Inducible Nitric Oxide Synthase (iNOS): Why a Different Production in COVID-19 Patients of the Two Waves?
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Methods
2.3. Statistical Analysis
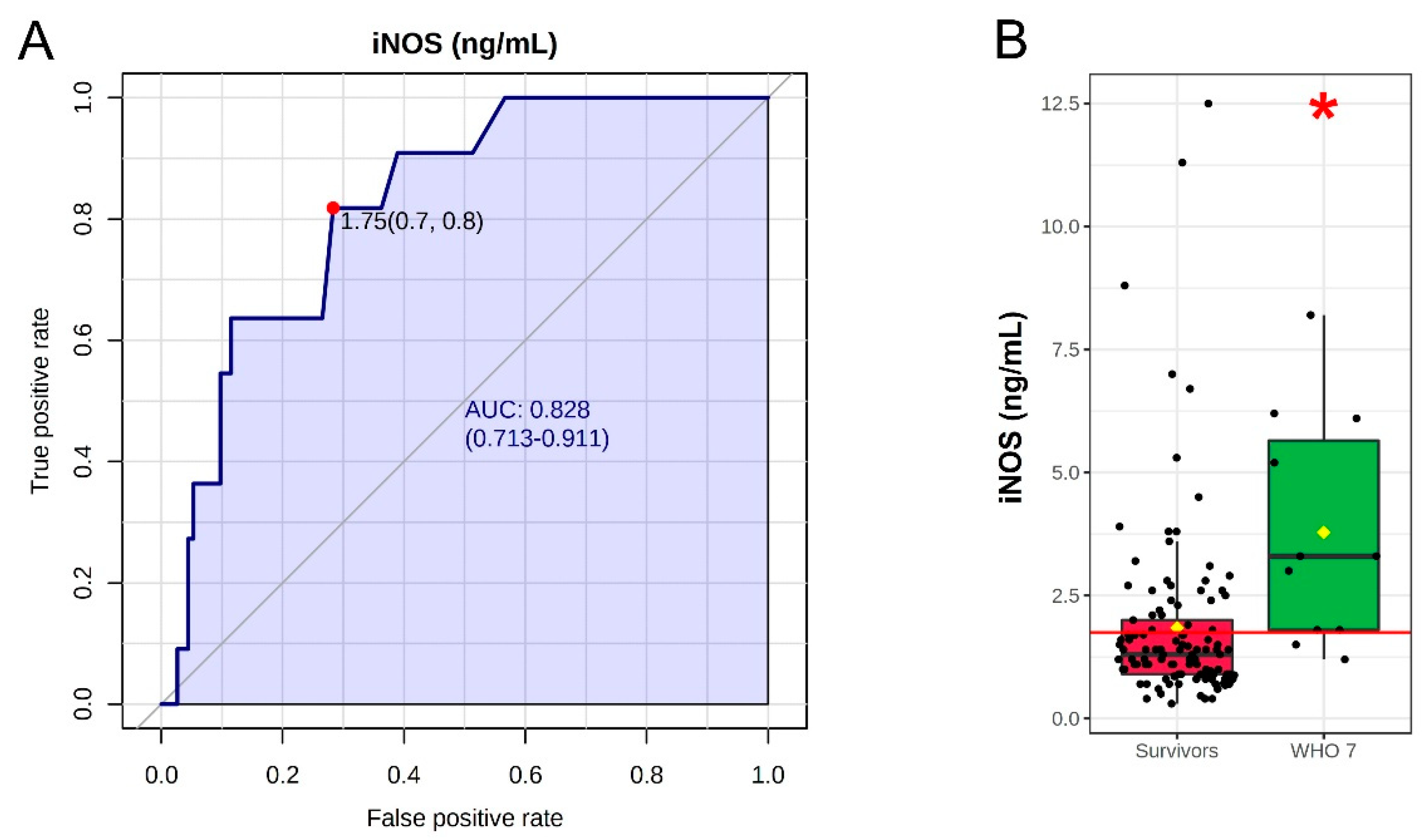
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gandhi, R.T.; Lynch, J.B.; Del Rio, C. Mild or moderate COVID-19. N. Engl. J. Med. 2020, 383, 1757–1766. [Google Scholar] [CrossRef] [PubMed]
- Berlin, D.A.; Gulick, R.M.; Martinez, F.J. Severe COVID-19. N. Engl. J. Med. 2020, 383, 2451–2460. [Google Scholar] [CrossRef] [PubMed]
- Gottlieb, R.L.; Vaca, C.E.; Paredes, R.; Mera, J.; Webb, B.J.; Perez, G.; Oguchi, G.; Ryan, P.; Nielsen, B.U.; Brown, M.; et al. Early Remdesivir to Prevent Progression to Severe COVID-19 in Outpatients. N. Engl. J. Med. 2022, 386, 305–315. [Google Scholar] [CrossRef] [PubMed]
- Jayk Bernal, A.; Gomes da Silva, M.M.; Musungaie, D.B.; Kovalchuk, E.; Gonzalez, A.; Delos Reyes, V.; Martín-Quirós, A.; Caraco, Y.; Williams-Diaz, A.; Brown, M.L.; et al. Molnupiravir for Oral Treatment of COVID-19 in Nonhospitalized Patients. N. Engl. J. Med. 2022, 386, 509–520. [Google Scholar] [CrossRef] [PubMed]
- Mahase, E. COVID-19: Pfizer’s paxlovid is 89% effective in patients at risk of serious illness, company reports. BMJ 2021, 375, n2713. [Google Scholar] [CrossRef]
- Lin, W.T.; Hung, S.H.; Lai, C.C.; Wang, C.Y.; Chen, C.H. The impact of neutralizing monoclonal antibodies on the outcomes of COVID-19 outpatients: A systematic review and meta-analysis of randomized controlled trials. J. Med. Virol. 2022; epub ahead of print. [Google Scholar]
- Powell, K.L.; Baylis, S.A. The antiviral effects of nitric oxide. Trends Microbiol. 1995, 3, 81–82. [Google Scholar] [CrossRef]
- Lirk, P.; Hoffmann, G.; Rieder, J. Indicible nitric oxide synthase—Time for reappraisal. Curr. Drug Targets 2002, 1, 89–108. [Google Scholar] [CrossRef]
- Yu, B.; Ichinose, F.; Bloch, D.B.; Zapol, W.M. Inhaled nitric oxide. Br. J. Pharmacol. 2019, 176, 246–255. [Google Scholar] [CrossRef] [Green Version]
- Alvarez, R.A.; Berra, L.; Giadwin, M.T. Home nitric oxide therapy for COVID-19. Am. J. Respir. Crit. Care Med. 2020, 202, 16–20. [Google Scholar] [CrossRef]
- Abou-Arab, O.; Huette, P.; Debouvries, F.; Dupont, H.; Jounieaux, V.; Mahjoub, Y. Inhaled nitric oxide for critically ill COVID-19 patients: A prospective study. Crit. Care 2020, 24, 645. [Google Scholar] [CrossRef]
- Fakhr, B.S.; Wiegand, S.B.; Pinciroli, R.; Gianni, S.; Morais, C.C.; Ikeda, T.; Miyazaki, Y.; Marutani, E.; Di Fenza, R.; Larson, G.M.; et al. High concentrations of nitric oxide inhalation therapy in pregnant patients with severe coronavirus disease 2019 (COVID-19). Obstet. Ginecol. 2020, 136, 1109–1113. [Google Scholar] [CrossRef] [PubMed]
- Cau, S.B.; Carneiro, F.S.; Tostes, R.C. Differential modulation of nitric oxide synthases in aging: Therapeutic opportunities. Front. Physiol. 2012, 3, 218. [Google Scholar] [CrossRef] [Green Version]
- Escames, G.; López, L.C.; Ortiz, F.; Ros, E.; Acuña-Castroviejo, D. Age-dependent lipopolysaccharide-induced iNOS expression and multiorgan failure in rats: Effects of melatonin treatment. Exp. Gerontol. 2006, 41, 1165–1173. [Google Scholar] [CrossRef] [PubMed]
- Sugiura, H.; Ichinose, M. Nitrative stress in inflammatory lung diseases. Nitric Oxide 2011, 25, 138–444. [Google Scholar] [CrossRef]
- Pautz, A.; Art, J.; Hahn, S.; Nowag, S.; Voss, C.; Kleinert, H. Regulation of the expression of inducible nitric oxide synthase. Nitric Oxide 2010, 23, 75–93. [Google Scholar] [CrossRef]
- Cunha, F.Q.; Moncada, S.; Liew, F.Y. Interleukin-10 (IL-10) inhibits the induction of nitric oxide synthase by interferon-gamma in murine macrophages. Biochem. Biophys. Res. Commun. 1992, 182, 1155–1159. [Google Scholar] [CrossRef]
- Borghesi, A.; Golemi, S.; Carapella, N.; Zigliani, A.; Farina, D.; Maroldi, R. Lombardy, Northern Italy: COVID-19 second wave less severe and deadly than the first? A preliminary investigation. Infect. Dis. (Lond.) 2021, 53, 370–375. [Google Scholar] [CrossRef] [PubMed]
- Ioannidis, J.P.A.; Axfors, C.; Contopoulos-Ioannidis, D.G. Second versus first wave of COVID-19 deaths: Shifts in age distribution and in nursing home fatalities. Environ. Res. 2021, 195, 110856. [Google Scholar] [CrossRef] [PubMed]
- Palmieri, L.; Palmer, K.; Lo Noce, C.; Meli, P.; Giuliano, M.; Floridia, M.; Tamburo de Bella, M.; Piccioli, A.; Brusaferro, S.; Onder, G. Differences in the clinical characteristics of COVID-19 patients who died in hospital during different phases of the pandemic: National data from Italy. Aging Clin. Exp. Res. 2021, 33, 193–199. [Google Scholar] [CrossRef]
- De Natale, G.; De Natale, L.; Troise, C.; Marchitelli, V.; Coviello, A.; Holmberg, K.G.; Somma, R. The Evolution of COVID-19 in Italy after the Spring of 2020: An Unpredicted Summer Respite Followed by a Second Wave. Int. J. Environ. Res. Public Health 2020, 17, 8708. [Google Scholar] [CrossRef]
- Cacciapuoti, S.; De Rosa, A.; Gelzo, M.; Megna, M.; Raia, M.; Pinchera, B.; Pontarelli, A.; Scotto, R.; Scala, E.; Scarano, F.; et al. Immunocytometric analysis of COVID patients: A contribution to personalized therapy? Life Sci. 2020, 261, 118355. [Google Scholar] [CrossRef] [PubMed]
- von Cube, M.; Grodd, M.; Wolkewitz, M.; Hazard, D.; Wengenmayer, T.; Canet, E.; Lambert, J. Harmonizing Heterogeneous Endpoints in Coronavirus Disease 2019 Trials Without Loss of Information. Crit. Care Med. 2021, 49, e11–e19. [Google Scholar] [CrossRef] [PubMed]
- Marshall, J.C.; Murthy, S.; Diaz, J.; Adhikari, N.K.; Angus, D.C.; Arabi, Y.M.; Baillie, K.; Bauer, M.; Berry, S.; Blackwood, B.; et al. WHO Working Group on the Clinical Characterization and Management of COVID-19 infection. A minimal common outcome measure set for COVID-19 clinical research. Lancet Infect. Dis. 2020, 20, e192–e197. [Google Scholar] [CrossRef]
- Handelsman, D.J.; Ly, L.P. An accurate substitution method to minimize left censoring bias in serum steroid measurements. Endocrinology 2019, 160, 2395–2400. [Google Scholar] [CrossRef] [PubMed]
- Jones, C.M.; Athanasiou, T. Summary receiver operating characteristic curve analysis techniques in the evaluation of diagnostic tests. Ann. Thorac. Surg. 2005, 79, 16–20. [Google Scholar] [CrossRef]
- The RECOVERY Collaborative Group Dexamethasone in hospitalized patients with COVID-19—Preliminary report. N. Engl. J. Med. 2020, 384, 693–704.
- Sultana, J.; Cutroneo, P.M.; Crisafulli, S.; Puglisi, G.; Caramori, G.; Trifirò, G. Azithromycin in COVID-19 patients: Pharmacological mechanism clinical evidence and prescribing guidelines. Drug Saf. 2020, 43, 691–698. [Google Scholar] [CrossRef]
- Capoluongo, E.D.; Amato, F.; Castaldo, G. The friendly use of chloroquine in the COVID-19 disease: A warning for the G6PD-deficient males and for the unaware carriers of pathogenic alterations of the G6PD gene. Clin. Chem. Lab. Med. 2020, 58, 1162–1164. [Google Scholar] [CrossRef]
- Ansari, A.W.; Sharif-Askari, F.S.; Jayakumar, M.N.; Mohammed, A.K.; Sharif-Askari, N.S.; Venkatachalam, T.; Mahboub, B.; Schmidt, R.E.; Hamoudi, R.A.; Halwani, R.; et al. Azithromycin differentially alters TCR-activated helper T cell subset phenotype and effector function. Front. Immunol. 2020, 11, 556579. [Google Scholar] [CrossRef]
- Zimmermann, P.; Ziesenitz, V.C.; Curtis, N.; Ritz, N. The immunomodulatory effects of macrolides-a systematic review of the underlying mechanisms. Front. Immunol. 2018, 9, 302. [Google Scholar] [CrossRef] [Green Version]
- Xiang, Z.; Liu, J.; Shi, D.; Chen, W.; Li, J.; Yan, R.; Bi, Y.; Hu, W.; Zhu, Z.; Yu, Y.; et al. Glucocorticoids improve severe or critical COVID-19 by activating ACE2 and reducing IL-6 levels. Int. J. Biol. Sci. 2020, 16, 2382–2391. [Google Scholar] [CrossRef] [PubMed]
- Leonard, N.; Bishop, A.E.; Polak, J.M.; Talbot, C. Expression of nitric oxide synthase in inflammatory bowel disease is not affected by corticosteroid treatment. J. Clin. Pathol. 1998, 51, 750–753. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Adusumilli, N.C.; Zhang, D.; Friedman, J.M.; Friedman, A.J. Harnessing nitric oxide for preventing, limiting and treating the severe pulmonary consequences of COVID-19. Nitric Oxide 2020, 103, 4–8. [Google Scholar] [CrossRef]
- Frostell, C.G.; Hedenstierna, G. Nitric oxide and COVID-19: Dose, timing and how to administer it might be crucial. Acta Anaesthesiol. Scand. 2021, 65, 576–577. [Google Scholar] [CrossRef] [PubMed]

| Wave | All | WHO 3 | WHO 4 | WHO 5–7 | Multiple Comparison | |
|---|---|---|---|---|---|---|
| N | 1st | 35 | 7 (20) | 20 (57) | 8 (23) | - |
| 2nd | 153 | 57 (37) | 58 (38) | 38 (25) | - | |
| 1st vs. 2nd | - | 0.052 | 0.037 | 0.806 | ||
| Age | 1st | 62 (50–73) | 60 (39–62) | 64 (51–73) | 75 (58–80) | 0.068 |
| (years) | 2nd | 48 (33–63) | 34 (29–43) | 53 (38–64) a | 56 (48–73) b | <0.0001 |
| 1st vs. 2nd | 0.0003 | 0.062 | 0.019 | 0.074 | ||
| Males | 1st | 27 (77) | 4 (57) | 16 (80) | 7 (88) | - |
| (n, %) | 2nd | 75 (49) | 12 (21) | 35 (60) a | 28 (74) b | - |
| 1st vs. 2nd | 0.003 | 0.037 | 0.111 | 0.405 | ||
| IL-6 | 1st | 171 (94–397) | 130 (92–223) | 198 (86–375) | 292 (53–769) b | 0.021 |
| (pg/mL) | 2nd | 22 (16–30) | 26 (21–35) | 19 (13–25) a | 24 (17–36) c | 0.0002 |
| 1st vs. 2nd | <0.0001 | 0.0004 | <0.0001 | 0.0002 | ||
| IL-10 | 1st | 10.1 (5.1–24) | 5.4 (4.3–9.1) | 13.5 (4.5–24.2) | 23.5 (9.7–90.8) b | 0.037 |
| (pg/mL) | 2nd | 5.5 (1.13–8.1) | 6.5 (5.3–8.2) | 2.6 (1.13–7.4) a | 2.8 (1.13–8.6) | 0.011 |
| 1st vs. 2nd | <0.0001 | 0.656 | <0.0001 | <0.0001 | ||
| iNOS | 1st | 2.9 (2.3–5.3) | 2.3 (1.4–2.6) | 2.9 (2.5–4.4) a | 6.2 (3.8–7.8) b | 0.007 |
| (ng/mL) | 2nd | 1.1 (0.8–1.4) | 0.9 (0.7–1.3) | 1.2 (0.8–1.5) | 1.1 (0.9–1.6) | 0.104 |
| 1st vs. 2nd | <0.0001 | 0.0002 | <0.0001 | <0.0001 |
| Wave | WHO 3 | WHO 4 | WHO 5–7 | Multiple Comparison | |
|---|---|---|---|---|---|
| N | untreated | 48 | 20 | 12 | - |
| treated | 9 | 38 | 26 | - | |
| Age | untreated | 33 (28–40) | 37 (32–61) | 49 (41–56) a | 0.012 |
| (years) | treated | 37 (28–63) | 57 (48–64) | 61 (51–74) a | 0.011 |
| p value | 0.443 | 0.003 | 0.043 | ||
| Males | untreated | 8 (17) | 6 (30) | 9 (75) a | - |
| (n, %) | treated | 4 (44) | 29 (76) | 19 (73) | - |
| p value | 0.061 | 0.0006 | 0.900 | ||
| IL-6 | untreated | 27.3 (22.4–39.8) | 22.8 (18.3–28.8) b | 22.6 (14.5–36.8) a,c | <0.0001 |
| (pg/mL) | treated | 17.6 (12.0–20.5) | 16.6 (13.0–20.4) | 24.3 (18.1–35.7) a,c | 0.001 |
| p value | 0.0002 | 0.010 | 0.582 | ||
| IL-10 | untreated | 6.9 (5.5–8.3) | 6.0 (1.9–9.3) | 5.4 (1.13–9.9) | 0.724 |
| (pg/mL) | treated | 1.13 (1.13–6.6) | 1.13 (1.13–4.8) | 2.8 (1.13–8.1) | 0.260 |
| p value | 0.028 | 0.046 | 0.540 | ||
| iNOS | untreated | 0.9 (0.7–1.4) | 1.2 (0.8–1.5) | 1.6 (1.1–2.0) a | 0.005 |
| (ng/mL) | treated | 0.8 (0.6–1.2) | 1.2 (0.8–1.6) | 1.0 (0.8–1.1) | 0.131 |
| p value | 0.258 | 0.768 | 0.005 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gelzo, M.; Scialò, F.; Cacciapuoti, S.; Pinchera, B.; De Rosa, A.; Cernera, G.; Comegna, M.; Tripodi, L.; Schiano Moriello, N.; Mormile, M.; et al. Inducible Nitric Oxide Synthase (iNOS): Why a Different Production in COVID-19 Patients of the Two Waves? Viruses 2022, 14, 534. https://doi.org/10.3390/v14030534
Gelzo M, Scialò F, Cacciapuoti S, Pinchera B, De Rosa A, Cernera G, Comegna M, Tripodi L, Schiano Moriello N, Mormile M, et al. Inducible Nitric Oxide Synthase (iNOS): Why a Different Production in COVID-19 Patients of the Two Waves? Viruses. 2022; 14(3):534. https://doi.org/10.3390/v14030534
Chicago/Turabian StyleGelzo, Monica, Filippo Scialò, Sara Cacciapuoti, Biagio Pinchera, Annunziata De Rosa, Gustavo Cernera, Marika Comegna, Lorella Tripodi, Nicola Schiano Moriello, Mauro Mormile, and et al. 2022. "Inducible Nitric Oxide Synthase (iNOS): Why a Different Production in COVID-19 Patients of the Two Waves?" Viruses 14, no. 3: 534. https://doi.org/10.3390/v14030534
APA StyleGelzo, M., Scialò, F., Cacciapuoti, S., Pinchera, B., De Rosa, A., Cernera, G., Comegna, M., Tripodi, L., Schiano Moriello, N., Mormile, M., Fabbrocini, G., Parrella, R., Corso, G., Gentile, I., & Castaldo, G. (2022). Inducible Nitric Oxide Synthase (iNOS): Why a Different Production in COVID-19 Patients of the Two Waves? Viruses, 14(3), 534. https://doi.org/10.3390/v14030534








