Stillbirth after COVID-19 in Unvaccinated Mothers Can Result from SARS-CoV-2 Placentitis, Placental Insufficiency, and Hypoxic Ischemic Fetal Demise, Not Direct Fetal Infection: Potential Role of Maternal Vaccination in Pregnancy
Abstract
:1. Introduction
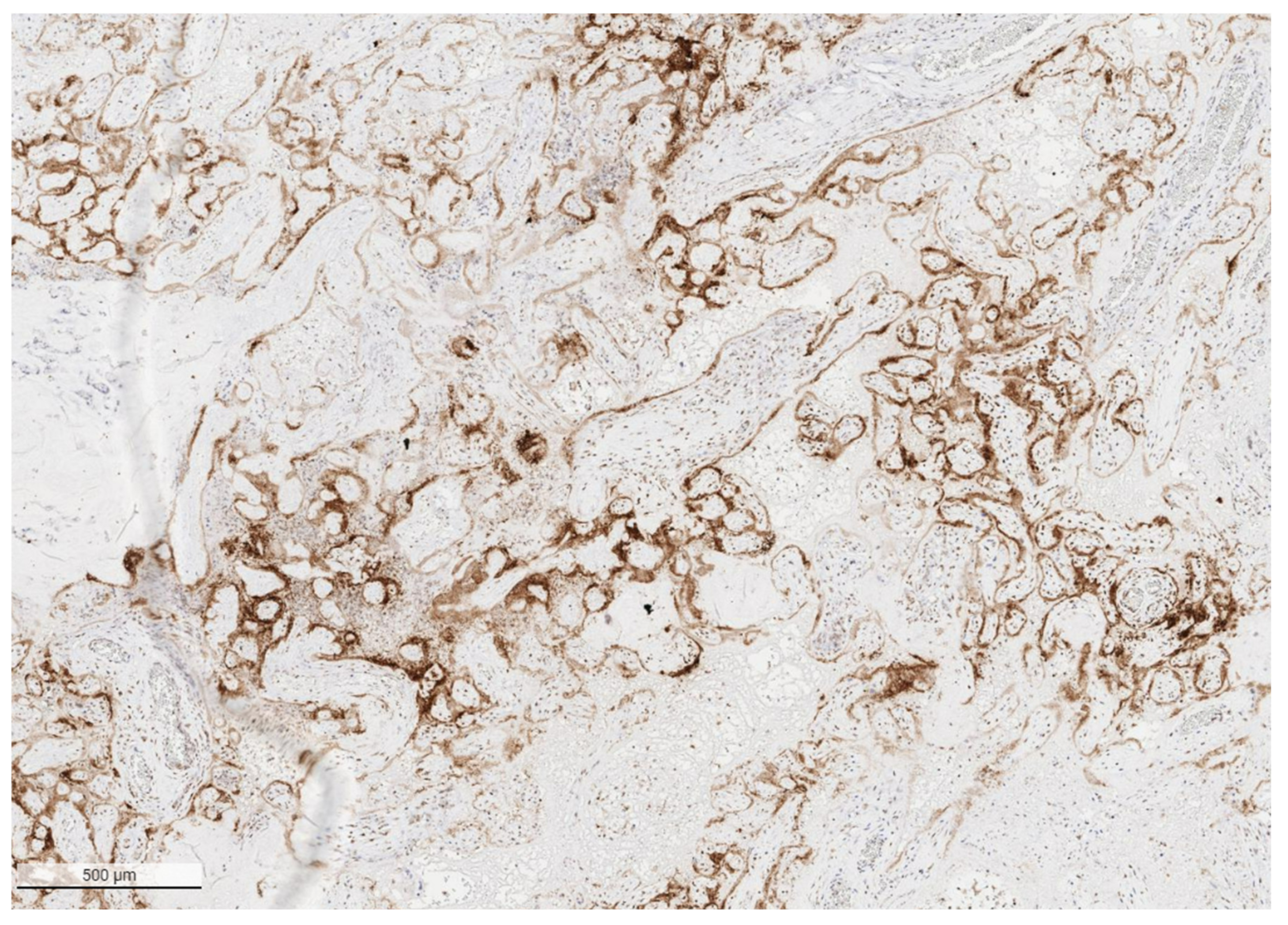
2. SARS-CoV-2 Placentitis
3. Placental Insufficiency and Stillbirth
4. COVID-19 Infection and Stillbirth
5. Potential Importance of Vaccination of Pregnant Women to Reduce Risk of Viremia and Stillbirth
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Schwartz, D.A.; Graham, A.L. Potential maternal and infant outcomes from coronavirus 2019-nCoV (SARS-CoV-2) infecting pregnant women: Lessons from SARS, MERS, and other human coronavirus infections. Viruses 2020, 12, 194. [Google Scholar] [CrossRef] [Green Version]
- Schwartz, D.A.; Dhaliwal, A. Infections in pregnancy with COVID-19 and other respiratory RNA virus diseases are rarely, if ever, transmitted to the fetus: Experiences with coronaviruses, parainfluenza, metapneumovirus respiratory syncytial virus, and influenza. Arch. Pathol. Lab. Med. 2020, 144, 920–928. [Google Scholar] [CrossRef]
- Schwartz, D.A.; Dhaliwal, A. Coronavirus diseases in pregnant women, the placenta, fetus, and neonate. Adv. Exp. Med. Biol. 2021, 1318, 223–241. [Google Scholar] [CrossRef]
- Wong, S.F.; Chow, K.M.; de Swiet, M. Severe acute respiratory syndrome and pregnancy. BJOG 2003, 110, 641–642. [Google Scholar] [CrossRef]
- Wong, S.F.; Chow, K.M.; Leung, T.N.; Ng, W.F.; Ng, T.K.; Shek, C.C.; Ng, P.C.; Lam, P.W.; Ho, L.C.; To, W.W.; et al. Pregnancy and perinatal outcomes of women with severe acute respiratory syndrome. Am. J. Obstet. Gynecol. 2004, 191, 292–297. [Google Scholar] [CrossRef] [Green Version]
- Payne, D.C.; Iblan, I.; Alqasrawi, S.; Al Nsour, M.; Rha, B.; Tohme, R.A.; Abedi, G.R.; Farag, N.H.; Haddadin, A.; Al Sanhouri, T.; et al. Stillbirth during infection with Middle East respiratory syndrome coronavirus. J. Infect. Dis. 2014, 209, 1870–1872. [Google Scholar] [CrossRef]
- Assiri, A.; Abedi, G.R.; Almasry, M.; Bin Saeed, A.; Gerber, S.I.; Watson, J.T. Middle East respiratory syndrome coronavirus infection during pregnancy: A report of 5 cases from Saudi Arabia. Clin. Infect. Dis. 2016, 63, 951–953. [Google Scholar] [CrossRef] [Green Version]
- Zhu, N.; Zhang, D.; Wang, W.; Li, X.; Yang, B.; Song, J.; Zhao, X.; Huang, B.; Shi, W.; Lu, R.; et al. A novel coronavirus from patients with pneumonia in China, 2019. N. Engl. J. Med. 2020, 382, 727–733. [Google Scholar] [CrossRef]
- Li, Q.; Guan, X.; Wu, P.; Wang, X.; Zhou, L.; Tong, Y.; Ren, R.; Leung, K.S.M.; Lau, E.H.Y.; Wong, J.Y.; et al. Early transmission dynamics in Wuhan, China, of novel coronavirus-infected pneumonia. N. Engl. J. Med. 2020, 382, 1199–1207. [Google Scholar] [CrossRef]
- Schwartz, D.A. The effects of pregnancy on women with COVID-19: Maternal and infant outcomes. Clin. Infect. Dis. 2020, 71, 2042–2044. [Google Scholar] [CrossRef]
- Chen, H.; Guo, J.; Wang, C.; Luo, F.; Yu, X.; Zhang, W.; Li, J.; Zhao, D.; Xu, D.; Gong, Q.; et al. Clinical characteristics and intrauterine vertical transmission potential of COVID-19 infection in nine pregnant women: A retrospective review of medical records. Lancet 2020, 395, 809–815. [Google Scholar] [CrossRef] [Green Version]
- Qiancheng, X.; Jian, S.; Lingling, P.; Lei, H.; Xiaogan, J.; Weihua, L.; Gang, Y.; Shirong, L.; Zhen, W.; GuoPing, X.; et al. Coronavirus disease 2019 in pregnancy. Int. J. Infect. Dis. 2020, 95, 376–383. [Google Scholar] [CrossRef]
- Schwartz, D.A. An analysis of 38 pregnant women with COVID-19, their newborn infants, and maternal-fetal transmission of SARS-CoV-2: Maternal coronavirus infections and pregnancy outcomes. Arch. Pathol. Lab. Med. 2020, 144, 799–805. [Google Scholar] [CrossRef] [Green Version]
- Zaigham, M.; Andersson, O. Maternal and perinatal outcomes with COVID-19: A systematic review of 108 pregnancies. Acta Obstet. Gynecol. Scand. 2020, 99, 823–829. [Google Scholar] [CrossRef] [Green Version]
- Schwartz, D.A.; Mohagheghi, P.; Beig, B.; Zafaranloo, N.; Moshfegh, F.; Yazdani, A. Spectrum of neonatal COVID-19 in Iran: 19 infants with SARS-CoV-2 perinatal infections with varying test results, clinical findings and outcomes. J. Matern. Fetal Neonatal Med. 2020, 12, 1–10. [Google Scholar] [CrossRef]
- Zeng, L.; Xia, S.; Yuan, W.; Yan, K.; Xiao, F.; Shao, J.; Zhou, W. Neonatal early-onset infection with SARS-CoV-2 in 33 neonates born to mothers with COVID-19 in Wuhan, China. JAMA Pediatr. 2020, 174, 722–725. [Google Scholar] [CrossRef] [Green Version]
- Savasi, V.M.; Parisi, F.; Patanè, L.; Ferrazzi, E.; Frigerio, L.; Pellegrino, A.; Spinillo, A.; Tateo, S.; Ottoboni, M.; Veronese, P.; et al. Clinical findings and disease severity in hospitalized pregnant women with coronavirus disease 2019 (COVID-19). Obstet. Gynecol. 2020, 136, 252–258. [Google Scholar] [CrossRef]
- Meslin, P.; Guiomard, C.; Chouakria, M.; Porcher, J.; Duquesne, F.; Tiprez, C.; Zemouri, N. Coronavirus disease 2019 in newborns and very young infants: A series of six patients in France. Pediatr. Infect. Dis. J. 2020, 39, e145–e147. [Google Scholar] [CrossRef]
- Raschetti, R.; Vivanti, A.J.; Vauloup-Fellous, C.; Loi, B.; Benachi, A.; de Luca, D. Synthesis and systematic review of reported neonatal SARS-CoV-2 infections. Nat. Commun. 2020, 11, 5164. [Google Scholar] [CrossRef]
- Patanè, L.; Morotti, D.; Giunta, M.R.; Sigismondi, C.; Piccoli, M.G.; Frigerio, L.; Mangili, G.; Arosio, M.; Cornolti, G. Vertical transmission of coronavirus disease 2019: Severe acute respiratory syndrome coronavirus 2 RNA on the fetal side of the placenta in pregnancies with coronavirus disease 2019-positive mothers and neonates at birth. Am. J. Obstet. Gynecol. MFM 2020, 2, 100145. [Google Scholar] [CrossRef]
- Facchetti, F.; Bugatti, M.; Drera, E.; Tripodo, C.; Sartori, E.; Cancila, V.; Papaccio, M.; Castellani, R.; Casola, S.; Boniotti, M.B.; et al. SARS-CoV-2 vertical transmission with adverse effects on the newborn revealed through integrated immunohistochemical, electron microscopy and molecular analyses of placenta. EBioMedicine 2020, 59, 102951. [Google Scholar] [CrossRef]
- Vivanti, A.J.; Vauloup-Fellous, C.; Prevot, S.; Zupan, V.; Suffee, C.; do Cao, J.; Benachi, A.; de Luca, D. Transplacental transmission of SARS-CoV-2 infection. Nat. Commun. 2020, 11, 3572. [Google Scholar] [CrossRef]
- Schwartz, D.A.; Morotti, D. Placental pathology of COVID-19 with and without fetal and neonatal infection: Trophoblast necrosis and chronic histiocytic intervillositis as risk factors for transplacental transmission of SARS-CoV-2. Viruses 2020, 12, 1308. [Google Scholar] [CrossRef]
- Sisman, J.; Jaleel, M.A.; Moreno, W.; Rajaram, V.; Collins, R.R.J.; Savani, R.C.; Rakheja, D.; Evans, A.S. Intrauterine transmission of SARS-CoV-2 infection in a preterm infant. Pediatr. Infect. Dis. J. 2020, 39, e265–e267. [Google Scholar] [CrossRef]
- Schwartz, D.A.; Morotti, D.; Beigi, B.; Moshfegh, F.; Zafaranloo, N.; Patanè, L. Confirming vertical fetal infection with coronavirus disease 2019: Neonatal and pathology criteria for early onset and transplacental transmission of severe acute respiratory syndrome coronavirus 2 from infected pregnant mothers. Arch. Pathol. Lab. Med. 2020, 144, 1451–1456. [Google Scholar] [CrossRef]
- Schwartz, D.A.; Baldewijns, M.; Benachi, A.; Bugatti, M.; Bulfamante, G.; Cheng, K.; Collins, R.R.J.; Debelenko, L.; de Luca, D.; Facchetti, F.; et al. Hofbauer cells and COVID-19 in pregnancy. Molecular pathology analysis of villous macrophages, endothelial cells, and placental findings from 22 placentas infected by SARS-CoV-2 with and without fetal transmission. Arch. Pathol. Lab. Med. 2021, 145, 1328–1340. [Google Scholar] [CrossRef]
- Arnaez, J.; Ochoa-Sangrador, C.; Caserío, S.; Gutiérrez, E.P.; Jiménez, M.D.P.; Castañón, L.; Benito, M.; Peña, A.; Hernández, N.; Hortelano, M.; et al. Lack of changes in preterm delivery and stillbirths during COVID-19 lockdown in a European region. Eur. J. Pediatr. 2021, 180, 1997–2002. [Google Scholar] [CrossRef]
- Hedley, P.L.; Hedermann, G.; Hagen, C.M.; Bækvad-Hansen, M.; Hjalgrim, H.; Rostgaard, K.; Laksafoss, A.D.; Hoffmann, S.; Jensen, J.S.; Breindahl, M.; et al. Preterm birth, stillbirth and early neonatal mortality during the Danish COVID-19 lockdown. Eur. J. Pediatr. 2021, 1–10. [Google Scholar] [CrossRef]
- Kniffka, M.S.; Nitsche, N.; Rau, R.; Kühn, M. Stillbirths in Germany: On the rise, but no additional increases during the first COVID-19 lockdown. Int. J. Gynaecol. Obstet. 2021, 155, 483–489. [Google Scholar] [CrossRef]
- Royal College of Physicians of Ireland. COVID Placentitis: Statement from the RCPI Faculty of Pathology and the Institute of Obstetricians and Gynaecologists. Available online: https://www.rcpi.ie/news/releases/covid-placentitis-statement-from-the-rcpi-faculty-of-pathology-and-the-institute-of-obstetricians-and-gynaecologists/ (accessed on 19 February 2021).
- Gurol-Urganci, I.; Jardine, J.E.; Carroll, F.; Draycott, T.; Dunn, G.; Fremeaux, A.; Harris, T.; Hawdon, J.; Morris, E.; Muller, P.; et al. Maternal and perinatal outcomes of pregnant women with SARS-CoV-2 infection at the time of birth in England: National cohort study. Am. J. Obstet. Gynecol. 2021, 225, 522.e1–522.e11. [Google Scholar] [CrossRef]
- DeSisto, C.L.; Wallace, B.; Simeone, R.M.; Polen, K.; Ko, J.Y.; Meaney-Delman, D.; Ellington, S.R. Risk for stillbirth among women with and without COVID-19 at delivery hospitalization—United States, March 2020–September 2021. MMWR Morb. Mortal. Wkly. Rep. 2021, 70, 1640–1645. [Google Scholar] [CrossRef]
- Schwartz, D.A. Viral infection, proliferation, and hyperplasia of Hofbauer cells and absence of inflammation characterize the placental pathology of fetuses with congenital Zika virus infection. Arch Gynecol. Obstet. 2017, 295, 1361–1368. [Google Scholar] [CrossRef]
- Muehlenbachs, A.; de la Rosa Vázquez, O.; Bausch, D.G.; Schafer, I.J.; Paddock, C.D.; Nyakio, J.P.; Lame, P.; Bergeron, E.; McCollum, A.M.; Goldsmith, C.S.; et al. Ebola virus disease in pregnancy: Clinical, histopathologic, and immunohistochemical findings. J. Infect. Dis. 2017, 215, 64–69. [Google Scholar] [CrossRef] [Green Version]
- Gao, L.; Ren, J.; Xu, L.; Ke, X.; Xiong, L.; Tian, X.; Fan, C.; Yan, H.; Yuan, J. Placental pathology of the third trimester pregnant women from COVID-19. Diagn. Pathol. 2021, 16, 8. [Google Scholar] [CrossRef]
- Prabhu, M.; Cagino, K.; Matthews, K.C.; Friedlander, R.L.; Glynn, S.M.; Kubiak, J.M.; Yang, Y.J.; Zhao, Z.; Baergen, R.N.; DiPace, J.I.; et al. Pregnancy and postpartum outcomes in a universally tested population for SARS-CoV-2 in New York City: A prospective cohort study. BJOG 2020, 127, 1548–1556. [Google Scholar] [CrossRef]
- Shanes, E.D.; Mithal, L.B.; Otero, S.; Azad, H.A.; Miller, E.S.; Goldstein, J.A. Placental pathology in COVID-19. Am. J. Clin. Pathol. 2020, 154, 23–32. [Google Scholar] [CrossRef]
- Sharps, M.C.; Hayes, D.; Lee, S.; Zou, Z.; Brady, C.A.; Almoghrabi, Y.; Kerby, A.; Tamber, K.K.; Jones, C.J.; Adams Waldorf, K.M.; et al. A structured review of placental morphology and histopathological lesions associated with SARS-CoV-2 infection. Placenta 2020, 101, 13–29. [Google Scholar] [CrossRef]
- Baergen, R.N.; Heller, D.S. Placental pathology in COVID-19 positive mothers: Preliminary findings. Pediatr. Dev. Pathol. 2020, 23, 177–180. [Google Scholar] [CrossRef]
- Gulersen, M.; Prasannan, L.; Tam Tam, H.; Metz, C.N.; Rochelson, B.; Meirowitz, N.; Shan, W.; Edelman, M.; Millington, K.A. Histopathologic evaluation of placentas after diagnosis of maternal severe acute respiratory syndrome coronavirus 2 infection. Am. J. Obstet. Gynecol. MFM 2020, 2, 100211. [Google Scholar] [CrossRef]
- Hecht, J.L.; Quade, B.; Deshpande, V.; Mino-Kenudson, M.; Ting, D.T.; Desai, N.; Dygulska, B.; Heyman, T.; Salafia, C.; Shen, D.; et al. SARS-CoV-2 can infect the placenta and is not associated with specific placental histopathology: A series of 19 placentas from COVID-19-positive mothers. Mod. Pathol. 2020, 33, 2092–2103. [Google Scholar] [CrossRef]
- Schwartz, D.A.; Thomas, K.M. Characterizing COVID-19 maternal-fetal transmission and placental infection using comprehensive molecular pathology. EBioMedicine 2020, 60, 102983. [Google Scholar] [CrossRef]
- Kirtsman, M.; Diambomba, Y.; Poutanen, S.M.; Malinowski, A.K.; Vlachodimitropoulou, E.; Parks, W.T.; Erdman, L.; Morris, S.K.; Shah, P.S. Probable congenital SARS-CoV-2 infection in a neonate born to a woman with active SARS-CoV-2 infection. CMAJ 2020, 192, E647–E650. [Google Scholar] [CrossRef]
- Hosier, H.; Farhadian, S.F.; Morotti, R.A.; Deshmukh, U.; Lu-Culligan, A.; Campbell, K.H.; Yasumoto, Y.; Vogels, C.B.; Casanovas-Massana, A.; Vijayakumar, P.; et al. SARS-CoV-2 infection of the placenta. J. Clin. Investig. 2020, 130, 4947–4953. [Google Scholar] [CrossRef]
- Pulinx, B.; Kieffer, D.; Michiels, I.; Petermans, S.; Strybol, D.; Delvaux, S.; Baldewijns, M.; Raymaekers, M.; Cartuyvels, R.; Maurissen, W. Vertical transmission of SARS-CoV-2 infection and preterm birth. Eur. J. Clin. Microbiol. Infect. Dis. 2020, 39, 2441–2445. [Google Scholar] [CrossRef]
- Debelenko, L.; Katsyv, I.; Chong, A.M.; Peruyero, L.; Szabolcs, M.; Uhlemann, A.C. Trophoblast damage with acute and chronic intervillositis: Disruption of the placental barrier by severe acute respiratory syndrome coronavirus 2. Hum. Pathol. 2020, 109, 69–79. [Google Scholar] [CrossRef]
- Schoenmakers, S.; Snijder, P.; Verdijk, R.M.; Kuiken, T.; Kamphuis, S.; Koopman, L.P.; Krasemann, T.B.; Rousian, M.; Broekhuizen, M.; Steegers, E.; et al. Severe acute respiratory syndrome coronavirus 2 placental infection and inflammation leading to fetal distress and neonatal multiorgan failure in an asymptomatic woman. J. Pediatr. Infect. Dis. Soc. 2020, 10, 556–561. [Google Scholar] [CrossRef]
- Schwartz, D.A.; Baldewijns, M.; Benachi, A.; Bugatti, M.; Collins, R.R.J.; de Luca, D.; Facchetti, F.; Linn, R.L.; Marcelis, L.; Morotti, D.; et al. Chronic histiocytic intervillositis with trophoblast necrosis is a risk factor associated with placental infection from coronavirus disease 2019 (COVID-19) and intrauterine maternal-fetal severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) transmission in live-born and stillborn infants. Arch. Pathol. Lab. Med. 2021, 145, 517–528. [Google Scholar] [CrossRef]
- Watkins, J.C.; Torous, V.F.; Roberts, D.J. Defining severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) placentitis: A report of 7 cases with confirmatory in situ hybridization, distinct histomorphologic features, and evidence of complement deposition. Arch. Pathol. Lab. Med. 2021, 145, 1341–1349. [Google Scholar] [CrossRef]
- Schwartz, D.A.; Levitan, D. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infecting pregnant women and the fetus, intrauterine transmission, and placental pathology during the coronavirus disease 2019 (COVID-19) pandemic: It’s complicated. Arch. Pathol. Lab. Med. 2021, 145, 925–928. [Google Scholar] [CrossRef]
- Schwartz, D.A.; Bugatti, M.; Santoro, A.; Facchetti, F. Molecular pathology demonstration of SARS-CoV-2 in cytotrophoblast from placental tissue with chronic histiocytic intervillositis, trophoblast necrosis and COVID-19. J. Dev. Biol. 2021, 9, 33. [Google Scholar] [CrossRef]
- Nye, G.A.; Ingram, E.; Johnstone, E.D.; Jensen, O.E.; Schneider, H.; Lewis, R.M.; Chernyavsky, I.L.; Brownbill, P. Human placental oxygenation in late gestation: Experimental and theoretical approaches. J. Physiol. 2018, 596, 5523–5534. [Google Scholar] [CrossRef]
- Dellschaft, N.S.; Hutchinson, G.; Shah, S.; Jones, N.W.; Bradley, C.; Leach, L.; Platt, C.; Bowtell, R.; Gowland, P.A. The haemodynamics of the human placenta in utero. PLoS Biol. 2020, 18, e3000676. [Google Scholar] [CrossRef]
- Manktelow, B.N.; Smith, L.K.; Evans, T.A.; Hyman-Taylor, P.; Kurinczuk, J.J.; Field, D.J.; Smith, P.W.; Mielewczyk, F.; Draper, E.S.; on behalf of the MBRRACE-UK collaboration. MBRRACE-UK Perinatal Mortality Surveillance Report. UK Perinatal Death for Births from January to December 2013. Supplementary Report. UK Trusts and Health Boards; The Infant Mortality and Morbidity Studies Group, Department of Health Sciences, University of Leicester: Leicester, UK, 2015. [Google Scholar]
- Gibbins, K.J.; Pinar, H.; Reddy, U.M.; Saade, G.R.; Goldenberg, R.L.; Dudley, D.J.; Drews-Botsch, C.; Freedman, A.A.; Daniels, L.M.; Parker, C.B.; et al. Findings in stillbirths associated with placental disease. Am. J. Perinatol. 2020, 37, 708–715. [Google Scholar] [CrossRef]
- Man, J.; Hutchinson, J.C.; Heazell, A.E.; Ashworth, M.; Jeffrey, I.; Sebire, N.J. Stillbirth and intrauterine fetal death: Role of routine histopathological placental findings to determine cause of death. Ultrasound Obstet. Gynecol. 2016, 48, 579–584. [Google Scholar] [CrossRef] [Green Version]
- Graham, N.; Heazell, A.E.P. When the fetus goes still and the birth is tragic: The role of the placenta in stillbirths. Obstet. Gynecol. Clin. N. Am. 2020, 47, 183–196. [Google Scholar] [CrossRef]
- Mazarico, E.; Molinet-Coll, C.; Martinez-Portilla, R.J.; Figueras, F. Heparin therapy in placental insufficiency: Systematic review and meta-analysis. Acta. Obstet. Gynecol. Scand. 2020, 99, 167–174. [Google Scholar] [CrossRef]
- Gagnon, R. Placental insufficiency and its consequences. Eur. J. Obstet. Gynecol. Reprod. Biol. 2003, 110 (Suppl. 1), S99–S107. [Google Scholar] [CrossRef]
- Hunt, K.; Kennedy, S.H.; Vatish, M. Definitions and reporting of placental insufficiency in biomedical journals: A review of the literature. Eur. J. Obstet. Gynecol. Reprod. Biol. 2016, 205, 146–149. [Google Scholar] [CrossRef]
- Wardinger, J.E.; Ambati, S. Placental Insufficiency. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2021. Available online: https://www.ncbi.nlm.nih.gov/books/NBK563171/ (accessed on 19 February 2022).
- Megli, C.J.; Coyne, C.B. Infections at the maternal-fetal interface: An overview of pathogenesis and defence. Nat. Rev. Microbiol. 2022, 20, 67–82. [Google Scholar] [CrossRef]
- Arora, N.; Sadovsky, Y.; Dermody, T.S.; Coyne, C.B. Microbial vertical transmission during human pregnancy. Cell Host Microbe 2017, 21, 561–567. [Google Scholar] [CrossRef]
- Jaan, A.; Rajnik, M. TORCH complex. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2022. Available online: https://www.ncbi.nlm.nih.gov/books/NBK560528/ (accessed on 19 February 2022).
- Handley, S.C.; Mullin, A.M.; Elovitz, M.A.; Gerson, K.D.; Montoya-Williams, D.; Lorch, S.A.; Burris, H.H. Changes in preterm birth phenotypes and stillbirth at 2 Philadelphia hospitals during the SARS-CoV-2 pandemic, March–June 2020. JAMA 2021, 325, 87–89. [Google Scholar] [CrossRef]
- Stowe, J.; Smith, H.; Thurland, K.; Ramsay, M.E.; Andrews, N.; Ladhani, S.N. Stillbirths during the COVID-19 pandemic in England, April–June 2020. JAMA 2021, 325, 86–87. [Google Scholar] [CrossRef]
- Pasternak, B.; Neovius, M.; Söderling, J.; Ahlberg, M.; Norman, M.; Ludvigsson, J.F.; Stephansson, O. Preterm birth and stillbirth during the COVID-19 pandemic in Sweden: A nationwide cohort study. Ann. Intern. Med. 2021, 174, 873–875. [Google Scholar] [CrossRef]
- Mor, M.; Kugler, N.; Jauniaux, E.; Betser, M.; Wiener, Y.; Cuckle, H.; Maymon, R. Impact of the COVID-19 pandemic on excess perinatal mortality and morbidity in Israel. Am. J. Perinatol. 2021, 38, 398–403. [Google Scholar] [CrossRef]
- Khalil, A.; von Dadelszen, P.; Draycott, T.; Ugwumadu, A.; O’Brien, P.; Magee, L. Change in the incidence of stillbirth and preterm delivery during the COVID-19 pandemic. JAMA 2020, 324, 705–706. [Google Scholar] [CrossRef]
- De Curtis, M.; Villani, L.; Polo, A. Increase of stillbirth and decrease of late preterm infants during the COVID-19 pandemic lockdown. Arch. Dis. Child. Fetal Neonatal Ed. 2021, 106, 456. [Google Scholar] [CrossRef]
- Kc, A.; Gurung, R.; Kinney, M.V.; Sunny, A.K.; Moinuddin, M.; Basnet, O.; Paudel, P.; Bhattarai, P.; Subedi, K.; Shrestha, M.P.; et al. Effect of the COVID-19 pandemic response on intrapartum care, stillbirth, and neonatal mortality outcomes in Nepal: A prospective observational study. Lancet Glob. Health 2020, 8, e1273–e1281. [Google Scholar] [CrossRef]
- King, A. Doctors Investigate Several Stillbirths among Moms with COVID-19. New Scientist. 2021. Available online: https://www.the-scientist.com/news-opinion/doctors-investigate-several-stillbirths-among-moms-with-covid-19-68703 (accessed on 19 February 2022).
- Six Stillbirths and One Miscarriage ‘Caused by COVID’ Since January, Says RCPI. RTÉ. 13 April 2021. Available online: https://www.rte.ie/news/2021/0413/1209701-covid-stillbirths-rcpi/ (accessed on 19 February 2022).
- Irish Health Authorities Investigating Four Stillbirth Cases with Potential COVID Link. EuroNews. 5 March 2021. Available online: https://www.euronews.com/my-europe/2021/03/04/irish-health-authorities-investigating-four-stillbirth-cases-with-potential-covid-link (accessed on 19 February 2022).
- Wadman, M. Studies reveal dangers of SARS-CoV-2 infection in pregnancy. Science 2022, 375, 253. [Google Scholar] [CrossRef]
- Stock, S.J.; Carruthers, J.; Calvert, C.; Denny, C.; Donaghy, J.; Goulding, A.; Hopcroft, L.; Hopkins, L.; McLaughlin, T.; Pan, J.; et al. SARS-CoV-2 infection and COVID-19 vaccination rates in pregnant women in Scotland. Nat. Med. 2022. [Google Scholar] [CrossRef]
- Rad, H.S.; Röhl, J.; Stylianou, N.; Allenby, M.C.; Bazaz, S.R.; Warkiani, M.E.; Guimaraes, F.; Clifton, V.L.; Kulasinghe, A. The effects of COVID-19 on the placenta during pregnancy. Front Immunol. 2021, 12, 743022. [Google Scholar] [CrossRef]
- Fitzgerald, B.; O’Donoghue, K.; McEntagart, N.; Gillan, J.E.; Kelehan, P.; O’Leary, J.; Downey, P.; Dean, J.; de Gascun, C.F.; Bermingham, J.; et al. Fetal deaths in Ireland due to SARS-CoV-2 placentitis caused by SARS-CoV-2 Alpha. Arch. Pathol. Lab. Med. 2022. [Google Scholar] [CrossRef]
- Di Gioia, C.; Zullo, F.; Bruno Vecchio, R.C.; Pajno, C.; Perrone, G.; Galoppi, P.; Pecorini, F.; di Mascio, D.; Carletti, R.; Prezioso, C.; et al. Stillbirth and fetal capillary infection by SARS-CoV-2. Am. J. Obstet. Gynecol. MFM 2021, 4, 100523. [Google Scholar] [CrossRef]
- Biringer, K.; Sivakova, J.; Marcinek, J.; Pribulova, T.; Rokos, T.; Kozubik, E.; Kudela, E.; Planket, L. Placental pathology concerning sudden foetal demise in SARS-CoV-2 positive asymptomatic pregnant female. Biomed. Pap. Med. Fac. Univ. Palacky Olomouc Czech Repub. 2021, 165, 328–331. [Google Scholar] [CrossRef]
- Lesieur, E.; Torrents, J.; Fina, F.; Zandotti, C.; Blanc, J.; Collardeau-Frachon, S.; Gazin, C.; Sirgant, D.; Mezouar, S.; Otmani Idrissi, M.; et al. Congenital infection of SARS-CoV-2 with intrauterine foetal death: A clinicopathological study with molecular analysis. Clin. Infect. Dis. 2021, ciab840. [Google Scholar] [CrossRef]
- Garrido-Pontnou, M.; Navarro, A.; Camacho, J.; Crispi, F.; Alguacil-Guillén, M.; Moreno-Baró, A.; Hernandez-Losa, J.; Sesé, M.; Ramón, Y.; Cajal, S.; et al. Diffuse trophoblast damage is the hallmark of SARS-CoV-2-associated fetal demise. Mod. Pathol. 2021, 34, 1704–1709. [Google Scholar] [CrossRef]
- Babal, P.; Krivosikova, L.; Sarvaicova, L.; Deckov, I.; Szemes, T.; Sedlackova, T.; Palkovic, M.; Kalinakova, A.; Janega, P. Intrauterine fetal demise after uncomplicated COVID-19: What can we learn from the case? Viruses 2021, 13, 2545. [Google Scholar] [CrossRef]
- Libbrecht, S.; van Cleemput, J.; Vandekerckhove, L.; Colman, S.; Padalko, E.; Verhasselt, B.; van de Vijver, K.; Dendooven, A.; Dehaene, I.; van Dorp, J. A rare but devastating cause of twin loss in a near-term pregnancy highlighting the features of severe SARS-CoV-2 placentitis. Histopathology 2021, 79, 674–676. [Google Scholar] [CrossRef]
- Marton, T.; Hargitai, B.; Hunter, K.; Pugh, M.; Murray, P. Massive perivillous fibrin deposition and chronic histiocytic intervillositis a complication of SARS-CoV-2 Infection. Pediatr. Dev. Pathol. 2021, 24, 450–454. [Google Scholar] [CrossRef]
- Marinho, P.S.; da Cunha, A.; Chimelli, L.; Avvad-Portari, E.; Andreiuolo, F.; de Oliveira-Szejnfeld, P.S.; Mendes, M.A.; Gomes, I.C.; Souza, L.; Guimarães, M.Z.; et al. Case report: SARS-CoV-2 mother-to-child transmission and fetal death associated with severe placental thromboembolism. Front. Med. 2021, 8, 677001. [Google Scholar] [CrossRef]
- Nizyaeva, N.V.; Lomova, N.A.; Dolgopolova, E.L.; Petrova, U.L.; Karapetyan, T.E.; Shmakov, R.G.; Frankevich, V.E. Effect of novel coronavirus infection COVID-19 on the mother-placenta-fetus system. Vestnik RSMU 2021, 2, 27–34. [Google Scholar] [CrossRef]
- Richtmann, R.; Torloni, M.R.; Oyamada Otani, A.R.; Levi, J.E.; Crema Tobara, M.; de Almeida Silva, C.; Dias, L.; Miglioli-Galvão, L.; Martins Silva, P.; Macoto Kondo, M. Fetal deaths in pregnancies with SARS-CoV-2 infection in Brazil: A case series. Case Rep. Womens Health 2020, 27, e00243. [Google Scholar] [CrossRef]
- Bewley, D.J.; Lee, J.; Popescu, O.; Oviedo, A. SARS-CoV-2 placental infection in an unvaccinated mother resulting in fetal demise. Cureus 2021, 13, e20833. [Google Scholar] [CrossRef] [PubMed]
- Bouachba, A.; Allias, F.; Nadaud, B.; Massardier, J.; Mekki, Y.; Bouscambert Duchamp, M.; Fourniere, B.; Huissoud, C.; Trecourt, A.; Collardeau-Frachon, S. Placental lesions and SARS-CoV-2 infection: Diffuse placenta damage associated to poor fetal outcome. Placenta 2021, 112, 97–104. [Google Scholar] [CrossRef] [PubMed]
- Zaigham, M.; Gisselsson, D.; Sand, A.; Wikström, A.-K.; von Wowern, E.; Schwartz, D.A.; Iorizzo, L.; Nelander, M. Clinical and pathological features of SARS-CoV-2 infected placentas in pregnancies with impaired foetal outcome: A case-series of 13 placentas with and without vertical transmission. BJOG 2022, in press. [Google Scholar]
- Dubucs, C.; Groussolles, M.; Ousselin, J.; Sartor, A.; van Acker, N.; Vayssière, C.; Pasquier, C.; Reyre, J.; Batlle, L.; Favarel, S.; et al. Severe placental lesions due to maternal SARS-CoV-2 infection associated to intrauterine fetal death. Hum. Pathol. 2022. [Google Scholar] [CrossRef]
- Schwartz, D.A.; Avvad-Portari, E.; Babál, P.; Baldewijns, M.; Blomberg, M.; Bouachba, A.; Camacho, J.; Collardeau-Frachon, S.; Colson, A.; Dehaene, I.; et al. Placental tissue destruction and insufficiency from COVID-19 causes stillbirth and neonatal death from hypoxic-ischemic injury: A study of 68 cases with SARS-CoV-2 placentitis from 12 countries. Arch. Pathol. Lab. Med. 2022. [Google Scholar] [CrossRef]
- Pereira, L. Congenital viral infection: Traversing the uterine-placental interface. Annu. Rev. Virol. 2018, 5, 273–299. [Google Scholar] [CrossRef] [Green Version]
- Lamouroux, A.; Attie-Bitach, T.; Martinovic, J.; Leruez-Ville, M.; Ville, Y. Evidence for and against vertical transmission for severe acute respiratory syndrome coronavirus 2. Am. J. Obstet Gynecol. 2020, 223, 91.E1–91.E4. [Google Scholar] [CrossRef]
- Jamieson, D.J.; Rasmussen, S.A. An update on COVID-19 and pregnancy. Am. J. Obstet Gynecol. 2022, 226, 177–186. [Google Scholar] [CrossRef]
- Shook, L.L.; Brigida, S.; Regan, J.; Flynn, J.P.; Mohammadi, A.; Etemad, B.; Siegel, M.R.; Clapp, M.A.; Li, J.Z.; Roberts, D.J.; et al. SARS-CoV-2 placentitis associated with B.1.617.2 (Delta) variant and fetal distress or demise. J. Infect. Dis. 2022, jiac008. [Google Scholar] [CrossRef]
- Guan, M.; Johannesen, E.; Tang, C.Y.; Hsu, A.L.; Barnes, C.L.; Burnam, M.; McElroy, J.A.; Wan, X.-F. Intrauterine fetal demise in the third trimester of pregnancy associated with mild infection with the SARS-CoV-2 Delta variant without protection from vaccination. J. Infect. Dis. 2022, jiac007. [Google Scholar] [CrossRef] [PubMed]
- Bleier, B.S.; Ramanathan, M., Jr.; Lane, A.P. COVID-19 Vaccines may not prevent nasal SARS-CoV-2 infection and asymptomatic transmission. Otolaryngol. Head Neck Surg. 2021, 164, 305–307. [Google Scholar] [CrossRef] [PubMed]
- Tiboni, M.; Casettari, L.; Illum, L. Nasal vaccination against SARS-CoV-2: Synergistic or alternative to intramuscular vaccines? Int. J. Pharm. 2021, 603, 120686. [Google Scholar] [CrossRef] [PubMed]
- Yewdell, J.W. Individuals cannot rely on COVID-19 herd immunity: Durable immunity to viral disease is limited to viruses with obligate viremic spread. PLoS Pathog. 2021, 17, e1009509. [Google Scholar] [CrossRef]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schwartz, D.A. Stillbirth after COVID-19 in Unvaccinated Mothers Can Result from SARS-CoV-2 Placentitis, Placental Insufficiency, and Hypoxic Ischemic Fetal Demise, Not Direct Fetal Infection: Potential Role of Maternal Vaccination in Pregnancy. Viruses 2022, 14, 458. https://doi.org/10.3390/v14030458
Schwartz DA. Stillbirth after COVID-19 in Unvaccinated Mothers Can Result from SARS-CoV-2 Placentitis, Placental Insufficiency, and Hypoxic Ischemic Fetal Demise, Not Direct Fetal Infection: Potential Role of Maternal Vaccination in Pregnancy. Viruses. 2022; 14(3):458. https://doi.org/10.3390/v14030458
Chicago/Turabian StyleSchwartz, David A. 2022. "Stillbirth after COVID-19 in Unvaccinated Mothers Can Result from SARS-CoV-2 Placentitis, Placental Insufficiency, and Hypoxic Ischemic Fetal Demise, Not Direct Fetal Infection: Potential Role of Maternal Vaccination in Pregnancy" Viruses 14, no. 3: 458. https://doi.org/10.3390/v14030458





