Abstract
Feline infectious peritonitis (FIP) is a systemic immune-mediated inflammatory perivasculitis that occurs in a minority of cats infected with feline coronavirus (FCoV). Various therapies have been employed to treat this condition, which was previously usually fatal, though no parameters for differentiating FIP recovery from remission have been defined to enable clinicians to decide when it is safe to discontinue treatment. This retrospective observational study shows that a consistent reduction of the acute phase protein alpha-1 acid glycoprotein (AGP) to within normal limits (WNL, i.e., 500 μg/mL or below), as opposed to duration of survival, distinguishes recovery from remission. Forty-two cats were diagnosed with FIP: 75% (12/16) of effusive and 54% (14/26) of non-effusive FIP cases recovered. Presenting with the effusive or non-effusive form did not affect whether or not a cat fully recovered (p = 0.2). AGP consistently reduced to WNL in 26 recovered cats but remained elevated in 16 cats in remission, dipping to normal once in two of the latter. Anaemia was present in 77% (23/30) of the cats and resolved more quickly than AGP in six recovered cats. The presence of anaemia did not affect the cat’s chances of recovery (p = 0.1). Lymphopenia was observed in 43% (16/37) of the cats and reversed in nine recovered cats but did not reverse in seven lymphopenic cats in the remission group. Fewer recovered cats (9/24: 37%) than remission cats (7/13: 54%) were lymphopenic, but the difference was not statistically different (p = 0.5). Hyperglobulinaemia was slower than AGP to return to WNL in the recovered cats. FCoV antibody titre was high in all 42 cats at the outset. It decreased significantly in 7 recovered cats but too slowly to be a useful parameter to determine discontinuation of antiviral treatments. Conclusion: a sustained return to normal levels of AGP was the most rapid and consistent indicator for differentiating recovery from remission following treatment for FIP. This study provides a useful model for differentiating recovery from chronic coronavirus disease using acute phase protein monitoring.
1. Introduction
Feline coronavirus (FCoV) is a positive strand RNA virus belonging to the order Nidovirales, genus Coronavirus, and family Coronaviridae, subfamily alphacoronavirus. The FCoV species is further divided into two types: I and II, the first type being wholly feline, and type II FCoV arising from recombination events with canine coronavirus [1,2].
Feline infectious peritonitis (FIP) is an immune-mediated perivascular pyogranulomatous [3] disease which affects 5–10% of cats infected with FCoV [4]. FIP can present acutely, with effusions in one or more body cavity, or as a disease of chronic inflammation, with cachexia and variable organ damage depending on the sites of pyogranuloma formation.
The initial target for FCoV is the epithelial cells of the small intestine [5]; from there, macrophages engulf the virus and transport it to the mesenteric lymph nodes (MLN), and a brief systemic phase of infection follows when the monocyte is the target cell.
FCoV is highly prevalent in the cat population, but FIP is relatively rare. There are two theories for why FIP occurs; the first is the internal mutation theory, postulating that some mutation is required in an otherwise benign virus to permit replication in monocytes [6]. Spike gene mutations known as M1058L or S1060A were considered to be virulence markers and evidence for FIP diagnosis [7,8], though it has been argued that these mutations are only indicative of the systemic spread of the virus [9], and current expert opinion is that they are not useful diagnostic tests [10,11,12,13]. The second theory is the circulating virulent–avirulent theory which states that distinct benign and virulent, FIP-causing strains circulate in the feline population [14,15]. Whichever theory is true, the rôle of virus load is critical but often overlooked. Pedersen & Black found that kittens infected with a small amount of virulent laboratory strains of FCoV did not develop FIP. At medium doses, FIP was sporadic, but higher doses were almost always lethal [16]. This pattern of sporadic deaths and occasional FIP outbreaks reflects what is seen in real life, where outbreaks of FIP occur in environments where a high environmental virus burden would be expected, such as in rescue or breeding catteries [2,15,17,18,19]. The stress of entering a rescue cattery increases virus load in some individual cats [20], and stress is reported to precede the onset of FIP [21,22].
Progression to FIP is primarily driven by viral replication [23]. The ability of the virus to replicate to high titres in monocytes depends upon the host rather than the virus strain. Some host monocytes do not permit viral replication regardless of how virulent is the strain of FCoV [24].
In FIP, coronavirus-infected monocytes/macrophages release matrix metalloproteinase-9 (MMP-9) [3]. MMP-9 attacks the collagen IV in the vascular basal lamina allowing extravasation of FCoV-infected monocytes (which differentiate into macrophages) [3] and leakage of protein-rich plasma (i.e., a modified transudate) into body cavities such as the peritoneal or pleural cavities, pericardial sac [13,15,25], or scrotal sac [26] in effusive (or wet) FIP. Plasma leakage into the brain’s ventricles leads to hydrocephalus [27,28]. B cells gradually replace macrophages in more chronic perivascular pyogranulomata [29] typical of non-effusive FIP. FCoV-infected macrophages release a storm of cytokines and chemokines, including tumor necrosis factor-alpha (TNF-α) [3,30,31]. and interleukin-6 (IL-6) [32].
TNF-α is a major contributor to the inflammatory response and pathogenesis of FIP. FCoV-infected cells release a substance that causes apoptosis in nearby lymphocytes [33]: the mystery substance is probably TNF-α [30]: around 50% of cats with FIP are lymphopenic [22,34,35]. In an experimental infection of specific pathogen-free cats, lymphopenia began around 2–4 weeks post-infection and correlated with the disease course: the earlier post-infection that lymphopenia occurred, the more rapid the progression of the disease [36].
Cats with FIP typically have the non-regenerative normocytic normochromic anaemia of chronic disease [13]. A study of 51 cats with FIP found that a decreasing red blood cell count was a significant prognostic indicator: a packed cell volume of under 20% (along with other prognostic indicators such as elevated bilirubin and aspartate aminotransferase, low potassium, and sodium) heralded a survival of less than three days [34].
IL-6 stimulates hepatocytes to release acute phase proteins [32], such as alpha-1 acid glycoprotein (AGP) and serum amyloid A (SAA). AGP levels rise in all acute viral and bacterial infections [37,38], Mycoplasma hemofelis infection [39], and after trauma. AGP was first reported to be elevated in FIP cases in 1997 [40], but it also rises transiently post FCoV-infection even in cats who do not develop FIP [41,42]. Raised AGP is superior to biopsy histopathology [43], SAA, and haptoglobin [44] in differentiating FIP from similarly-presenting cases. In a cat with an effusion, AGP levels above 1550 μg/mL in cats were 93% specific for FIP [44]. In non-effusive FIP, AGP levels tend to be above normal, but often not markedly so [Addie, personal observation], presumably because non-effusive FIP is a more chronic presentation. A normal AGP level usually rules out FIP [11]. Monitoring AGP is an accurate predictor of survival in humans with sepsis [45].
Many treatments, including prednisolone, feline interferon omega (rFeIFN omega) [46], polyprenyl immunostimulant [47], meloxicam [48], and most recently specific antivirals [49,50,51,52,53] have been used. Those treatments involved in the present study are detailed in Table 1 and Table 2.
There are three possible outcomes following the diagnosis of FIP and treatment: death due to FIP-related causes; total recovery; or remission, defined as an intermediate stage between cure and death and carrying the specter of relapse. This latter state is a source of considerable stress for cat guardians, so it would bring great reassurance to people to know that their cats are cured of FIP rather than being in remission.
Veterinary surgeons seek a criterion to be assured that the cat has recovered to know when to cease treatment. Markers for poor prognosis in FIP have been well defined [34], yet no guidelines have been established to define recovery from FIP, probably because, until recently, it was widely assumed that recovery from FIP could not occur.
Prior to the advent of antivirals specific to coronavirus, the average survival time of cats with effusive FIP was 21 days [34], while cats with non-effusive FIP tended to survive longer, on average 38 days [34]. No survival time point after which a cat can be said to have recovered from FIP has been established. In their report of their seminal study of successful treatment of FIP with an antiviral drug, Pedersen et al. wrote: “It raises the question of how long remission must be sustained to declare the disease cured, rather than in a sustained remission.” [49] The purpose of this paper was to attempt to answer that question, but as opposed to duration of survival, we found that a return of elevated AGP to normal levels is a rapid and definitive marker for recovery from FIP.
2. Materials and Methods
2.1. Selection of Cases
This study retrospectively evaluated the medical records of 42 cats diagnosed with FIP between January 2004 and March 2021. Cats were selected for this study if sequential laboratory test results were available, including AGP measurements, a reasonable certainty that the FIP diagnosis was correct, and whose survival outcomes were known.
2.2. FIP Diagnosis
Diagnosis of FIP was by histopathology, immunohistochemistry (IHC); detection of FCoV RNA as demonstrated by RT-PCR in effusions, [54,55,56] or mesenteric lymph node (MLN) FNA [57]; messenger RNA (mRNA) [58,59] or three prime–untranslated region (3′-UTR) RNA [60,61] in peripheral blood mononuclear cells (PBMC); FCoV spike gene mutations M1058L, S1060A [7,8]; and FIP profile based on an algorithm consisting of history, clinical signs and clinicopathological abnormalities considered consistent with FIP, including high FCoV antibody titre, raised AGP, hyperglobulinaemia, anaemia, and lymphopenia [62,63].
2.3. Treatment
Treatment choice was at the discretion of the cat’s attending veterinary surgeon or, more recently, the cat’s guardian, with two exceptions. Previously, prednisolone was considered an integral part of FIP treatment [64], but systemic corticosteroids were subsequently found to decrease survival time in cats that were being treated concurrently with polyprenyl immunostimulant (PI, VetImmune, Sass & Sass, Inc., Oak Ridge, TN, USA) [47]. Consequently, in 2017 one author (DDA) recommended stopping (or not commencing) the use of corticosteroids and using meloxicam instead (after a suitable wash out period and provided blood pressure and kidney function were normal). The second exception was that clients whose cats were treated with adenosine nucleoside analogue drugs were recommended to supplement with S-adenosyl-L-methionine (SAMe) and silybin phosphatidycholine complex, (Denamarin, Nutramax Laboratories, Inc., Lancaster, SC, USA) for liver support.
2.4. Outcomes: Death, Recovery, or Remission
Recovery was defined as the resolution of all the clinical signs attributable to FIP and reversal of anaemia, lymphopenia, and hyperglobulinaemia, if present. AGP was not included in the decision to classify a cat as recovered or only in remission because the purpose of this study was to assess whether or not AGP reduction was an indicator of clinical recovery. Clinical scores are shown in Table 3 and Table 4.
2.5. AGP
AGP was measured by enzyme-linked immunosorbent assay (ELISA) (Avacta Animal Health, Wetherby, Yorkshire, UK) at the University of Glasgow Veterinary Diagnostic Services (VDS) in all cases except seven: the samples of Basil 1; Amy; Brook; Bugsy; Daisy; Roxanne and Pip were measured by radial immunodiffusion as previously described [40].
2.6. Statistics
Due to the small sample size, Fisher’s exact test was used with significance set at <0.05.
3. Results
3.1. FIP Diagnosis
The laboratory records of effusive (n = 16) and non-effusive (n = 26) FIP cases were reviewed. As shown in Table 1 and Table 2, the diagnosis of FIP was by histopathology (n = 13), of which two cases had positive immunohistochemistry (IHC). However, the histopathology of biopsies was inconclusive in four cases (Elmo, Harry, Pharaoh, Ragamuffin) and was reported non-specifically as “pyogranulomatous inflammation”.
FIP diagnosis was by detection of FCoV RNA as demonstrated by RT-PCR in effusions [54,55,56,60] (n = 10), or mesenteric lymph node (MLN) FNA [57] (n = 10); messenger RNA (mRNA) [58,59] or 3′-UTR [60,61] in PBMC (n = 4). One of two cats in which the mutation tests [7,8] were performed was positive for the M1058L mutation, and neither cat was positive for the S1060A mutation. Some cats fulfilled multiple diagnostic criteria for FIP: seven cats had both positive FCoV RT-PCR tests and histopathology. All 42 cats were positive for at least some components of an FIP profile consisting of typical clinical signs, FCoV antibody titre, raised AGP, hyperglobulinaemia, anaemia, and lymphopenia (Table 3 and Table 4). In 11 cats, the FIP profile was the sole evidence for its diagnosis, although a positive response to an anti-coronavirus drug was thought to corroborate the FIP diagnosis. AGP was raised in all cats except two, where a pre-treatment test was not done.

Table 1.
How FIP was diagnosed, survival time, and treatment details of 26 cats who recovered.
Table 1.
How FIP was diagnosed, survival time, and treatment details of 26 cats who recovered.
| Cat | FIP Presentation | How FIP Diagnosed | Survival in Years or Months | Time to Normal AGP | Treatments | Prednisolone/Corticosteroids | |
|---|---|---|---|---|---|---|---|
| 1 | Basil 1 | Non-effusive (icterus) | mRNA RT-PCR on PBMC positive thrice. | 11y † (Died of chronic kidney disease aged 15y.) | <22d | 5 × 104 units of rFeIFN-ω (Virbagen Omega, Virbac, Nice, France) per os q24h for 13m until FCoV antibody titre reduced from >1280 to <1:10. | 2 mg/kg q24h for 7d then 1 mg/kg for 7d |
| 2 | Boris | Non-effusive but initially effusive FIP suspected | RT-qPCR on MLN FNA CT 33.7. RT-qPCR on ascites negative. | >2.2y | <3m | 1 MU units rFeIFN-ω per os q24h. PI 3 mg/kg per os q72h. Weekly cobalamin injections. Effusion was negative on RT-PCR and was found to be due to cardiomyopathy. | No |
| 3 | Mars | Non-effusive | RT-qPCR on MLN FNA CT 30. | >5.5y | <6m | PI 3 mg/kg twice per week. | No |
| 4 | Chester | Effusive (pleural effusion) | RT-qPCR on pleural effusion CT 34. | >3.1y | <8m | 1 MU/kg rFeIFN-ω s/c q48h reduced to q4d then 1 × 105 units per os q24h for 28m. Meloxicam. SAMe (Denamarin®, Nutramax Laboratories, Inc., Lancaster, SC, USA) Cobalamin (Cobalaplex, Protexin® Veterinary, Somerset, UK). Tramadol hydrochloride (Bova Specials, London, UK) 10 mg/cat q24h per os. [48] Liquorice tea was attempted but cat did not like it. | 1 mg/kg q12h for 2w, weaned off for another 3w and replaced by meloxicam |
| 5 | Amy | Non-effusive | FIP profile. | >1.3y | <4m | 1 MU units rFeIFN-ω s/c q48h. | Sliding doses |
| 6 | Brook | Non-effusive | FIP profile. | >1.1y | <41d | Unknown. | Unknown |
| 7 | Basil 2 | Effusive (ascites) | RT-qPCR on ascites positive. | >3.0y | <10w | 1 MU/kg rFeIFN-ω s/c q24h for 36d, reducing to every 3d, followed by 1 × 105 units of rFeIFN-ω per os q24h for 2y. PI at 3 mg/kg q48h during 10d. Mirtazapine (Summit Veterinary Pharmaceuticals, Kidlington, UK.) Ursodeoxychloic acid. Cobalamin injections weekly. Itraconazole 10 mg/kg from days 4–87. One Darbepoetin injection. GC-376 s/c Days 17–100. Doxycycline 10 mg/kg bid per os from d.32 for 30d (to treat haemotropic mycoplasmosis). | Only for 3d: meloxicam used in preference from Day 4 |
| 8 | Kitten 2 | Effusive (ascites) then non-effusive | FIP Profile. | >2y | <6m | 57d of Mutian X (Xraphconn®, Mutian Biotechnology Co., Ltd., Nantong, China) at 80 mg/kg. Following her neurological relapse, she was re-treated with 160 mg/kg for 2 m then 1 × 105 units of rFeIFN-ω per os q24h. | No |
| 9 | Skywise | Non-effusive | RT-qPCR on MLN FNA positive for mutation M1058L, negative for S1060A. | >2.0y | <35d | 50d Mutian X starting 160 mg/kg q24h per os in divided doses, reduced to 120 mg/kg on Day 25; followed by 1 × 105 units of rFeIFN-ω per os q24h. Cobalamin (Cobalaplex). | 6d per os, then in eye drops for 14d |
| 10 | Betsy | Effusive (ascites) | RT-qPCR on ascites CT 25.6. | >1.5y | <39d | 38d Mutian X 80 mg/kg per os for 31d and 160 mg/kg for 7d followed by 1 × 105 units of rFeIFN-ω per os q24h for 1y. Doxycycline. | 5 mg/cat q24h for 10d replaced by meloxicam |
| 11 | Dante | Colonic FIP | Histopath of biopsies positive. | >7m | <28d | 84d of Mutian X 80 mg/kg per os. Protexin pro-kolin enterogenic probiotics 2 mL per os q12h. Cobalamin (Cobalaplex). | No: gabapentin 25 mg q8h |
| 12 | Elmo | Non-effusive | Biopsy histopath reported pyogranuloma. | >17m | <31d | 54d Mutian X (160 mg/kg for 37d, then 80 mg/kg) followed by 1 × 105 units of rFeIFN-ω per os q24h. | One injection and one 5 mg pill given once only |
| 13 | Lyra | Effusive (ascites) | RT-qPCR on ascites: low positive CT 33. | >13m | <20d | 47d Mutian X 80 mg/kg q24h (divided doses) per os followed by 1 × 105 units of rFeIFN-ω per os q24h for 5m. | No: meloxicam given instead |
| 14 | Molly | Non-effusive | FIP profile. RT-PCR positive feces over 24m. | >3.3y | <16m | 1 × 105 units of rFeIFN-ω per os q24h, gabapentin, cefovecin (Convenia, Zoetis, Surrey, UK), Synbiotic D-C probiotics (Protexin). | Sliding doses (unspecified) then meloxicam |
| 15 | Bea | Effusive (ascites) | RT-PCR on ascites positive. Histopath biopsy positive. | 13m † (died of cancer, aged 8y) | <69d | 94d of Mutian X: 40 mg/kg (i.e., half dose) for 4d, then 80 mg/kg for 90d, except for one week of double dose, followed by 1 × 105 units of rFeIFN-ω per os q24h for 6 m. Robenacoxib (Onsior, Elanco Animal Health, Hampshire, UK) given once only. | No |
| 16 | Buddie | Effusive (ascites) | RT-PCR on ascites: CT 33. Histopath of MLN, spleen, liver biopsies positive. | >14m | <51d | 69d of Mutian X 80 mg/kg with 160 mg/kg in the 3rd week of treatment; followed by 1 × 105 units of rFeIFN-ω per os q24h for 6m. Cobalamin (Cobalaplex); SAMe (Denamarin). | One dexamethasone injection only |
| 17 | Nelson | Non-effusive becoming effusive | IHC of MLN biopsy positive. | >6m | <36d | 52d of Mutian X: 7d at 160 mg/kg, reduced to 120 mg/kg then 80 mg/kg; followed by 1 × 105 units of rFeIFN-ω per os q24h. Also cobalamin (Cobalaplex); amoxycillin and clavulanic acid (Synulox, Zoetis, Surrey, UK), mirtazapine; cobalamin (Cobalaplex); SAMe (Denamarin). | No: meloxicam instead |
| 18 | Wish | Non-effusive | IHC of MLN biopsy positive, RT-PCR positive (negative for mutations). | >12m | <13d but no initial test, so no proof it was ever raised | 29d of Mutian X: 7d at 160 mg/kg and 22d at 80 mg/kg; followed by 1 × 105 units of rFeIFN-ω per os q24h. Cobalamin (Cobalaplex); Denamarin. | For 7d only |
| 19 | Mike | Non-effusive (colonic) | Biopsy histopath positive. | >2.5y | unknown: AGP tested only once after 1 y of treatment | 1 × 105 units rFeIFN-ω per os q24h for 5m, meloxicam and PI, followed by 12 w of GS-441524 injections (DC Chemicals, USA), followed by 1 × 105 units of rFeIFN-ω per os q24h, and PI. | No |
| 20. | Chynah | Effusive (pleural effusion) | RT-PCR on pleural effusion CT 33. | >7m | remained raised > 3m | rFeIFN-ω 1 MU/kg s/c q48h, reducing to twice weekly for 5m. Repeated drainage of effusion. | 2.5 mg/cat q24h sliding doses |
| 21 | Tabitha | Non-effusive | FIP profile. | >12m | <29d | 58d of Mutian X starting at 160 mg/kg for 8d, then 120 mg/kg for 20d then 80 mg/kg; followed by 1 × 105 units of rFeIFN-ω per os q24h. Protexin pro-kolin enterogenic probiotics. | No: meloxicam |
| 22 | Harry | Small amount of ascites and enlarged MLN | FCoV RT-PCR positive. Cytology and IHC MLN biopsy inconclusive. | >8m | <30d | 50d of Mutian X: 43d at 80 mg/kg then 7d at 160 mg/kg; followed by 1 × 105 units of rFeIFN-ω per os q24h. Cobalamin, SAMe. | 5.0 mg /cat q12h for 5d |
| 23 | Mr Twinkles | Effusive (ascites) | FIP profile. | >8m | <117d | 7 mg/kg Spark [53] injection s/c for 10d, followed by 4 Spark 5 mg tablets (i.e., 7 mg/kg) per os q24h for 84d. Cobalamin (Cobalaplex). Denamarin. | No |
| 24 | Tyra | Non-effusive (uveitis) | FIP profile. | >10m | <68d | Pine and Lucky 9 [53] injections (12–14 mg/kg): Pine for 4d, Lucky injections for 68d. Then 9 Lucky red pills per os q24h for 13d. | No |
| 25 | Munchie | Effusive (ascites) | FIP profile. | >1.5y | <108d | Spark 8 mg/kg per os q24h for 84d. | No |
| 26 | Edward | Effusive (pleural effusion) | FIP profile incuding cytology. | 10m († RTA) | <7m | rFeIFN-ω 1 MU/kg s/c q48h, meloxicam per os q24h. | No |
> indicates that survival was over this period † indicates death. AGP—alpha-1 acid glycoprotein. CT—Cycle threshold. FCoV—feline coronavirus. FNA—fine needle aspirate. GC376—a 3c-like protease inhibitor antiviral drug [49]. GS-441524—a nucleoside analogue antiviral drug [50]. d—days: h—hours: m—months: w—weeks: y—years IHC—immunohistochemistry. MLN—mesenteric lymph node. mRNA—messenger RNA. MU—million units. Mutian X—Mutian Xraphconn® (Mutian Biotechnology Co., Ltd., Nantong, China), an adenosine nucleoside analog [52,65]. PBMC—peripheral blood mononuclear cells. PI—polyprenyl immunostimulant (PI, VetImmune, Sass & Sass, Oak Ridge, USA). Pos—positive. q—indicating how often, i.e., q24h is daily; q12h indicates twice a day, etc. rFeIFN-ω—recombinant feline interferon omega (Virbac, Nice, France). RTA—road traffic accident. RT-PCR—reverse transcriptase polymerase chain reaction. RT-qPCR—reverse transcriptase quantitative polymerase chain reaction. SAMe—S-adenosyl-L-methionine s/c—subcutaneously.
This table shows the clinical presentation of FIP (effusive, non-effusive, colonic, etc.) and what evidence there was for FIP diagnosis (CT results given when available). Twenty-three of the 26 recovered cats are alive and well at the time of writing; therefore, the over sign (>) precedes their survival duration. The time from the onset of treatment to the first normal AGP test is given in the 6th column. The exact interval from FIP treatment onset to normal AGP level could not be accurately determined because it depended on the frequency of the cat being blood tested. We were only able to determine that it was less than (represented by the < sign) however many days from the start of treatment to the first AGP test WNL. The 7th column lists the treatments given, and the 8th column lists whether corticosteroids were used or not.

Table 2.
How FIP was diagnosed, survival time, and treatment details of 16 cats who went into remission or died.
Table 2.
How FIP was diagnosed, survival time, and treatment details of 16 cats who went into remission or died.
| Cat | FIP Presentation | How FIP Diagnosed | Survival in Months | Treatments | Prednisolone/Corticosteroids | |
|---|---|---|---|---|---|---|
| 1 | Yrael | Effusive | RT-qPCR on effusion CT 32. | 1.5 † | Coconut oil and draining the effusion. | Unknown |
| 2 | Charlie Chaplin | Non-effusive | RT-qPCR on MLN FNA CT 25. Histopath positive. | 1.5 † | 1 × 105 rFeIFN-ω per os q24h. Clindamycin for toxoplasmosis co-infection. | ✓ |
| 3 | Smokey | Non-effusive | RT-qPCR on MLN FNA CT 28. Histopath positive. | 1.8 † | Adipose stem cell therapy. | Unknown |
| 4 | Rowley | Effusive (ascites), then non-effusive (uveitis, finally severe haemolytic anaemia) | RT-qPCR on ascites CT 28.4. Histopath positive. | 2.25 † | 1 MU/kg rFeIFN-ω s/c q48h resulted in resolution of his ascites then 1 × 105 units per os q24h. Mirtazapine 3 times. Anti-TNF-alpha monoclonal antibody infliximab, (Remicade®, MSD, London, UK) 4 mg/kg in a 0.9% saline infusion over a 4h period. Type B blood transfusion. | ✓ 5 mg q12h, dose not reduced |
| 5 | Claude | Effusive (pleural effusion initially then also ascites) | Partial FIP profile. | 3.0 † | 1 MU/kg rFeIFN-ω s/c q48h. Thoracentesis. | ✓ 1 mg/kg sliding doses |
| 6 | Alfie | Non-effusive (chronic diarrhoea, enlarged MLN) | RT-qPCR on MLN FNA CT 31, on feces CT 29. Histopath positive. | 3.5 † | rFeIFN-ω given per os, but dose not recorded. Fortiflora probiotics (Purina). | ✓ dose not recorded |
| 7 | Holly | Non-effusive to effusive ascites | mRNA and 3′UTR RT-PCR on ascites positive. | 4 † | 5 × 104 rFeIFN-ω per os. Colloidal silver (dose unknown). | Unknown |
| 8 | Bugsy | Non-effusive | FIP Profile. | 6 † | 1 MU/kg rFeIFN-ω s/c q48h then 1 × 105 units per os q24h. | ✓5 mg eod |
| 9 | Daisy | Non-effusive | RT-qPCR on MLN FNA CT 27. | 5 † | 1 MU/kg rFeIFN-ω s/c q48h then once a week, then 5 × 104 units rFeIFN-ω per os q24h. | ✓ higher dose reduced to 0.5 mg/kg q24h after 14d |
| 10 | Levi | Effusive (ascites) | RT-qPCR on ascites CT 33. | 5.5 † | 1 MU/kg rFeIFN-ω s/c twice a week. Ascites drained. | ✓dose not recorded |
| 11 | Roxanne | Non-effusive and FGS | RT-qPCR on PBMC CT 42 (very low positive), on faeces CT 23. | 11 † | 1 MU/kg rFeIFN-ω s/c twice weekly. Clindamycin (Antirobe, Zoetis, Surrey, UK), meloxicam. | No: meloxicam |
| 12 | Pip | Non-effusive | RT-qPCR on PBMC CT 40 (very low positive) twice. | >12 | 5 × 104 units rFeIFN-ω per os q24h. | ✓10 mg/cat pred q24h reducing to 5 mg q24h |
| 13 | Pharaoh | Non-effusive | RT-PCR on MLN biopsy CT 27. MLN biopsy inconclusive. | 8 † | Adipose stem cell therapy. | Unknown |
| 14 | Maximus | Non-effusive | RT-PCR on MLN FNA positive. | 14 † | 1 MU /kg rFeIFN-ω s/c q48h | 5 mg/cat q24h |
| 15 | Ragamuffin | Non-effusive | Biopsy MLN & intestine inconclusive. | >60 | 1 MU/kg rFeIFN-ω s/c q48h then 1 × 105 units per os q24h then 1 MU/kg rFeIFN-ω twice a week | ✓Sliding doses |
| 16 | Tinkerbell | Non-effusive | FIP Profile. RT-qPCR on feces CT 31–34. | >36 | 1 MU/kg rFeIFN-ω s/c q48h then 1 × 105 units per os q24h. PI. Mirtazapine 2 mg/cat q48h. | ✓For over 1y at 2 mg/kg q24h |
✓—present. > indicates that survival was over this period † indicates death. 3′-UTR—three prime untranslated regions of the FCoV gene. AGP—alpha-1 acid glycoprotein. CT—Cycle threshold. FCoV—feline coronavirus. FGS—feline chronic gingivostomatitis. FNA—fine needle aspirate. h—hours: m—months: y—years. IHC—immunohistochemistry. MLN—mesenteric lymph node. mRNA—messenger RNA. MU—million units. PI—polyprenyl immunostimulant (VetImmune, Sass & Sass, Oak Ridge, USA). PBMC—peripheral blood mononuclear cells. Pos—positive. q—indicating how often, i.e., q24h is daily; q12h indicates twice a day, etc. rFeIFN-ω—recombinant feline interferon omega (Virbac, Nice, France). RT-PCR—reverse transcriptase polymerase chain reaction. RT-qPCR—reverse transcriptase quantitative polymerase chain reaction. SAMe—S-adenosyl-L-methionine. s/c—subcutaneously. This table of 16 cats that only experienced remission shows the clinical presentation of FIP (effusive, non-effusive, colonic, etc.) in the 3rd column; the 4th column gives the evidence for an FIP diagnosis. The 5th column shows the cats’ survival in months: three were lost to follow up. The 6th column lists the treatments given, and the 7th column lists whether corticosteroids were used or not. Their AGP results are shown in Figure 2.

Table 3.
FIP score before and after treatment in the recovered group.
Table 3.
FIP score before and after treatment in the recovered group.
| Cat | Clinical Signs | FCoV Antibody Titre (Interval First to Last) | Anaemia i.e., Hct <30% | Lymphopenia <1.5 × 109/L | Hyperglobulinaemia i.e., >45 g/L | Score | ||
|---|---|---|---|---|---|---|---|---|
| Before | After | |||||||
| 1 | Basil 1 | Icterus | 1280/10 (13m) | ✓/✗ (22d) | ✓/✗ | ✓/✗ (63–43) | 5 | 0 |
| 2 | Boris | Poor appetite, diarrhoea | >1280/160 (4m) | ✗ | ✗ | ✗ (34–40) | 2 | 1 |
| 3 | Mars | Anorexia | >1280/320 (18m) | ✗ | ✓/✗ | ✓/✗ (69–42) | 4 | 1 |
| 4 | Chester | Pleural effusion, pyrexia | >1280/1280 (28m) | ✗ * | ✗ | NA/✗ (NA–43) | 2 | 1 |
| 5 | Amy | Weight loss, pyrexia, tender abdomen. Icteric plasma. | 1280/640 (16m) | ✗ | ✓/✗ | ✓/✗ (55–38) | 4 | 1 |
| 6 | Brook | Recurring pyrexia | 1280/0 (13m) | ✓/✗ (33d) | ✓/✗ | ✓/✓ (57–51) | 5 | 1 |
| 7 | Basil 2 | Ascites | >1280/>1280 (27m) | ✓/✗ ** | ✓/✗ | ✓/✗ (63–42) | 5 | 1 |
| 8 | Kitten 2 | Ascites. Relapse: painful tail, ataxia, seizures. Full recovery. | >1280/640 (8m) | ✗ | ✗ | ✓/✗ (58–39) | 5 | 1 |
| 9 | Skywise | Weight loss, uveitis | >10,240/640 (9m) | ✓/✗ (12d) | ✓/✗ | ✓/✗ (91–47) | 5 | 0 |
| 10 | Betsy | Ascites, profound anaemia (Hct 14%), underweight: BCS 2/9. | >1280/>1280 (2m) | ✓/✗ (20d) | ✓/✗ | ✗ (41–44) | 4 | 1 |
| 11 | Dante | Vomiting and diarrhoea | 1280/>1280 (2m) | ✗/✗ | ✗ | ✓/✓ (68–53) | 3 | 1 |
| 12 | Elmo | Quiet purr, poor appetite, listless. Pyrexia, mesenteric lymph node enlargement, anaemia. | >10,240/0 (8m) | ✓/✗ (31d) | ✗ | ✓/✗ (72–42) | 4 | 0 |
| 13 | Lyra | Ascites | >10,240/2560 (8m) | ✓/✗ (47d) | ✓/✗ | ✓/✗ (69–35) | 5 | 0 |
| 14 | Molly | Uveitis, chronic diarrhoea: carrier cat shedding virus in faeces >23m | >1280/1280 (15m) | ✗ | ✗/✗ (low) | ✓/✗ (57–56) | 3 | 2 |
| 15 | Bea | Ascites, sudden weakness in limbs. Trichobezoar caused vomiting. | >1280/640 (11m) | ✓/✗ (26d) | ✗ | ✓/✓ (78–53) | 4 | 1 |
| 16 | Buddie | Ascites | >1280/1280 (7m) | ✓/✗ (26d) | 2 of 7 samples | ✓/✓ (106–74) | 4 | 1 |
| 17 | Nelson | Dull, reduced appetite, enlarged MLN, ascites after biopsy. | 640/>1280 (6m) | ✗ | ✗ | ✓/✗ (79–33) | 3 | 1 |
| 18 | Wish | Enlarged MLN, chronic diarrhoea | >1280/not re-tested yet | ✗ | ✓/✗ | ✓/✓ (85–51) | 4 | 2 |
| 19 | Mike | Chronic diarrhea, haematochezia, continuous virus shedding two years later although cat is well. | >1280/not re-tested | ✓/✗ (154d) | ✓/✗ | ✓/✗ (105–45) | 5 | 2 |
| 20 | Chynah | Cough, pneumonia, nasal discharge, dyspnoea, pleural effusion. | 1280/>1280 (8m) | NA | NA | ✓/✓ (64–47) | 3 | 2 |
| 21 | Tabitha | Underweight, pyrexic, watery diarrhoea, ataxic episode, possible uveitis. | >1280/not re-tested | ✗ * | ✗ (but initial count low at 1.89) | ✓/✓ (88–59) | 3 | 2 |
| 22 | Harry | Small amount of ascites and enlarged MLN | >1280/>1280 (2m) | ✓/✗ (30d) | ✓/✗ | ✓/✓ (99–54) | 5 | 2 |
| 23 | Mr Twinkles | Ascites | >1280/1280 (6m) | ✓/✗ (57d) | ✓/✗ | ✓/✓ (58–51) | 5 | 2 |
| 24 | Tyra | Uveitis | >1280/320 (5m) | ✗ | ✗ | ✓/✓ (58–54) | 3 | 1 |
| 25 | Munchie | Ascites, pyrexia, lethargy | >1280/>1280 (4m) | ✗ | ✗ | ✓/✗ (51–38) | 3 | 1 |
| 26 | Edward | Pleural effusion | >1280/>1280 (7m) | ✗ but 1st count 2m into treatment | ✗ but 1st count 2m into treatment | ✓/✗ (59–44) | 3 | 1 |
| Summary | 7 of 26 FCoV antibody titres reduced significantly (at least 3-fold) | 12 of 25 cats anaemic: anaemia resolved in all | 9 of 24 cats lymphopenic on at least one test | 22/25 cats had raised globulins, one NA. Reduced in all 22 and to WNL in 15 cats. | ||||
✓—present ✗—absent <—under >—over. Hct—haematocrit. d—days m—months. NA—not available. WNL—within normal limits. * Only one result just under the Hct cut-off of 30% (i.e., 29% on one occasion). ** Number of days not given because this case was complicated by concurrent infectious anaemia.
Table 3 shows the clinical signs before treatment; FCoV antibody titres first and most recent, with the interval in parenthesis; Hct before and after with the time taken for anaemia to resolve in parenthesis; lymphopenia; hyperglobulinaemia, showing a reduction in parenthesis. Anaemia was defined as Hct <30%, lymphopenia as a lymphocyte count under 1.5 × 109 per liter, and hyperglobulinaemia as over 45 g per liter (g/L).
The Summary row gives the totals for how many FCoV antibody titres reduced significantly, how many cats were anemic before and after treatment, how many cats were lymphopenic, and to what extent the globulins reduced. The last two columns are simply an addition of the FIP features in each row before treatment and after being declared recovered.

Table 4.
FIP profile parameters at FIP diagnosis and at the last available sample in the remission group.
Table 4.
FIP profile parameters at FIP diagnosis and at the last available sample in the remission group.
| Cat | Clinical Signs | FCoV Antibody Titre | Anaemia i.e., Hct < 30% | Lymphopenia <1.5 × 109/L | Hyperglobulinaemia i.e., >45 g/L | Score | ||
|---|---|---|---|---|---|---|---|---|
| First | Last | |||||||
| 1 | Yrael | Effusion | >1280/>1280 | NA | NA | NA | 2 | 2 |
| 2 | Charlie Chaplin | Neurological signs. Toxoplasmosis co-infection. | >1280/>1280 | NA/✓ | NA/✓ | ✓/✗ | insufficient data | |
| 3 | Smokey | Failure to gain weight and enlarged MLN. | >1280/>1280 | ✓/✓ | ✓/✓ | ✓/✓ | 5 | 5 |
| 4 | Rowley | Ascites, then uveitis, then haemolytic anaemia, euthanasia. | >1280/640 | ✓/✓ | ✓/✓ | ✓/✗ | 4 | 3 |
| 5 | Claude | Pleural effusion, deteriorated and ascites appeared. | NA | NA | NA | NA | insufficient data | |
| 6 | Alfie | Vomiting but bright initially, chronic diarrhoea, enlarged MLN. | >1280/1280 | NA | NA | NA | insufficient data | |
| 7 | Holly | Persistent pyrexia, weight loss then ascites. | >1280/1280 | ✗/✗ | ✗/✗ | ✓ /✗ | 3 | 2 |
| 8 | Bugsy | Pyrexia, poor body condition, variable appetite. | >1280/>1280 | ✗/✓ | ✗/✓ | ✗/✓ | 2 | 5 |
| 9 | Daisy | Weight loss, intestinal granuloma, raised MLN. Intestinal granuloma resolved with treatment, but cat still died. | >1280/>1280 | ✓/✓ | ✓/✓ | ✓/✓ | 5 | 5 |
| 10 | Levi | Ascites. | >1280/not repeated | ✓/✓ (improved though) | ✓/✓ | ✓/✗ | 5 | 4 |
| 11 | Roxanne | Chronic gingivostomatitis, poor condition, poor appetite, jaundice which resolved, but she suddenly developed ataxia and was euthanased. | >1280/320 | ✓/✓ | ✓/✓ | ✓/✓ | 5 | 4 |
| 12 | Pip | Biopsied 2004. February 2005: enlarged MLN, weight loss, anaemia, reported well in February 2006 although pyrexic (39.2 °C). | >1280/>1280 | ✓/✓ | low/✗ | ✓/✓ | 5 | 4 |
| 13 | Pharaoh | Weight loss, gut biopsy showed inflammation. | 640/>1280 | ✓/✓ | ✗/✓ | ✗/✓ | 3 | 5 |
| 14 | Maximus | Chronic diarrhoea, poor appetite. Collapsed and was euthanased. | >1280/>1280 | ✓/✓ | ✗/✗ | ✓/✓ | 4 | 4 |
| 15 | Ragamuffin | Weight loss. MLN enlarged, diarrhoea, always shed low amounts of virus in faeces, chronic anaemia. | >1280/>1280 | ✗/✓ | ✓/✓ (sometimes low normal) | ✓/✓ | 4 | 5 |
| 16 | Tinkerbell | Underweight, chronic poor appetite. | >1280/1280 | ✓/✗ | ✗/✗ | ✓/✓ | 4 | 3 |
| Summary | 15 of 15/ 13 of 14 | 9 of 12/ 11 of 13 | 6 of 11/ 10 of 13 | 11 of 13/ 9 of 13 | ||||
✓—present ✗—absent >—over. NA: not available.
Table 4 shows the clinical signs and scores before treatment and at death or last sampling prior to death or loss to follow up, showing that treatment had not greatly improved the cat’s score, and in some cases it worsened. First and latest FCoV antibody titres, presence or absence of anaemia, lymphopenia, and hyperglobulinaemia are shown in the 4th, 5th, 6th, and 7th columns, respectively. In the last row, a summary of how many cats were anemic, lymphopenic, and hyperglobulinaemic at the first and last samples are shown. FCoV antibody titres were available for 15 of 16 cats, and all were high. 13 of 14 last available samples had high antibody titres, one cat’s titre had reduced three-fold from >1280 to 320; 9 of 12 cats were anemic at the outset, and at the last sample, 11 of 13 were anemic. Six of 11 cats were lymphopenic, rising to 10 of 13; 11 of 13 cats were hyperglobulinaemic at the outset, globulin levels reduced in four cats, probably due to corticosteroids. FIP scores worsened in 3 cats, stayed the same in 4 cats, and improved slightly in 6 cats. The last two columns are simply an addition of the FIP features in the row at the time of FIP diagnosis and the last available sample prior to death or loss to follow-up.
3.2. FIP Treatment
Treatment protocols were variable: treatment choice was at the discretion of the cat’s attending veterinary surgeon or, more recently, the cat’s guardian, and treatments are described to the best of our ability for each cat in Table 1 and Table 2. They most commonly included recombinant feline interferon omega (rFeIFN-ω, Virbac, France) by subcutaneous injection (for effusive FIP) or diluted and administered per os daily (for non-effusive FIP or as follow up to other therapies) (n = 20 recovered and 13 remission cats). Twelve cats were treated with a 5% oral adenosine nucleoside analogue (Mutian Xraphconn®, Mutian Biotechnology Co., Ltd., Nantong, China) [52,65], now known to contain GS-441524 [52], and three cats were treated with online products of unknown active ingredient but suspected to contain GS-441524.
Supportive treatment included vitamin B12 (cobalamin) either as weekly injections or daily pills (Cobalaplex, Protexin Veterinary, Somerset, UK); and Pro-kolin enterogenic probiotics (Protexin Veterinary, Somerset, UK); prednisolone (n = 11/25 recovered and 11/12 remission cats for whom use of corticosteroids was known). The use of corticosteroids was recommended to be discontinued or not started by one author (DDA) from 2017, and to use meloxicam per os daily instead (after a suitable wash out period and provided blood pressure and kidney function were normal).
3.3. Recovery, Remission, and Death
Case 1 (Basil 1) set the benchmark for defining recovery from FIP against which other cases were compared. This cat was treated and was followed up for eleven years without relapsing and died of chronic kidney disease at 15 years of age. Based on this case, the criteria for declaring a cat recovered from FIP included the following:
- The cat returned to clinical normality, specifically resolution of the clinical signs of FIP.
- Globulin levels were reduced to normal (≤45 g/L) or at least significantly reduced (often by over 15 g/L as shown in Table 3).
- Resolution of lymphopenia, where present, (normal defined as ≥1.5 × 109/L).
- Haematocrit level increased to normal (defined as ≥30%), with reversal of non-regenerative anaemia where present.
- At least three-fold reduction in FCoV antibody titre.
- AGP levels returned to normal levels (≤500 μg/mL) [40].
Cats were deemed to be in remission if only some of the criteria listed above were met: for example, remission cat 15 (Ragamuffin) survived over 5 years, but she was never clinically well during that time, required continuous treatment, and unfortunately, she was lost to follow up.
As shown in Table 1 and Table 2, 26 cats recovered from FIP, with follow-up periods of up to 11 years. Death due to a non-FIP reason occurred in three cats who had recovered from FIP: Basil 1, Bea, and Edward died of chronic kidney disease, cancer, and a road accident aged 15, 8, and 3 years, respectively.
Of the recovered cases, 12/26 (46%) had effusive FIP, and 14/26 (54%) were non-effusive. One cat (Boris) had non-effusive FIP, initially thought to be effusive, but later established to be a cardiogenic effusion (FCoV RT-PCR on his effusion was negative). One cat (Nelson) with non-effusive FIP developed an effusion following biopsy. Two of the non-effusive FIP cases were colonic presentations.
Sixteen cats experienced remissions of 1.5 to over 60 months. Median remission was 4.5 months (not including the outlier of Ragamuffin, who was lost to follow up after 5 years because her result would skew the median). Thirteen cats died or were euthanased, and three were lost to follow up.
Amongst the cats who experienced remission, four (25%) had effusive FIP (one—Rowley—became non-effusive), and 12 (75%) had non-effusive FIP (one—Holly—became effusive).
Twelve of 16 (75%) effusive cases and 14/26 (54%) non-effusive FIP cases recovered. The initial effusive or non-effusive form did not affect whether or not a cat fully recovered (Fisher’s exact, p = 0.21).
3.4. AGP Levels
AGP levels of recovered cats and those who experienced remission are shown in Figure 1a–c and Figure 2 respectively. AGP levels were elevated (i.e., above 500 μg/mL) in all FIP cases except in the cases of Wish and Mike, where the first AGP test was taken after treatment had started, so the pre-treatment AGP results were unknown. The median AGP level in effusive FIP was 4330 μg/mL, and for non-effusive FIP, 2600 μg/mL. Five cats with non-effusive FIP had levels below 1500 μg/mL.
The time for AGP levels to return to normal in recovered cats varied from a minimum of less than 13, 20, and 22 days (Wish, Lyra, and Basil 1) to over 16 months from onset of treatment, possibly depending on the treatment being used (Table 1). The AGP levels of one recovered cat (Chynah) did not return to normal, and with the benefit of hindsight, this cat should perhaps have been in the remission group, although her veterinary surgeon reported that she was clinically recovered, and unfortunately, she was lost to follow up.
Figure 2 shows the AGP levels of cats in remission and illustrates that cats who did not fully recover from FIP retained high AGP levels: in Ragamuffin’s case, very high levels. The AGP levels in these cats were often suppressed by prednisolone treatment.
Two of the cats (Holly and Daisy) who did not recover had AGP levels that were reduced to under 500 μg/mL on one occasion each, and AGP levels were reduced to under 1000 μg/mL in two more cats. Therefore, it is important that two consecutive normal AGP results at least one week apart be obtained to ensure that recovery from FIP has occurred.
Of particular interest was the AGP level of Kitten 2 (Figure 1c), who was diagnosed with effusive FIP in October 2019. Her guardian stopped the oral adenosine nucleoside analogue (Mutian Xraphconn) treatment on 5 January 2020, following an AGP result of 709 μg/mL. This young cat presented with an FIP relapse in February 2020, manifesting as hyperesthesia of the tail, which progressed to ataxia, then seizures. A second course was administered, this time at a double dose (one Mutian X 200 pill per kg) which is a level that enables sufficient antiviral drug to cross the blood-brain barrier (Tony Xue, Mutian CEO, personal communication). The cat improved within 24 h, and the second course of Mutian X was continued until AGP reached below 500 μg/mL: she is alive and well two years later. After this event, FIP cat guardians were recommended to give a double dose of oral (not injectable) Mutian (i.e., Mutian X, not Mutian II) for 7–10 days to clear the virus from the brain to prevent neurological relapse.
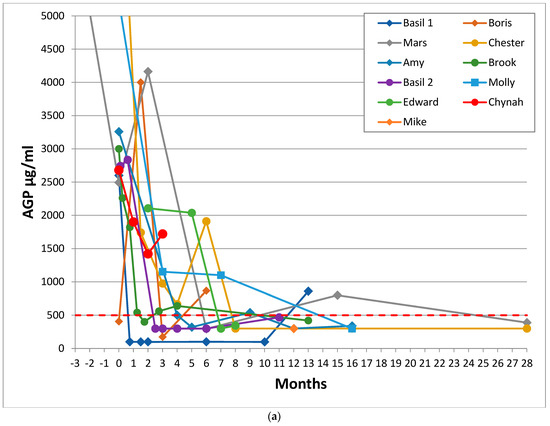
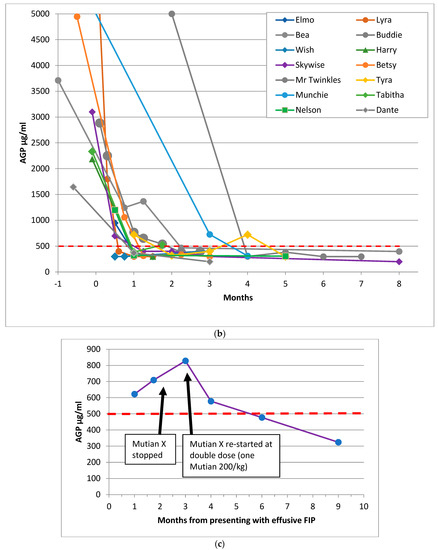
Figure 1.
Sequential AGP results of 26 cats who recovered from FIP. These graphs show the time in months for AGP levels to return to normal for the cured cats, with Day 0 being the first day of a specific (as opposed to supportive) FIP treatment (where known: if unknown then Day 0 was the first AGP test). (a) shows the AGP levels of 11 recovered cats treated before adenosine nucleoside analog antiviral drugs were available (but including Mike, for whom GS-441524 injections became available 8.5 months after he began treatment with oral of rFeIFN-ω and meloxicam), (b) shows the AGP levels of 14 recovered cats treated with products believed to be adenosine nucleoside analog antiviral drugs and (c) shows the AGP level and timeline of the 26th recovered cat, Kitten 2, who suffered a neurological FIP relapse when treatment was stopped before her AGP level had fully returned to less than 500 μg/mL; she was re-treated and made a full recovery. The red dashed line indicates the normal AGP cut-off at 500 μg/mL. The y axis of (a) and (b) was cut-off at 5000 to facilitate reading of the graphs. Cats with effusive FIP are represented by circle markers, cats with non-effusive FIP are shown in diamonds/squares. No cat had more than one AGP test before treatment began, which is why it appears as if AGP was decreasing prior to treatment—in reality, it would have been increasing. These graphs show that the AGP levels of recovered cats reduced to 500 μg/mL or below, whereas the AGP levels of cats in remission shown in Figure 2 stayed above that level.
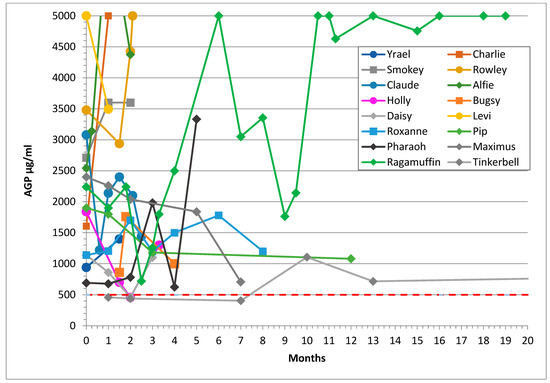
Figure 2.
Sequential AGP results of 16 cats who experienced remission from FIP showing that their AGP levels did not reduce to less than 500 μg/mL (shown as the dashed red line) despite treatment. The y axis was cut-off at 5000 to facilitate reading of the graphs. Their lines terminate at the last AGP level before death except for the three cats lost to follow up. Median survival time was 4.5 months.
3.5. Haematocrit
A lower cut-off of 30% was our definition of normal, below which a cat was said to be anemic. The value was determined by the University of Glasgow Veterinary Diagnostic Services (VDS) Laboratory.
Haematocrit was available for 25 of 26 recovered cats and 13 of 16 cats who went into remission (Table 3 and Table 4). One recovered cat (Chynah) and 3 remission cats (Yrael, Claude, and Alfie) had no haematology results. In five recovered cats (Dante, Molly, Nelson, Edward, Kitten 2), records were not available until after treatment began—too late to determine whether or not they had been anemic because by that time they were not anemic. In two cats, Hct was only marginally below normal (Tabitha 29.4% and Chester: 28.9%) on one occasion only; therefore, they were not seriously anemic and were discounted from analyses. Basil 2′s anaemia was complicated by concurrent haemoplasmosis infection (Figure 3). This left usable hematocrit records for 17 recovered cats and 13 cats who went into remission: 7 of the 17 recovered cats had effusive FIP, and 10 had non-effusive FIP; 2 remission cats had effusive FIP, and 11 remission cats had non-effusive FIP.
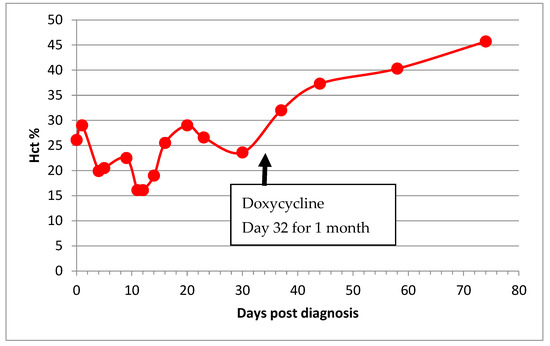
Figure 3.
Haematocrit (Hct) of a cat (Basil 2) with both FIP and haemotropic mycoplasmosis illustrating the typical wave pattern with a periodicity of 7–10 days due to the cyclical parasitemia of haemotropic mycoplasma infection. The co-infection compounded the effect of the anaemia of FIP, doxycycline was introduced on Day 32, and his anaemia normalised thereafter.
Eight of 9 (89%) cats with effusive FIP and 15 of 21 (71%) cats with non-effusive FIP were anemic (Table 3, Table 4 and Table 5). Whether FIP was effusive or non-effusive made no statistical difference to the likelihood of the cat being anemic (p = 0.70).

Table 5.
Summary of anaemia.
Eleven of 17 (65%) cured cats and 12 of 13 (92%) cats in remission were anemic: the presence of anaemia did not affect the cat’s chances of recovery (p = 0.10).
Two remission cats became anaemic after FIP diagnosis, and one cat’s anaemia resolved. Anaemia resolved in all of the cats who recovered, but Basil 2 required a one-month course of doxycycline for concurrent haemotropic mycoplasma infection for his anaemia to resolve (Figure 3).
While the average time for resolution of purely FIP related anaemia was 30.4 days in 10 cats (range 12 to 57 days: Table 3), from initiation of treatment, the average time for AGP to return to WNL was 45 days (range 20–117 days, not counting the two cats for whom there was no pre-treatment AGP). The outlier cat, Mike, was not counted in this analysis because there was no initial AGP for comparison: his anaemia resolved 154 days after treatment began.
3.6. Lymphopenia
A lymphocyte count of 1.5 × 109/L was considered the lowest level at which lymphocyte count could be considered normal: this is the cut-off set by the VDS laboratory. Sequential lymphocyte counts were available for 25 of 26 recovered cats and 13 of 16 remission cats, but the first count was two months after treatment began for one recovered cat (Edward). Sixteen (43%) of the remaining 37 cats with FIP were lymphopenic on the first available lymphocyte count closest to FIP diagnosis. The prevalence of lymphopenia was 38.5% (5/13) in effusive cases and 45.8% (11/24) in non-effusive cases.
It appeared that fewer recovered cats (9/24: 37%) than remission cats (7/13: 54%) were lymphopenic, but the difference was not statistically different (p = 0.5). Although the median lymphocyte count was slightly higher for recovered cats (1.833 vs. 1.366 × 109/L), the difference was not significantly different between the recovered or remission groups (p = 0.47).
Four recovered cats had very low lymphocyte counts but within the normal range (1.5 to 7.0 × 109/L): one cat (Buddie) was lymphopenic on 2 of 7 samples, and another cat (Molly) had low lymphocyte counts (below 2 × 109/L, but above 1.5 × 109/L which is considered the lower cut-off).
Lymphopenia resolved in all recovered cats. In the remission group, three cats became lymphopenic, one cat’s lymphocyte count increased to 1.9 × 109/L, and the status of the other cats remained the same (Table 4).
3.7. Hyperglobulinaemia
Globulin levels were available for all the 26 recovered cats and for 13 of 16 remission cats, but there was no pre-treatment sample for one of the recovered cats, and by the time he was tested, his globulin levels were normal. Twenty-three of the remaining 25 recovered cats were hyperglobulinemic: globulin levels reduced in all 23 cats, to WNL in 16 cats, but were still elevated in 7 cats.
Eleven of 13 remission cats were hyperglobulinemic, and globulin levels were reduced in 4 cats.
AGP was a more accurate prognostic indicator than globulin reduction because globulins were slower to reduce than AGP levels in recovered cats (7 of 23 cats still had elevated globulin levels once recovered), and they reduced in 4 of 11 remission cases.
3.8. FCoV Antibody Titre
As shown in Table 3 and Table 4, all of the cats had very high FCoV antibody titres, being on or above the upper cut-off point for the laboratory to which samples had been sent. Two exceptions were Nelson, who recovered, and the remission cat Pharaoh, whose FCoV antibody titre was only moderately high at 640 at the time of diagnosis, but subsequently became very high. FCoV antibody titres remained high in almost all recovered cats for a very long period, even years (Table 3). The earliest that a significant reduction in FCoV antibody titre was seen was at 4 months post-diagnosis, and one cat became seronegative at 8 months.
FCoV antibody titres in the remission group did not decrease, except for Rowley, whose titre decreased from >1280 to 640, likely due to immunosuppressive amounts of prednisolone with which he was being treated.
4. Discussion
Recovery implies that the disease is finished once and for all, whereas remission is defined as the reduction or diminution of clinical signs, implying that the disease could reappear, i.e., relapse. The state of remission from FIP in our series usually culminated in death, mainly by euthanasia due to a prolonged cachexic state, or sometimes acutely, presenting as acute haemolytic anaemia, collapse, or the onset of neurological signs. Neurological relapses were a problem in previous studies [49,50,51]. While parameters and a scoring system for predicting imminent death have been well documented [34], no such system has been previously defined to differentiate recovery from remission. We present the first documentation of sequential AGP testing of cats being treated for FIP and present evidence that a consistent reduction in AGP to normal levels is the most useful marker for differentiating recovery from remission, and is a clear indicator that it is safe to stop administering adenosine nucleoside analogue antiviral drugs.
Raised AGP was shown to be 100% and 93% sensitive in FIP diagnosis [40,43,44] but is not specific: it rises in both transient FCoV infection [41,42] and in other infections [37,38] (e.g., bacterial peritonitis or pleurisy; Mycoplasma haemofelis infection [39]). However, the sensitivity of AGP measurement in cats with FIP in the brain only has not been established: Rissi et al., 2018 [66] reported that five of 22 cats with neurological FIP had no FIP lesions in other organs, and it is possible that in those cats AGP measurement would not have been raised. Raised AGP was more sensitive than histopathology for diagnosing FIP in challenging cases [43], and this was also true in our series, where histopathology—especially of the MLN—was frequently non-specific and reported as simply pyogranulomatous inflammation.
AGP was the most useful parameter for assessing recovery from FIP because it was raised in all our cases, whereas the resolution of anaemia, lymphopenia, and hyperglobulinaemia were not consistently found. Anaemia resolved more quickly than did AGP in five of six of the nine anaemic cats in which the time to return to normal was not the same for both AGP and Hct (in three cats, the identical intervals presumably reflected the intervals between blood samplings). Consequently, where AGP measurement is not possible and if the cat is anaemic purely due to FIP (e.g., uncomplicated by concurrent haemoplasmosis), then the return of Hct to over 30% appears to be a good indicator of recovery, although our numbers were small. However, the anaemia of one of the cats in the remission group also resolved, so the resolution of anaemia alone is not a guarantee of recovery.
The exact interval from the start of FIP treatment to normal AGP could not be accurately determined because it depended on the frequency of the cat being blood tested, which was at the discretion of the cat’s guardian. We were only able to determine that it was less than however many days from the start of treatment to the first AGP test WNL. However, the intervals appeared noticeably shorter after 2019 (Figure 1b vs. Figure 1a), when adenosine nucleoside analogue antivirals were introduced [50]. The earliest that AGP returned to normal was around two to three weeks from the start of treatment. Another acute phase protein, SAA, was also shown to reduce rapidly in cured cats after the onset of Mutian X treatment in a study of 18 cats [52].
The prevalence of lymphopenia in our study (43%) was similar to that of other studies: 49.5% of 184 cats with FIP [22], 50% of 106 cats [35], and 64% of 45 cats [34]. In the study by Riemer et al. 2016 [22], lymphopenia was observed significantly more often in 139 cats with effusion and was documented in only 26.8% of 41 cats without effusion. Our results differed: 38.5% of effusive cases were lymphopenic compared with 48.8% of non-effusive cases, but our cohort of cats was much smaller than that of Riemer et al., 2016 [22].
In the study of naturally infected cats by Tsai et al., 2011, the prevalence of lymphopenia was 64% at initial presentation and increased to 91.7% from zero to three days before death [34]. In an experimental infection of 20 cats, the absolute lymphocyte count in blood was the most accurate predictor of disease outcome [36]; therefore, it was surprising that in our study absence of lymphopenia was not a predictor of which cat would recover. However, in our study, lymphopenia reversed in the lymphopenic cats who recovered, and two cats in the remission group became lymphopenic. Accordingly, the development of lymphopenia in a cat with FIP who had not previously been lymphopenic is a poor prognostic sign. Our study could not assess the utility of documenting the reversal of lymphopenia as a marker for recovery from FIP, because unfortunately during most of this study prednisolone was given to treat FIP, which would have interfered with (i.e., decreased or suppressed) the lymphocyte count. Further studies will be required to assess the utility of lymphopenia reversal as an indicator of FIP recovery, although given that around half of cats with FIP are not lymphopenic, it will clearly be less useful than AGP, which is consistently elevated in cats with FIP.
The definitive marker for recovery from FCoV infection is the reduction of FCoV antibody titre to undetectable levels, signifying that viral antigen no longer remains in the body to stimulate an immune response; but this takes many months to achieve and consequently it is not a useful marker to determine when to stop nucleoside analogue treatment (though we used falling FCoV antibody titres to determine when to stop the oral interferon omega follow up). In FIP recovered cats, significant reduction of FCoV antibody titre takes so long to achieve (usually over one year) that it would be inadvisable to sustain nucleoside analogue antiviral treatment long term due to the risk of toxic adverse effects to the kidneys and liver. In contrast, antibody levels in FCoV infected cats without FIP usually decline within a few months of the virus being eliminated from the intestine and faeces (data not shown).
We observed two relapsed cases who presented with a painful tail: Kitten 2, described in this paper, and another cat for whom we had no AGP results and was not documented here. The occurrence of idiopathic painful tail syndrome has been reported previously [67], and André et al., 2019 [68] described a neurological FIP case that began as plegia of the tail. FIP is the major cause of hydrocephalus in young cats [27,28,66]. Cerebrospinal fluid (CSF) drains from the thecal sac at the level of the second sacral vertebra. It is likely that the build-up of CSF in this area pressed on the nerves of the cauda equina, causing an initial presentation of sensitivity and pain in the tail. As CSF built up in the ventricular system of the central nervous system, the two cats progressed to show hind limb ataxia, then seizures. Fortunately, prompt administration of antiviral pills to Kitten 2 saved her life; the other cat was treated with an injectable nucleoside analogue (Mutian II) but died. Two other cases (outwith this study) treated with injectable nucleoside analogues have presented with neurological relapses, but made partial recoveries after a course of double dose pills: it seems counterintuitive for an oral formulation to cross the blood-brain barrier more effectively than the injectable formulations, but that is our experience. Following the Kitten 2 relapse, we recommend giving a 7–10-day double dose of oral GS-441524 early in the treatment to clear the brain of the virus.
It might be wondered why there was only one relapse amongst the 16 cats (6%) which were treated with nucleoside analogue drugs when in the first study where nucleoside analogues were used, there were eight relapses in 26 cats (31%) [50]. We believe that one of the most important reasons was that 12 cats were treated with an oral adenosine nucleoside analogue, which accesses the site of viral replication in the intestine and stopped virus shedding in faeces in all cases, rather than an injectable version of the same drug. In contrast, one cat (Mike) treated with injectable GS-441524 continued shedding virus two years after diagnosis. A similar problem has been encountered in humans treated with Remdesivir (Gilead Sciences, Foster City, CA, USA), the GS-441524 pro-drug (with or without convalescent plasma), where prolonged virus shedding was reported in 13 patients [69]. It may be that this antiviral fails to adequately reach every organ, allowing pockets of the virus to survive and recrudesce [70]. One patient not in our series (because AGP was not monitored) was presented with a relapse of FIP pleural effusion. Initially, that cat had been treated with ten days of Mutian II injections which may have failed to properly clear the virus from his lungs: this was seen in an immunocompromised human COVID19 patient treated with Remdesivir [70].
Other factors which contributed to a successful outcome included being able to differentiate accurately between recovery and remission using AGP levels, therefore knowing when it was safe to discontinue the antiviral drug. Secondly, antiviral treatment was followed up with long-term oral feline interferon, which has antiviral and immunomodulatory activity. Thirdly, in-contact cats were tested for FCoV shedding, and if positive, they were treated to stop virus shedding [65]; thus, re-infection was prevented (presumably, re-infection could appear as a relapse). Using antivirals in subclinically infected cats parallels SARS-CoV2 infection, where prophylactic ivermectin reduced the risk of COVID-19 disease by 83% in the following month in health care workers in India [71] and reduced COVID-19 mortality in Brazil by 68% [72].
It should always be considered that the appearance of relapse or failure to respond to treatment may, in reality, be due to secondary conditions. For example, the anaemia of Basil 2 was due to concurrent haemoplasmosis but initially appeared to be a lack of response to FIP treatment.
It is likely that immunosuppression due to FIP-induced lymphopenia leads to secondary infections in FIP. Thus, in addition to infectious anaemia, we have observed toxoplasmosis and clinical signs attributable to recrudescent latent feline herpesvirus infection, such as epiphora and sneezing. One relatively frequent curious condition was trichobezoar which is not explicable by immunosuppression. In most cases, this was easily dealt with using a psyllium-containing dry cat food (Aging 12+, Royal Canin, Aimargues, France), but in one cat, who was not included in this series due to lack of AGP results, surgery was required to remove a large furball.
A failing of our study was the inability to compare weight gain or loss in the two groups. In a previous study, weight gain was an important measurement of treatment success [50]. Unfortunately, in our study, weight records were only available for the most recent recovered cases and none in the remission group.
Another limitation of the study was the small number of cats involved. However, our figures are in the same ballpark as other recently published FIP treatment studies, which featured 20 [49], 18 [52], and 31 [50] FIP cats, respectively. Since the advent of effective treatments for FIP, it is hoped that our control group of remission cats will not be increased.
The data presented here were collected over almost two decades. As new tests became available, they were applied to the cases; consequently, confirmation of FIP diagnosis of individual cats was not uniform: obtaining reliable and complete data is one of the drawbacks of a retrospective study such as ours. Demonstration of FCoV in biopsy or post mortem material by immunohistochemistry is deemed the gold standard for FIP diagnosis, and this study might be criticised for a lack of histopathological confirmation of FIP in many of the cases. Since the cats were field cases, it would not have been ethical to ask for biopsies to be performed for this study, and most guardians did not elect to have histopathological confirmation of the diagnosis in those cats who died. Many of the cats had positive FCoV RT-PCR tests of effusion [54,55,56] or MLN FNA [57], and such evidence has been accepted as diagnostic of FIP in other therapeutic studies [49,50]. Demonstration of replicating FCoV by messenger RNA RT-PCR [58] in PBMC was considered diagnostic of FIP, but it was borne in mind that FCoV mRNA had been detected in 5% of cats without FIP [58,59], and during this study, we found that the primers cross-reacted with human DNA, giving false-positive results on some samples (therefore we confirmed positive PBMC mRNA results by a 3′ UTR RT-PCR [60]).
There remained some cats who were diagnosed only by circumstantial evidence, but in all cases, elevated FCoV antibody titres and AGP levels were documented, and response to anti-coronavirus drugs was further corroboration of a correct FIP diagnosis.
5. Conclusions
As new FIP treatments become available, we have shown that AGP measurement will be a useful parameter to assess their efficacy. Increasing AGP can indicate that a cat’s condition is deteriorating, but a return to 500 μg/mL or less sustained for at least one week indicated recovery from FIP and differentiated recovered cats from those in remission. It is inadvisable to reduce the dosage or frequency of treatment, even if clinical signs appear to have resolved, until AGP levels are once again normal.
Author Contributions
Conceptualization, D.D.A.; methodology, D.D.A.; validation, D.D.A., C.S., C.A., P.B., J.C.-R., C.F., M.F., C.G., K.M., E.P. and E.R.; formal analysis, D.D.A., C.S., C.A., P.B., C.F., M.F., C.G., J.M., E.P. and E.R.; investigation, C.S., C.A., P.B., J.C.-R., C.F., M.F., C.G., J.M., K.M., E.P. and E.R.; data curation, D.D.A., C.S., C.A., P.B., J.C.-R., C.F., M.F., C.G., K.M., J.M., E.P. and E.R.; writing—original draft preparation, D.D.A.; writing—review and editing, D.D.A., C.S., C.A., P.B., J.C.-R., C.F., M.F., C.G., K.M., E.P. and E.R.; visualization, D.D.A.; supervision, D.D.A.; project administration, C.S., C.A., P.B., J.C.-R., C.F., M.F., C.G., K.M., E.P. and E.R.; funding acquisition, D.D.A., K.M. and J.C.-R. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding, but D.D.A. gratefully acknowledges support from donors and subscribers to www.catvirus.com (accessed on 23 February 2022).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Informed consent was obtained from the cat guardians for publication of their pet’s data.
Data Availability Statement
All data generated or analysed during this study are included in this published article.
Ethical Approval
All applicable international, national and institutional guidelines for the care of animals were followed. All procedures performed in studies involving animals were in accordance with the ethical standards of the governing bodies of the veterinary surgeons of the countries in which the studies were conducted.
Acknowledgments
We are very grateful to the guardians of these cats for consenting to the publication of their cases, amongst whom were Helen Alford, James Arnold, Kirsty Bedford, Nancy Court, Diana Donciu, Sarah Edwards, Evans, Franklin, Jake Goldstein, Peter and Rochelle Harisis, Bianca Houlgrave, Eric and Carrie Johnson, Lake, Karen Major, Claire Neller-Clements, Tanya Osborne, Patrick Slocombe, Sarah Stoddart, Sarah Stokes. The authors sincerely thank the following veterinary surgeons for providing information and samples: David Abratt, Andrew Armitage, Roger Bralow, Colin Dick, Rebecca Gibb, Stuart Hills, Hannah Johnston, Colin Lyons, Carol Mason, Andy Nelson, Graham Pursey, Fiona Thomson, Jason Way. We sincerely thank Oswald Jarrett, Flora Bellini, and Ben Crowe for scientific editing. We are grateful to the technicians, office, and veterinary staff of Glasgow University Veterinary Diagnostic Services Laboratory, with special thanks to Leigh Marshall for AGP and FCoV antibody testing, and Dawn Dunbar and Mike McDonald for FCoV RT-PCR testing. We are deeply indebted to the Angelica Trust donors to the www.catvirus.com (accessed on 23 February 2022) website for support of D.D.A. during the writing of this paper and for covering publication fees.
Conflicts of Interest
D.D.A. is a former employee of VDS. She received commissions from Virbac in 2004 and 2021 and Royal Canin in 2021. She is a member of the European Advisory Board on Cat Diseases (ABCD), which is sponsored by Boehringer Ingelheim and Virbac: ABCD members receive no remuneration. No commercial company was involved at any stage of this study, or had any influence on the decision to publish the findings. None of the other authors declare any potential competing interests.
References
- Herrewegh, A.A.P.M.; Smeenk, I.; Horzinek, M.C.; Rottier, P.J.M.; de Groot, R.J. Feline coronavirus type II strains 79-1683 and 79-1146 originate from a double recombination between feline coronavirus type I and canine coronavirus. J. Virol. 1998, 72, 4508–4514. [Google Scholar] [CrossRef] [Green Version]
- Terada, Y.; Matsui, N.; Noguchi, K.; Kuwata, R.; Shimoda, H.; Soma, T.; Mochizuki, M.; Maeda, K. Emergence of pathogenic coronaviruses in cats by homologous recombination between feline and canine coronaviruses. PLoS ONE 2014, 9, e106534. [Google Scholar] [CrossRef] [Green Version]
- Kipar, A.; May, H.; Menger, S.; Weber, M.; Leukert, W.; Reinacher, M. Morphologic features and development of granulomatous vasculitis in feline infectious peritonitis. Vet. Pathol. 2005, 42, 321–330. [Google Scholar] [CrossRef]
- Addie, D.D.; Toth, S.; Murray, G.D.; Jarrett, O. The risk of feline infectious peritonitis in cats naturally infected with feline coronavirus. Am. J. Vet. Res. 1995, 56, 429–434. [Google Scholar]
- Pedersen, N.C.; Boyle, J.F.; Floyd, K.; Fudge, A.; Barker, J. An enteric coronavirus infection of cats and its relationship to feline infectious peritonitis. Am. J. Vet. Res. 1981, 42, 368–377. [Google Scholar]
- Pedersen, N.C.; Liu, H.; Dodd, K.A.; Pesavento, P.A. Significance of coronavirus mutants in feces and diseased tissues of cats suffering from feline infectious peritonitis. Viruses 2009, 1, 166–184. [Google Scholar] [CrossRef] [Green Version]
- Chang, H.W.; Egberink, H.F.; Halpin, R.; Spiro, D.J.; Rottier, P.J. Spike protein fusion peptide and feline coronavirus virulence. Emerg. Infect. Dis. 2012, 18, 1089–1095. [Google Scholar] [CrossRef]
- Felten, S.; Leutenegger, C.M.; Balzer, H.J.; Pantchev, N.; Matiasek, K.; Wess, G.; Egberink, H.; Hartmann, K. Sensitivity and specificity of a real-time reverse transcriptase polymerase chain reaction detecting feline coronavirus mutations in effusion and serum/plasma of cats to diagnose feline infectious peritonitis. BMC Vet. Res. 2017, 13, 228. [Google Scholar] [CrossRef]
- Porter, E.; Tasker, S.; Day, M.J.; Harley, R.; Kipar, A.; Siddell, S.G.; Helps, C.R. Amino acid changes in the spike protein of feline coronavirus correlate with systemic spread of virus from the intestine and not with feline infectious peritonitis. Vet. Res. 2014, 45, 49. [Google Scholar] [CrossRef] [Green Version]
- Barker, E.N.; Stranieri, A.; Helps, C.R.; Porter, E.L.; Davidson, A.D.; Day, M.J.; Knowles, T.; Kipar, A.; Tasker, S. Limitations of using feline coronavirus spike protein gene mutations to diagnose feline infectious peritonitis. Vet. Res. 2017, 48, 60. [Google Scholar] [CrossRef] [Green Version]
- Stranieri, A.; Giordano, A.; Paltrinieri, S.; Giudice, C.; Cannito, V.; Lauzi, S. Comparison of the performance of laboratory tests in the diagnosis of feline infectious peritonitis. J. Vet. Diagn. Investig. 2018, 30, 459–463. [Google Scholar] [CrossRef] [Green Version]
- Decaro, N.; Mari, V.; Lanave, G.; Lorusso, E.; Lucente, M.S.; Desario, C.; Colaianni, M.L.; Elia, G.; Ferringo, F.; Alfano, F.; et al. Mutation analysis of the spike protein in Italian feline infectious peritonitis virus and feline enteric coronavirus sequences. Res. Vet. Sci. 2021, 135, 15–19. [Google Scholar] [CrossRef]
- Tasker, S. Diagnosis of feline infectious peritonitis: Update on evidence supporting available tests. J. Feline Med. Surg. 2018, 20, 228–243. [Google Scholar] [CrossRef] [Green Version]
- Brown, M.A.; Troyer, J.L.; Pecon-Slattery, J.; Roelke, M.; O’Brien, S.J. Genetics and pathogenesis of feline infectious peritonitis virus. Emerg. Infect. Dis. 2009, 15, 1445–1452. [Google Scholar] [CrossRef]
- Healey, E.A.; Andre, N.M.; Miller, A.D.; Whitaker, G.R.; Berliner, E.A. Outbreak of feline infectious peritonitis (FIP) in shelter-housed cats: Molecular analysis of the feline coronavirus S1/S2 cleavage site consistent with a ‘circulating virulent-avirulent theory’ of FIP pathogenesis. JFMS Open Rep. 2022, 8. [Google Scholar] [CrossRef]
- Pedersen, N.C.; Black, J.W. Attempted immunization of cats against feline infectious peritonitis, using avirulent live virus or sublethal amounts of virulent virus. Am. J. Vet. Res. 1983, 44, 229–234. [Google Scholar]
- Barker, E.N.; Tasker, S.; Gruffydd-Jones, T.J.; Tuplin, C.K.; Burton, K.; Porter, E.; Day, M.J.; Harley, R.; Fews, D.; Helps, C.R.; et al. Phylogenetic analysis of feline coronavirus strains in an epizootic outbreak of feline infectious peritonitis. J. Vet. Intern. Med. 2013, 27, 445–450. [Google Scholar] [CrossRef] [Green Version]
- Graham, E.M.; Went, K.; Serra, F.; Dunbar, D.; Fuentes, M.; McDonald, M.; Jackson, M.W. Early molecular diagnosis of an effusive FIP outbreak in antibody-negative kittens. J. Feline Med. Surg. 2012, 14, 652. [Google Scholar]
- Wang, Y.T.; Su, B.L.; Hsieh, L.E.; Chueh, L.L. An outbreak of feline infectious peritonitis in a Taiwanese shelter: Epidemiologic and molecular evidence for horizontal transmission of a novel type II feline coronavirus. Vet. Res. 2013, 44, 57. [Google Scholar] [CrossRef] [Green Version]
- Pedersen, N.C.; Sato, R.; Foley, J.E.; Poland, A.M. Common virus infections in cats, before and after being placed in shelters, with emphasis on Feline Enteric Coronavirus. J. Feline Med. Surg. 2004, 6, 83–88. [Google Scholar] [CrossRef] [Green Version]
- Rohrer, C.; Suter, P.F.; Lutz, H. The diagnosis of feline infectious peritonitis (FIP): A retrospective and prospective study. Kleinterpraxis 1993, 38, 379–389. [Google Scholar]
- Riemer, F.; Kuehner, K.A.; Ritz, S.; Sauter-Louis, C.; Hartmann, K. Clinical and laboratory features of cats with feline infectious peritonitis—A retrospective study of 231 confirmed cases (2000–2010). J. Feline Med. Surg. 2016, 18, 348–356. [Google Scholar] [CrossRef] [Green Version]
- De Groot-Mijnes, J.D.; van Dun, J.M.; van der Most, R.G.; de Groot, R.J. Natural history of a recurrent feline coronavirus infection and the role of cellular immunity in survival and disease. J. Virol. 2005, 79, 1036–1044. [Google Scholar] [CrossRef] [Green Version]
- Dewerchin, H.L.; Cornelissen, E.; Nauwynck, H.J. Replication of feline coronaviruses in peripheral blood monocytes. Arch. Virol. 2005, 150, 2483–2500. [Google Scholar] [CrossRef] [Green Version]
- Fischer, Y.; Wess, G.; Hartmann, K. Perikarderguss bei einer Katze mit feliner infektiöser Peritonitis [Pericardial effusion in a cat with feline infectious peritonitis]. Schweiz. Arch. Tierheilkd. 2012, 154, 27–31. [Google Scholar] [CrossRef]
- Pedersen, N.C. A review of feline infectious peritonitis virus infection: 1963–2008. J. Feline Med. Surg. 2009, 11, 225–258. [Google Scholar] [CrossRef]
- Penderis, J. The Wobbly Cat: Diagnostic and therapeutic approach to generalised ataxia. J. Feline Med. Surg. 2009, 11, 349–359. [Google Scholar] [CrossRef]
- Crawford, A.H.; Stoll, A.L.; Sanchez-Masian, D.; Shea, A.; Michaels, J.; Fraser, A.R.; Beltran, E. Clinicopathologic Features and Magnetic Resonance Imaging Findings in 24 Cats with Histopathologically Confirmed Neurologic Feline Infectious Peritonitis. J. Vet. Intern. Med. 2017, 31, 1477–1486. [Google Scholar] [CrossRef]
- Kipar, A.; Bellman, S.; Kremendahl, J.; Kohler, K.; Reinacher, M. Cellular composition, coronavirus antigen expression and production of specific antibodies in lesions in feline infectious peritonitis. Vet. Immunol. Immunopathol. 1998, 65, 243–257. [Google Scholar] [CrossRef]
- Takano, T.; Hohdatsu, T.; Hashida, Y.; Kaneko, Y.; Tanabe, M.; Koyama, H. A “possible” involvement of TNF-alpha in apoptosis induction in peripheral blood lymphocytes of cats with feline infectious peritonitis. Vet. Microbiol. 2007, 119, 121–131. [Google Scholar] [CrossRef]
- Takano, T.; Azuma, N.; Satoh, M.; Toda, A.; Hashida, Y.; Satoh, R.; Hohdatsu, T. Neutrophil survival factors (TNF-alpha, GM-CSF, and G-CSF) produced by macrophages in cats infected with feline infectious peritonitis virus contribute to the pathogenesis of granulomatous lesions. Arch. Virol. 2009, 154, 775–781. [Google Scholar] [CrossRef] [PubMed]
- Goitsuka, R.; Ohashi, T.; Ono, K.; Yasukawa, K.; Koishibara, Y.; Fukui, H.; Ohsugi, Y.; Hasegawa, A. IL-6 activity in feline infectious peritonitis. J. Immunol. 1990, 144, 2599–2603. [Google Scholar] [PubMed]
- Haagmans, B.L.; Egberink, H.F.; Horzinek, M.C. Apoptosis and T-cell depletion during feline infectious peritonitis. J. Virol. 1996, 70, 8977–8983. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tsai, H.Y.; Chueh, L.L.; Lin, C.N.; Su, B.L. Clinicopathological findings and disease staging of feline infectious peritonitis: 51 cases from 2003 to 2009 in Taiwan. J. Feline Med. Surg. 2011, 13, 74–80. [Google Scholar] [CrossRef]
- Yin, Y.; Li, T.; Wang, C.; Liu, X.; Ouyang, H.; Ji, W.; Liu, J.; Liao, X.; Li, J.; Hu, C. A retrospective study of clinical and laboratory features and treatment on cats highly suspected of feline infectious peritonitis in Wuhan, China. Nat. Sci. Rep. 2021, 11, 5208. [Google Scholar] [CrossRef]
- Pedersen, N.C.; Eckstrand, C.; Liu, H.; Leutenegger, C.; Murphy, B. Levels of feline infectious peritonitis virus in blood, effusions, and various tissues and the role of lymphopenia in disease outcome following experimental infection. Vet. Microbiol. 2015, 175, 157–166. [Google Scholar] [CrossRef]
- Paltrinieri, S.; Giordano, A.; Tranquillo, V.; Guazzetti, S. Critical assessment of the diagnostic value of feline alpha1-acid glycoprotein for feline infectious peritonitis using the likelihood ratios approach. J. Vet. Diagn. Investig. 2007, 19, 266–272. [Google Scholar]
- Paltrinieri, S. The feline acute phase reaction. Vet. J. 2008, 177, 26–35. [Google Scholar] [CrossRef]
- Korman, R.M.; Cerón, J.J.; Knowles, T.G.; Barker, E.N.; Eckersall, P.D.; Tasker, S. Acute phase response to Mycoplasma haemofelis and ‘Candidatus Mycoplasma haemominutum’ infection in FIV-infected and non-FIV-infected cats. Vet. J. 2012, 193, 433–438. [Google Scholar] [CrossRef] [Green Version]
- Duthie, S.; Eckersall, P.D.; Addie, D.D.; Lawrence, C.E.; Jarrett, O. Value of α1-acid glycoprotein in the diagnosis of feline infectious peritonitis. Vet. Rec. 1997, 141, 299–303. [Google Scholar] [CrossRef]
- Giordano, A.; Spagnolo, V.; Colombo, A.; Paltrinieri, S. Changes in some acute phase protein and immunoglobulin concentrations in cats affected by feline infectious peritonitis (FIP) or exposed to feline coronavirus infection. Vet. J. 2004, 167, 38–44. [Google Scholar] [CrossRef]
- Paltrinieri, S.; Metzger, C.; Battilani, M.; Pocacqua, V.; Gelain, M.E.; Giordano, A. Serum alpha1-acid glycoprotein (AGP) concentration in non-symptomatic cats with feline coronavirus (FCoV) infection. J. Feline Med. Surg. 2007, 9, 271–277. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Giori, L.; Giordano, A.; Giudice, C.; Grieco, V.; Paltrinieri, S. Performances of different diagnostic tests for feline infectious peritonitis in challenging clinical cases. J. Small Anim. Pract. 2011, 52, 152–157. [Google Scholar] [CrossRef]
- Hazuchova, K.; Held, S.; Neiger, R. Usefulness of acute phase proteins in differentiating between feline infectious peritonitis and other diseases in cats with body cavity effusions. J. Feline Med. Surg. 2017, 19, 809–816. [Google Scholar] [CrossRef] [PubMed]
- Hsiao, S.Y.; Lai, Y.R.; Kung, C.T.; Tsai, N.W.; Su, C.M.; Huang, C.C.; Wang, H.C.; Cheng, B.C.; Su, Y.J.; Lin, W.C.; et al. α-1-Acid Glycoprotein Concentration as an Outcome Predictor in Adult Patients with Sepsis. Biomed. Res. Int. 2019, 3174896. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ishida, T.; Shibanai, A.; Tanaka, S.; Uchida, K.; Mochizuki, M. Use of recombinant feline interferon and glucocorticoid in the treatment of feline infectious peritonitis. J. Feline Med. Surg. 2004, 6, 107–109. [Google Scholar] [CrossRef] [PubMed]
- Legendre, A.M.; Kuritz, T.; Galyon, G.; Baylor, V.M.; Heidel, R.E. Polyprenyl Immunostimulant Treatment of Cats with Presumptive Non-Effusive Feline Infectious Peritonitis in a Field Study. Front. Vet. Sci. 2017, 4, 7. [Google Scholar] [CrossRef] [Green Version]
- Hugo, T.B.; Heading, K.L. Prolonged survival of a cat diagnosed with feline infectious peritonitis by immunohistochemistry. Can. Vet. J. 2015, 56, 53–58. [Google Scholar]
- Pedersen, N.C.; Kim, Y.; Liu, H.; Galasiti Kankanamalage, A.C.; Eckstrand, C.; Groutas, W.C.; Bannasch, M.; Meadows, J.M.; Chang, K.O. Efficacy of a 3C-like protease inhibitor in treating various forms of acquired feline infectious peritonitis. J. Feline Med. Surg. 2018, 20, 378–392. [Google Scholar] [CrossRef]
- Pedersen, N.C.; Perron, M.; Bannasch, M.; Montgomery, E.; Murakami, E.; Liepnieks, M.; Liu, H. Efficacy and safety of the nucleoside analog GS-441524 for treatment of cats with naturally occurring feline infectious peritonitis. J. Feline Med. Surg. 2019, 21, 271–281. [Google Scholar] [CrossRef] [Green Version]
- Dickinson, P.J.; Bannasch, M.; Thomasy, S.M.; Murthy, V.D.; Vernau, K.M.; Liepnieks, M.; Montgomery, E.; Knickelbein, K.E.; Murphy, B.; Pedersen, N.C. Antiviral treatment using the adenosine nucleoside analogue GS-441524 in cats with clinically diagnosed neurological feline infectious peritonitis. J. Vet. Intern. Med. 2020, 34, 1587–1593. [Google Scholar] [CrossRef] [PubMed]
- Krentz, D.; Zenger, K.; Alberer, M.; Felten, S.; Bergmann, M.; Dorsch, R.; Matiasek, K.; Kolberg, L.; Hofmann-Lehmann, R.; Meli, M.L.; et al. Curing Cats with Feline Infectious Peritonitis with an Oral Multi-Component Drug Containing GS-441524. Viruses 2021, 13, 2228. [Google Scholar] [CrossRef] [PubMed]
- Jones, S.; Novicoff, W.; Nadeau, J.; Evans, S.W. Unlicensed GS-441524-Like Antiviral Therapy Can Be Effective for at-Home Treatment of Feline Infectious Peritonitis. Animals 2021, 11, 2257. [Google Scholar] [CrossRef] [PubMed]
- Doenges, S.J.; Weber, K.; Dorsch, R.; Fux, R.; Hartmann, K. Comparison of real-time reverse transcriptase polymerase chain reaction of peripheral blood mononuclear cells, serum and cell-free body cavity effusion for the diagnosis of feline infectious peritonitis. J. Feline Med. Surg. 2017, 19, 344–350. [Google Scholar] [CrossRef] [PubMed]
- Longstaff, L.; Porter, E.; Crossley, V.J.; Hayhow, S.E.; Helps, C.R.; Tasker, S. Feline coronavirus quantitative reverse transcriptase polymerase chain reaction on effusion samples in cats with and without feline infectious peritonitis. J. Feline Med. Surg. 2017, 19, 240–245. [Google Scholar] [CrossRef] [Green Version]
- Yen, S.-J.; Chen, H.-W. Feline Coronaviruses Identified in Feline Effusions in Suspected Cases of Feline Infectious Peritonitis. Microorganisms 2021, 9, 1801. [Google Scholar] [CrossRef]
- Dunbar, D.; Kwok, W.; Graham, E.; Armitage, A.; Irvine, R.; Johnston, P.; McDonald, M.; Montgomery, D.; Nicolson, L.; Robertson, E.; et al. Diagnosis of non-effusive feline infectious peritonitis by reverse transcriptase quantitative polymerase chain reaction from mesenteric lymph node fine needle aspirates. J. Feline Med. Surg. 2019, 21, 910–921. [Google Scholar] [CrossRef] [Green Version]
- Simons, F.A.; Vennema, H.; Rofina, J.E.; Pol, J.M.; Horzinek, M.C.; Rottier, P.J.; Egberink, H.F. A mRNA PCR for the diagnosis of feline infectious peritonitis. J. Virol. Methods 2005, 124, 111–116. [Google Scholar] [CrossRef] [Green Version]
- Fish, E.J.; Diniz, P.P.V.; Juan, Y.C.; Bossong, F.; Collisson, E.W.; Drechsler, Y.; Kaltenboeck, B. Cross-sectional quantitative RT-PCR study of feline coronavirus viremia and replication in peripheral blood of healthy shelter cats in Southern California. J. Feline Med. Surg. 2018, 20, 295–301. [Google Scholar] [CrossRef] [Green Version]
- Herrewegh, A.A.P.M.; de Groot, R.J.; Cepica, A.; Egberink, H.F.; Horzinek, M.C.; Rottier, P.J.M. Detection of feline coronavirus RNA in feces, tissue, and body fluids of naturally infected cats by reverse transcriptase PCR. J. Clin. Microbiol. 1995, 33, 684–689. [Google Scholar] [CrossRef] [Green Version]
- Gut, M.; Leutenegger, C.M.; Huder, J.; Pedersen, N.C.; Lutz, H. One-tube fluorogenic reverse transcription-polymerase chain reaction for the quantitation of feline coronaviruses. J. Virol. Methods 1999, 77, 37–46. [Google Scholar] [CrossRef]
- Addie, D.; Belak, S.; Boucrat-Baralon, C.; Egberink, H.; Frymus, T.; Gruffydd-Jones, T.; Hartmann, K.; Hosie, M.J.; Marsilio, F.; Lloret, A.; et al. Feline infectious peritonitis. ABCD guidelines on prevention and management. J. Feline Med. Surg. 2009, 11, 594–604. [Google Scholar] [CrossRef]
- Addie, D.D. Available online: www.catvirus.com (accessed on 29 January 2022).
- Hartmann, K.; Ritz, S. Treatment of cats with feline infectious peritonitis. Vet. Immunol. Immunopathol. 2008, 123, 172–175. [Google Scholar] [CrossRef]
- Addie, D.D.; Curran, S.; Bellini, F.; Crowe, B.; Sheehan, E.; Ukrainchuk, L.; Decaro, N. Oral Mutian® X stopped faecal feline coronavirus shedding by naturally infected cats. Res. Vet. Sci. 2020, 130, 222–229. [Google Scholar] [CrossRef]
- Rissi, D.R. A retrospective study of the neuropathology and diagnosis of naturally occurring feline infectious peritonitis. J. Vet. Diagn. Investig. 2018, 30, 392–399. [Google Scholar] [CrossRef]
- Amengual Batle, P.; Rusbridge, C.; Nuttall, T.; Heath, S.; Marioni-Henry, K. Feline hyperaesthesia syndrome with self-trauma to the tail: Retrospective study of seven cases and proposal for an integrated multidisciplinary diagnostic approach. J. Feline Med. Surg. 2019, 21, 178–185. [Google Scholar] [CrossRef]
- André, N.M.; Cossic, B.; Davies, E.; Miller, A.D.; Whittaker, G.R. Distinct mutation in the feline coronavirus spike protein cleavage activation site in a cat with feline infectious peritonitis-associated meningoencephalomyelitis. JFMS Open Rep. 2019, 5. [Google Scholar] [CrossRef] [Green Version]
- Drancourt, M.; Cortaredona, S.; Melenotte, C.; Amrane, S.; Eldin, C.; La Scola, B.; Parola, P.; Million, M.; Lagier, J.C.; Raoult, D.; et al. SARS-CoV-2 Persistent Viral Shedding in the Context of Hydroxychloroquine-Azithromycin Treatment. Viruses 2021, 13, 890. [Google Scholar] [CrossRef]
- Choi, B.; Choudhary, M.C.; Regan, J.; Sparks, J.A.; Padera, R.F.; Qiu, X.; Solomon, I.H.; Kuo, H.H.; Boucau, J.; Bowman, K.; et al. Persistence and Evolution of SARS-CoV-2 in an Immunocompromised Host. N. Engl. J. Med. 2020, 383, 2291–2293. [Google Scholar] [CrossRef]
- Behera, P.; Patro, B.K.; Padhy, B.M.; Mohapatra, P.R.; Bal, S.K.; Chandanshive, P.D.; Mohanty, R.R.; Ravikumar, S.R.; Singh, A.K.; Singh, S.R.; et al. Prophylactic Role of Ivermectin in Severe Acute Respiratory Syndrome Coronavirus 2 Infection Among Healthcare Workers. Cureus 2021, 13, e16897. [Google Scholar] [CrossRef]
- Kerr, L.; Cadegiani, F.A.; Baldi, F.; Lobo, R.B.; Assagra, W.L.O.; Proença, F.C.; Kory, P.; Hibberd, J.A.; Chamie-Quintero, J.J. Ivermectin Prophylaxis Used for COVID-19: A Citywide, Prospective, Observational Study of 223, 128 Subjects Using Propensity Score Matching. Cureus 2022, 14, e21272. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).