Characterization of H9N2 Avian Influenza Viruses Isolated from Poultry Products in a Mouse Model
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cells
2.2. Viruses
2.3. Viral Genome Sequencing
2.4. Viral Replication Assay
2.5. Mouse Experiments
2.6. Statistical Analysis
3. Results
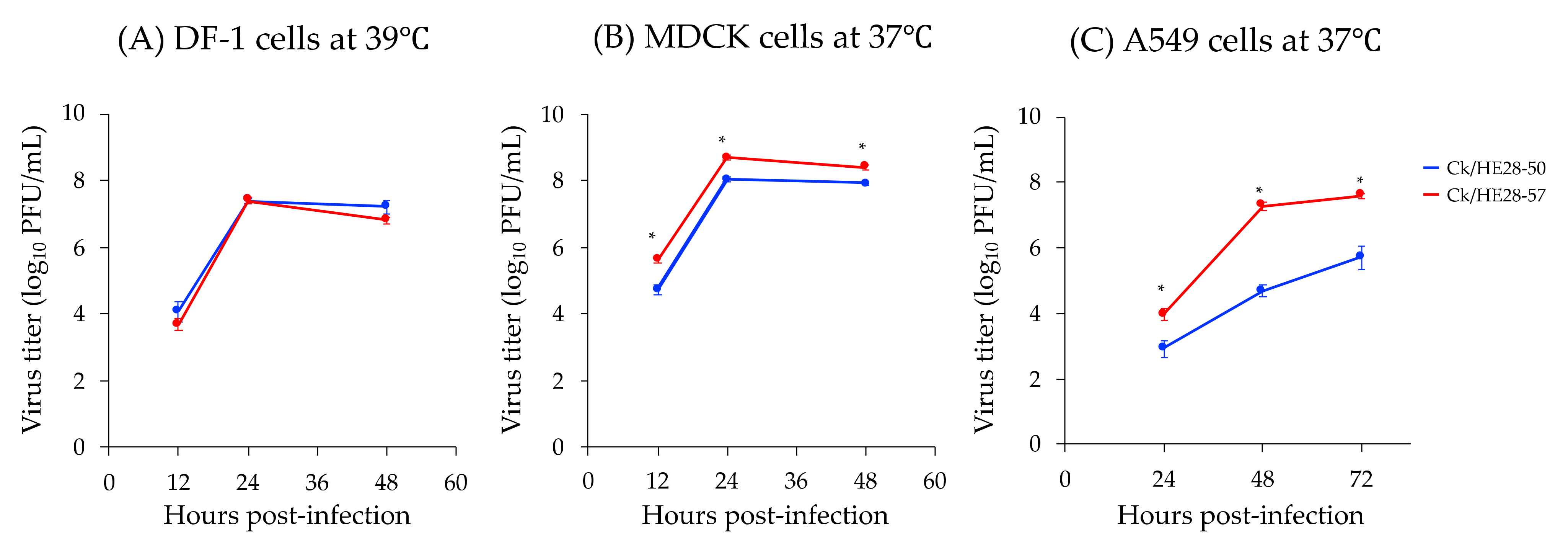
3.1. Replicative Ability of Two H9N2 Avian Influenza Viruses In Vitro Subsection
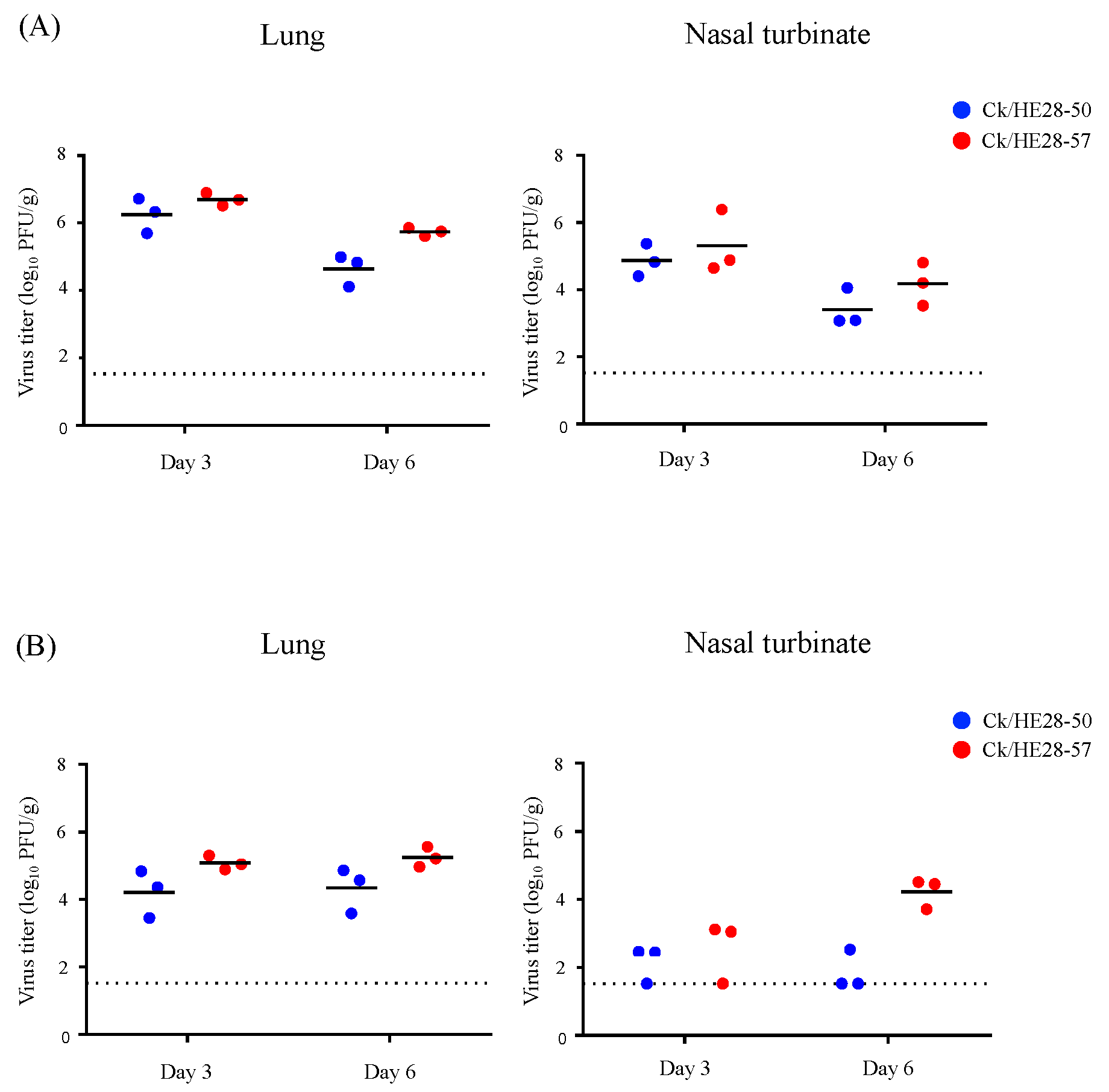
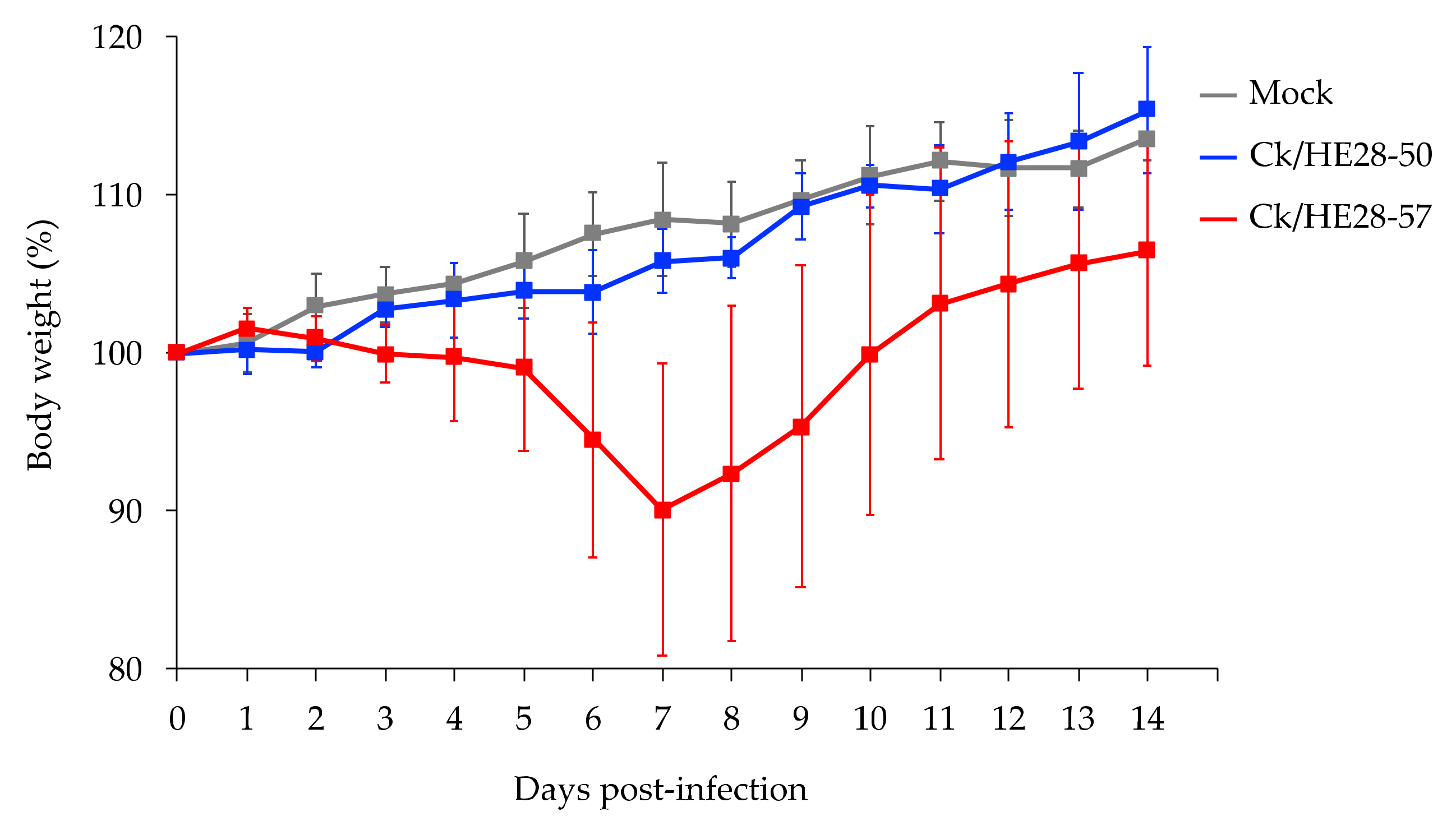
3.2. Replicative Ability and Pathogenicity of Two H9N2 Avian Influenza Viruses in Mice
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Acknowledgments
Conflicts of Interest
References
- Hassan, M.S.H.; Abdul-Careem, M.F. Avian Viruses that Impact Table Egg Production. Animals 2020, 10, 1747. [Google Scholar] [CrossRef]
- Smithies, L.K.; Radloff, D.B.; Friedell, R.W.; Albright, G.W.; Misner, V.E.; Easterday, B.C. Two Different Type A Influenza Virus Infections in Turkeys in Wisconsin, I. 1965–66 Outbreak. Avian Dis. 1969, 13, 603. [Google Scholar] [CrossRef]
- Homme, P.J.; Easterday, B.C. Avian Influenza Virus Infections. I. Characteristics of Influenza A/Turkey/Wisconsin/1966 Virus. Avian Dis. 1970, 14, 66. [Google Scholar] [CrossRef] [PubMed]
- Carnaccini, S.; Perez, D.R. H9 Influenza Viruses: An Emerging Challenge. Cold Spring Harb Perspect Med. 2020, 10, a038588. [Google Scholar] [CrossRef] [PubMed]
- Peiris, M.; Yuen, K.; Leung, C.; Chan, K.; Ip, P.; Lai, R.; Orr, W.; Shortridge, K. Human infection with influenza H9N2. Lancet 1999, 354, 916–917. [Google Scholar] [CrossRef]
- World Health Organization. Influenza at the Human-Animal Interface Summary and Assessment, from 21 January to 28 February 2020; World Health Organization: Geneva, Switzerland, 2020; pp. 1–2. [Google Scholar]
- World Health Organization. Influenza at the Human-Animal Interface Summary and Assessment, from 28 September 2019 to 25 November 2019; World Health Organization: Geneva, Switzerland, 2019; pp. 1–2. [Google Scholar]
- Guan, Y.; Shortridge, K.F.; Krauss, S.; Chin, P.S.; Dyrting, K.C.; Ellis, T.M.; Webster, R.G.; Peiris, M. H9N2 Influenza Viruses Possessing H5N1-Like Internal Genomes Continue To Circulate in Poultry in Southeastern China. J. Virol. 2000, 74, 9372–9380. [Google Scholar] [CrossRef] [Green Version]
- Lin, Y.P.; Shaw, M.; Gregory, V.; Cameron, K.; Lim, W.; Klimov, A.; Subbarao, K.; Guan, Y.; Krauss, S.; Shortridge, K.; et al. Avian-to-human transmission of H9N2 subtype influenza A viruses: Relationship between H9N2 and H5N1 human isolates. Proc. Natl. Acad. Sci. USA 2000, 97, 9654–9658. [Google Scholar] [CrossRef] [Green Version]
- To, K.K.; Chan, J.F.; Chen, H.; Li, L.; Yuen, K.-Y. The emergence of influenza A H7N9 in human beings 16 years after influenza A H5N1: A tale of two cities. Lancet Infect. Dis. 2013, 13, 809–821. [Google Scholar] [CrossRef]
- Su, S.; Bi, Y.; Wong, G.; Gray, G.C.; Gao, G.F.; Li, S. Epidemiology, Evolution, and Recent Outbreaks of Avian Influenza Virus in China. J. Virol. 2015, 89, 8671–8676. [Google Scholar] [CrossRef] [Green Version]
- Guo, Y.J.; Krauss, S.; Senne, D.A.; Mo, I.P.; Lo, K.S.; Xiong, X.P.; Norwood, M.; Shortridge, K.F.; Webster, R.G.; Guan, Y. Characterization of the Pathogenicity of Members of the Newly Established H9N2 Influenza Virus Lineages in Asia. Virology 2000, 267, 279–288. [Google Scholar] [CrossRef] [Green Version]
- Guan, Y.; Shortridge, K.F.; Krauss, S.; Webster, R.G. Molecular characterization of H9N2 influenza viruses: Were they the donors of the “internal” genes of H5N1 viruses in Hong Kong? Proc. Natl. Acad. Sci. USA 1999, 96, 9363–9367. [Google Scholar] [CrossRef] [Green Version]
- Shibata, A.; Okamatsu, M.; Sumiyoshi, R.; Matsuno, K.; Wang, Z.-J.; Kida, H.; Osaka, H.; Sakoda, Y. Repeated detection of H7N9 avian influenza viruses in raw poultry meat illegally brought to Japan by international flight passengers. Virology 2018, 524, 10–17. [Google Scholar] [CrossRef]
- Shibata, A.; Hiono, T.; Fukuhara, H.; Sumiyoshi, R.; Ohkawara, A.; Matsuno, K.; Okamatsu, M.; Osaka, H.; Sakoda, Y. Isolation and characterization of avian influenza viruses from raw poultry products illegally imported to Japan by international flight passengers. Transbound Emerg. Dis. 2018, 65, 465–475. [Google Scholar] [CrossRef]
- Hoffmann, E.; Stech, J.; Guan, Y.; Webster, R.G.; Perez, D.R. Universal primer set for the full-length amplification of all influenza A viruses. Arch. Virol. 2001, 146, 2275–2289. [Google Scholar] [CrossRef]
- Belser, J.A.; Gustin, K.M.; Pearce, M.B.; Maines, T.R.; Zeng, H.; Pappas, C.; Sun, X.; Carney, P.J.; Villanueva, J.M.; Stevens, J.; et al. Pathogenesis and transmission of avian influenza A (H7N9) virus in ferrets and mice. Nature 2013, 501, 556–559. [Google Scholar] [CrossRef]
- Chen, L.-M.; Blixt, O.; Stevens, J.; Lipatov, A.S.; Davis, C.T.; Collins, B.E.; Cox, N.J.; Paulson, J.C.; Donis, R.O. In vitro evolution of H5N1 avian influenza virus toward human-type receptor specificity. Virology 2012, 422, 105–113. [Google Scholar] [CrossRef] [Green Version]
- Shinya, K.; Ebina, M.; Yamada, S.; Ono, M.; Kasai, N.; Kawaoka, Y. Influenza virus receptors in the human airway. Nature 2006, 440, 435–436. [Google Scholar] [CrossRef]
- Li, J.; Ishaq, M.; Prudence, M.; Xi, X.; Hu, T.; Liu, Q.; Guo, D. Single mutation at the amino acid position 627 of PB2 that leads to increased virulence of an H5N1 avian influenza virus during adaptation in mice can be compensated by multiple mutations at other sites of PB2. Virus Res. 2009, 144, 123–129. [Google Scholar] [CrossRef]
- Song, J.; Xu, J.; Shi, J.; Li, Y.; Chen, H. Synergistic Effect of S224P and N383D Substitutions in the PA of H5N1 Avian Influenza Virus Contributes to Mammalian Adaptation. Sci. Rep. 2015, 5, 10510. [Google Scholar] [CrossRef] [Green Version]
- Rogers, G.N.; Paulson, J.C.; Daniels, R.S.; Skehel, J.J.; Wilson, I.A.; Wiley, D.C. Single amino acid substitutions in influenza haemagglutinin change receptor binding specificity. Nature 1983, 304, 76–78. [Google Scholar] [CrossRef]
- Wan, H.; Perez, D.R. Amino Acid 226 in the Hemagglutinin of H9N2 Influenza Viruses Determines Cell Tropism and Replication in Human Airway Epithelial Cells. JVI 2007, 81, 5181–5191. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Teng, Q.; Xu, D.; Shen, W.; Liu, Q.; Rong, G.; Li, X.; Yan, L.; Yang, J.; Chen, H.; Yu, H.; et al. A Single Mutation at Position 190 in Hemagglutinin Enhances Binding Affinity for Human Type Sialic Acid Receptor and Replication of H9N2 Avian Influenza Virus in Mice. J. Virol. 2016, 90, 9806–9825. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- H5N1 Virus Attachment to Lower Respiratory Tract. Science 2006, 312, 399. [CrossRef] [Green Version]
- Suzuki, Y.; Ito, T.; Suzuki, T.; Holland, R.E.; Chambers, T.M.; Kiso, M.; Ishida, H.; Kawaoka, Y. Sialic Acid Species as a Determinant of the Host Range of Influenza A Viruses. J. Virol. 2000, 74, 11825–11831. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Receptor determinants of human and animal influenza virus isolates: Differences in receptor specificity of the H3 hemagglutinin based on species of origin. Virology 1983, 127, 361–373. [CrossRef]
- Wang, W.; Lu, B.; Zhou, H.; Suguitan, A.L.; Cheng, X.; Subbarao, K.; Kemble, G.; Jin, H. Glycosylation at 158N of the Hemagglutinin Protein and Receptor Binding Specificity Synergistically Affect the Antigenicity and Immunogenicity of a Live Attenuated H5N1 A/Vietnam/1203/2004 Vaccine Virus in Ferrets. J. Virol. 2010, 84, 6570–6577. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kaverin, N.V.; Rudneva, I.A.; Ilyushina, N.A.; Lipatov, A.S.; Krauss, S.; Webster, R.G. Structural Differences among Hemagglutinins of Influenza A Virus Subtypes Are Reflected in Their Antigenic Architecture: Analysis of H9 Escape Mutants. J. Virol. 2004, 78, 240–249. [Google Scholar] [CrossRef] [Green Version]
- Kamiki, H.; Matsugo, H.; Kobayashi, T.; Ishida, H.; Takenaka-Uema, A.; Murakami, S.; Horimoto, T. A PB1-K577E Mutation in H9N2 Influenza Virus Increases Polymerase Activity and Pathogenicity in Mice. Viruses 2018, 10, 653. [Google Scholar] [CrossRef] [Green Version]
- Hu, M.; Chu, H.; Zhang, K.; Singh, K.; Li, C.; Yuan, S.; Chow, B.K.C.; Song, W.; Zhou, J.; Zheng, B.-J. Amino acid substitutions V63I or A37S/I61T/V63I/V100A in the PA N-terminal domain increase the virulence of H7N7 influenza A virus. Sci. Rep. 2016, 6, 37800. [Google Scholar] [CrossRef] [Green Version]
- Yamayoshi, S.; Fukuyama, S.; Yamada, S.; Zhao, D.; Murakami, S.; Uraki, R.; Watanabe, T.; Tomita, Y.; Neumann, G.; Kawaoka, Y. Amino acids substitutions in the PB2 protein of H7N9 influenza A viruses are important for virulence in mammalian hosts. Sci. Rep. 2015, 5, 8039. [Google Scholar] [CrossRef]
- Xu, G.; Zhang, X.; Gao, W.; Wang, C.; Wang, J.; Sun, H.; Sun, Y.; Guo, L.; Zhang, R.; Chang, K.-C.; et al. Prevailing PA Mutation K356R in Avian Influenza H9N2 Virus Increases Mammalian Replication and Pathogenicity. J. Virol. 2016, 90, 8105–8114. [Google Scholar] [CrossRef] [Green Version]
- Feng, X.; Wang, Z.; Shi, J.; Deng, G.; Kong, H.; Tao, S.; Li, C.; Liu, L.; Guan, Y.; Chen, H. Glycine at Position 622 in PB1 Contributes to the Virulence of H5N1 Avian Influenza Virus in Mice. J. Virol. 2016, 90, 1872–1879. [Google Scholar] [CrossRef] [Green Version]
- Kandeil, A.; El-Shesheny, R.; Maatouq, A.M.; Moatasim, Y.; Shehata, M.M.; Bagato, O.; Rubrum, A.; Shanmuganatham, K.; Webby, R.J.; Ali, M.A.; et al. Genetic and antigenic evolution of H9N2 avian influenza viruses circulating in Egypt between 2011 and 2013. Arch. Virol. 2014, 159, 2861–2876. [Google Scholar] [CrossRef]
- Xiao, C.; Ma, W.; Sun, N.; Huang, L.; Li, Y.; Zeng, Z.; Wen, Y.; Zhang, Z.; Li, H.; Li, Q.; et al. PB2-588 V promotes the mammalian adaptation of H10N8, H7N9 and H9N2 avian influenza viruses. Sci. Rep. 2016, 6, 19474. [Google Scholar] [CrossRef] [Green Version]
- Gao, W.; Zu, Z.; Liu, J.; Song, J.; Wang, X.; Wang, C.; Liu, L.; Tong, Q.; Wang, M.; Sun, H.; et al. Prevailing I292V PB2 mutation in avian influenza H9N2 virus increases viral polymerase function and attenuates IFN-β induction in human cells. J. Gen. Virol. 2019, 100, 1273–1281. [Google Scholar] [CrossRef]
- Wan, H.; Sorrell, E.M.; Song, H.; Hossain, M.J.; Ramirez-Nieto, G.; Monne, I.; Stevens, J.; Cattoli, G.; Capua, I.; Chen, L.-M.; et al. Replication and Transmission of H9N2 Influenza Viruses in Ferrets: Evaluation of Pandemic Potential. PLoS ONE 2008, 3, e2923. [Google Scholar] [CrossRef] [Green Version]
- Li, X.; Shi, J.; Guo, J.; Deng, G.; Zhang, Q.; Wang, J.; He, X.; Wang, K.; Chen, J.; Li, Y.; et al. Genetics, Receptor Binding Property, and Transmissibility in Mammals of Naturally Isolated H9N2 Avian Influenza Viruses. PLoS Pathog 2014, 10, e1004508. [Google Scholar] [CrossRef]
| Protein | Amino Acid | Viruses Tested in This Study | Phenotype | Subtypes Tested | ||||
|---|---|---|---|---|---|---|---|---|
| Residue | Avian- like Motif | Mammalian-like Motif | Ck/HE28-50 | Ck/HE28-57 | ||||
| PB2 | 292 | I | V | I | V | Increased polymerase activity in mammalian and avian cell lines, increased virulence in mice | H9N2 | |
| Increased polymerase activity in mammalian cell line | H10N8 | |||||||
| 504 | I | V | V | V | Increased virulence in mice | H9 | ||
| 588 | A | V | V | V | Increased polymerase activity and replication in mammalian and avian cell lines, increased virulence in mice | H7N9, H9N2, H10N8 | ||
| 89, 309 | 89 | L | V | V | V | Increased polymerase activity and replication in mammalian cells, increased virulence in mice | H5N1 | |
| 309 | G | D | D | D | ||||
| 340,588 | 340 | R | K | R | K | Transmission in guinea pigs | H9N2 | |
| 588 | A | V | V | V | ||||
| PB1 | 622 | D | G | G | G | Increased polymerase activity and enhanced replication in mammalian cell line, increased virulence in mice | H5N1 | |
| PA | 63 | V | I | I | I | Increased polymerase activity and enhanced replication in mammalian cell line, increased virulence in mice | H7N7 | |
| 356 | K | R | R | R | Increased polymerase activity and replication in mammalian cell line, increased virulence in mice | H9N2 | ||
| 383 | N | D | D | D | Increased polymerase activity in avian and mammalian cell lines | H5N1 | ||
| HA | 159 | S | N | N | N | Increased virus binding to α2-6SA | H5N1 | |
| 160 | T | A | T | A | Increased virus binding to α2-6SA, increased transmission ability in guinea pigs | H5N1 | ||
| 190 | T | V | V | V | Enhanced binding affinity to mammalian cells and replication in mammalian cells | H9N2 | ||
| 198 | N | T | T | T | Increase replication and transmission in ferrets | H9 | ||
| 226 | Q | L | L | L | Increased virus binding to α2-6SA, enhanced replication in mammalian cells and ferrets, enhanced contact transmission in ferrets | H9N2 | ||
| M1 | 30 | N | D | D | D | Increased virulence in mice | H5N1 | |
| 43 | I | M | M | M | Increased virulence in mice, chickens, and ducks | H5N1 | ||
| 215 | T | A | A | A | Increased virulence in mice | H5N1 | ||
| NS | 42 | P | S | S | S | Increased virulence in mice | H5N1 | |
| 138 | C | F | F | F | Increased replication mammalian cells, decreased interferon response | H5N1 | ||
| 149 | V | A | A | A | Increased virulence and decreased interferon response | H5N1 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Murakami, J.; Shibata, A.; Neumann, G.; Imai, M.; Watanabe, T.; Kawaoka, Y. Characterization of H9N2 Avian Influenza Viruses Isolated from Poultry Products in a Mouse Model. Viruses 2022, 14, 728. https://doi.org/10.3390/v14040728
Murakami J, Shibata A, Neumann G, Imai M, Watanabe T, Kawaoka Y. Characterization of H9N2 Avian Influenza Viruses Isolated from Poultry Products in a Mouse Model. Viruses. 2022; 14(4):728. https://doi.org/10.3390/v14040728
Chicago/Turabian StyleMurakami, Jurika, Akihiro Shibata, Gabriele Neumann, Masaki Imai, Tokiko Watanabe, and Yoshihiro Kawaoka. 2022. "Characterization of H9N2 Avian Influenza Viruses Isolated from Poultry Products in a Mouse Model" Viruses 14, no. 4: 728. https://doi.org/10.3390/v14040728
APA StyleMurakami, J., Shibata, A., Neumann, G., Imai, M., Watanabe, T., & Kawaoka, Y. (2022). Characterization of H9N2 Avian Influenza Viruses Isolated from Poultry Products in a Mouse Model. Viruses, 14(4), 728. https://doi.org/10.3390/v14040728





