Venous Thrombosis within 30 Days after Vaccination against SARS-CoV-2 in a Multinational Venous Thromboembolism Registry
Abstract
:1. Introduction
2. Methods
2.1. Data Source
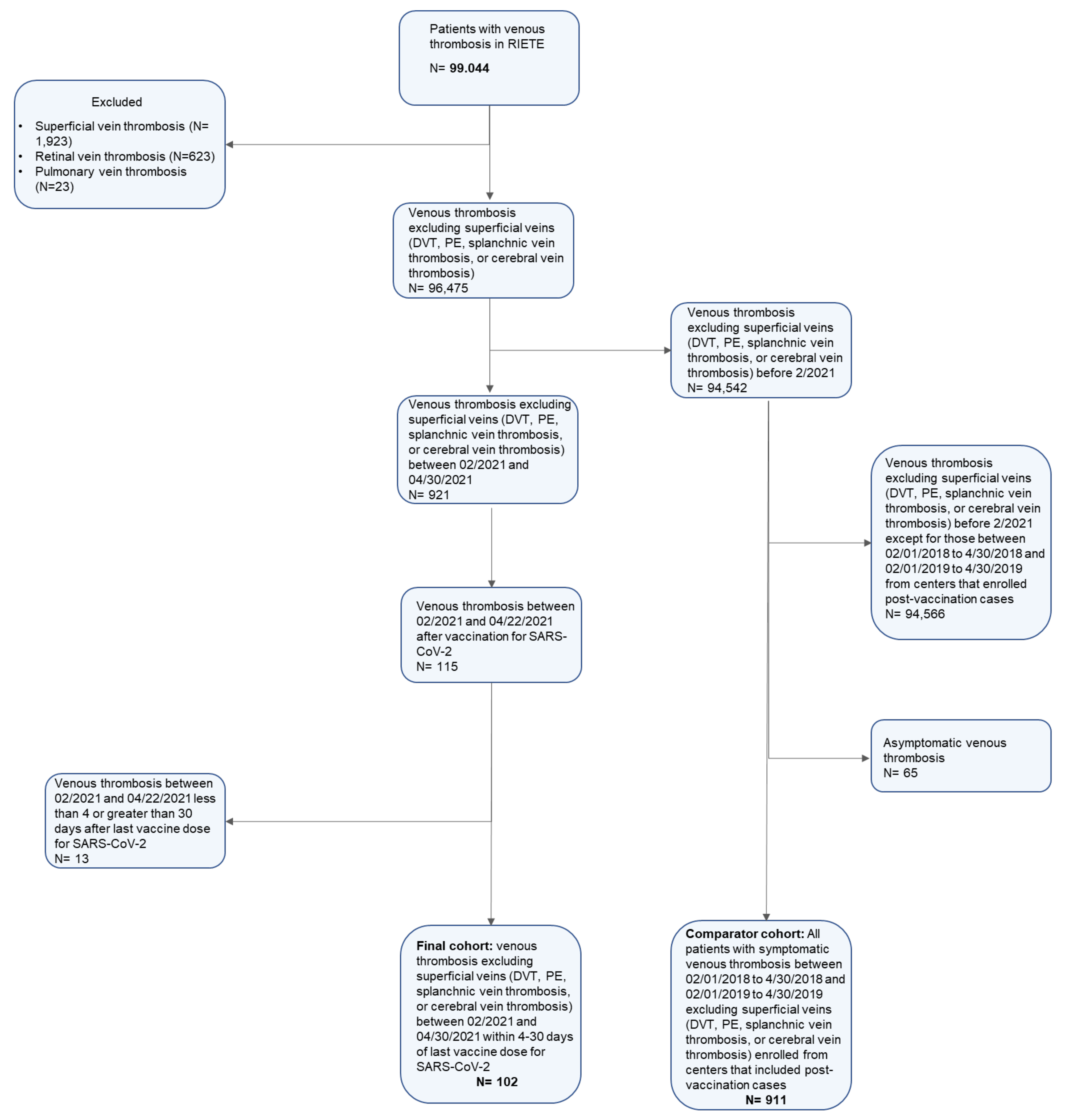
2.2. Patients
2.3. Exposure Variable and Other Variables of Interest
2.4. Outcomes
2.5. Statistical Analysis
3. Results
3.1. VTE Presentation and Clinical Characteristics
3.2. Initial Treatment
3.3. Clinical Outcomes
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Disclosures
Appendix A
References
- Gebru, A.A.; Birhanu, T.; Wendimu, E.; Ayalew, A.F.; Mulat, S.; Abasimel, H.Z.; Kazemi, A.; Tadesse, B.A.; Gebru, B.A.; Deriba, B.S.; et al. Global burden of COVID-19: Situational analyis and review. Hum. Antibodies 2021, 29, 139–148. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.; Madhavan, M.V.; Sehgal, K.; Nair, N.; Mahajan, S.; Sehrawat, T.S.; Bikdeli, B.; Ahluwalia, N.; Ausiello, J.C.; Wan, E.Y.; et al. Extrapulmonary manifestations of COVID-19. Nat. Med. 2020, 26, 1017–1032. [Google Scholar] [CrossRef] [PubMed]
- Bikdeli, B.; Madhavan, M.V.; Jimenez, D.; Chuich, T.; Dreyfus, I.; Driggin, E.; Nigoghossian, C.; Ageno, W.; Madjid, M.; Guo, Y.; et al. COVID-19 and Thrombotic or Thromboembolic Disease: Implications for Prevention, Antithrombotic Therapy, and Follow-Up: JACC State-of-the-Art Review. J. Am. Coll. Cardiol. 2020, 75, 2950–2973. [Google Scholar] [CrossRef] [PubMed]
- Jiménez, D.; García-Sanchez, A.; Rali, P.; Muriel, A.; Bikdeli, B.; Ruiz-Artacho, P.; Le Mao, R.; Rodríguez, C.; Hunt, B.J.; Monreal, M. Incidence of VTE and Bleeding Among Hospitalized Patients with Coronavirus Disease 2019: A Systematic Review and Meta-analysis. Chest 2021, 159, 1182–1196. [Google Scholar] [CrossRef]
- Piazza, G.; Morrow, D.A. Diagnosis, Management, and Pathophysiology of Arterial and Venous Thrombosis in COVID-19. JAMA 2020, 324, 2548. [Google Scholar] [CrossRef]
- Sadoff, J.; Gray, G.; Vandebosch, A.; Cárdenas, V.; Shukarev, G.; Grinsztejn, B.; Goepfert, P.A.; Truyers, C.; Fennema, H.; Spiessens, B.; et al. Safety and Efficacy of Single-Dose Ad26.COV2.S Vaccine against COVID-19. N. Engl. J. Med. 2021, 384, 2187–2201. [Google Scholar] [CrossRef]
- Baden, L.R.; El Sahly, H.M.; Essink, B.; Kotloff, K.; Frey, S.; Novak, R.; Diemert, D.; Spector, S.A.; Rouphael, N.; Creech, C.B.; et al. Efficacy and Safety of the mRNA-1273 SARS-CoV-2 Vaccine. N. Engl. J. Med. 2021, 384, 403–416. [Google Scholar] [CrossRef]
- Polack, F.P.; Thomas, S.J.; Kitchin, N.; Absalon, J.; Gurtman, A.; Lockhart, S.; Perez, J.L.; Pérez Marc, G.; Moreira, E.D.; Zerbini, C.; et al. Safety and efficacy of the BNT162b2 mRNA COVID-19 vaccine. N. Engl. J. Med. 2020, 383, 2603–2615. [Google Scholar] [CrossRef]
- Voysey, M.; Clemens, S.A.C.; Madhi, S.A.; Weckx, L.Y.; Folegatti, P.M.; Aley, P.K.; Angus, B.; Baillie, V.L.; Barnabas, S.L.; Bhorat, Q.E.; et al. Single-dose administration and the influence of the timing of the booster dose on immunogenicity and efficacy of ChAdOx1 nCoV-19 (AZD1222) vaccine: A pooled analysis of four randomised trials. Lancet 2021, 397, 881–891. [Google Scholar] [CrossRef]
- Cines, D.B.; Bussel, J.B. SARS-CoV-2 Vaccine-Induced Immune Thrombotic Thrombocytopenia. N. Engl. J. Med. 2021, 384, 2254–2256. [Google Scholar] [CrossRef]
- Ledford, H. How could a COVID vaccine cause blood clots? Scientists race to investigate. Nature 2021, 592, 334–335. [Google Scholar] [CrossRef] [PubMed]
- Schultz, N.H.; Sørvoll, I.H.; Michelsen, A.E.; Munthe, L.A.; Lund-Johansen, F.; Ahlen, M.T.; Wiedmann, M.; Aamodt, A.-H.; Skattør, T.H.; Tjønnfjord, G.E.; et al. Thrombosis and Thrombocytopenia after ChAdOx1 nCoV-19 Vaccination. N. Engl. J. Med. 2021, 384, 2124–2130. [Google Scholar] [CrossRef] [PubMed]
- Scully, M.; Singh, D.; Lown, R.; Poles, A.; Solomon, T.; Levi, M.; Goldblatt, D.; Kotoucek, P.; Thomas, W.; Lester, W. Pathologic Antibodies to Platelet Factor 4 after ChAdOx1 nCoV-19 Vaccination. N. Engl. J. Med. 2021, 384, 2202–2211. [Google Scholar] [CrossRef] [PubMed]
- Muir, K.-L.; Kallam, A.; Koepsell, S.A.; Gundabolu, K. Thrombotic Thrombocytopenia after Ad26.COV2.S Vaccination. N. Engl. J. Med. 2021, 384, 1964–1965. [Google Scholar] [CrossRef]
- Greinacher, A.; Thiele, T.; Warkentin, T.E.; Weisser, K.; Kyrle, P.A.; Eichinger, S. Thrombotic Thrombocytopenia after ChAdOx1 nCov-19 Vaccination. N. Engl. J. Med. 2021, 384, 2092–2101. [Google Scholar] [CrossRef]
- See, I.; Su, J.R.; Lale, A.; Woo, E.J.; Guh, A.Y.; Shimabukuro, T.T.; Streiff, M.B.; Rao, A.K.; Wheeler, A.P.; Beavers, S.F.; et al. US Case Reports of Cerebral Venous Sinus Thrombosis With Thrombocytopenia After Ad26.COV2.S Vaccination, March 2 to April 21, 2021. JAMA 2021, 325, 2448–2456. [Google Scholar] [CrossRef]
- Bikdeli, B.; Chatterjee, S.; Arora, S.; Monreal, M.; Jimenez, D.; Krumholz, H.M.; Goldhaber, S.Z.; Elkind, M.S.V.; Piazza, G. Cerebral Venous Sinus Thrombosis in the US Population, after Adenovirus-based SARS-CoV-2 Vaccination, and After COVID-19. J. Am. Coll. Cardiol. 2021, 78, 408–411. [Google Scholar] [CrossRef]
- Konstantinides, S.V. Thrombotic complications of vaccination against SARS-CoV-2: What pharmacovigilance reports tell us—And what they don’t. Eur. Respir. J. 2021, 58, 2101111. [Google Scholar] [CrossRef] [PubMed]
- Pavord, S.; Scully, M.; Hunt, B.J.; Lester, W.; Bagot, C.; Craven, B.; Rampotas, A.; Ambler, G.; Makris, M. Clinical Features of Vaccine-Induced Immune Thrombocytopenia and Thrombosis. N. Engl. J. Med. 2021, 385, 1680–1689. [Google Scholar] [CrossRef]
- Nazy, I.; Sachs, U.J.; Arnold, D.M.; McKenzie, S.E.; Choi, P.; Althaus, K.; Ahlen, M.T.; Sharma, R.; Grace, R.F.; Bakchoul, T. Recommendations for the clinical and laboratory diagnosis of vaccine-induced immune thrombotic thrombocytopenia (VITT) for SARS-CoV-2 infections: Communication from the ISTH SSC Subcommittee on Platelet Immunology. J. Thromb. Haemos. 2021, 19, 1585–1588. [Google Scholar] [CrossRef]
- Bikdeli, B.; Jimenez, D.; Hawkins, M.; Ortíz, S.; Prandoni, P.; Brenner, B.; Decousus, H.; Masoudi, F.A.; Trujillo-Santos, J.; Krumholz, H.M.; et al. Rationale, Design and Methodology of the Computerized Registry of Patients with Venous Thromboembolism (RIETE). Thromb. Haemost. 2018, 118, 214–224. [Google Scholar] [CrossRef] [PubMed]
- Schulman, S.; Kearon, C.; The Subcommittee on Control of Anticoagulation of the Scientific and Standardization Committee of the International Society on Thrombosis and Haemostasis. Definition of major bleeding in clinical investigations of antihemostatic medicinal products in non-surgical patients. J. Thromb. Haemost. 2005, 3, 692–694. [Google Scholar] [CrossRef] [PubMed]
- Smadja, D.M.; Yue, Q.-Y.; Chocron, R.; Sanchez, O.; Louet, A.L.-L. Vaccination against COVID-19: Insight from arterial and venous thrombosis occurrence using data from VigiBase. Eur. Respir. J. 2021, 58, 2100956. [Google Scholar] [CrossRef]
- Guijarro, R.; Montes, J.; Sanromán, C.; Monreal, M. Venous thromboembolism in Spain. Comparison between an administrative database and the RIETE registry. Eur. J. Intern. Med. 2008, 19, 443–446. [Google Scholar] [CrossRef]

| Adenovirus-Based Vaccines (Astrazeneca, Johnson and Johnsson) | mRNA-Based Vaccines (Pfizer and Moderna) | Controls from 2018–2019 a | |
|---|---|---|---|
| Number of patients | 28 | 74 | 911 |
| Most common location of venous thrombosis | |||
| Pulmonary embolism with or without DVT | 11 (39.3%) | 48 (64.9%) | 476 (52.3%) |
| Isolated DVT | 7 (25.0%) | 21 (28.4%) | 405 (44.5%) |
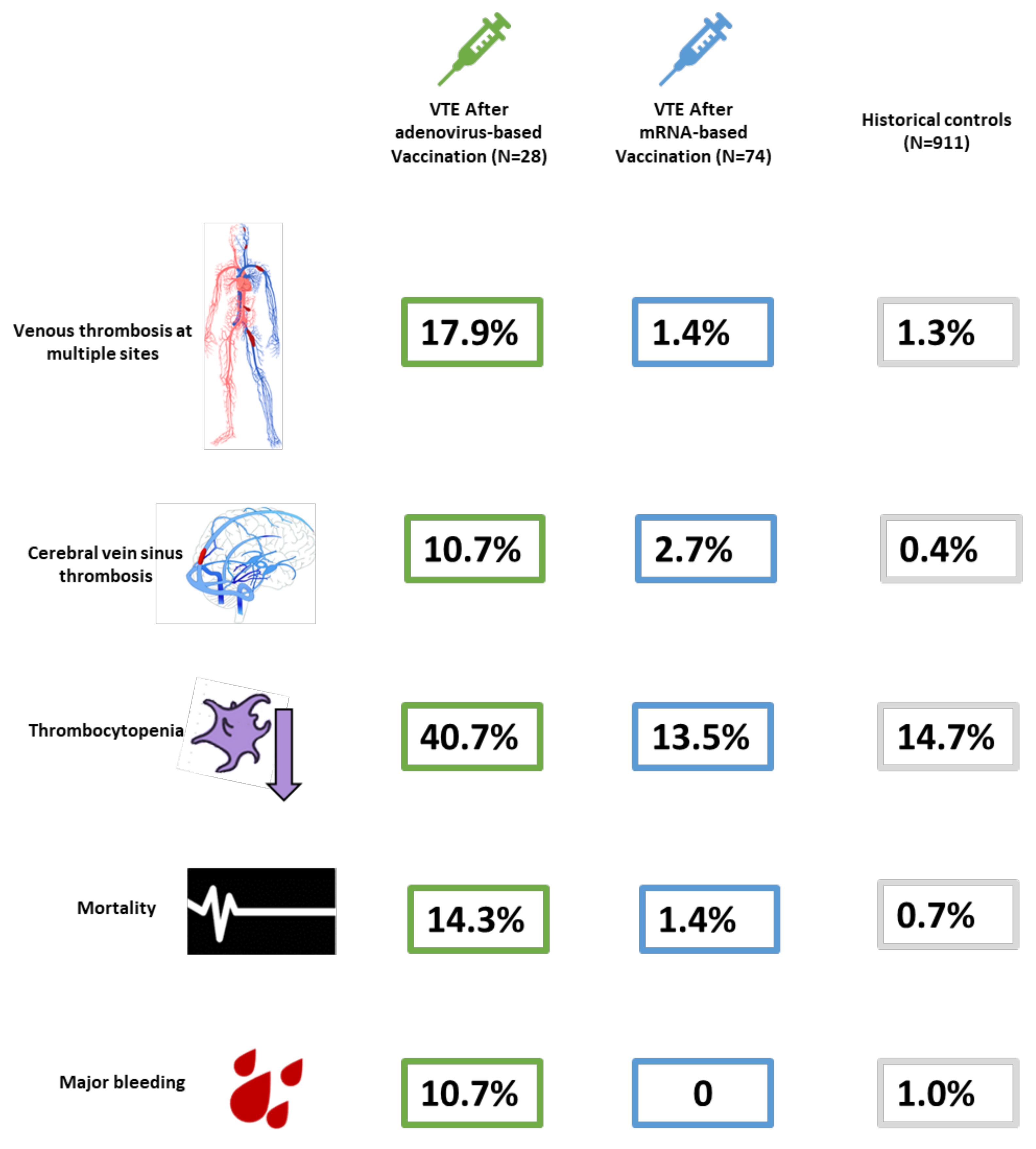
| Cerebral venous sinus thrombosis only | 3 (10.7%) | 2 (2.7%) | 4 (0.4%) |
| Splanchnic vein thrombosis only | 2 (7.1%) | 2 (2.7%) | 14 (1.5%) |
| Venous thrombosis in >1 territory b | 5 (17.9%) | 1 (1.4%) | 12 (1.3%) |
| Time of first vaccine dose (days) | 14.2 ± 1.1 | 22.8 ± 1.85 | - |
| Time of second vaccine dose (days) | - | 10.8 ± 2.7 | - |
| Sex, women | 11 (39.3%) | 50 (67.6%) | 470 (51.6%) |
| Mean age—years (SEM) | 53.8 ± 2.53 | 78.3 ± 1.72 | 65.5 ± 0.56 |
| Age < 50 years N, % | 12 (42.9%) | 6 (8.1%) | 172 (18.9%) |
| Major risk factors | |||
| Active cancer | 1 (3.6%) | 9 (12.2%) | 136 (14.9%) |
| Major surgery within 30 days | 1/27 (3.7%) | 1 (1.4%) | 105 (11.5%) |
| Medical hospitalization > 24hours within 30 days | 0 | 1 (1.4%) | 62 (6.8%) |
| Recent immobilization for ≥ 4 days within 30 days | 2/27 (7.4%) | 14 (18.9%) | 192 (21.1%) |
| Pregnancy, puerperium, assisted reproductive therapy or contraceptive hormonal therapies | 0 | 3 (4.1%) | 74 (8.1%) |
| None of the above | 24 (85.7%) | 49 (66.2%) | 462 (50.7%) |
| Co-morbidities and other risk factors | |||
| Chronic lung disease | 3 (10.7%) | 5 (6.8%) | 107 (11.7%) |
| Heart failure | 1/26 (3.8%) | 10/73 (13.7%) | 57/905 (6.3%) |
| Coronary or peripheral arterial disease or ischemic stroke | 3 (10.7%) | 9 (12.2%) | 116 (12.7%) |
| Hypertension | 10 (35.7%) | 37 (50.0%) | 426 (46.8%) |
| Personal history of VTE | 3/27 (11.1%) | 15 (20.3%) | 121 (13.3%) |
| Family history of VTE | 3 (10.7%) | 0 | 81 (8.9%) |
| Known thrombophilia c | 1/24 (4.2%) | 2/62 (3.2%) | 19/820 (2.3%) |
| Major bleeding in the past 30 days | 1 (3.6%) | 2 (2.7%) | 23 (2.5%) |
| Laboratory Tests | |||
| D-dimer (Positive) d (N = 746) | 19/21 (100.0%) | 55/56 (98.2%) | 651/669 (97.3%) |
| D-dimer levels > 5× upper limit d | 9/21 (42.9%) | 36/55 (65.5%) | 402/651 (61.8%) |
| D-dimer levels > 10× upper limit d | 4/21 (19.0%) | 28/55 (50.9%) | 237/651 (36.4%) |
| Fibrinogen (mg/dL) | 423 ± 41.0 | 393 ± 17.9 | 408 ± 5.35 |
| Fibrinogen < 150 mg/dL | 2 (7.1%) | 0 | 3 (0.3%) |
| Abnormal INR (as reported by sites) | 4 (14.3%) | 6 (8.1%) | 75 (8.2%) |
| INR values (N = 983) | 1.02 ± 0.03 | 1.02 ± 0.01 | (N = 884) 1.02 ± 0 |
| Platelet count (/fL) | 164.2 ± 19.07 | 226.3 ± 8.73 | 229.8 ± 2.95 |
| Platelet count (/fL) Median (Q1, Q3) | 192 (62–244) | 218 (187–250) | 217 (174–268) |
| Platelet count < 150,000/fL | 11/27 (40.7%) | 10 (13.5%) | 134/910 (14.7%) |
| Platelet count < 50,000/fL | 5/27 (18.5%) | 0 | 1/910 (0.1%) |
| PF-4 antibody tested (Yes/No) | 5/9 (55.6%) | 0 | 0 |
| PF-4 antibody above normal limit | 5 (100.0%) | – | – |
| AST | (N = 16) 65.7 ± 23.0 | (N = 43) 40.8 ± 9.5 | (N = 459) 31.9 ± 1.9 |
| ALT | (N = 15) 85.1 ± 38.4 | (N = 51) 38.6 ± 9.68 | (N = 596) 32.0 ± 1.54 |
| SARS-CoV2 Status | |||
| Infected | 1 (3.6%) | 4 (5.4%) | 0 |
| Not infected or not tested | 27 (96.4%) | 70 (94.6%) | 911 (100.0%) |
| Countries | |||
| Spain | 17 (60.7%) | 51 (68.9%) | 701 (76.9%) |
| Austria | 2 (7.1%) | 1 (1.4%) | 0 |
| Czech Republic | 2 (7.1%) | 3 (4.1%) | 2 (0.2%) |
| France | 3 (10.7%) | 3 (4.1%) | 69 (7.6%) |
| Israel | 0 | 6 (8.1%) | 29 (3.2%) |
| Italy | 4 (14.3%) | 8 (10.8%) | 56 (6.1%) |
| USA | 0 | 2 (2.7%) | 54 (5.9%) |
| Adenovirus-Based Vaccines (Astrazeneca, Johnson and Johnsson) | mRNA-Based Vaccines (Pfizer and Moderna) | Controls from 2018–2019 a | |
|---|---|---|---|
| Total Number of patients | 28 | 74 | 911 |
| Pulmonary Embolism or Deep Vein Thrombosis | 18 | 69 | 881 |
| Initial treatment | |||
| Unfractionated heparin | 1 (5.6%) | 1 (1.4%) | 52 (5.9%) |
| Low-molecular weight heparin | 8 (44.4%) | 62 (89.9%) | 713 (80.9%) |
| Argatroban/bivalirudin/danaparoid/fondaparinux | 2 (11.1%) | 0 | 15 (1.7%) |
| Direct oral anticoagulants | 3 (16.7%) | 1 (1.4%) | 78 (8.9%) |
| No anticoagulation | 4 (22.2%) | 4 (5.8%) | 6 (0.7%) |
| Vitamin K antagonists | 0 | 0 | 2 (0.2%) |
| Fibrinolytic therapy | 0 | 1 (1.4%) | 15 (1.7%) |
| Surgical or percutaneous thrombectomy | 0 | 1 (1.4%) | 10 (1.1%) |
| Inferior vena cava filters | 0 | 1 (1.4%) | 23 (2.6%) |
| CVST | 3 | 2 | 4 |
| Initial treatment | |||
| Unfractionated heparin | 0 | 0 | 1 (25.0%) |
| Low-molecular weight heparin | 2 (66.7%) | 2 (100.0%) | 2 (50.0%) |
| Argatroban/bivalirudin/danaparoid/fondaparinux | 1 (33.3%) | 0 | 0 |
| Direct oral anticoagulants | 0 | 0 | 0 |
| No anticoagulation | 0 | 0 | 1 (25.0%) |
| Fibrinolytic therapy | 0 | 0 | 0 |
| Splanchnic Vein Thrombosis | 2 | 2 | 14 |
| Inpatient treatment | |||
| Unfractionated heparin | 0 | 0 | 1 (7.1%) |
| Low-molecular weight heparin | 1 (50%) | 2 (100%) | 12 (85.7%) |
| Argatroban/bivalirudin/danaparoid/fondaparinux | 0 | 0 | 0 |
| Direct oral anticoagulants | 0 | 0 | 0 |
| No anticoagulation | 1 (50%) | 0 | 1 (7.4%) |
| Fibrinolytic therapy | 0 | 0 | 0 |
| Thrombosis At Multiple Sites b | 5 | 1 | 12 |
| Inpatient treatment | |||
| Unfractionated heparin | 0 | 0 | 2 (16.7%) |
| Low-molecular weight heparin | 3 (60.0%) | 1 (100.0%) | 6 (50.0%) |
| Argatroban/bivalirudin/danaparoid/fondaparinux | 1 (20.0%) | 0 | 0 |
| Direct oral anticoagulants | 1 (20.0%) | 0 | 2 (16.7%) |
| No anticoagulation | 0 | 0 | 1 (8.3%) |
| Vitamin K antagonists | 0 | 0 | 1 (8.3%) |
| Fibrinolytic therapy | 0 | 0 | 0 |
| Adenovirus-Based Vaccines (Astrazeneca, Johnson and Johnsson) | mRNA-Based Vaccines (Pfizer and Moderna) | Controls from 2018–2019 a | |
|---|---|---|---|
| Total Number of patients | 28 | 74 | 911 |
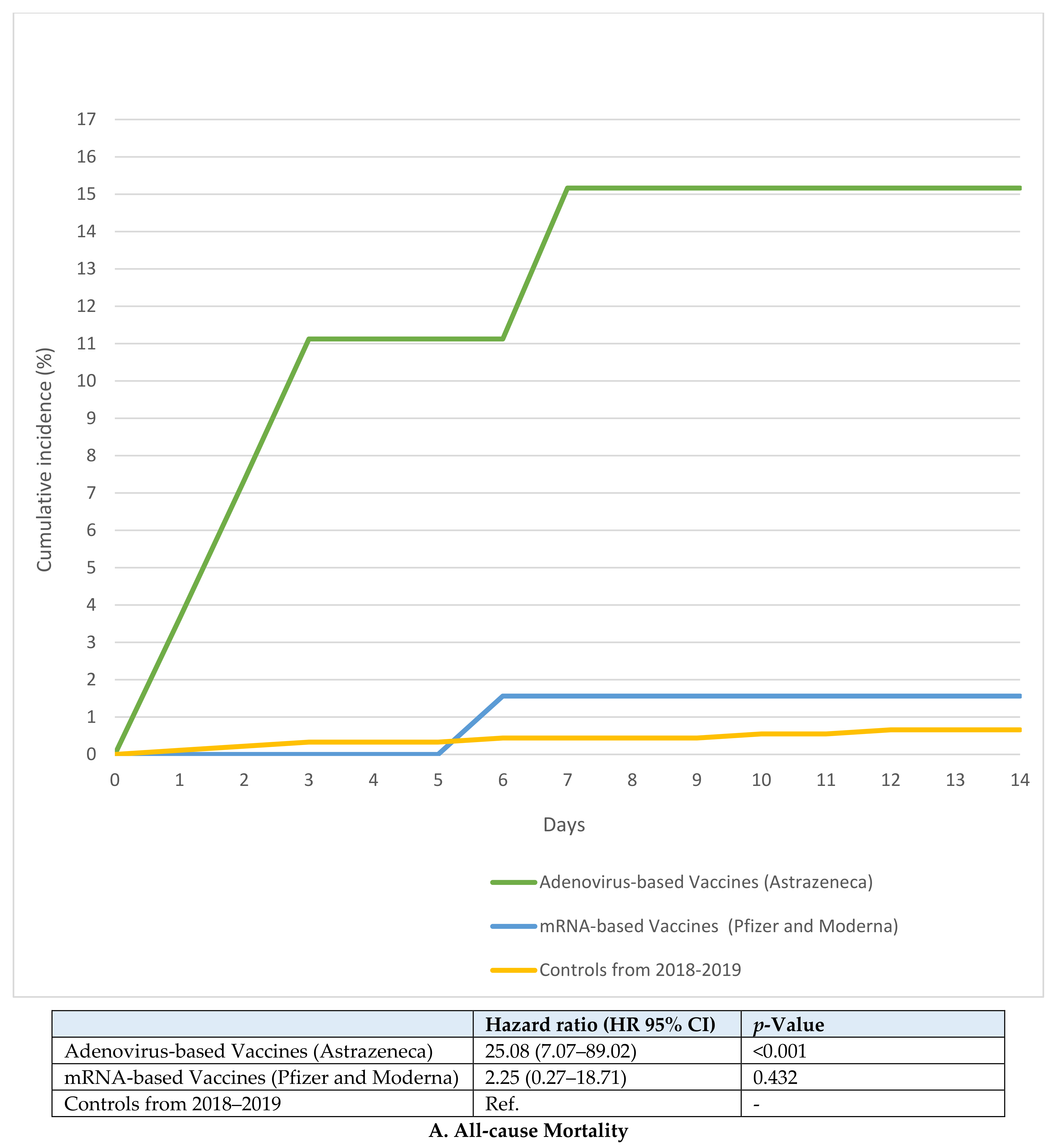
| All-cause mortality N, % | 4 (14.3%) | 1 (1.4%) | 6 (0.7%) |
| Unadjusted odds ratio | 25.14 (6.66–94.91) | 2.07 (0.25–17.39) | Ref. |
| Adjusted odds ratio b | 13.24 (1.44–121.3) | 1.05 (0.11–10.16) | Ref. |
| Major bleeding N, % | 3 (10.7%) | 0 | 9 (1.0%) |
| Unadjusted odds ratio | 12.03 (3.07–47.13) | - | Ref. |
| Adjusted odds ratio b | 9.03 (1.07–76.13) | - | Ref. |
| Pulmonary Embolism or Deep Vein Thrombosis | 18 | 69 | 881 |
| All-cause mortality N, % | 0 | 1 (1.4%) | 5 (0.6%) |
| Unadjusted odds ratio | - | 2.58 (0.30–22.37) | Ref. |
| Adjusted odds ratio | - | 1.56 (0.18–13.77) | Ref. |
| Major bleeding N, % | 0 | 0 | 9 (1.0%) |
| Unadjusted odds ratio | - | - | Ref. |
| Adjusted odds ratio | - | - | Ref. |
| CVST | 3 | 2 | 4 |
| All-cause mortality N, % | 2 (66.7%) | 0 | 0 |
| Major bleeding N, % | 1 (33.3%) | 0 | 0 |
| Unadjusted odds ratio | - | - | - |
| Splanchnic Vein Thrombosis | 2 | 2 | 14 |
| All-cause mortality N, % | 0 | 0 | 0 |
| Major bleeding N, % | 0 | 0 | 0 |
| Unadjusted odds ratio | - | - | - |
| Thrombosis At Multiple Sites c | 5 | 1 | 12 |
| All-cause mortality N, % | 2 (40.0%) | 0 | 1 (8.3%) |
| Unadjusted odds ratio | 7.33 (0.48–111.2) | - | Ref. |
| Major bleeding N, % | 2 (40.0%) | 0 | 0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bikdeli, B.; Jiménez, D.; Demelo-Rodriguez, P.; Galeano-Valle, F.; Porras, J.A.; Barba, R.; Ay, C.; Malý, R.; Braester, A.; Imbalzano, E.; et al. Venous Thrombosis within 30 Days after Vaccination against SARS-CoV-2 in a Multinational Venous Thromboembolism Registry. Viruses 2022, 14, 178. https://doi.org/10.3390/v14020178
Bikdeli B, Jiménez D, Demelo-Rodriguez P, Galeano-Valle F, Porras JA, Barba R, Ay C, Malý R, Braester A, Imbalzano E, et al. Venous Thrombosis within 30 Days after Vaccination against SARS-CoV-2 in a Multinational Venous Thromboembolism Registry. Viruses. 2022; 14(2):178. https://doi.org/10.3390/v14020178
Chicago/Turabian StyleBikdeli, Behnood, David Jiménez, Pablo Demelo-Rodriguez, Francisco Galeano-Valle, José Antonio Porras, Raquel Barba, Cihan Ay, Radovan Malý, Andrei Braester, Egidio Imbalzano, and et al. 2022. "Venous Thrombosis within 30 Days after Vaccination against SARS-CoV-2 in a Multinational Venous Thromboembolism Registry" Viruses 14, no. 2: 178. https://doi.org/10.3390/v14020178
APA StyleBikdeli, B., Jiménez, D., Demelo-Rodriguez, P., Galeano-Valle, F., Porras, J. A., Barba, R., Ay, C., Malý, R., Braester, A., Imbalzano, E., Rosa, V., Lecumberri, R., Siniscalchi, C., Fidalgo, Á., Ortiz, S., Monreal, M., & for the RIETE Investigators. (2022). Venous Thrombosis within 30 Days after Vaccination against SARS-CoV-2 in a Multinational Venous Thromboembolism Registry. Viruses, 14(2), 178. https://doi.org/10.3390/v14020178










