Prognosis of Indolent Adult T-Cell Leukemia/Lymphoma
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Statistical Analysis
3. Results
3.1. Patient Characteristics
3.2. Disease Progression and Therapy
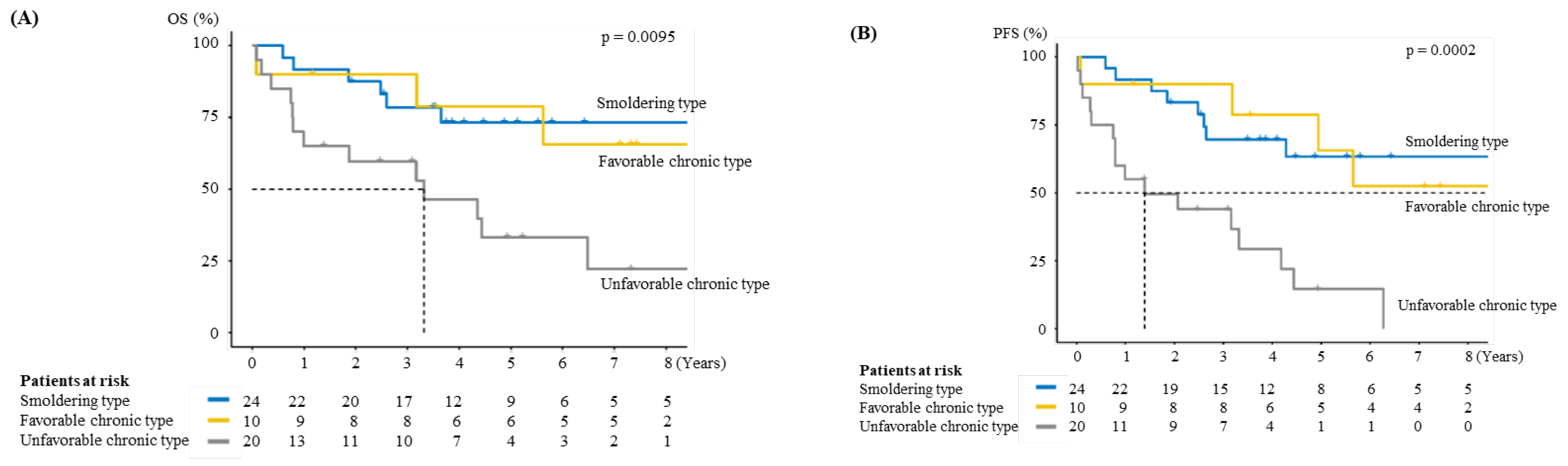
3.3. Prognosis
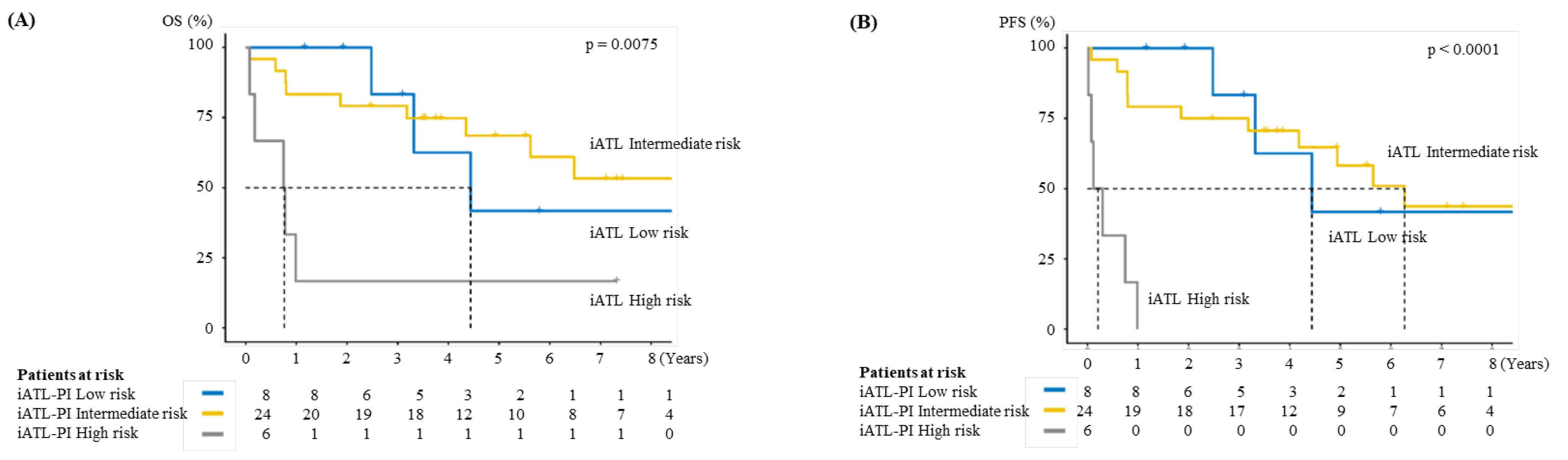
3.4. Validation of Indolent ATL-Prognostic Index (iATL-PI)
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Uchiyama, T.; Yodoi, J.; Sagawa, K.; Takatsuki, K.; Uchino, H. Adult T-cell leukemia: Clinical and hematologic features of 16 cases. Blood 1977, 50, 481–492. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vose, J.; Armitage, J.; Weisenburger, D.; International, T.C.L.P. International peripheral T-cell and natural killer/T-cell lymphoma study: Pathology findings and clinical outcomes. J. Clin. Oncol. 2008, 26, 4124–4130. [Google Scholar] [CrossRef] [PubMed]
- Swerdlow, S.H.; Campo, E.; Harris, N.L.; Jaffe, E.S.; Pileri, S.A.; Stein, H.; Thiele, J.; Vardiman, J.W. (Eds.) WHO Classification of Tumors of Haematopoietic and Lymphoid Tissues. In Adult T-Cell Leukaemia/Lymphoma; IARC Press: Lyon, France, 2008; pp. 281–284. [Google Scholar]
- Shimoyama, M. Diagnostic criteria and classification of clinical subtypes of adult T-cell leukaemia-lymphoma. A report from the Lymphoma Study Group (1984–1987). Br. J. Haematol. 1991, 79, 428–437. [Google Scholar] [CrossRef] [PubMed]
- Shimoyama, M. Chemotherapy of ATL. In Adult T-Cell Leukemia; Takatsuki, K., Ed.; Oxford University Press: Oxford, UK, 1994; pp. 221–227. [Google Scholar]
- Katsuya, H.; Shimokawa, M.; Ishitsuka, K.; Kawai, K.; Amano, M.; Utsunomiya, A.; Hino, R.; Hanada, S.; Jo, T.; Tsukasaki, K.; et al. Prognostic index for chronic- and smoldering-type adult T-cell leukemia-lymphoma. Blood 2017, 130, 39–47. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Imaizumi, Y.; Iwanaga, M.; Nosaka, K.; Ishitsuka, K.; Ishizawa, K.; Ito, S.; Amano, M.; Ishida, T.; Uike, N.; Utsunomiya, A.; et al. Prognosis of patients with adult T-cell leukemia/lymphoma in Japan: A nationwide hospital-based study. Cancer Sci. 2020, 111, 4567–4580. [Google Scholar] [CrossRef] [PubMed]
- Takasaki, Y.; Iwanaga, M.; Imaizumi, Y.; Tawara, M.; Joh, T.; Kohno, T.; Yamada, Y.; Kamihira, S.; Ikeda, S.; Miyazaki, Y.; et al. Long-term study of indolent adult T-cell leukemia-lymphoma. Blood 2010, 115, 4337–4343. [Google Scholar] [CrossRef] [PubMed]
- Sekine, M.; Kameda, T.; Shide, K.; Maeda, K.; Toyama, T.; Kawano, N.; Takeuchi, M.; Kawano, H.; Sato, S.; Ishizaki, J.; et al. Higher average chemotherapy dose intensity improves prognosis in patients with aggressive adult T-cell leukemia/lymphoma. Eur. J. Haematol. 2021, 106, 398–407. [Google Scholar] [CrossRef]
- Katsuya, H.; Ishitsuka, K.; Utsunomiya, A.; Hanada, S.; Eto, T.; Moriuchi, Y.; Saburi, Y.; Miyahara, M.; Sueoka, E.; Uike, N.; et al. Treatment and survival among 1594 patients with ATL. Blood 2015, 126, 2570–2577. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Katsuya, H.; Yamanaka, T.; Ishitsuka, K.; Utsunomiya, A.; Sasaki, H.; Hanada, S.; Eto, T.; Moriuchi, Y.; Saburi, Y.; Miyahara, M.; et al. Prognostic index for acute- and lymphoma-type adult T-cell leukemia/lymphoma. J. Clin. Oncol. 2012, 30, 1635–1640. [Google Scholar] [CrossRef] [PubMed]
- Tsukasaki, K.; Marcais, A.; Nasr, R.; Kato, K.; Fukuda, T.; Hermine, O.; Bazarbachi, A. Diagnostic Approaches and Established Treatments for Adult T Cell Leukemia Lymphoma. Front. Microbiol. 2020, 11, 1207. [Google Scholar] [CrossRef] [PubMed]
| Variable | Smoldering Type | Chronic Type | p |
|---|---|---|---|
| n | 24 | 30 | |
| Age (median [IQR]) | 75.5 (65.5, 80.5) | 73.0 [66.0, 81.0) | 0.882 |
| Sex, Female/Male, no (%) | 8/16 (33.3/66.7) | 18/12 (60.0/40.0) | 0.094 |
| ECOG PS, no (%) | 0.219 | ||
| 0 | 15 (62.5) | 15 (50.0) | |
| 1 | 6 (25.0) | 7 (23.3) | |
| 2 | 3 (12.5) | 2 (6.7) | |
| 3 | 0 (0.0) | 5 (16.7) | |
| 4 | 0 (0.0) | 1 (3.3) | |
| Skin lesion, absent/present, no (%) | 17/7 (70.8/29.2) | 21/9 (70.0/30.0) | 1 |
| Lung involvement, absent/present, no (%) | 23/1 (95.8/4.2) | 29/1 (96.7/3.3) | 1 |
| WBC count, ×109/L, median [IQR] | 6.73 (5.74, 7.48) | 12.70 (9.43, 15.19) | <0.001 |
| Neutrophil count, ×109/L, median [IQR] | 3.56 (3.01, 4.82) | 3.64 (2.72, 4.22) | 0.657 |
| Lymphocyte count, ×109/L, median [IQR] | 1.63 (1.20, 2.54) | 3.22 (1.92, 4.59) | 0.006 |
| Abnormal lymphocyte proportion, %, (median [IQR]) | 5.25 (3.00, 8.62) | 31.3 (13.3, 53.5) | <0.001 |
| Abnormal lymphocyte count, ×109/L, median [IQR] | 0.37 (0.15, 0.60) | 3.21 (2.49, 7.08) | <0.001 |
| Hemoglobin level, g/dL, median [IQR] | 12.8 (11.7, 14.3) | 12.4 (11.5, 14.0) | 0.662 |
| Platelet count, ×109/L, median [IQR] | 20.0 (17.0, 24.5) | 17.5 (13.0, 22.0) | 0.055 |
| Serum TP, g/dL, median [IQR] | 6.95 (6.68, 7.53) | 6.84 (6.30, 7.27) | 0.388 |
| Serum Alb, g/dL, median [IQR] | 3.95 (3.70, 4.15) | 4.00 (3.70, 4.21) | 0.752 |
| BUN, mg/dL, median [IQR] | 15.1 (11.0, 17.3) | 15.2 (12.6, 19.9) | 0.329 |
| Cre, mg/dL, median [IQR] | 0.80 (0.64, 1.00) | 0.70 (0.60, 0.83) | 0.251 |
| Ca, mg/dL, median [IQR] | 9.15 (9.00, 9.53) | 9.30 (9.03, 9.40) | 0.6 |
| T-Bil, mg/dL, median [IQR] | 0.60 (0.50, 0.70) | 0.70 (0.60, 0.90) | 0.056 |
| ALT, IU/L, median [IQR] | 17.5 (13.8, 24.3) | 20.0 (12.0, 27.8) | 0.889 |
| LDH, IU/L, median [IQR] | 199 (183,220) | 228 (198,291) | 0.119 |
| CRP, mg/dL, median [IQR] | 0.46 (0.10, 1.53) | 0.10 (0.04, 0.40) | 0.028 |
| sIL-2R, U/mL, median [IQR] | 1180 (930,1730) | 2190 (1300, 5040) | 0.015 |
| iATL-PI, no (%) | 0.121 | ||
| Low | 4 (30.8) | 4 (16.0) | |
| Intermediate | 9 (69.2) | 15 (60.0) | |
| High | 0 (0.0) | 6 (24.0) |
| Variable | Favorable Chronic Type | Unfavorable Chronic Type | p |
|---|---|---|---|
| n | 10 | 20 | |
| Age (median [IQR]) | 70.5 (64.0, 79.0) | 74.5 (68.0, 81.0) | 0.441 |
| Sex, Female/Male, no (%) | 8/2 (80.0/20.0) | 10/10 (50.0/50.0) | 0.236 |
| ECOG PS, no (%) | 0.312 | ||
| 0 | 7 (70.0) | 8 (40.0) | |
| 1 | 2 (20.0) | 5 (25.0) | |
| 2 | 1 (10.0) | 1 (5.0) | |
| 3 | 0 (0.0) | 5 (25.0) | |
| 4 | 0 (0.0) | 1 (5.0) | |
| Skin lesion, absent/present, no (%) | 9/1 (90.0/10.0) | 12/8 (60.0/40.0) | 0.205 |
| Lung involvement, absent/present, no (%) | 10/0 (100/0) | 19/1 (95.0/5.0) | 1 |
| WBC count, ×109/L, median [IQR] | 10.9 (8.75, 17.8) | 13.5 (9.88, 14.7) | 0.792 |
| Neutrophil count, ×109/L, median [IQR] | 3.14 (2.72, 3.99) | 3.74 (2.96, 4.37) | 0.481 |
| Lymphocyte count, ×109/L, median [IQR] | 3.84 (2.30, 5.09) | 3.03 (1.39, 4.53) | 0.455 |
| Abnormal lymphocyte proportion, %, (median [IQR]) | 23.0 (8.6, 31.6) | 40.0 (19.3, 58.0) | 0.179 |
| Abnormal lymphocyte count, ×109/L, median [IQR] | 2.72 (1.12, 4.01) | 3.83 (2.50, 7.29) | 0.356 |
| Hemoglobin level, g/dL, median [IQR] | 13.8 (11.8, 14.2) | 12.0 (11.6, 13.2) | 0.185 |
| Platelet count, ×109/L, median [IQR] | 20.0 (17.0, 22.0) | 14.5 (12.7, 20.0) | 0.333 |
| Serum TP, g/dL, median [IQR] | 7.20 (6.93, 7.38) | 6.70 (6.20, 7.03) | 0.025 |
| Serum Alb, g/dL, median [IQR] | 4.20 (4.00, 4.57) | 3.80 (3.55, 4.03) | 0.012 |
| BUN, mg/dL, median [IQR] | 12.7 (10.2, 15.3) | 18.5 (13.0, 22.9) | 0.014 |
| Cre, mg/dL, median [IQR] | 0.60 (0.52, 0.70) | 0.80 (0.67, 0.86) | 0.039 |
| Ca, mg/dL, median [IQR] | 9.25 (9.00, 9.30) | 9.30 (9.10, 9.43) | 0.58 |
| T-Bil, mg/dL, median [IQR] | 0.64 (0.60, 0.78) | 0.70 (0.65, 0.92) | 0.34 |
| ALT, IU/L, median [IQR] | 14.0 (12.0, 26.0) | 20.5 (13.5, 28.5) | 0.481 |
| LDH, IU/L, median [IQR] | 187 (161,202) | 260 (231,326) | <0.001 |
| CRP, mg/dL, median [IQR] | 0.04 (0.01, 0.06) | 0.13 (0.10, 0.57) | 0.021 |
| sIL-2R, U/mL, median [IQR] | 1595 (1308, 2020) | 4840 (1735, 9570) | 0.052 |
| iATL-PI, no (%) | 0.03 | ||
| Low | 1 (10.0) | 3 (20.0) | |
| Intermediate | 9 (90.0) | 6 (40.0) | |
| High | 0 (0.0) | 6 (40.0) |
| Variable | Univariate | Multivariate | ||
|---|---|---|---|---|
| HR (95% CI) | p | HR (95% CI) | p | |
| Age | 1.05 (0.99–1.12) | 0.110 | ||
| Sex, male vs. female | 1.02 (0.39–2.66) | 0.968 | ||
| Subtype, chronic vs. smoldering | 2.65 (0.76–9.23) | 0.126 | ||
| ECOG PS | 1.47 (1.02–2.14) | 0.041 | 1.03 (0.63–1.70) | 0.898 |
| Skin lesion, present vs. absent | 0.59 (0.19–1.82) | 0.356 | ||
| WBC count, ×109/L # | 1.06 (0.97–1.15) | 0.203 | ||
| Neutrophil count, ×109/L # | 1.08 (0.93–1.25) | 0.325 | ||
| Lymphocyte count, ×109/L # | 0.94 (0.78–1.13) | 0.509 | ||
| Abnormal lymphocyte count, ×109/L # | 1.12 (1.00–1.25) | 0.050 | 1.07 (0.90–1.27) | 0.434 |
| Hemoglobin level, g/dL | 0.89 (0.69–1.15) | 0.370 | ||
| Platelet count, ×109/L # | 1.02 (0.96–1.07) | 0.562 | ||
| Serum TP, g/dL | 0.64 (0.31–1.34) | 0.238 | ||
| Serum Alb, g/dL | 0.45 (0.18–1.14) | 0.093 | 0.42 (0.13–1.44) | 0.168 |
| BUN, mg/dL | 1.02 (0.95–1.10) | 0.586 | ||
| Cre, mg/dL | 0.91 (0.32–2.61) | 0.861 | ||
| Ca, mg/dL | 0.93 (0.40–2.18) | 0.866 | ||
| T-Bil, mg/dL | 0.72 (0.11–4.52) | 0.723 | ||
| ALT, IU/L | 1.02 (0.99–1.05) | 0.277 | ||
| LDH, ×102 IU/L # | 1.50 (0.93–2.42) | 0.101 | ||
| sIL2-R, ×103 U/mL # | 1.07 (1.03–1.11) | 0.001 | 1.06 (1.01–1.11) | 0.010 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kameda, T.; Shide, K.; Tahira, Y.; Sekine, M.; Sato, S.; Ishizaki, J.; Takeuchi, M.; Akizuki, K.; Kamiunten, A.; Shimoda, H.; et al. Prognosis of Indolent Adult T-Cell Leukemia/Lymphoma. Viruses 2022, 14, 710. https://doi.org/10.3390/v14040710
Kameda T, Shide K, Tahira Y, Sekine M, Sato S, Ishizaki J, Takeuchi M, Akizuki K, Kamiunten A, Shimoda H, et al. Prognosis of Indolent Adult T-Cell Leukemia/Lymphoma. Viruses. 2022; 14(4):710. https://doi.org/10.3390/v14040710
Chicago/Turabian StyleKameda, Takuro, Kotaro Shide, Yuki Tahira, Masaaki Sekine, Seiichi Sato, Junzo Ishizaki, Masanori Takeuchi, Keiichi Akizuki, Ayako Kamiunten, Haruko Shimoda, and et al. 2022. "Prognosis of Indolent Adult T-Cell Leukemia/Lymphoma" Viruses 14, no. 4: 710. https://doi.org/10.3390/v14040710
APA StyleKameda, T., Shide, K., Tahira, Y., Sekine, M., Sato, S., Ishizaki, J., Takeuchi, M., Akizuki, K., Kamiunten, A., Shimoda, H., Toyama, T., Maeda, K., Yamashita, K., Kawano, N., Kawano, H., Hidaka, T., Yamaguchi, H., Kubuki, Y., Kitanaka, A., ... Shimoda, K. (2022). Prognosis of Indolent Adult T-Cell Leukemia/Lymphoma. Viruses, 14(4), 710. https://doi.org/10.3390/v14040710






