Abstract
Severe fever with thrombocytopenia syndrome (SFTS) is an emerging infectious disease caused by the SFTS virus. It involves multiple organ systems, including the lungs. However, the significance of the lung involvement in SFTS remains unclear. In the present study, we aimed to investigate the relationship between the clinical findings and abnormalities noted in the chest computed tomography (CT) of patients with SFTS. The medical records of 22 confirmed SFTS patients hospitalized in five hospitals in Nagasaki, Japan, between April 2013 and September 2019, were reviewed retrospectively. Interstitial septal thickening and ground-glass opacity (GGO) were the most common findings in 15 (68.1%) and 12 (54.5%) patients, respectively, and lung GGOs were associated with fatalities. The SFTS patients with a GGO pattern were elderly, had a disturbance of the conscious and tachycardia, and had higher c-reactive protein levels at admission (p = 0.009, 0.006, 0.002, and 0.038, respectively). These results suggested that the GGO pattern in patients with SFTS displayed disseminated inflammation in multiple organs and that cardiac stress was linked to higher mortality. Chest CT evaluations may be useful for hospitalized patients with SFTS to predict their severity and as early triage for the need of intensive care.
1. Introduction
Severe fever with thrombocytopenia syndrome (SFTS) is an emerging tick-borne infectious disease caused by a novel bunyavirus, the SFTS virus (SFTSV), which was first identified in China in 2011 [1]. As it continues to spread, SFTS is attracting increasing global attention, as relatively little is known about this disease, and no standardized treatment currently exists. In 2015, the World Health Organization compiled a list of priority pathogens with the potential to generate an international public health emergency, for which no insufficient, preventive, or curative solutions existed [2]. SFTS was subsequently placed on the list after an annual review in 2016 [3]. In Japan, SFTS was first identified in 2013 [4], and it has been designated as a Category IV disease since 2013 under Japan’s Infectious Diseases Control Law, mandating that physicians notify the authorities of all laboratory-confirmed cases [5]. SFTS exhibits high case fatality rates (CFRs) in humans (13–35%), and from national surveillance data, was found to be especially severe in people over 50 years of age, causing a higher fatality [6], and thus posing a particular threat to Japanese rapidly aging population. So far, SFTS cases have been mainly restricted to localities in the southwestern parts of the Japanese islands [7,8], similar to the distribution of Japanese spotted fever, another tick-borne disease [8].
SFTS involves multiple organs, including the liver, muscles, central nervous system, kidney, genital organs, and lungs [9,10,11]. It was reported that ∼9.6% to 28.7% of patients with SFTS experience respiratory symptoms, including cough, sputum, and dyspnea, with radiographic abnormalities being present in 29 to 45% of patients with SFTS [12]. However, there are limited data regarding the detailed lung involvement in SFTS [12,13,14], with no the studies discussing the relationship between lung imaging abnormalities and the clinical characteristics of patients with the SFTS. In this study, we conducted a retrospective investigation into the relationship between clinical findings and chest CT abnormalities in patients with SFTS that were identified in Nagasaki, a southwestern prefecture of Japan.
2. Materials and Methods
2.1. Study Population and Clinical Data
We performed a retrospective observational study of adult patients aged ≥ 20 years who were diagnosed with SFTS using a SFTSV PCR test, hospitalized, and underwent chest radiography and CT during the first three days of admission. The patients were registered at five institutions (Nagasaki University Hospital, Isahaya General Hospital, Sasebo City General Hospital, Sasebo Chuo Hospital, and Nagasaki Rosai Hospital) in Nagasaki Prefecture, Japan, between April 2013 and September 2019. Patient medical charts were reviewed retrospectively, and data on baseline characteristics, clinical presentations, laboratory findings, and radiological findings at admission were collected from the medical records. The in-hospital complications and outcomes were also assessed. The disseminated intravascular coagulation (DIC) score was calculated using the Japanese Association for Acute Medicine (JAAM) DIC scoring system [15]. The study protocol was approved by the Institutional Review Board of the Nagasaki University Hospital (approval number, 18121024), Isahaya General Hospital (approval number, 2020-21), Sasebo City General Hospital (approval number, 2020-A024), Sasebo Chuo Hospital (approval number, 2020-17), and Nagasaki Rosai Hospital (approval number: 02003).
2.2. SFTSV Real-Time PCR
Sera samples from patients with SFTS, collected at the Nagasaki University Hospital and Sasebo City General Hospital, were measured for the SFTSV viral load using a real-time PCR assay. The total RNA was extracted using a RNeasy Lipid Tissue Mini Kit (Qiagen, Hilden, Germany). The SFTSV-specific primers and probes were designed based on the RdRp region of the consensus sequence of the L segment. The forward primer was SFTS_QPCR_965F: 5′-GCRAGGAGCAACAARCAAACATC-3′, the reverse primer was SFTS_QPCR_1069R: 5′-GCCTGAGTCGGTCTTGATGTC-3′, and the PrimeTimes qPCR probe was FAM/5′-CTCCCRCCC-3′/ZEN/5′-TGGCTACCAAAGC-3′/IBFQ (Integrated DNA Technologies, Coralville, IA, USA) [16]). A real-time RT-PCR was performed using a One Step PrimeScript RT-PCR Kit (Takara Bio Inc., Shiga, Japan) and a 7500 Real-time RT-PCR System (Applied Biosystems). Copy numbers were calculated as the ratio of the copy numbers of the standard controls.
2.3. Evaluation of the Chest Radiograph and CT
Two Japanese Respiratory Society (JRS) board-certified pulmonologists, who had 15 and 8 years of experience, respectively, reviewed all chest radiographs and CT examinations independently and came to a consensus. Any disagreements with findings between the two readers were evaluated by a third reader with 15 years of experience as a JRS board-certified pulmonologist. Cardiomegaly was defined as a cardiothoracic (CTR) ratio > 0.50, based on posterior–anterior (PA) chest radiographs or a CTR ratio > 0.55 in anterior–posterior (AP) chest radiographs [17]. The abnormalities in chest CT were characterized by consolidation, ground-glass opacity (GGO), centrilobular nodules, interstitial septal thickening, and bronchial wall thickening. The presence of mediastinal lymph node enlargement (>10 mm along the short axis), pleural effusion, pericardial effusion (pericardial thickness of 4 mm or more [18]), hepatomegaly (diameter of >16.0 cm at craniocaudal line [19]), splenomegaly (width measurement of >10.5 cm [20]), and additional lung findings were also recorded.
2.4. Endpoint
The primary endpoint was the association between abnormal chest CT findings and the clinical characteristics of patients with SFTS. First, abnormal CT findings, which correlated with in-hospital fatality in patients with SFTS, were determined. Second, the underlying condition and clinical findings at admission, such as physical symptoms, vital signs, laboratory data, DIC scores, and qSOFA scores were evaluated for associations with abnormal chest CT findings connected to fatality in patients with SFTS. Finally, in-hospital complications and outcomes, such as in-hospital secondary infections (bacterial and/or fungal), ICU admission, and length of hospital stay, were evaluated for their association with abnormal chest CT findings related to fatality.
2.5. Statistical Analysis
The results are expressed as means ± standard deviation, medians, and as percentages. SPSS version 25.0 (SPSS, Inc., Chicago, IL, USA) was used to analyze the data. The categorical variables were analyzed using the Chi-square test or Fisher’s exact test, and the continuous variables were analyzed using the Mann–Whitney U test. All the tests were two-tailed and the differences were considered significant at a p-value of <0.05.
3. Results
3.1. Chest CT Findings in Fatal and Non-Fatal Hospitalized Patients with SFTS
A total of 24 hospitalized patients with SFTS during the study period were initially reviewed. Of these patients, two who did not undergo CT examinations were excluded. Finally, the findings were analyzed in 22 hospitalized patients with SFTS in the present study. All the patients were confirmed to be SFTSV-positive using RT-PCR. The chest CT scans were performed in the supine position with 1 mm (n = 17), 2 mm (n = 3), 3 mm (n = 1), or 5 mm (n = 1) slices. Chest CT scans were performed in these patients at an average of 4.95 days (range, 1–21 days) following symptom onset, and at an average of 0.14 days (range, 0–1 days) after admission (Table 1). Nineteen patients (86.4%) had abnormal chest CT findings. Interstitial septal thickening and GGO were the most frequently found chest CT abnormalities in 15 (68.1%) and 12 (54.5%) patients, respectively, followed by centrilobular nodules, bronchial wall thickening, and cardiomegaly, which were found in eight (36.4%) patients (Table 1). Hepatomegaly and splenomegaly were found in 27.3 and 13.6%, respectively, of the SFTS patients. Six patients (27.2%) died during hospitalization with the mean duration from disease onset until death being 17.2 days (range: 6–48 days). With regard to the relationship between chest CT abnormalities and fatality in patients with SFTS, GGO was the only finding that was significantly related to death (p = 0.015, Table 1).

Table 1.
Chest CT findings in fatal and non-fatal hospitalized patients with SFTS.
The typical GGO findings in patients with SFTS are shown in Figure 1. The patchy GGO shadows in the lungs worsened after a week of admission. Chest CT was followed up during hospitalization in seven SFTS patients, including six with GGO and one without GGO at admission. The SFTS patients without GGO at admission did not develop GGO during hospitalization for 30 days. In the SFTS patients with GGO findings at admission, GGO areas peaked at 10.8 (±3.4) days after admission, and these were prolonged until 27.0 (±9.0) days after admission. Table 2 shows the correlation between the GGOs and other abnormal chest CT findings in patients with SFTS. The GGO did not correlate with interstitial septal thickening or other findings, except for cardiomegaly (p = 0.031, Table 2). We considered that the GGO findings on the chest CT may correlate with the outcomes in patients with SFTS; therefore, we further investigated the clinical characteristics of patients with SFTS with or without GGO findings on chest CT.
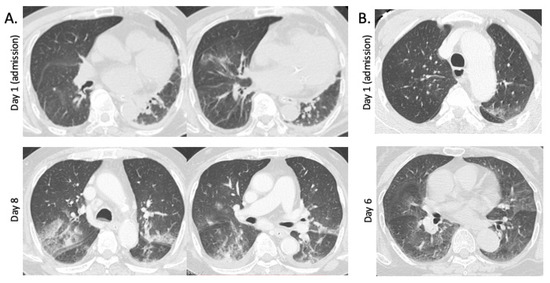
Figure 1.
Ground-glass opacities (GGOs) in chest CT in patients with SFTS. (A) Chest CT images in a 79-year-old nonfatal male patient. Focal GGOs in the lower lobes of the lungs, interstitial septal thickening, cardiomegaly, and pleural effusion were found at admission (2 days after onset). At day 8 of admission, multifocal GGOs with patchy consolidations are present in both lungs. (B) Chest CT images of a 73-year-old fatal female patient. Focal GGO is present at upper lobe of left lung at admission (3 days after onset). Multifocal GGOs, interstitial septal thickening, and cardiomegaly are found at day 6 of admission.

Table 2.
Correlation of GGO and other abnormal chest CT findings in patients with SFTS.
3.2. Patient Characteristics and Complications during Hospitalization
Table 3 shows the baseline characteristics and clinical symptoms in the patients with SFTS. The mean age of the patients was 71.4 ± 9.9 years and SFTS was more common in men (68.1%). The GGO was significantly more common in elderly patients (p = 0.009). Nineteen (86.3%) patients were farmers and hunters, living or working in wooded and hilly areas before the onset of disease. Seventeen (77.2%) patients had at least one underlying illness, and six (27.2%) patients were smokers. Including chronic lung, cardiovascular, and kidney diseases, the presence of comorbid conditions was comparable between the groups with or without the GGO pattern.

Table 3.
Underlying conditions, symptoms, and outcomes in patients with SFTS.
With regard to general symptoms, fever (81.8%) and fatigue (72.7%) were most frequently observed in patients with SFTS, while respiratory symptoms were present in 40.9% of the patients with dyspnea (31.8%) being the most frequent symptom. Respiratory symptoms, including cough and sputum, were comparable between the patients with and those without GGO patterns. Gastrointestinal symptoms were present in 81.8% of the patients with SFTS, with diarrhea being the most common symptom, which was significantly present in the patients without a GGO pattern. Skin rash and tick bites were found in >30% of the patients, and lymphadenopathy was present in 45.5% of the patients with SFTS.
Secondary bacterial or fungal infections occurred in five patients (22.7%) with SFTS during hospitalization. Fungal infections (invasive pulmonary aspergillus, candidemia, and disseminated trichosporonosis) occurred in three patients (13.6%) who presented with GGO in the lung, although the difference was not statistically significant. The length of hospital stay and intensive care unit (ICU) admission also tended to be higher in patients with GGO, although the difference was not statistically significant.
3.3. Laboratory Findings
Table 4 shows the vital signs and laboratory findings at admission. Impaired consciousness was observed in 63.6% of patients with SFTS and was significantly present in those with GGO in the lung (p = 0.006). The mean body temperature at admission was 38.3 °C in patients with SFTS and a significantly high heart rate (mean, 93.3/min) was observed in those with GGO findings (p = 0.002). There were 11 (50.0%) patients with hypoxemia at admission, although there were no differences in the SpO2 or SpO2/FiO2 ratios between the two groups. The qSOFA scores in patients with SFTS between the groups with or without GGO in the lung were comparable.

Table 4.
Vital signs and laboratory data on the admission of patients with SFTS.
Leukocytopenia and thrombocytopenia were also observed and were similar between the groups. Abnormal liver function tests, elevated creatine kinase and ferritin levels, renal dysfunction, and coagulation disturbances were generally observed in patients with SFTS, and these were comparable between groups. However, elevated c-reactive protein (CRP) levels were significantly observed in patients with GGO patterns (p = 0.038). The mean SFTSV viral titer was higher in patients with GGO patterns, although the difference was not statistically significant. SFTSV serial viral load was measured during hospitalization in nine patients (seven SFTS patients with GGO and two without GGO, Figure 2). Viremia was prolonged for between a week through 4 weeks after admission, though a trend of SFTS patients with GGO was not definable.
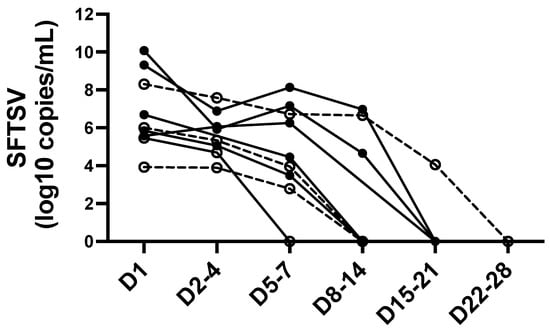
Figure 2.
Serial SFTSV viral load in patients with SFTS. Open circles with dotted lines represent the SFTS patients without GGO in chest CT at admission. Filled circles with solid lines represent the SFTS patients with GGO in chest CT at admission. Days (D) after admission are displayed on X-axis. Abbreviations: SFTSV, severe fever with thrombocytopenia syndrome virus.
4. Discussion
Since the first report of SFTS in Japan, in 2013, approximately 640 cases have been reported as of July 2021, with a high mortality rate (12%): 80 deaths [21]. In the present study, we found that GGOs were identified frequently in the lungs of patients with SFTS and correlated with poor outcomes. In a previous report from China, in 2013, chest radiograph (CR) abnormalities were found in 45% of 98 cases diagnosed with SFTS [13], which was lower than the figures in our report, and may be due to the differences in the sensitivities between CR and CT. These were supported by our data, which showed that CR could detect 8 out of 12 (66.6%) GGO findings compared to CT. Another report from Korea found abnormal chest CT findings in 62% of 21 hospitalized patients with SFTS, with a variable consolidation; GGOs were the most commonly identified abnormality (48%), which was consistent with the findings of the present study. From the viewpoint of pathogenesis, GGO in the lungs of patients with SFTS may be generated by lung edema [22] due to accelerated inflammation or disseminated SFTSV in the lung. While the initial CR may have been normal, bilateral pulmonary infiltration indicated the development of pulmonary edema [22]. Although serial follow-up CT was performed in only 7 (31.8%) patients in our study, GGO in the lungs of patients with SFTS became more prominent at approximately 1 to 2 weeks of onset (Figure 1) and was prolonged for a month. In our study, the GGO was correlated with tachycardia, indicating that cardiac stress may result in the generation of GGO in the lungs. The cardiomegaly that was found in 36.4% of our cases was more frequently reported in hospitalized patients with SFTS (90%) [12], which may support this hypothesis. The SFTSV viral titer was not correlated significantly with GGO abnormalities in our study; however, a quantitative RT-PCR was performed partially (72.7%) in two hospitals, and serial SFTSV follow-up measurement was performed in only 40.9% of all patients with SFTS; therefore, it was difficult to make a conclusion regarding this connection. Viral dissemination in the pulmonary alveoli was confirmed using bronchoalveolar lavage in one of our patients who presented with GGO in the lung [11], suggesting that the GGO pattern may be generated by viral dissemination and accelerated inflammation in the lung. Pleural effusion, pericardial effusion, and mediastinal lymph node enlargement in the present study (22.7%, 18.1%, and 13.6%, respectively) were similar to those in a previous study (38%, 24%, and 14%, respectively) [12]. Although SFTS causes various systemic symptoms, there is a significant disturbance in conscious among patients with GGO, which may indicate the risk of the dissemination of SFTS disease to multiple organs [10]. In addition, CRP levels were elevated significantly in patients with GGO, which may indicate an association with systemic inflammation.
The present study had several limitations that are characteristic of retrospective studies. First, symptoms and laboratory data may not have been comprehensively recorded. Second, patients with milder illnesses may not have presented at the hospital. Third, selection bias toward more severe disease with SFTS may have been included in the present study because the chest CT scans were included in the inclusion criteria. Fourth, the sample size may not have been large enough to draw a conclusion on an association between abnormal findings in chest CT and the clinical features in patients with SFTS.
Despite these limitations, to the best of our knowledge, the present study was the first to evaluate the significance of lung abnormalities in chest CT findings in patients with SFTS. Patients with SFTS showing GGO patterns in the lungs, may develop more severe diseases and require intensive care; therefore, attention to abnormal chest findings is important at the time of admission.
5. Conclusions
Abnormal lung GGOs were associated with fatalities in patients with SFTS. Pulmonary GGO findings were accompanied frequently with cardiomegaly and were mainly found in elderly patients with SFTS. Patients with SFTS with GGO findings in the lung often present with impaired consciousness, tachycardia, and high inflammation at admission. These results suggested that the GGO patterns in patients with SFTS had a more disseminated inflammation in multiple organs, and that cardiac stress was linked to a higher mortality. Chest CT evaluations may be useful for hospitalized patients with SFTS, in order to predict the severity of their condition and the need for early triage to meet their need for intensive care. Further studies are needed to determine the clinical impact of chest CT evaluations on the diagnosis and treatment of SFTS.
Author Contributions
Conceptualization, K.Y. (Kazuko Yamamoto) and H.M.; Data curation, H.A., K.Y. (Kazuko Yamamoto), M.S., S.I. (Shotaro Ide), A.U., M.Y., Y.F., T.K. and S.K. (Shungo Katoh); Formal analysis, S.M.; Funding acquisition, K.Y. (Kazuko Yamamoto) and K.I.; Investigation, N.A., K.T., N.I., T.T. (Takahiro Takazono), M.T., T.T. (Takeshi Tanaka), K.M. (Konosuke Morimoto) and S.K. (Shintaro Kurihara); Methodology, M.M.N.T., S.I. (Shingo Inoue) and K.M. (Kouichi Morita); Project administration, K.Y. (Kazuko Yamamoto) and H.M.; Resources, K.A. and H.M.; Supervision, K.I. and K.Y. (Katzunori Yanagihara); Validation, K.Y. (Kazuko Yamamoto), N.I. and N.S.; Writing—original draft, H.A.; Writing—review and editing, K.Y. (Kazuko Yamamoto). All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by the Non-Profit Organization aimed to support community medicine research in Nagasaki, and by AMED under grant number JP21fk0108081.
Institutional Review Board Statement
This multicenter study was conducted in compliance with the principles of the Declaration of Helsinki and was approved by the Ethics Committees of the participating institutions (approval number: 18121024; Nagasaki University Hospital, approval number: 2020-21; Sasebo City General Hospital, approval number: 2020-A024; Sasebo Chuo Hospital, approval number: 2020-17; Nagasaki Rosai Hospital, approval number: 02003).
Informed Consent Statement
The requirement for patient consent was waived owing to the retrospective nature of the study, which ensured anonymity.
Data Availability Statement
Data are contained within the article.
Acknowledgments
We would like to thank Akira Nishiyama, Department of General Internal Medicine, Nagasaki Rosai Hospital, Sasebo City, Japan, to input patients’ data.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Yu, X.J.; Liang, M.F.; Zhang, S.Y.; Liu, Y.; Li, J.D.; Sun, Y.L.; Zhang, L.; Zhang, Q.F.; Popov, V.L.; Li, C.; et al. Fever with Thrombocytopenia Associated with a Novel Bunyavirus in China. N. Engl. J. Med. 2011, 364, 1523–1532. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- World Health Organization. An, R.&D Blueprint for Action to Prevent Epidemics: Plan of Action. 2016. Available online: https://www.who.int/who-documents-detail/an-r-d-blueprint-for-action-to-prevent-epidemics (accessed on 14 May 2020).
- World Health Organization. An, R.&D Blueprint for Action to Prevent Epidemics: Funding and Coordination Models for Prepared and Response. 2016. Available online: https://www.who.int/blueprint/what/improving-coordination/workstream_5_document_on_financing.pdf?ua=1 (accessed on 14 May 2020).
- Saijo, M.; Shimojima, M.; Yamagishi, T.; Ohishi, K.; Morikawa, S.; Hasegawa, H.A. Severe Fever with Thrombocytopenia Syndrome (SFTS), a New Tick-Borne Virus Infection—The First Case Diagnosed in Japan, 2012. Infect. Agents Surveill. Rep. 2013, 34, 40–41. (In Japanese) [Google Scholar]
- National Institute for Infectious Diseases (NIID). Severe Fever with Thrombocytopenia Syndrome (SFTS) in Japan, 2013. IASR 2014, 35, 31–32. [Google Scholar]
- Kobayashi, Y.; Kato, H.; Yamagishi, T.; Shimada, T.; Matsui, T.; Yoshikawa, T.; Kurosu, T.; Shimojima, M.; Morikawa, S.; Hasegawa, H.; et al. Severe Fever with Thrombocytopenia Syndrome, Japan, 2013–2017. Emerg. Infect. Dis. 2020, 26, 692–699. [Google Scholar] [CrossRef] [Green Version]
- Crump, A.; Tanimoto, T. Severe Fever with Thrombocytopenia Syndrome: Japan under Threat from Life-Threatening Emerging Tick-Borne Disease. JMA J. 2020, 3, 295–302. [Google Scholar] [CrossRef] [PubMed]
- Yamaji, K.; Aonuma, H.; Kanuka, H. Distribution of tick-borne diseases in Japan: Past patterns and implications for the future. J. Infect. Chemother. 2018, 24, 499–504. [Google Scholar] [CrossRef] [Green Version]
- Nakamura, S.; Iwanaga, N.; Hara, S.; Shimada, S.; Kashima, Y.; Hayasaka, D.; Abe, K.; Izumikawa, K.; Yanagihara, K.; Miyazaki, Y.; et al. Viral Load and Inflammatory Cytokine Dynamics Associated with the Prognosis of Severe Fever with Thrombocytopenia Syndrome Virus Infection: An Autopsy Case. J. Infect. Chemother. 2019, 25, 480–484. [Google Scholar] [CrossRef] [PubMed]
- Koga, S.; Takazono, T.; Ando, T.; Hayasaka, D.; Tashiro, M.; Saijo, T.; Kurihara, S.; Sekino, M.; Yamamoto, K.; Imamura, Y.; et al. Severe Fever with Thrombocytopenia Syndrome Virus RNA in Semen, Japan. Emerg. Infect. Dis. 2019, 25, 2127–2128. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Akagi, K.; Miyazaki, T.; Oshima, K.; Umemura, A.; Shimada, S.; Morita, K.; Senju, H.; Tashiro, M.; Takazono, T.; Saijo, T.; et al. Detection of Viral RNA in Diverse Body Fluids in an SFTS Patient with Encephalopathy, Gastrointestinal Bleeding and Pneumonia: A Case Report and Literature Review. BMC Infect. Dis. 2020, 20, 281. [Google Scholar] [CrossRef]
- Yun, J.H.; Hwang, H.J.; Jung, J.; Kim, M.J.; Chong, Y.P.; Lee, S.O.; Choi, S.H.; Kim, Y.S.; Woo, J.H.; Kim, M.Y.; et al. Comparison of Chest Radiographic Findings between Severe Fever with Thrombocytopenia Syndrome and Scrub Typhus: Single Center Observational Cross-Sectional Study in South Korea. Medicine 2019, 98, e17701. [Google Scholar] [CrossRef] [Green Version]
- Deng, B.; Zhou, B.; Zhang, S.; Zhu, Y.; Han, L.; Geng, Y.; Jin, Z.; Liu, H.; Wang, D.; Zhao, Y.; et al. Clinical Features and Factors Associated with Severity and Fatality among Patients with Severe Fever with Thrombocytopenia Syndrome Bunyavirus Infection in Northeast China. PLoS ONE. 2013, 8, e80802. [Google Scholar] [CrossRef] [Green Version]
- Zhu, Y.; Wu, H.; Gao, J.; Zhou, X.; Zhu, R.; Zhang, C.; Bai, H.; Abdullah, A.S.; Pan, H. Two Confirmed Cases of Severe Fever with Thrombocytopenia Syndrome with Pneumonia: Implication for a Family Cluster in East China. BMC Infect. Dis. 2017, 17, 537. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gando, S.; Saitoh, D.; Ogura, H.; Fujishima, S.; Mayumi, T.; Araki, T.; Ikeda, H.; Kotani, J.; Kushimoto, S.; Miki, Y.; et al. A Multicenter, Prospective Validation Study of the Japanese Association for Acute Medicine Disseminated Intravascular Coagulation Scoring System in Patients with Severe Sepsis. Crit. Care. 2013, 17, R111. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shimada, S.; Posadas-Herrera, G.; Aoki, K.; Morita, K.; Hayasaka, D. Therapeutic Effect of Post-Exposure Treatment with Antiserum on Severe Fever with Thrombocytopenia Syndrome (SFTS) in a Mouse Model of SFTS Virus Infection. Virology 2015, 482, 19–27. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Truszkiewicz, K.; Poręba, R.; Gać, P. Radiological Cardiothoracic Ratio in Evidence-Based Medicine. J. Clin. Med. 2021, 10, 2016. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.J.; Reddy, G.P.; Gotway, M.B.; Yeh, B.M.; Hetts, S.W.; Higgins, C.B. CT and MR Imaging of Pericardial Disease. Radiographics. 2003, 23, S167–S180. [Google Scholar] [CrossRef] [PubMed]
- Muggli, D.; Müller, M.A.; Karlo, C.; Fornaro, J.; Marincek, B.; Frauenfelder, T. A Simple Method to Approximate Liver Size on Cross-Sectional Images Using Living Liver Models. Clin. Radiol. 2009, 64, 682–689. [Google Scholar] [CrossRef] [PubMed]
- Nuffer, Z.; Marini, T.; Rupasov, A.; Kwak, S.; Bhatt, S. The Best Single Measurement for Assessing Splenomegaly in Patients with Cirrhotic Liver Morphology. Acad. Radiol. 2017, 24, 1510–1516. [Google Scholar] [CrossRef] [PubMed]
- National Institute for Infectious Diseases (NIID). Severe Fever with Thrombocytopenia Syndrome (SFTS) in Japan. 2021. Available online: https://www.niid.go.jp/niid/ja/sfts/sfts-idwrs/7415-sfts-nesid.html (accessed on 1 December 2021). (In Japanese).
- Koo, H.J.; Lim, S.; Choe, J.; Choi, S.H.; Sung, H.; Do, K.H. Radiographic and CT Features of Viral Pneumonia. Radiographics 2018, 38, 719–739. [Google Scholar] [CrossRef] [PubMed] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).