Revisiting the MMTV Zoonotic Hypothesis to Account for Geographic Variation in Breast Cancer Incidence
Abstract
1. Introduction
2. Materials and Methods
2.1. Testing for Association of Breast Cancer and M. m. domesticus Populations
2.2. Correlation of Mouse Population Outbreaks and Annual Breast Cancer Incidence Rates
3. Results
3.1. Breast Cancer Incidence Rates Still Associate with M. m. domesticus Range in Europe
3.2. Breast Cancer Incidence Rates Are No Longer Segregated by M. m. domesticus Range outside of Europe
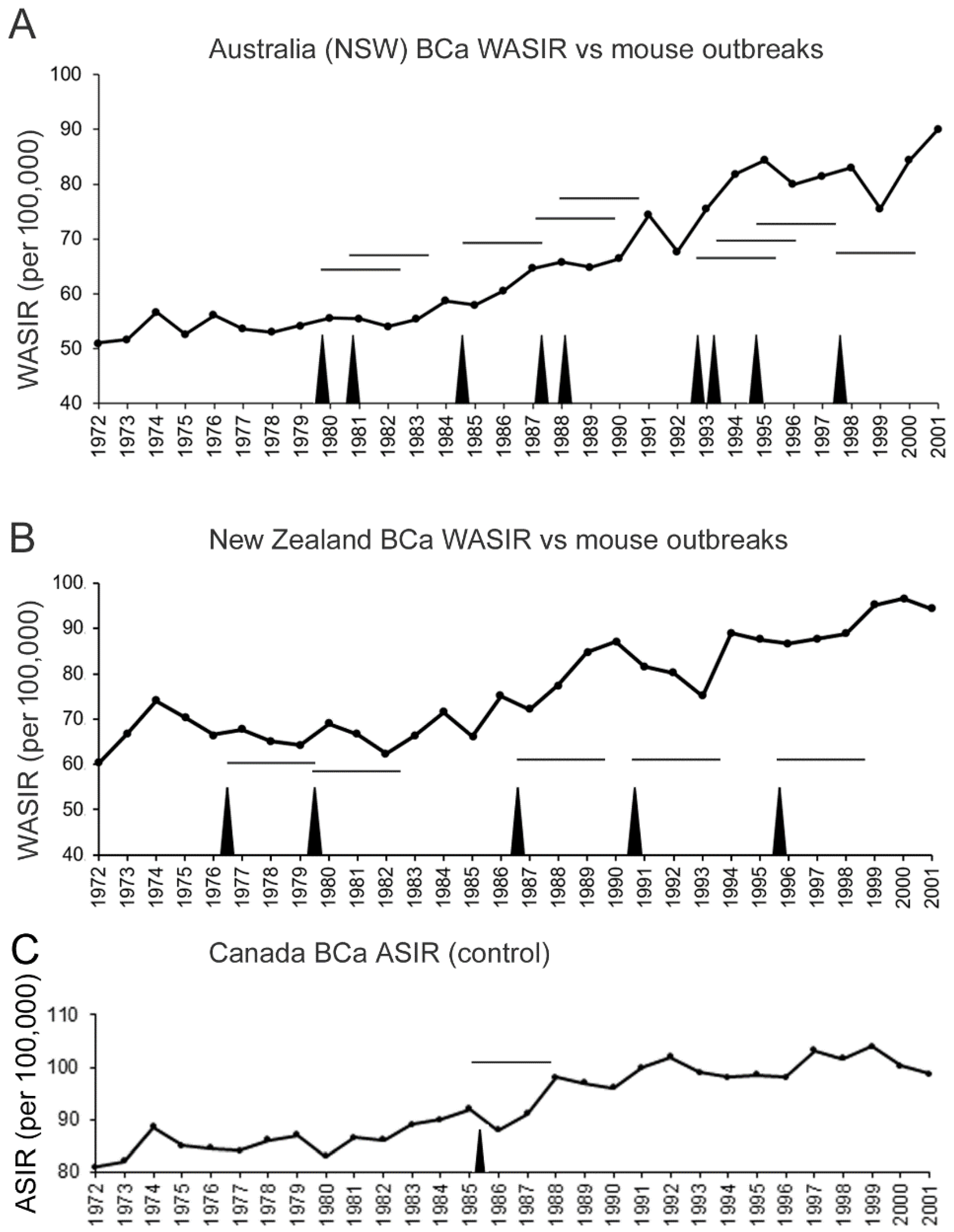
3.3. Cyclical Mouse Population Outbreaks Precede Increases in Breast Cancer Incidence Rates
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hunter, D.J.; Hankinson, S.E.; Laden, F.; Colditz, G.A.; Manson, J.E.; Willett, W.C.; Speizer, F.E.; Wolff, M.S. Plasma organochlorine levels and the risk of breast cancer. N. Engl. J. Med. 1997, 337, 1253–1258. [Google Scholar] [CrossRef] [PubMed]
- Parkin, D.M.; Whelan, S.L.; Ferlay, J.; Teppo, L.; Thomas, D.B.E. Cancer Incidence in Five Continents Volume VIII; IARC Scientific Publication: Lyon, France, 2002; p. 831. [Google Scholar]
- Ziegler, R.G.; Hoover, R.N.; Pike, M.C.; Hildesheim, A.; Nomura, A.M.Y.; West, D.W.; Wu-Williams, A.H.; Kolonel, L.N.; Horn-Ross, P.L.; Rosenthal, J.F.; et al. Migration patterns and breast cancer risk in asian-american women. J. Natl. Cancer Inst. 1993, 85, 1819–1827. [Google Scholar] [CrossRef] [PubMed]
- Iscovich, J.; Howe, G.R. Cancer incidence patterns (1972-91) among migrants from the Soviet Union to Israel. Cancer Causes Control. 1998, 9, 29–36. [Google Scholar] [CrossRef] [PubMed]
- Winter, H.; Cheng, K.; Cummins, C.; Maric, R.; Silcocks, P.; Varghese, C. Cancer incidence in the south Asian population of England (1990–92). Br. J. Cancer 1999, 79, 645–654. [Google Scholar] [CrossRef]
- Iwasaki, M.; Inoue, M.; Sasazuki, S.; Kurahashi, N.; Itoh, H.; Usuda, M.; Tsugane, S.; Japan Public Health Center-based Prospective Study Group. Plasma organochlorine levels and subsequent risk of breast cancer among Japanese women: A nested case–control study. Sci. Total Environ. 2008, 402, 176–183. [Google Scholar] [CrossRef] [PubMed]
- Hunter, D.J.; Spiegelman, D.; Adami, H.-O.; Beeson, L.; Brandt, P.A.V.D.; Folsom, A.R.; Fraser, G.E.; Goldbohm, R.A.; Graham, S.; Howe, G.R.; et al. Cohort studies of fat intake and the risk of breast cancer—A pooled analysis. N. Engl. J. Med. 1996, 334, 356–361. [Google Scholar] [CrossRef] [PubMed]
- Brown, L.M.; Gridley, G.; Wu, A.H.; Falk, R.T.; Hauptmann, M.; Kolonel, L.N.; West, D.W.; Nomura, A.M.Y.; Pike, M.C.; Hoover, R.N.; et al. Low level alcohol intake, cigarette smoking and risk of breast cancer in Asian-American women. Breast Cancer Res. Treat. 2010, 120, 203–210. [Google Scholar] [CrossRef] [PubMed]
- Slattery, M.L.; Kerber, R.A. A comprehensive evaluation of family history and breast cancer risk. The Utah population database. JAMA J. Am. Med. Assoc. 1993, 270, 1563–1568. [Google Scholar] [CrossRef]
- Colditz, G.A.; Willett, W.C.; Hunter, D.J.; Stampfer, M.J.; Manson, J.E.; Hennekens, C.H.; Rosner, B.A. Family history, age, and risk of breast cancer. Prospective data from the nurses’ health study. JAMA J. Am. Med. Assoc. 1993, 270, 338–343. [Google Scholar] [CrossRef]
- Michailidou, K.; Lindstrom, S.; Dennis, J.; Beesley, J.; Hui, S.; Kar, S.; Lemacon, A.; Soucy, P.; Glubb, D.; Rostamianfar, A.; et al. Association analysis identifies 65 new breast cancer risk loci. Nature 2017, 551, 92–94. [Google Scholar] [CrossRef]
- Zhang, H.; Ahearn, T.U.; Lecarpentier, J.; Barnes, D.; Beesley, J.; Qi, G.; Jiang, X.; O’Mara, T.A.; Zhao, N.; Bolla, M.K.; et al. Genome-wide association study identifies 32 novel breast cancer susceptibility loci from overall and subtype-specific analyses. Nat Genet 2020, 52, 572–581. [Google Scholar] [CrossRef] [PubMed]
- Mavaddat, N.; Pharoah, P.D.P.; Michailidou, K.; Tyrer, J.; Brook, M.; Bolla, M.K.; Wang, Q.; Dennis, J.; Dunning, A.M.; Shah, M.; et al. Prediction of breast cancer risk based on profiling with common genetic variants. J. Natl. Cancer Inst. 2015, 107, 5. [Google Scholar] [CrossRef] [PubMed]
- Burchell, A.N.; Coutlée, F.; Tellier, P.-P.; Hanley, J.; Franco, E. Genital transmission of human papillomavirus in recently formed heterosexual couples. J. Infect. Dis. 2011, 204, 1723–1729. [Google Scholar] [CrossRef] [PubMed]
- Falcaro, M.; Castañon, A.; Ndlela, B.; Checchi, M.; Soldan, K.; Lopez-Bernal, J.; Elliss-Brookes, L.; Sasieni, P. The effects of the national HPV vaccination programme in England, UK, on cervical cancer and grade 3 cervical intraepithelial neoplasia incidence: A register-based observational study. Lancet 2021, 398, 2084–2092. [Google Scholar] [CrossRef]
- Stewart, T.H.M.; Sage, R.D.; Stewart, A.; Cameron, D.W. Breast cancer incidence highest in the range of one species of house mouse, Mus domesticus. Br. J. Cancer 2000, 82, 446–451. [Google Scholar] [CrossRef]
- Bittner, J.J. Some possible effects of nursing on the mammary gland tumor incidence in mice. Science 1936, 84, 162. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Holland, J.F.; Bleiweiss, I.J.; Melana, S.; Liu, X.; Pelisson, I.; Cantarella, A.; Stellrecht, K.; Mani, S.; Pogo, B.G. Detection of mammary tumor virus env gene-like sequences in human breast cancer. Cancer Res. 1995, 55, 5173–5179. [Google Scholar]
- Karapetian, O.; Shakhov, A.N.; Kraehenbuhl, J.P.; Acha-Orbea, H. Retroviral infection of neonatal Peyer’s patch lymphocytes: The mouse mammary tumor virus model. J. Exp. Med. 1994, 180, 1511–1516. [Google Scholar] [CrossRef]
- Golovkina, T.V.; Dudley, J.P.; Ross, S.R. B and T cells are required for mouse mammary tumor virus spread within the mammary gland. J. Immunol. 1998, 161, 2375–2382. [Google Scholar]
- Drickamer, L.C. Seasonal variation in litter size, bodyweight and sexual maturation in juvenile female house mice (Mus musculus). Lab. Anim. 1977, 11, 159–162. [Google Scholar] [CrossRef]
- Riley, V. Mouse mammary tumors: Alteration of incidence as apparent function of stress. Science 1975, 189, 465–467. [Google Scholar] [CrossRef] [PubMed]
- Trichopoulos, D.; MacMahon, B.; Cole, P. Menopause and breast cancer risk. J. Natl. Cancer Inst. 1972, 48, 605–613. [Google Scholar] [PubMed]
- Lessi, F.; Grandi, N.; Mazzanti, C.M.; Civita, P.; Scatena, C.; Aretini, P.; Bandiera, P.; Fornaciari, A.; Giuffra, V.; Fornaciari, G.; et al. A human MMTV-like betaretrovirus linked to breast cancer has been present in humans at least since the copper age. Aging 2020, 12, 15978–15994. [Google Scholar] [CrossRef] [PubMed]
- Etkind, P.R.; Stewart, A.F.; Wiernik, P.H. Mouse mammary tumor virus (MMTV)-like DNA sequences in the breast tumors of father, mother, and daughter. Infect. Agents Cancer 2008, 3, 2–11. [Google Scholar] [CrossRef]
- Blankenstein, T.; Coulie, P.G.; Gilboa, E.; Jaffee, E.M. The determinants of tumour immunogenicity. Nat. Rev. 2012, 12, 307–313. [Google Scholar] [CrossRef]
- Pulaski, B.A.; Ostrand-Rosenberg, S. Mouse 4T1 breast tumor model. Curr. Protoc. Immunol. 2000, 39, 20–22. [Google Scholar] [CrossRef]
- Stewart, T.H.M.; Orizaga, M. The presence of delayed hypersensitivity reactions in patients toward cellular extracts of their malignant tumors.3. The frequency, duration, and cross reactivity of this phenomenon in patients with breast cancer, and its correlation with survival. Cancer 1971, 28, 1472–1478. [Google Scholar] [CrossRef]
- Stewart, T.; Tsai, S.-C.; Grayson, H.; Henderson, R.; Opelz, G. Incidence of de-novo breast cancer in women chronically immunosuppressed after organ transplantation. Lancet 1995, 346, 796–798. [Google Scholar] [CrossRef]
- Wang, F.-L.; Zhang, X.-L.; Yang, M.; Lin, J.; Yue, Y.-F.; Li, Y.-D.; Wang, X.; Shu, Q.; Jin, H.-C. Prevalence and characteristics of mouse mammary tumor virus-like virus associated breast cancer in China. Infect. Agents Cancer 2021, 16, 47. [Google Scholar] [CrossRef]
- Singleton, G.; Krebs, C.J.; Davis, S.; Chambers, L.; Brown, P. Reproductive changes in fluctuating house mouse populations in southeastern Australia. Proc. Biol. Sci. 2001, 268, 1741–1748. [Google Scholar] [CrossRef]
- Saunders, G.; Robards, G. Economic considerations of mouse-plague control in irrigated sunflower crops. Crop. Prot. 1983, 2, 153–158. [Google Scholar] [CrossRef]
- Choquenot, D.; Ruscoe, W.A. Mouse population eruptions in New Zealand forests: The role of population density and seedfall. J. Anim. Ecol. 2001, 69, 1058–1070. [Google Scholar] [CrossRef]
- Searle, J.B.; Jamieson, P.M.; Gündüz, I.; Stevens, M.I.; Jones, E.P.; Gemmill, C.E.; King, C.M. The diverse origins of New Zealand house mice. Proc. Biol. Sci. 2009, 276, 209–217. [Google Scholar] [CrossRef] [PubMed]
- Bonhomme, F.; Orth, A.; Cucchi, T.; Rajabi-Maham, H.; Catalan, J.; Boursot, P.; Auffray, J.-C.; Britton-Davidian, J. Genetic differentiation of the house mouse around the Mediterranean basin: Matrilineal footprints of early and late colonization. Proc. Biol. Sci. 2010, 278, 1034–1043. [Google Scholar] [CrossRef] [PubMed]
- Gabriel, S.I.; Stevens, M.I.; Mathias, M.D.L.; Searle, J.B. Of mice and ‘convicts’: Origin of the Australian house mouse, mus musculus. PLoS ONE 2011, 6, e28622. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Tomich, P.Q. Mammals in Hawai’i: A Synopsis and Notational Bibliography, 2nd ed.; Bishop Museum Press: Honolulu, HI, USA, 1986; p. 375. [Google Scholar]
- Nowak, R.M. Mammals of the World, 6th ed.; The Johns Hopkins University Press: Baltimore, MD, USA, 1999. [Google Scholar]
- Falls, J.B.; Falls, E.A.; Fryxell, J.M. Fluctuations of deer mice in ontario in relation to seed crops. Ecol. Monogr. 2007, 77, 19–32. [Google Scholar] [CrossRef]
- National Cancer Institute of Canada. National Cancer Institute of Canada: Canadian Cancer Statistics 2001; National Cancer Institute of Canada: Toronto, ON, Canada, 2001; p. 90.
- Huang, Z.; Wen, W.; Zheng, Y.; Gao, Y.; Wu, C.; Bao, P.; Wang, C.; Gu, K.; Peng, P.; Gong, Y.; et al. Breast cancer incidence and mortality: Trends over 40 years among women in Shanghai, China. Ann. Oncol. 2016, 27, 1129–1134. [Google Scholar] [CrossRef]
- Chao, T.; Cai, H.; Zhou, Y.; Li, K.; Xiao, J. Distribution of Subspecies of the House Mouse, Mus musculus (Rodentia: Muridae) in East China as Inferred from Mitochondrial D-loop Sequences. Pak. J. Zool. 2017, 49, 1175–1184. [Google Scholar] [CrossRef]
- Orth, A.; Adama, T.; Din, W.; Bonhomme, F. Natural hybridization between two subspecies of the house mouse, Mus musculus domesticus and Mus musculus castaneus, near Lake Casitas, California. Genome 1998, 41, 104–110. [Google Scholar] [CrossRef]
- Chie, W.-C.; Chen, C.F.; Chen, C.J.; Chang, C.L.; Liaw, Y.P.; Lin, R.S. Geographic variation of breast cancer in Taiwan: International and migrant comparison. Anticancer Res. 1995, 15, 2745–2749. [Google Scholar]
- Liu, F.-C.; Lin, H.T.; Kuo, C.-F.; See, L.-C.; Chiou, M.-J.; Yu, H.-P. Epidemiology and survival outcome of breast cancer in a nationwide study. Oncotarget 2017, 8, 16939–16950. [Google Scholar] [CrossRef] [PubMed]
- Geraldes, A.; Basset, P.; Gibson, B.; Smith, K.L.; Harr, B.; Yu, H.-T.; Bulatova, N.; Ziv, Y.; Nachman, M.W. Inferring the history of speciation in house mice from autosomal, X-linked, Y-linked and mitochondrial genes. Mol. Ecol. 2008, 17, 5349–5363. [Google Scholar] [CrossRef]
- Kuo, C.C.; Wardrop, N.; Chang, C.T.; Wang, H.C.; Atkinson, P.M. Significance of major international seaports in the distribution of murine typhus in Taiwan. PLoS Negl. Trop. Dis. 2017, 11, e0005430. [Google Scholar]
- Li, C.; Chen, Q.; Zhang, X.; Li, H.; Liu, Q.; Fei, P.; Huang, L.; Yao, Z. Early life domestic pet ownership, and the risk of pet sensitization and atopic dermatitis in preschool children: A prospective birth cohort in Shanghai. Front. Pediatr. 2020, 8, 192. [Google Scholar] [CrossRef] [PubMed]
- Laumbacher, B.; Fellerhoff, B.; Herzberger, B.; Wank, R. Do dogs harbour risk factors for human breast cancer? Med. Hypotheses 2006, 67, 21–26. [Google Scholar] [CrossRef] [PubMed]
- Garcia, D.O.; Lander, E.M.; Wertheim, B.C.; Manson, J.E.; Volpe, S.L.; Chlebowski, R.T.; Stefanick, M.L.; Lessin, L.S.; Kuller, L.H.; Thomson, C.A. Pet ownership and cancer risk in the women’s health initiative. Cancer Epidemiol. Biomark. Prev. 2016, 25, 1311–1316. [Google Scholar] [CrossRef] [PubMed]
- Fiolet, T.; Srour, B.; Sellem, L.; Kesse-Guyot, E.; Alles, B.; Mejean, C.; Deschasaux, M.; Fassier, P.; Latino-Martel, P.; Beslay, M.; et al. Consumption of ultra-processed foods and cancer risk: Results from NutriNet-Sante prospective cohort. BMJ 2018, 360, k322. [Google Scholar] [CrossRef]
- Mertens, E.; Colizzi, C.; Peñalvo, J.L. Ultra-processed food consumption in adults across Europe. Eur. J. Nutr. 2021, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Dalecky, A.; Bâ, K.; Piry, S.; Lippens, C.; Diagne, C.A.; Kane, M.; Sow, A.; Diallo, M.; Niang, Y.; Koneczny, A.; et al. Range expansion of the invasive house mouse Mus musculus domesticus in Senegal, West Africa: A synthesis of trapping data over three decades, 1983-2014. Mammal Rev. 2015, 45, 176–190. [Google Scholar] [CrossRef]
- Okeoma, C.; Lovsin, N.; Peterlin, B.M.; Ross, S.R. APOBEC3 inhibits mouse mammary tumour virus replication in vivo. Nature 2007, 445, 927–930. [Google Scholar] [CrossRef]
- Okeoma, C.; Petersen, J.; Ross, S.R. Expression of murine APOBEC3 alleles in different mouse strains and their effect on mouse mammary tumor virus infection. J. Virol. 2009, 83, 3029–3038. [Google Scholar] [CrossRef] [PubMed]
- Sanville, B.; Dolan, M.A.; Wollenberg, K.; Yan, Y.; Martin, C.; Yeung, M.L.; Strebel, K.; Buckler-White, A.; Kozak, C.A. Adaptive evolution of Mus Apobec3 includes retroviral insertion and positive selection at two clusters of residues flanking the substrate groove. PLoS Pathog. 2010, 6, e1000974. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, N.H.; Golovkina, T.; Uz-Zaman, T. RIII/Sa mice with a high incidence of mammary tumors express two exogenous strains and one potential endogenous strain of mouse mammary tumor virus. J. Virol. 2004, 78, 1055–1062. [Google Scholar] [CrossRef] [PubMed]
- Niimi, N.; Wajjwalku, W.; Ando, Y.; Nakamura, N.; Ueda, M.; Yoshikai, Y. A novel V beta 2-specific endogenous mouse mammary tumor virus which is capable of producing a milk-borne exogenous virus. J. Virol. 1995, 69, 7269–7273. [Google Scholar] [CrossRef]
- Imai, S.; Okumoto, M.; Iwai, M.; Haga, S.; Mori, N.; Miyashita, N.; Moriwaki, K.; Hilgers, J.; Sarkar, N.H. Distribution of mouse mammary tumor virus in Asian wild mice. J. Virol. 1994, 68, 3437–3442. [Google Scholar] [CrossRef]
- Golovkina, T.V.; Piazzon, I.; Nepomnaschy, I.; Buggiano, V.; de Olano Vela, M.; Ross, S.R. Generation of a tumorigenic milk-borne mouse mammary tumor virus by recombination between endogenous and exogenous viruses. J. Virol. 1997, 71, 3895–3903. [Google Scholar] [CrossRef]
- Harris, R.S.; Liddament, M.T. Retroviral restriction by APOBEC proteins. Nat. Rev. Immunol. 2004, 4, 868–877. [Google Scholar] [CrossRef]
- McDonnell, M.M.; Karvonen, S.C.; Gaba, A.; Flath, B.; Chelico, L.; Emerman, M. Highly-potent, synthetic APOBEC3s restrict HIV-1 through deamination-independent mechanisms. PLoS Pathog. 2021, 17, e1009523. [Google Scholar] [CrossRef]
- Klonowska, K.; Kluzniak, W.; Rusak, B.; Jakubowska, A.; Ratajska, M.; Krawczynska, N.; Vasilevska, D.; Czubak, K.; Wojciechowska, M.; Cybulski, C.; et al. The 30 kb deletion in the APOBEC3 cluster decreases APOBEC3A and APOBEC3B expression and creates a transcriptionally active hybrid gene but does not associate with breast cancer in the European population. Oncotarget 2017, 8, 76357–76374. [Google Scholar] [CrossRef]
- Xuan, D.; Li, G.; Cai, Q.; Deming-Halverson, S.; Shrubsole, M.J.; Shu, X.-O.; Kelley, M.C.; Zheng, W.; Long, J. APOBEC3 deletion polymorphism is associated with breast cancer risk among women of European ancestry. Carcinogenesis 2013, 34, 2240–2243. [Google Scholar] [CrossRef]
- Revathidevi, S.; Manikandan, M.; Rao, A.K.D.M.; Vinothkumar, V.; Arunkumar, G.; Rajkumar, K.S.; Ramani, R.; Rajaraman, R.; Ajay, C.; Munirajan, A.K. Analysis of APOBEC3A/3B germline deletion polymorphism in breast, cervical and oral cancers from South India and its impact on miRNA regulation. Tumor Biol. 2016, 37, 11983–11990. [Google Scholar] [CrossRef] [PubMed]
- Gansmo, L.B.; Romundstad, P.; Hveem, K.; Vatten, L.; Nik-Zainal, S.; Lønning, P.E.; Knappskog, S. APOBEC3A/B deletion polymorphism and cancer risk. Carcinogenesis 2018, 39, 118–124. [Google Scholar] [CrossRef] [PubMed]
- Göhler, S.; Filho, M.I.D.S.; Johansson, R.; Enquist-Olsson, K.; Henriksson, R.; Hemminki, K.; Lenner, P.; Försti, A. Impact of functional germline variants and a deletion polymorphism in APOBEC3A and APOBEC3B on breast cancer risk and survival in a Swedish study population. J. Cancer Res. Clin. Oncol. 2016, 142, 273–276. [Google Scholar] [CrossRef] [PubMed]
- Wen, W.X.; Soo, J.S.-S.; Kwan, P.Y.; Hong, E.; Khang, T.F.; Mariapun, S.; Lee, C.; Hasan, S.N.; Rajadurai, P.; Yip, C.H.; et al. Germline APOBEC3B deletion is associated with breast cancer risk in an Asian multi-ethnic cohort and with immune cell presentation. Breast Cancer Res. 2016, 18, 56. [Google Scholar] [CrossRef]
- Long, J.; Delahanty, R.J.; Li, G.; Gao, Y.-T.; Lu, W.; Cai, Q.; Xiang, Y.-B.; Li, C.; Ji, B.-T.; Zheng, Y.; et al. A Common Deletion in the APOBEC3 Genes and Breast Cancer Risk. J. Natl. Cancer Inst. 2013, 105, 573–579. [Google Scholar] [CrossRef]
- Simard, E.P.; Pfeiffer, R.M.; Engels, E.A. Spectrum of cancer risk late after AIDS onset in the United States. Arch. Intern. Med. 2010, 170, 1337–1345. [Google Scholar] [CrossRef]
- Mahale, P.; Engels, E.A.; Coghill, A.E.; Kahn, A.R.; Shiels, M.S. Cancer risk in older persons living with human immunodeficiency virus infection in the United States. Clin. Infect. Dis. 2018, 67, 50–57. [Google Scholar] [CrossRef]
- Xu, L.; Shen, Z.; Guo, L.; Fodera, B.; Keogh, A.; Joplin, R.; O’Donnell, B.; Aitken, J.; Carman, W.; Neuberger, J.; et al. Does a betaretrovirus infection trigger primary biliary cirrhosis? Proc. Natl. Acad. Sci. USA 2003, 100, 8454–8459. [Google Scholar] [CrossRef]
- Sharon, D.; Chen, M.; Zhang, G.; Girgis, S.; Sis, B.; Graham, D.; McDougall, C.; Wasilenko, S.T.; Montano-Loza, A.; Mason, A.L. Impact of combination antiretroviral therapy in the NOD.c3c4 mouse model of autoimmune biliary disease. Liver Int. 2014, 35, 1442–1450. [Google Scholar] [CrossRef]

| WASIR 1997 | WASIR 2020 | ||
|---|---|---|---|
| M. m. domesticus | Iceland | 79 | 81 |
| Republic of Ireland | 64 | 90 | |
| UK | 69 | 88 | |
| Belgium | 92 | 113 | |
| Germany * | 62 | 82 | |
| France | 75 | 99 | |
| Spain | 46 | 78 | |
| Portugal | 53 | 71 | |
| Italy | 72 | 87 | |
| Netherlands | 101 | 101 | |
| Hybrid | Norway | 54 | 83 |
| Sweden | 73 | 84 | |
| Finland | 65 | 92 | |
| Denmark | 73 | 98 | |
| Croatia | 37 | 69 | |
| Austria | 69 | 70 | |
| M. m. musculus | Poland | 40 | 69 |
| Romania | 39 | 66 | |
| Hungary | NA | 77 | |
| Estonia | 36 | 63 | |
| Latvia | 34 | 63 | |
| Lithuania | 29 | 62 | |
| Belarus | 30 | 52 | |
| Ukraine | 39 | 44 | |
| Czech Republic | 45 | 72 | |
| Slovak Republic | 39 | 60 | |
| Slovenia | 46 | 69 |
| WASIR 1997 | WASIR 2020 | ||
|---|---|---|---|
| M. m. domesticus | Algeria | 10 | 55.8 |
| Ecuador | 27 | 38.2 | |
| Costa Rica | 29 | 47.5 | |
| Peru | 31 | 35.9 | |
| Columbia | 39 | 48.3 | |
| Brazil | 44 | 61.9 | |
| Puerto Rico | 46 | 68.2 | |
| Argentina | 60 | 73.1 | |
| Australia | 67 | 96 | |
| Canada | 77 | 82 | |
| New Zealand | 77 | 93 | |
| Israel | 77 | 78.3 | |
| USA | 79 | 90.3 | |
| Hawaii | 97 | 139 | |
| Uruguay | 93 | 65 | |
| Other mice | South Korea | 20.8 | 64.2 |
| Thailand | 12 | 37.8 | |
| Taiwan | 17 | 93 | |
| Vietnam | 18 | 34.2 | |
| India | 21 | 25.8 | |
| China | 26 | 39.1 | |
| Japan | 26 | 76.3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stewart, A.F.R.; Chen, H.-H. Revisiting the MMTV Zoonotic Hypothesis to Account for Geographic Variation in Breast Cancer Incidence. Viruses 2022, 14, 559. https://doi.org/10.3390/v14030559
Stewart AFR, Chen H-H. Revisiting the MMTV Zoonotic Hypothesis to Account for Geographic Variation in Breast Cancer Incidence. Viruses. 2022; 14(3):559. https://doi.org/10.3390/v14030559
Chicago/Turabian StyleStewart, Alexandre F. R., and Hsiao-Huei Chen. 2022. "Revisiting the MMTV Zoonotic Hypothesis to Account for Geographic Variation in Breast Cancer Incidence" Viruses 14, no. 3: 559. https://doi.org/10.3390/v14030559
APA StyleStewart, A. F. R., & Chen, H.-H. (2022). Revisiting the MMTV Zoonotic Hypothesis to Account for Geographic Variation in Breast Cancer Incidence. Viruses, 14(3), 559. https://doi.org/10.3390/v14030559







