Longitudinal Analysis of Neutralizing Potency against SARS-CoV-2 in the Recovered Patients after Treatment with or without Favipiravir
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Case and Control Definition
2.3. Sample Collection
2.4. Neutralizing Antibody Titer Measurement
2.5. ELISA
2.6. Quantification and Statistical Analysis
3. Results
3.1. Patients Characteristics
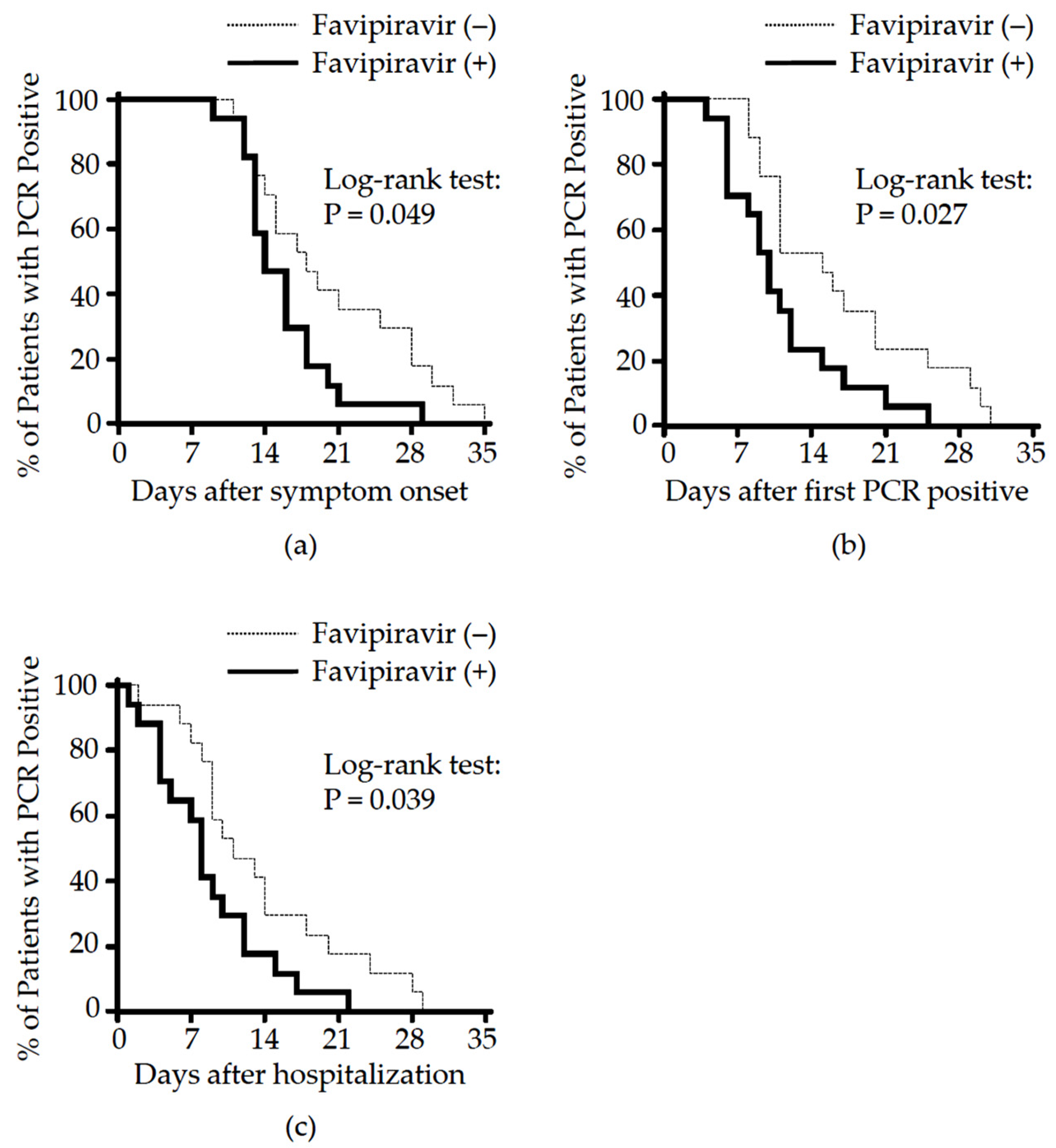
3.2. Effect of Favipiravir Treatment on Clinical Outcome
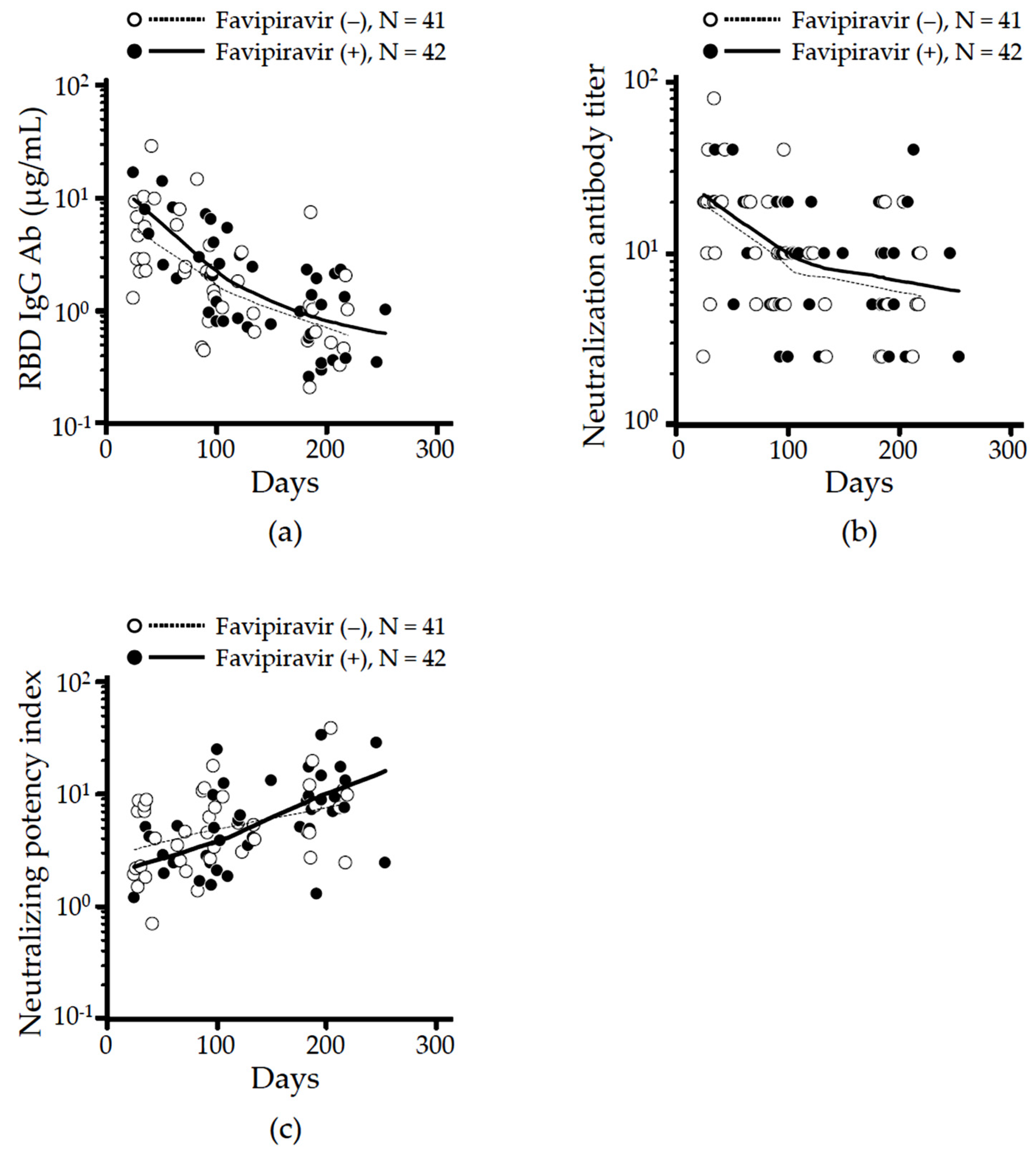
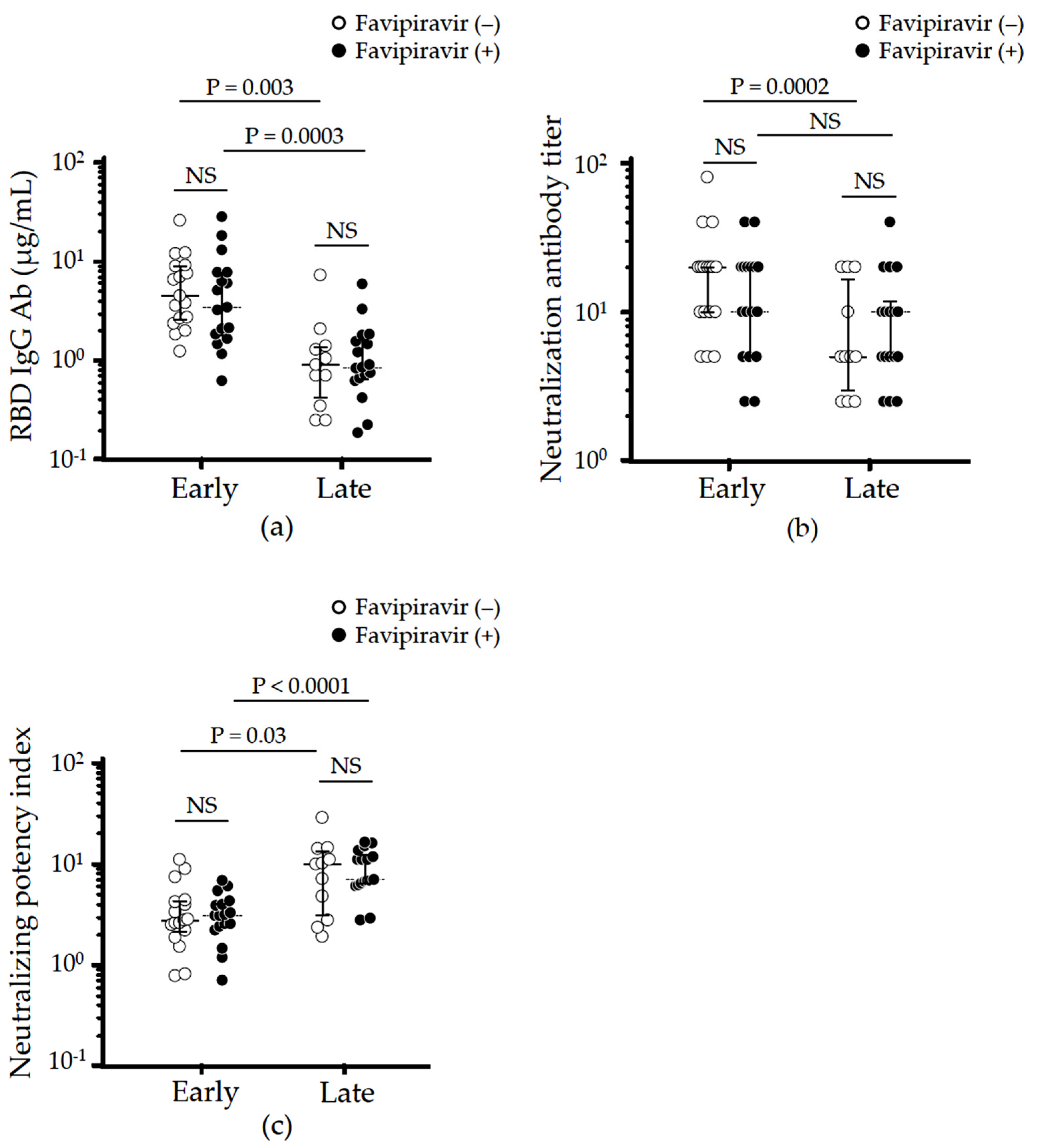
3.3. Effect of Favipiravir Treatment on Long-Term Neutralizing Antibodies Production
3.4. Effect of Favipiravir Treatment on Cross-Reactive Humoral Immunity against SARS-CoV-2 Variants
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization. COVID-19 Weekly Epidemiological Update. Available online: https://covid19.who.int/ (accessed on 30 June 2021).
- Altay, O.; Mohammadi, E.; Lam, S.; Turkez, H.; Boren, J.; Nielsen, J.; Uhlen, M.; Mardinoglu, A. Current Status of COVID-19 Therapies and Drug Repositioning Applications. iScience 2020, 23, 101303. [Google Scholar] [CrossRef] [PubMed]
- Cai, Q.; Yang, M.; Liu, D.; Chen, J.; Shu, D.; Xia, J.; Liao, X.; Gu, Y.; Cai, Q.; Yang, Y.; et al. Experimental Treatment with Favipiravir for COVID-19: An Open-Label Control Study. Engineering 2020, 6, 1192–1198. [Google Scholar] [CrossRef]
- Doi, Y.; Hibino, M.; Hase, R.; Yamamoto, M.; Kasamatsu, Y.; Hirose, M.; Mutoh, Y.; Homma, Y.; Terada, M.; Ogawa, T.; et al. A Prospective, Randomized, Open-Label Trial of Early versus Late Favipiravir Therapy in Hospitalized Patients with COVID-19. Antimicrob. Agents Chemother. 2020, 64, e01897-20. [Google Scholar] [CrossRef] [PubMed]
- ClinicalTrials.gov. Favipiravir for Patients With Mild to Moderate Disease from Novel Coronavirus (COVID-19). Available online: https://clinicaltrials.gov/ct2/show/NCT04600895 (accessed on 21 October 2021).
- Shinkai, M.; Tsushima, K.; Tanaka, S.; Hagiwara, E.; Tarumoto, N.; Kawada, I.; Hirai, Y.; Fujiwara, S.; Komase, Y.; Saraya, T.; et al. Efficacy and Safety of Favipiravir in Moderate COVID-19 Pneumonia Patients without Oxygen Therapy: A Randomized, Phase III Clinical Trial. Infect. Dis. Ther. 2021, 10, 2489–2509. [Google Scholar] [CrossRef] [PubMed]
- ClinicalTrials.gov. Available online: https://clinicaltrials.gov/ct2/results?cond=COVID-19&term=Favipiravir&cntry=&state=&city=&dist= (accessed on 17 March 2022).
- Hassanipour, S.; Arab-Zozani, M.; Amani, B.; Heidarzad, F.; Fathalipour, M.; Martinez-de-Hoyo, R. The efficacy and safety of Favipiravir in treatment of COVID-19: A systematic review and meta-analysis of clinical trials. Sci. Rep. 2021, 11, 11022. [Google Scholar] [CrossRef]
- FUJIFILM. Fujifilm Announces the Start of a New Phase III Clinical Trial of Anti-Influenza Drug Avigan® Tablet in Japan, Targeting COVID-19 Patients. Available online: https://www.fujifilm.com/jp/en/news/hq/6478 (accessed on 30 June 2021).
- Japan Registry of Clinical Trials. Phase III Clinical Trial of Favipiravir in Early-Onset COVID-19 Patients with Severe Risk Factors-Placebo-Controlled, Stratified Randomized, Multicenter, Double-Blind Study. Available online: https://jrct.niph.go.jp/en-latest-detail/jRCT2041210004 (accessed on 13 June 2021).
- Marois, I.; Cloutier, A.; Garneau, E.; Lesur, O.; Richter, M.V. The administration of oseltamivir results in reduced effector and memory CD8+ T cell responses to influenza and affects protective immunity. FASEB J. 2015, 29, 973–987. [Google Scholar] [CrossRef]
- Shinahara, W.; Takahashi, E.; Sawabuchi, T.; Arai, M.; Hirotsu, N.; Takasaki, Y.; Shindo, S.; Shibao, K.; Yokoyama, T.; Nishikawa, K.; et al. Immunomodulator clarithromycin enhances mucosal and systemic immune responses and reduces re-infection rate in pediatric patients with influenza treated with antiviral neuraminidase inhibitors: A retrospective analysis. PLoS ONE 2013, 8, e70060. [Google Scholar] [CrossRef] [Green Version]
- Takahashi, E.; Kataoka, K.; Fujii, K.; Chida, J.; Mizuno, D.; Fukui, M.; Hiro, O.I.; Fujihashi, K.; Kido, H. Attenuation of inducible respiratory immune responses by oseltamivir treatment in mice infected with influenza A virus. Microbes Infect. 2010, 12, 778–783. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Tostanoski, L.H.; Peter, L.; Mercado, N.B.; McMahan, K.; Mahrokhian, S.H.; Nkolola, J.P.; Liu, J.; Li, Z.; Chandrashekar, A.; et al. DNA vaccine protection against SARS-CoV-2 in rhesus macaques. Science 2020, 369, 806–811. [Google Scholar] [CrossRef]
- Gottlieb, R.L.; Nirula, A.; Chen, P.; Boscia, J.; Heller, B.; Morris, J.; Huhn, G.; Cardona, J.; Mocherla, B.; Stosor, V.; et al. Effect of Bamlanivimab as Monotherapy or in Combination With Etesevimab on Viral Load in Patients With Mild to Moderate COVID-19: A Randomized Clinical Trial. JAMA 2021, 325, 632–644. [Google Scholar] [CrossRef]
- Weinreich, D.M.; Sivapalasingam, S.; Norton, T.; Ali, S.; Gao, H.; Bhore, R.; Musser, B.J.; Soo, Y.; Rofail, D.; Im, J.; et al. REGN-COV2, a Neutralizing Antibody Cocktail, in Outpatients with COVID-19. N. Engl. J. Med. 2021, 384, 238–251. [Google Scholar] [CrossRef] [PubMed]
- Bosnjak, B.; Stein, S.C.; Willenzon, S.; Cordes, A.K.; Puppe, W.; Bernhardt, G.; Ravens, I.; Ritter, C.; Schultze-Florey, C.R.; Godecke, N.; et al. Low serum neutralizing anti-SARS-CoV-2 S antibody levels in mildly affected COVID-19 convalescent patients revealed by two different detection methods. Cell. Mol. Immunol. 2021, 18, 936–944. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Liang, B.; Chen, C.; Wang, H.; Fang, Y.; Shen, S.; Yang, X.; Wang, B.; Chen, L.; Chen, Q.; et al. SARS-CoV-2 infection induces sustained humoral immune responses in convalescent patients following symptomatic COVID-19. Nat. Commun. 2021, 12, 1813. [Google Scholar] [CrossRef]
- Garcia-Beltran, W.F.; Lam, E.C.; Astudillo, M.G.; Yang, D.; Miller, T.E.; Feldman, J.; Hauser, B.M.; Caradonna, T.M.; Clayton, K.L.; Nitido, A.D.; et al. COVID-19-neutralizing antibodies predict disease severity and survival. Cell 2021, 184, 476–488.e11. [Google Scholar] [CrossRef] [PubMed]
- Moriyama, S.; Adachi, Y.; Sato, T.; Tonouchi, K.; Sun, L.; Fukushi, S.; Yamada, S.; Kinoshita, H.; Nojima, K.; Kanno, T.; et al. Temporal maturation of neutralizing antibodies in COVID-19 convalescent individuals improves potency and breadth to circulating SARS-CoV-2 variants. Immunity 2021, 54, 1841–1852.e4. [Google Scholar] [CrossRef] [PubMed]
- Ong, S.W.X.; Chiew, C.J.; Ang, L.W.; Mak, T.M.; Cui, L.; Toh, M.; Lim, Y.D.; Lee, P.H.; Lee, T.H.; Chia, P.Y.; et al. Clinical and virological features of SARS-CoV-2 variants of concern: A retrospective cohort study comparing B.1.1.7 (Alpha), B.1.315 (Beta), and B.1.617.2 (Delta). Clin. Infect. Dis. 2021; in press. [Google Scholar] [CrossRef]
- Tegally, H.; Wilkinson, E.; Giovanetti, M.; Iranzadeh, A.; Fonseca, V.; Giandhari, J.; Doolabh, D.; Pillay, S.; San, E.J.; Msomi, N.; et al. Detection of a SARS-CoV-2 variant of concern in South Africa. Nature 2021, 592, 438–443. [Google Scholar] [CrossRef] [PubMed]
- Torjesen, I. COVID-19: Omicron may be more transmissible than other variants and partly resistant to existing vaccines, scientists fear. BMJ 2021, 375, n2943. [Google Scholar] [CrossRef]
- Volz, E.; Mishra, S.; Chand, M.; Barrett, J.C.; Johnson, R.; Geidelberg, L.; Hinsley, W.R.; Laydon, D.J.; Dabrera, G.; O’Toole, A.; et al. Assessing transmissibility of SARS-CoV-2 lineage B.1.1.7 in England. Nature 2021, 593, 266–269. [Google Scholar] [CrossRef] [PubMed]
- Ministry of Health Labour and Welfare. Clinical Management of Patients with COVID-19: A Guide for Front-Line Healthcare Workers (Version 2.1). Available online: https://www.mhlw.go.jp/content/000646531.pdf (accessed on 12 June 2021).
- Japan Pharmaceutical Information Center. Multicenter, Adaptive, Randomized, Placebo-Controlled, Comparative Study to Evaluate the Efficacy and Safety of Favipiravir in Patients with COVID-19 Non-Severe Pneumonia. Available online: https://www.clinicaltrials.jp/cti-user/trial/ShowDirect.jsp?japicId=JapicCTI-205238 (accessed on 30 June 2021).
- Centers for Disease Control and Prevention. Underlying Medical Conditions Associated with High Risk for Severe COVID-19: Information for Healthcare Providers. Available online: https://www.cdc.gov/coronavirus/2019-ncov/hcp/clinical-care/underlyingconditions.html (accessed on 12 June 2021).
- Williamson, E.J.; Walker, A.J.; Bhaskaran, K.; Bacon, S.; Bates, C.; Morton, C.E.; Curtis, H.J.; Mehrkar, A.; Evans, D.; Inglesby, P.; et al. Factors associated with COVID-19-related death using OpenSAFELY. Nature 2020, 584, 430–436. [Google Scholar] [CrossRef]
- Edalatifard, M.; Akhtari, M.; Salehi, M.; Naderi, Z.; Jamshidi, A.; Mostafaei, S.; Najafizadeh, S.R.; Farhadi, E.; Jalili, N.; Esfahani, M.; et al. Intravenous methylprednisolone pulse as a treatment for hospitalised severe COVID-19 patients: Results from a randomised controlled clinical trial. Eur. Respir. J. 2020, 56, 2002808. [Google Scholar] [CrossRef]
- Salton, F.; Confalonieri, P.; Meduri, G.U.; Santus, P.; Harari, S.; Scala, R.; Lanini, S.; Vertui, V.; Oggionni, T.; Caminati, A.; et al. Prolonged Low-Dose Methylprednisolone in Patients With Severe COVID-19 Pneumonia. Open Forum Infect. Dis. 2020, 7, ofaa421. [Google Scholar] [CrossRef]
- Alqahtani, J.S.; Oyelade, T.; Aldhahir, A.M.; Alghamdi, S.M.; Almehmadi, M.; Alqahtani, A.S.; Quaderi, S.; Mandal, S.; Hurst, J.R. Prevalence, Severity and Mortality associated with COPD and Smoking in patients with COVID-19: A Rapid Systematic Review and Meta-Analysis. PLoS ONE 2020, 15, e0233147. [Google Scholar] [CrossRef]
- Ranjbar, K.; Moghadami, M.; Mirahmadizadeh, A.; Fallahi, M.J.; Khaloo, V.; Shahriarirad, R.; Erfani, A.; Khodamoradi, Z.; Gholampoor Saadi, M.H. Methylprednisolone or dexamethasone, which one is superior corticosteroid in the treatment of hospitalized COVID-19 patients: A triple-blinded randomized controlled trial. BMC Infect. Dis. 2021, 21, 337. [Google Scholar] [CrossRef]
- Recovery Collaborative Group; Horby, P.; Lim, W.S.; Emberson, J.R.; Mafham, M.; Bell, J.L.; Linsell, L.; Staplin, N.; Brightling, C.; Ustianowski, A.; et al. Dexamethasone in Hospitalized Patients with COVID-19. N. Engl. J. Med. 2021, 384, 693–704. [Google Scholar] [CrossRef]
- Jeronimo, C.M.P.; Farias, M.E.L.; Val, F.F.A.; Sampaio, V.S.; Alexandre, M.A.A.; Melo, G.C.; Safe, I.P.; Borba, M.G.S.; Netto, R.L.A.; Maciel, A.B.S.; et al. Methylprednisolone as Adjunctive Therapy for Patients Hospitalized With Coronavirus Disease 2019 (COVID-19; Metcovid): A Randomized, Double-blind, Phase IIb, Placebo-controlled Trial. Clin. Infect. Dis. 2021, 72, e373–e381. [Google Scholar] [CrossRef]
- Gluck, V.; Grobecker, S.; Tydykov, L.; Salzberger, B.; Gluck, T.; Weidlich, T.; Bertok, M.; Gottwald, C.; Wenzel, J.J.; Gessner, A.; et al. SARS-CoV-2-directed antibodies persist for more than six months in a cohort with mild to moderate COVID-19. Infection 2021, 49, 739–746. [Google Scholar] [CrossRef] [PubMed]
- Lau, E.H.Y.; Tsang, O.T.Y.; Hui, D.S.C.; Kwan, M.Y.W.; Chan, W.H.; Chiu, S.S.; Ko, R.L.W.; Chan, K.H.; Cheng, S.M.S.; Perera, R.; et al. Neutralizing antibody titres in SARS-CoV-2 infections. Nat. Commun. 2021, 12, 63. [Google Scholar] [CrossRef]
- Udwadia, Z.F.; Singh, P.; Barkate, H.; Patil, S.; Rangwala, S.; Pendse, A.; Kadam, J.; Wu, W.; Caracta, C.F.; Tandon, M. Efficacy and safety of favipiravir, an oral RNA-dependent RNA polymerase inhibitor, in mild-to-moderate COVID-19: A randomized, comparative, open-label, multicenter, phase 3 clinical trial. Int. J. Infect. Dis. 2021, 103, 62–71. [Google Scholar] [CrossRef] [PubMed]
- Goto, A.; Go, H.; Miyakawa, K.; Yamaoka, Y.; Ohtake, N.; Kubo, S.; Jeremiah, S.S.; Mihara, T.; Senuki, K.; Miyazaki, T.; et al. Sustained Neutralizing Antibodies 6 Months Following Infection in 376 Japanese COVID-19 Survivors. Front. Microbiol. 2021, 12, 661187. [Google Scholar] [CrossRef]

| Characteristics | All Included Patients (n = 34) | Case (n = 17) | Control (n = 17) | p Value * |
|---|---|---|---|---|
| Age, mean (SD), yrs | 49.8 (14.4) | 49.6 (14.8) | 50.1 (14.5) | 0.926 a |
| Gender, male/female, number (%) | 26 (76.5)/8 (23.5) | 13 (76.5)/4 (23.5) | 13 (76.5)/4 (23.5) | 0.686 b |
| Body mass index, mean (SD), kg/m2 | 23.8 (3.1) | 23.9 (3.2) | 23.7 (3.1) | 0.897 a |
| Smoking status: current and former/never, number (%) | 10 (29.4)/24 (70.6) | 5 (29.4)/12 (70.6) | 5 (29.4)/12 (70.6) | 0.707 b |
| Comorbidities, any of the listed conditions, number (%) | 10 (29.4) | 5 (29.4) | 5 (29.4) | 0.707 b |
| Hypertension, number (%) | 7 (20.6) | 4 (23.5) | 3 (17.6) | 1.000 c |
| Diabetes, number (%) | 4 (11.7) | 3 (17.6) | 1 (5.9) | 0.601 c |
| Hyperlipidemia, number (%) | 3 (8.8) | 2 (11.8) | 1 (5.9) | 1.000 c |
| Hyperuricemia, number (%) | 2 (5.9) | 1 (5.9) | 1 (5.9) | 1.000 c |
| Hyperthyroidism, number (%) | 1 (2.9) | 0 (0) | 1 (5.9) | 1.000 c |
| Disease severity, moderate I/II, number (%) | 27 (79.4)/7 (20.6) | 12 (70.6)/5 (29.4) | 15 (88.2)/2 (11.8) | 0.398 c |
| Admission oxygen saturation, SpO2, median (IQR), % | 97.0 (96.0, 98.0) | 97.0 (96.8, 98.0) | 97.0 (95.0, 98.0) | 0.479 d |
| The time from symptom onset to diagnosis, mean (SD), days | 4.2 (2.3) | 4.7 (2.3) | 3.8 (2.2) | 0.238 a |
| The time from symptom onset to initiate favipiravir, mean (SD), days | 8.9 (2.0) | NA |
| Outcome | All Included Patients (n = 34) | Case (n = 17) | Control (n = 17) | p Value * |
|---|---|---|---|---|
| Total amount of methylprednisolone used, median (IQR), mg | 400.0 (120.0, 800.0) | 400.0 (150.0, 1070.0) | 400.0 (90.0, 660.0) | 0.835 a |
| The time from symptom onset to hospital discharge, median (IQR), days | 17.5 (15.0, 21.0) | 17.0 (15.8, 21.3) | 18.0 (14.0, 21.5) | 0.855 b |
| Hazard ratio (95% CI) | 1.070 (0.519, 2.208) | |||
| The time for hospitalization, median (IQR), days | 10.5 (8.0, 14.0) | 10.0 (7.8, 14.8) | 11.0 (8.3, 14.3) | 0.831 b |
| Hazard ratio (95% CI) | 0.925 (0.452, 1.893) | |||
| The time from symptom onset to PCR conversion, median (IQR), days | 16.0 (13.0, 21.0) | 14.0 (13.0, 18.0) | 18.0 (13.8, 28.0) | 0.049 b |
| Hazard ratio (95% CI) | 0.457 (0.209, 0.998) | |||
| The time from hospitalization to PCR conversion, median (IQR), days | 9.0 (7.0, 14.0) | 8.0 (4.0, 12.0) | 11.0 (8.8, 18.5) | 0.039 b |
| Hazard ratio (95% CI) | 0.448 (0.209, 0.960) | |||
| The time for PCR positive duration, median (IQR), days | 11.0 (9.0, 17.0) | 10.0 (6.0, 12.8) | 15.0 (10.5, 21.3) | 0.027 b |
| Hazard ratio (95% CI) | 0.412 (0.188, 0.902) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shinada, K.; Sato, T.; Moriyama, S.; Adachi, Y.; Shinoda, M.; Ota, S.; Morikawa, M.; Mineshita, M.; Matsumura, T.; Takahashi, Y.; et al. Longitudinal Analysis of Neutralizing Potency against SARS-CoV-2 in the Recovered Patients after Treatment with or without Favipiravir. Viruses 2022, 14, 670. https://doi.org/10.3390/v14040670
Shinada K, Sato T, Moriyama S, Adachi Y, Shinoda M, Ota S, Morikawa M, Mineshita M, Matsumura T, Takahashi Y, et al. Longitudinal Analysis of Neutralizing Potency against SARS-CoV-2 in the Recovered Patients after Treatment with or without Favipiravir. Viruses. 2022; 14(4):670. https://doi.org/10.3390/v14040670
Chicago/Turabian StyleShinada, Kanako, Takashi Sato, Saya Moriyama, Yu Adachi, Masahiro Shinoda, Shinichiro Ota, Miwa Morikawa, Masamichi Mineshita, Takayuki Matsumura, Yoshimasa Takahashi, and et al. 2022. "Longitudinal Analysis of Neutralizing Potency against SARS-CoV-2 in the Recovered Patients after Treatment with or without Favipiravir" Viruses 14, no. 4: 670. https://doi.org/10.3390/v14040670
APA StyleShinada, K., Sato, T., Moriyama, S., Adachi, Y., Shinoda, M., Ota, S., Morikawa, M., Mineshita, M., Matsumura, T., Takahashi, Y., & Shinkai, M. (2022). Longitudinal Analysis of Neutralizing Potency against SARS-CoV-2 in the Recovered Patients after Treatment with or without Favipiravir. Viruses, 14(4), 670. https://doi.org/10.3390/v14040670







