 12, 2914. [Google Scholar] [CrossRef] [PubMed]
- Clé, M.; Eldin, P.; Briant, L.; Lannuzel, A.; Simonin, Y.; Van De Perre, P.; Cabié, A.; Salinas, S. Neurocognitive impacts of arbovirus infections. J. Neuroinflamm. 2020, 17, 233. [Google Scholar] [CrossRef] [PubMed]
- Clé, M.; Desmetz, C.; Barthelemy, J.; Martin, M.F.; Constant, O.; Maarifi, G.; Foulongne, V.; Bolloré, K.; Glasson, Y.; De Bock, F.; et al. Zika Virus Infection Promotes Local Inflammation, Cell Adhesion Molecule Upregulation, and Leukocyte Recruitment at the Blood-Brain Barrier. MBio 2020, 11, e01183-20. [Google Scholar] [CrossRef]
- Clé, M.; Barthelemy, J.; Desmetz, C.; Foulongne, V.; Lapeyre, L.; Bolloré, K.; Tuaillon, E.; Erkilic, N.; Kalatzis, V.; Lecollinet, S.; et al. Study of Usutu virus neuropathogenicity in mice and human cellular models. PLoS Negl. Trop. Dis. 2020, 14, e0008223. [Google Scholar] [CrossRef] [Green Version]
- Habarugira, G.; Suen, W.W.; Hobson-Peters, J.; Hall, R.A.; Bielefeldt-Ohmann, H. West nile virus: An update on pathobiology, epidemiology, diagnostics, control and “One health” implications. Pathogens 2020, 9, 589. [Google Scholar] [CrossRef]
- Long, D.; Deng, X.; Singh, P.; Loeb, M.; Lauring, A.S.; Seielstad, M. Identification of genetic variants associated with susceptibility to West Nile virus neuroinvasive disease. Genes Immun. 2016, 17, 298–304. [Google Scholar] [CrossRef] [Green Version]
- Desai, P.; Janova, H.; White, J.P.; Reynoso, G.V.; Hickman, H.D.; Baldridge, M.T.; Urban, J.F.; Stappenbeck, T.S.; Thackray, L.B.; Diamond, M.S. Enteric helminth coinfection enhances host susceptibility to neurotropic flaviviruses via a tuft cell-IL-4 receptor signaling axis. Cell 2021, 184, 1214. [Google Scholar] [CrossRef]
- Lanteri, M.C.; O’Brien, K.M.; Purtha, W.E.; Cameron, M.J.; Lund, J.M.; Owen, R.E.; Heitman, J.W.; Custer, B.; Hirschkorn, D.F.; Tobler, L.H.; et al. Tregs control the development of symptomatic West Nile virus infection in humans and mice. J. Clin. Investig. 2009, 119, 3266–3277. [Google Scholar] [CrossRef] [Green Version]
- Ramos, H.J.; Lanteri, M.C.; Blahnik, G.; Negash, A.; Suthar, M.S.; Brassil, M.M.; Sodhi, K.; Treuting, P.M.; Busch, M.P.; Norris, P.J.; et al. IL-1β signaling promotes CNS-intrinsic immune control of West Nile virus infection. PLoS Pathog. 2012, 8, e1003039. [Google Scholar] [CrossRef]
- Zidovec-Lepej, S.; Vilibic-Cavlek, T.; Barbic, L.; Ilic, M.; Savic, V.; Tabain, I.; Ferenc, T.; Grgic, I.; Gorenec, L.; Bogdanic, M.; et al. Antiviral Cytokine Response in Neuroinvasive and Non-Neuroinvasive West Nile Virus Infection. Viruses 2021, 13, 342. [Google Scholar] [CrossRef]
- Durrant, D.M.; Robinette, M.L.; Klein, R.S. IL-1R1 is required for dendritic cell-mediated T cell reactivation within the CNS during west nile virus encephalitis. J. Exp. Med. 2013, 210, 503–516. [Google Scholar] [CrossRef] [Green Version]
- Bardina, S.V.; Brown, J.A.; Michlmayr, D.; Hoffman, K.W.; Sum, J.; Pletnev, A.G.; Lira, S.A.; Lim, J.K. Chemokine Receptor Ccr7 Restricts Fatal West Nile Virus Encephalitis. J. Virol. 2017, 91, e02409-16. [Google Scholar] [CrossRef] [Green Version]
- Wynn, T.A. Type 2 cytokines: Mechanisms and therapeutic strategies. Nat. Rev. Immunol. 2015, 15, 271–282. [Google Scholar] [CrossRef]
- Weatherhead, J.E.; Miller, V.E.; Garcia, M.N.; Hasbun, R.; Salazar, L.; Dimachkie, M.M.; Murray, K.O. Long-term neurological outcomes in West Nile virus-infected patients: An observational study. Am. J. Trop. Med. Hyg. 2015, 92, 1006–1012. [Google Scholar] [CrossRef]
- Varatharaj, A.; Galea, I. The blood-brain barrier in systemic inflammation. Brain Behav. Immun. 2017, 60, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Kumar, M.; Verma, S.; Nerurkar, V.R. Pro-inflammatory cytokines derived from West Nile virus (WNV)-infected SK-N-SH cells mediate neuroinflammatory markers and neuronal death. J. Neuroinflamm. 2010, 7, 73. [Google Scholar] [CrossRef] [Green Version]
- Leonard, B.E. Inflammation and depression: A causal or coincidental link to the pathophysiology? Acta Neuropsychiatr. 2018, 30, 1–16. [Google Scholar] [CrossRef] [Green Version]
- Khovidhunkit, W.; Kim, M.S.; Memon, R.A.; Shigenaga, J.K.; Moser, A.H.; Feingold, K.R.; Grunfeld, C. Effects of infection and inflammation on lipid and lipoprotein metabolism: Mechanisms and consequences to the host. J. Lipid Res. 2004, 45, 1169–1196. [Google Scholar] [CrossRef] [Green Version]
- Cunningham, C. Microglia and neurodegeneration: The role of systemic inflammation. Glia 2013, 61, 71–90. [Google Scholar] [CrossRef]
- Patel, H.; Sander, B.; Nelder, M.P. Long-term sequelae of West Nile virus-related illness: A systematic review. Lancet Infect. Dis. 2015, 15, 951–959. [Google Scholar] [CrossRef]
- Leis, A.A.; Grill, M.F.; Goodman, B.P.; Sadiq, S.B.; Sinclair, D.J.; Vig, P.J.S.; Bai, F. Tumor Necrosis Factor-Alpha Signaling May Contribute to Chronic West Nile Virus Post-infectious Proinflammatory State. Front. Med. 2020, 7, 164. [Google Scholar] [CrossRef]
- Janigro, D.; Bailey, D.M.; Lehmann, S.; Badaut, J.; O’Flynn, R.; Hirtz, C.; Marchi, N. Peripheral Blood and Salivary Biomarkers of Blood–Brain Barrier Permeability and Neuronal Damage: Clinical and Applied Concepts. Front. Neurol. 2021, 11, 577312. [Google Scholar] [CrossRef]
- Le, N.D.; Muri, L.; Grandgirard, D.; Kuhle, J.; Leppert, D.; Leib, S.L. Evaluation of neurofilament light chain in the cerebrospinal fluid and blood as a biomarker for neuronal damage in experimental pneumococcal meningitis. J. Neuroinflamm. 2020, 17, 293. [Google Scholar] [CrossRef] [PubMed]
- Grygorczuk, S.; Parczewski, M.; Świerzbińska, R.; Czupryna, P.; Moniuszko, A.; Dunaj, J.; Kondrusik, M.; Pancewicz, S. The increased concentration of macrophage migration inhibitory factor in serum and cerebrospinal fluid of patients with tick-borne encephalitis. J. Neuroinflamm. 2017, 14, 126. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Canet, G.; Dias, C.; Gabelle, A.; Simonin, Y.; Gosselet, F.; Marchi, N.; Makinson, A.; Tuaillon, E.; Van de Perre, P.; Givalois, L.; et al. HIV Neuroinfection and Alzheimer’s Disease: Similarities and Potential Links? Front. Cell. Neurosci. 2018, 12, 307. [Google Scholar] [CrossRef] [PubMed]
- De Chiara, G.; Marcocci, M.E.; Sgarbanti, R.; Civitelli, L.; Ripoli, C.; Piacentini, R.; Garaci, E.; Grassi, C.; Palamara, A.T. Infectious Agents and Neurodegeneration. Mol. Neurobiol. 2012, 46, 614–638. [Google Scholar] [CrossRef] [Green Version]
- Ayub, M.; Jin, H.K.; Bae, J.-S. The blood cerebrospinal fluid barrier orchestrates immunosurveillance, immunoprotection, and immunopathology in the central nervous system. BMB Rep. 2021, 54, 196–202. [Google Scholar] [CrossRef]
- Brown, L.S.; Foster, C.G.; Courtney, J.M.; King, N.E.; Howells, D.W.; Sutherland, B.A. Pericytes and neurovascular function in the healthy and diseased brain. Front. Cell. Neurosci. 2019, 13, 282. [Google Scholar] [CrossRef] [Green Version]
- Neumann, M.; Sampathu, D.M.; Kwong, L.K.; Truax, A.C.; Micsenyi, M.C.; Chou, T.T.; Bruce, J.; Schuck, T.; Grossman, M.; Clark, C.M.; et al. Ubiquitinated TDP-43 in Frontotemporal Lobar Degeneration and Amyotrophic Lateral Sclerosis. Science 2006, 314, 130–133. [Google Scholar] [CrossRef] [Green Version]
- Cairns, N.J.; Bigio, E.H.; Mackenzie, I.R.A.; Neumann, M.; Lee, V.M.Y.; Hatanpaa, K.J.; White, C.L.; Schneider, J.A.; Grinberg, L.T.; Halliday, G.; et al. Neuropathologic diagnostic and nosologic criteria for frontotemporal lobar degeneration: Consensus of the Consortium for Frontotemporal Lobar Degeneration. Acta Neuropathol. 2007, 114, 5–22. [Google Scholar] [CrossRef] [Green Version]
- De Boer, E.M.J.; Orie, V.K.; Williams, T.; Baker, M.R.; De Oliveira, H.M.; Polvikoski, T.; Silsby, M.; Menon, P.; Van Den Bos, M.; Halliday, G.M.; et al. TDP-43 proteinopathies: A new wave of neurodegenerative diseases. J. Neurol. Neurosurg. Psychiatry 2021, 92, 86–95. [Google Scholar] [CrossRef]
- James, B.D.; Wilson, R.S.; Boyle, P.A.; Trojanowski, J.Q.; Bennett, D.A.; Schneider, J.A. TDP-43 stage, mixed pathologies, and clinical Alzheimer’s-type dementia. Brain 2016, 139, 2983–2993. [Google Scholar] [CrossRef] [Green Version]
- Fung, G.; Shi, J.; Deng, H.; Hou, J.; Wang, C.; Hong, A.; Zhang, J.; Jia, W.; Luo, H. Cytoplasmic translocation, aggregation, and cleavage of TDP-43 by enteroviral proteases modulate viral pathogenesis. Cell Death Differ. 2015, 22, 2087–2097. [Google Scholar] [CrossRef] [Green Version]
- Østergaard, C.; Johansen, J.S.; Benfield, T.; Price, P.A.; Lundgren, J.D. YKL-40 is elevated in cerebrospinal fluid from patients with purulent meningitis. Clin. Diagn. Lab. Immunol. 2002, 9, 598–604. [Google Scholar] [CrossRef] [Green Version]
- Craig-Schapiro, R.; Perrin, R.J.; Roe, C.M.; Xiong, C.; Carter, D.; Cairns, N.J.; Mintun, M.A.; Peskind, E.R.; Li, G.; Galasko, D.R.; et al. YKL-40: A novel prognostic fluid biomarker for preclinical Alzheimer’s disease. Biol. Psychiatry 2010, 68, 903–912. [Google Scholar] [CrossRef] [Green Version]
- Hellwig, K.; Kvartsberg, H.; Portelius, E.; Andreasson, U.; Oberstein, T.J.; Lewczuk, P.; Blennow, K.; Kornhuber, J.; Maler, J.M.; Zetterberg, H.; et al. Neurogranin and YKL-40: Independent markers of synaptic degeneration and neuroinflammation in Alzheimer’s disease. Alzheimer’s Res. Ther. 2015, 7, 4–11. [Google Scholar] [CrossRef] [Green Version]
- Czupryna, P.; Kulczyńka-Przybik, A.; Mroczko, B.; Wondim, M.A.; Grygorczuk, S.; Borawski, K.; Pancewicz, S.; Moniuszko-Malinowska, A. Assessment of the YKL-40 concentration in patients with tick-borne encephalitis. Ticks Tick-Borne Dis. 2022, 13, 101895. [Google Scholar] [CrossRef]
- Schoneveld, L.; Ladang, A.; Henket, M.; Frix, A.N.; Cavalier, E.; Guiot, J.; Schoneveld, L.; Ladang, A.; Henket, M.; Frix, A.; et al. YKL-40 as a new promising prognostic marker of severity in COVID infection. Crit. Care 2021, 25, 66. [Google Scholar] [CrossRef]
- Fraisier, C.; Papa, A.; Granjeaud, S.; Hintzen, R.; Martina, B.; Camoin, L.; Almeras, L. Cerebrospinal fluid biomarker candidates associated with human WNV neuroinvasive disease. PLoS ONE 2014, 9, e93637. [Google Scholar] [CrossRef] [Green Version]
- Cicognola, C.; Janelidze, S.; Hertze, J.; Zetterberg, H.; Blennow, K.; Mattsson-Carlgren, N.; Hansson, O. Plasma glial fibrillary acidic protein detects Alzheimer pathology and predicts future conversion to Alzheimer dementia in patients with mild cognitive impairment. Alzheimer’s Res. Ther. 2021, 13, 68. [Google Scholar] [CrossRef] [PubMed]
- Sporer, B.; Missler, U.; Magerkurth, O.; Koedel, U.; Wiesmann, M.; Pfister, H.W. Evaluation of CSF glial fibrillary acidic protein (GFAP) as a putative marker for HIV-associated dementia. Infection 2004, 32, 20–23. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; He, J.J. HIV-1 Tat Induces Unfolded Protein Response and Endoplasmic Reticulum Stress in Astrocytes and Causes Neurotoxicity through Glial Fibrillary Acidic Protein (GFAP) Activation and Aggregation. J. Biol. Chem. 2016, 291, 22819. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mella, C.; Figueroa, C.D.; Otth, C.; Ehrenfeld, P. Involvement of Kallikrein-Related Peptidases in Nervous System Disorders. Front. Cell. Neurosci. 2020, 14, 166. [Google Scholar] [CrossRef] [PubMed]
- Phipps, H.W.; Longo, L.M.; Blaber, S.I.; Blaber, M.; Vanlandingham, J.W. Kallikrein-related peptidase 6: A biomarker for traumatic brain injury in the rat. Brain Inj. 2013, 27, 1698–1706. [Google Scholar] [CrossRef] [PubMed]
- Bando, Y.; Hagiwara, Y.; Suzuki, Y.; Yoshida, K.; Aburakawa, Y.; Kimura, T.; Murakami, C.; Ono, M.; Tanaka, T.; Jiang, Y.P.; et al. Kallikrein 6 secreted by oligodendrocytes regulates the progression of experimental autoimmune encephalomyelitis. Glia 2018, 66, 359–378. [Google Scholar] [CrossRef] [PubMed]
- Patra, K.; Soosaipillai, A.; Sando, S.B.; Lauridsen, C.; Berge, G.; Møller, I.; Grøntvedt, G.R.; Bråthen, G.; Begcevic, I.; Moussaud, S.; et al. Assessment of kallikrein 6 as a cross-sectional and longitudinal biomarker for Alzheimer’s disease. Alzheimer’s Res. Ther. 2018, 10, 9. [Google Scholar] [CrossRef] [Green Version]
- Caucheteux, S.M.; Piguet, V. Kallikrein-6–Regulated Pathways Shed Light on New Potential Targets in Varicella Zoster Virus Infection. J. Investig. Dermatol. 2020, 140, 741–742. [Google Scholar] [CrossRef] [Green Version]
- Kong, L.; Wang, J.; Cheng, J.; Zang, C.; Chen, F.; Wang, W.; Zhao, H.; Wang, Y.; Wang, D. Comprehensive Identification of the Human Secretome as Potential Indicators in Treatment Outcome of HPV-Positive and -Negative Cervical Cancer Patients. Gynecol. Obstet. Investig. 2020, 85, 405–415. [Google Scholar] [CrossRef]
- Hermansson, L.; Yilmaz, A.; Axelsson, M.; Blennow, K.; Fuchs, D.; Hagberg, L.; Lycke, J.; Zetterberg, H.; Gisslén, M. Cerebrospinal fluid levels of glial marker YKL-40 strongly associated with axonal injury in HIV infection. J. Neuroinflamm. 2019, 16, 16. [Google Scholar] [CrossRef]
- Drucker, K.L.; Gianinni, C.; Decker, P.A.; Diamandis, E.P.; Scarisbrick, I.A. Prognostic significance of multiple kallikreins in high-grade astrocytoma. BMC Cancer 2015, 15, 565. [Google Scholar] [CrossRef] [Green Version]
- Morichi, S.; Kashiwagi, Y.; Takekuma, K.; Hoshika, A.; Kawashima, H. Expressions of brain-derived neurotrophic factor (BDNF) in cerebrospinal fluid and plasma of children with meningitis and encephalitis/encephalopathy. Int. J. Neurosci. 2013, 123, 17–23. [Google Scholar] [CrossRef]
- Soung, A.; Klein, R.S. Viral Encephalitis and Neurologic Diseases: Focus on Astrocytes. Trends Mol. Med. 2018, 24, 950–962. [Google Scholar] [CrossRef]
- Colucci-D’amato, L.; Speranza, L.; Volpicelli, F. Neurotrophic factor bdnf, physiological functions and therapeutic potential in depression, neurodegeneration and brain cancer. Int. J. Mol. Sci. 2020, 21, 7777. [Google Scholar] [CrossRef]
- Li, G.; Peskind, E.R.; Millard, S.P.; Chi, P.; Sokal, I.; Yu, C.E.; Bekris, L.M.; Raskind, M.A.; Galasko, D.R.; Montine, T.J. Cerebrospinal fluid concentration of brain-derived neurotrophic factor and cognitive function in non-demented subjects. PLoS ONE 2009, 4, e5424. [Google Scholar] [CrossRef] [Green Version]
- Kim, H.J.; Tsao, J.W.; Stanfill, A.G. The current state of biomarkers of mild traumatic brain injury. JCI Insight 2018, 3, e97105. [Google Scholar] [CrossRef]
- Solár, P.; Zamani, A.; Kubíčková, L.; Dubový, P.; Joukal, M. Choroid plexus and the blood-cerebrospinal fluid barrier in disease. Fluids Barriers CNS 2020, 17, 35. [Google Scholar] [CrossRef]
- Tokunaga, Y.; Kira, R.; Takahata, Y.; Gondo, K.; Mizuno, Y.; Aoki, T.; Hara, T. Neurotrophin-4 and glial cell line-derived neurotrophic factor in cerebrospinal fluid from meningitis/encephalitis patients. Pediatr. Neurol. 2002, 27, 102–105. [Google Scholar] [CrossRef]
- Nosheny, R.L.; Bachis, A.; Aden, S.A.; De Bernardi, M.A.; Mocchetti, I. Intrastriatal administration of human immunodeficiency virus-1 glycoprotein 120 reduces glial cell-line derived neurotrophic factor levels and causes apoptosis in the substantia nigra. J. Neurobiol. 2006, 66, 1311–1321. [Google Scholar] [CrossRef]
- Xiang, Y.; Xin, J.; Le, W.; Yang, Y. Neurogranin: A Potential Biomarker of Neurological and Mental Diseases. Front. Aging Neurosci. 2020, 12, 311. [Google Scholar] [CrossRef]
- Guha, D.; Wagner, M.C.E.; Ayyavoo, V. Human immunodeficiency virus type 1 (HIV-1)-mediated neuroinflammation dysregulates neurogranin and induces synaptodendritic injury. J. Neuroinflamm. 2018, 15, 126. [Google Scholar] [CrossRef] [Green Version]
- Stark, K.; Eckart, A.; Haidari, S.; Tirniceriu, A.; Lorenz, M.; Von Brühl, M.L.; Gärtner, F.; Khandoga, A.G.; Legate, K.R.; Pless, R.; et al. Capillary and arteriolar pericytes attract innate leukocytes exiting through venules and “instruct” them with pattern-recognition and motility programs. Nat. Immunol. 2013, 14, 41–51. [Google Scholar] [CrossRef]
- Wang, W.; Wang, L.; Luo, J.; Xi, Z.; Wang, X.; Chen, G.; Chu, L. Role of a neural cell adhesion molecule found in cerebrospinal fluid as a potential biomarker for epilepsy. Neurochem. Res. 2012, 37, 819–825. [Google Scholar] [CrossRef]
- Laudanski, K.; Yakhkind, A.; Restrepo, M.; Draham, L.; Lang, A.E. Guillain–Barré Syndrome in COVID-19—The Potential Role of NCAM-1 and Immunotherapy. BioMed 2021, 1, 80–92. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Constant, O.; Barthelemy, J.; Nagy, A.; Salinas, S.; Simonin, Y. West Nile Virus Neuroinfection in Humans: Peripheral Biomarkers of Neuroinflammation and Neuronal Damage. Viruses 2022, 14, 756. https://doi.org/10.3390/v14040756
Constant O, Barthelemy J, Nagy A, Salinas S, Simonin Y. West Nile Virus Neuroinfection in Humans: Peripheral Biomarkers of Neuroinflammation and Neuronal Damage. Viruses. 2022; 14(4):756. https://doi.org/10.3390/v14040756
Chicago/Turabian StyleConstant, Orianne, Jonathan Barthelemy, Anna Nagy, Sara Salinas, and Yannick Simonin. 2022. "West Nile Virus Neuroinfection in Humans: Peripheral Biomarkers of Neuroinflammation and Neuronal Damage" Viruses 14, no. 4: 756. https://doi.org/10.3390/v14040756
APA StyleConstant, O., Barthelemy, J., Nagy, A., Salinas, S., & Simonin, Y. (2022). West Nile Virus Neuroinfection in Humans: Peripheral Biomarkers of Neuroinflammation and Neuronal Damage. Viruses, 14(4), 756. https://doi.org/10.3390/v14040756






