Maternal Immunization Using a Protein Subunit Vaccine Mediates Passive Immunity against Zaire ebolavirus in a Murine Model
Abstract
1. Introduction
2. Materials and Methods
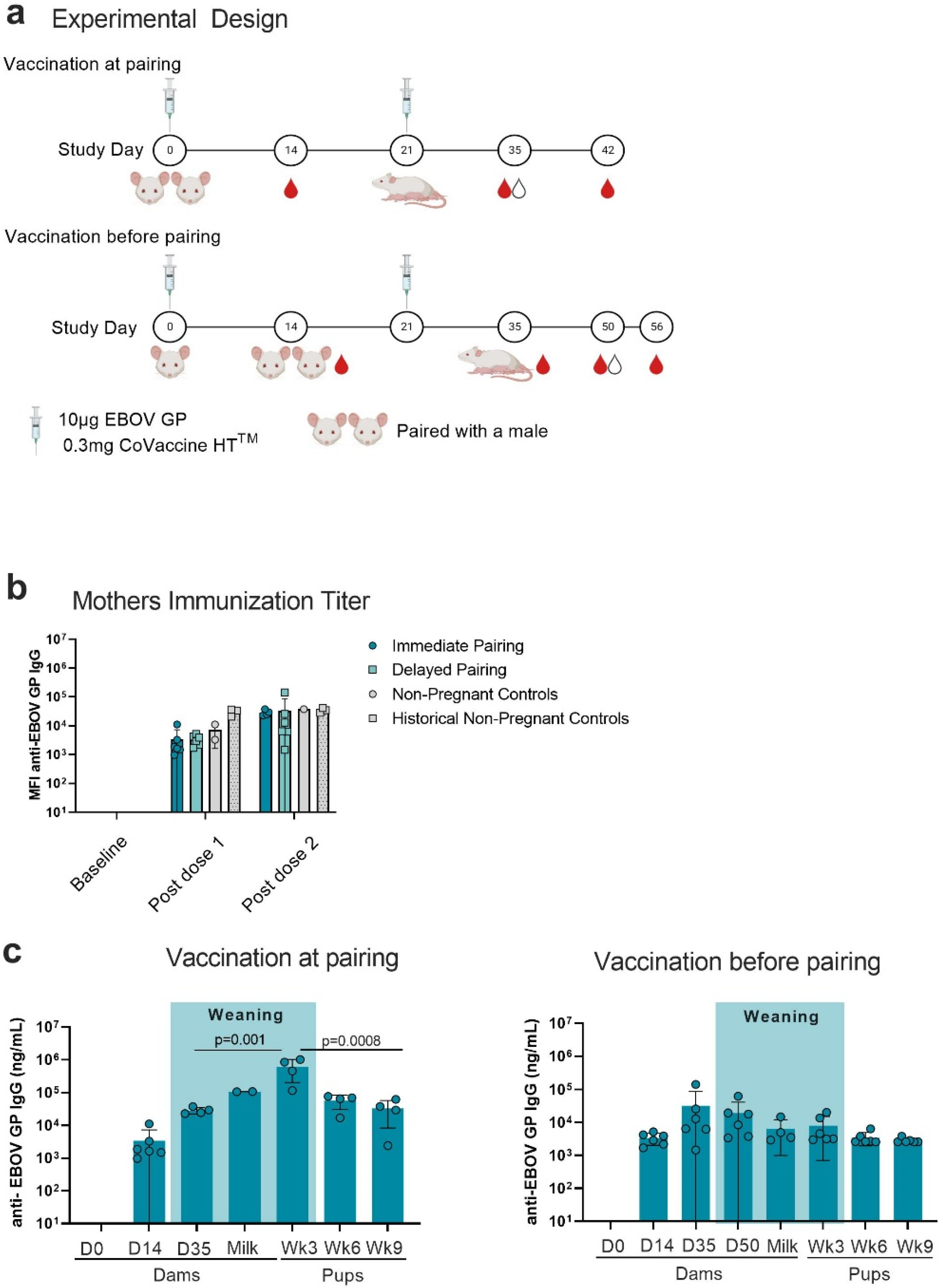
2.1. Vaccination and Serum Collection
2.2. Mouse Cohorts
2.3. Milk Collection and Delipidization
2.4. Multiplex Assays
2.5. Surrogate Neutralization Assays
2.6. Statistical Analysis
3. Results
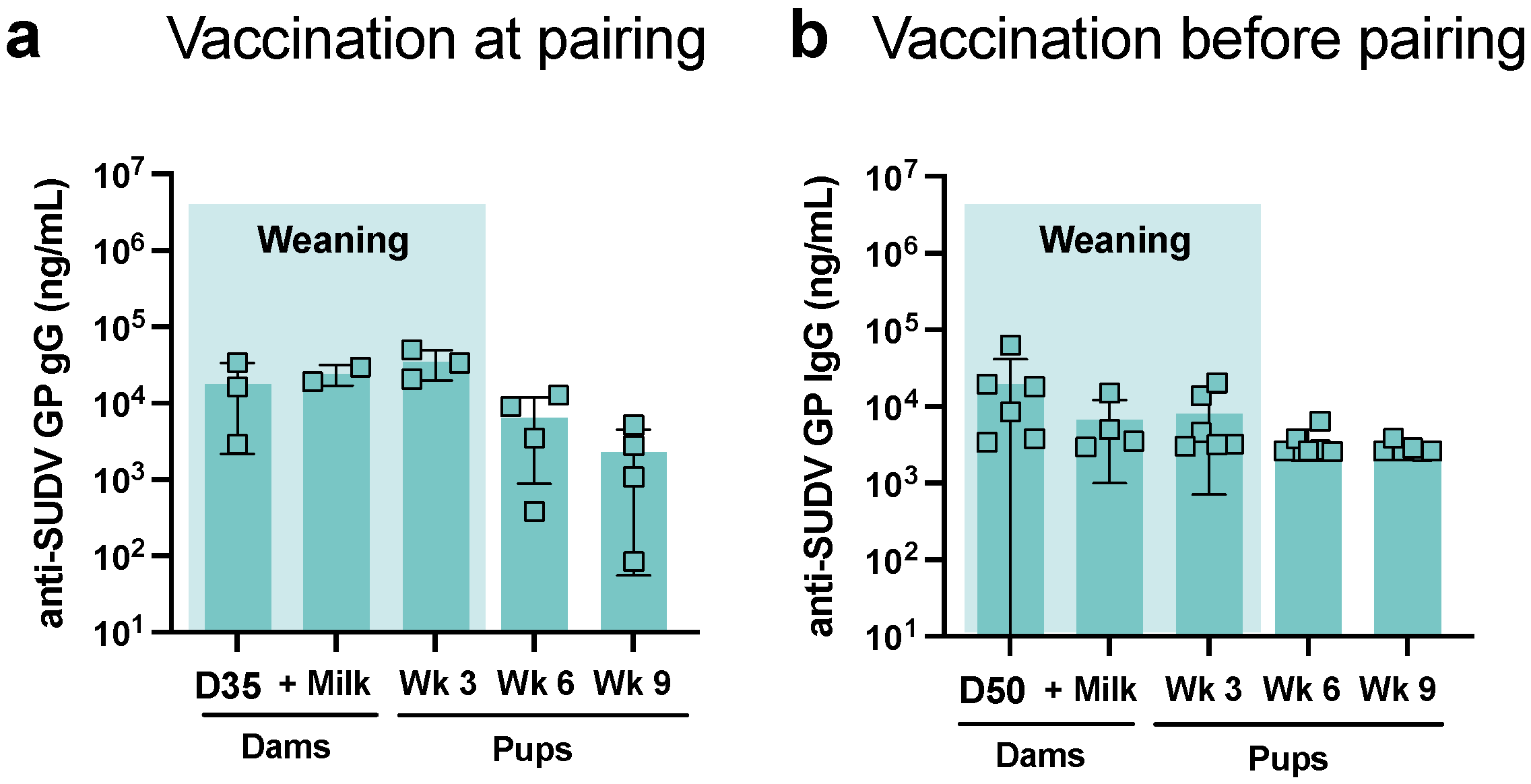
3.1. Antigen-Specific IgG in Mothers and Pups
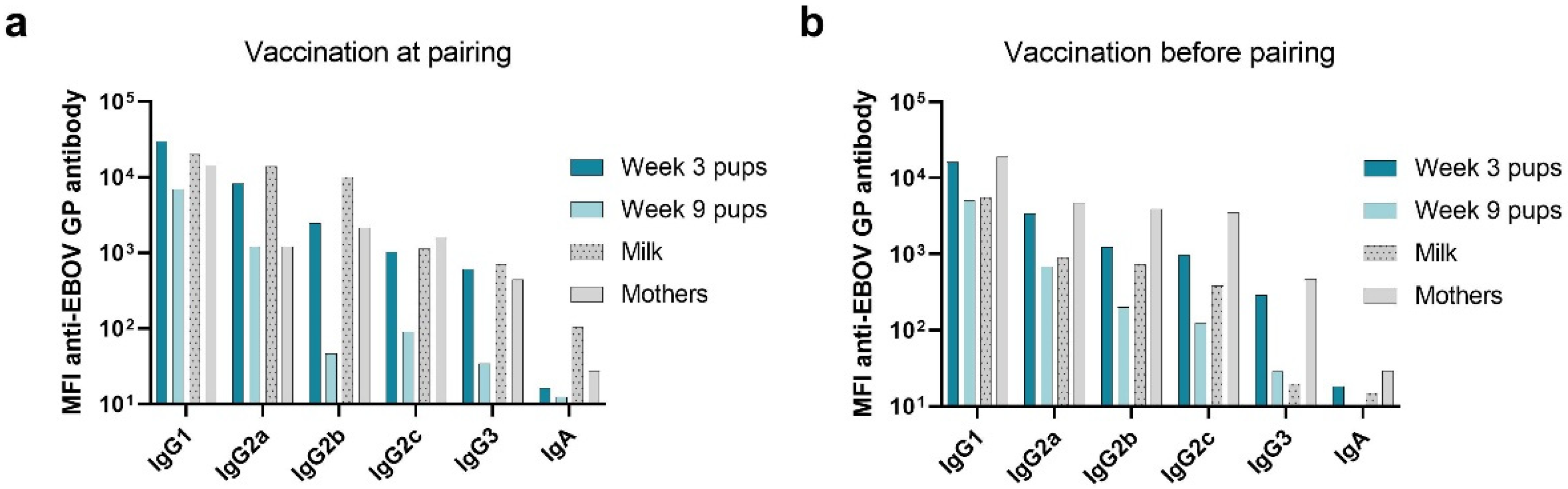
3.2. Immunoglobulin Isotype and IgG Subclass
3.3. Neutralization of rVSV-EBOV-GFP
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bausch, D.G.; Towner, J.S.; Dowell, S.F.; Kaducu, F.; Lukwiya, M.; Sanchez, A.; Nichol, S.T.; Ksiazek, T.G.; Rollin, P.E. Assessment of the Risk of Ebola Virus Transmission from Bodily Fluids and Fomites. J. Infect. Dis. 2007, 196, S142–S147. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Guidelines for the Management of Pregnant and Breastfeeding Women in the Context of Ebola Virus Disease Licence: CC BY-NC-SA 3.0 IGO; World Health Organization: Geneva, Switzerland, 2020. [Google Scholar]
- Foeller, M.E.; Carvalho Ribeiro do Valle, C.; Foeller, T.M.; Oladapo, O.T.; Roos, E.; Thorson, A.E. Pregnancy and breastfeeding in the context of Ebola: A systematic review. Lancet Infect. Dis. 2020, 20, e149–e158. [Google Scholar] [CrossRef] [PubMed]
- Erland, E.; Dahl, B. Midwives’ experiences of caring for pregnant women admitted to Ebola centres in Sierra Leone. Midwifery 2017, 55, 23–28. [Google Scholar] [CrossRef]
- Lehrer, A.T.; Chuang, E.; Namekar, M.; Williams, C.A.; Wong, T.A.S.; Lieberman, M.M.; Granados, A.; Misamore, J.; Yalley-Ogunro, J.; Andersen, H.; et al. Recombinant Protein Filovirus Vaccines Protect Cynomolgus Macaques From Ebola, Sudan, and Marburg Viruses. Front. Immunol. 2021, 12, 703986. [Google Scholar] [CrossRef] [PubMed]
- Lehrer, A.T.; Wong, T.S.; Lieberman, M.M.; Humphreys, T.; Clements, D.E.; Bakken, R.R.; Hart, M.K.; Pratt, W.D.; Dye, J.M. Recombinant proteins of Zaire ebolavirus induce potent humoral and cellular immune responses and protect against live virus infection in mice. Vaccine 2018, 36, 3090–3100. [Google Scholar] [CrossRef] [PubMed]
- Lehrer, A.T.; Wong, T.S.; Lieberman, M.M.; Johns, L.; Medina, L.; Feldmann, F.; Feldmann, H.; Marzi, A. Recombinant subunit vaccines protect guinea pigs from lethal Ebola virus challenge. Vaccine 2019, 37, 6942–6950. [Google Scholar] [CrossRef]
- Jamieson, D.J.; Rasmussen, S.A. The safety of adjuvants in influenza vaccines during pregnancy: What do we know and why do we need them? Am. J. Obstet. Gynecol. 2012, 207, 145–146. [Google Scholar] [CrossRef] [PubMed]
- Labayo, H.K.M.; Pajuelo, M.J.; Tohma, K.; Ford-Siltz, L.A.; Gilman, R.H.; Cabrera, L.; Mayta, H.; Sanchez, G.J.; Cornejo, A.T.; Bern, C.; et al. Norovirus-specific immunoglobulin A in breast milk for protection against norovirus-associated diarrhea among infants. EClinicalMedicine 2020, 27, 100561. [Google Scholar] [CrossRef]
- Jang, M.J.; Kim, Y.J.; Hong, S.; Na, J.; Hwang, J.H.; Shin, S.M.; Ahn, Y.M. Positive association of breastfeeding on respiratory syncytial virus infection in hospitalized infants: A multicenter retrospective study. Clin. Exp. Pediatr. 2020, 63, 135–140. [Google Scholar] [CrossRef]
- Preston, K.B.; Wong, T.A.S.; To, A.; Tashiro, T.E.; Lieberman, M.M.; Granados, A.; Feliciano, K.; Harrison, J.; Yalley-Ogunro, J.; Elyard, H.A.; et al. Single-vial filovirus glycoprotein vaccines: Biophysical characteristics and immunogenicity after co-lyophilization with adjuvant. Vaccine 2021, 39, 5650–5657. [Google Scholar] [CrossRef]
- Willis, E.; Pardi, N.; Parkhouse, K.; Mui, B.L.; Tam, Y.K.; Weissman, D.; Hensley, S.E. Nucleoside-modified mRNA vaccination partially overcomes maternal antibody inhibition of de novo immune responses in mice. Sci. Transl. Med. 2020, 12, eaav5701. [Google Scholar] [CrossRef] [PubMed]
- Johnson, M.S.; Thomson, S.C.; Speakman, J.R. Limits to sustained energy intake. I. Lactation in the laboratory mouse Mus musculus. J. Exp. Biol. 2001, 204, 1925–1935. [Google Scholar] [CrossRef] [PubMed]
- Willingham, K.; McNulty, E.; Anderson, K.; Hayes-Klug, J.; Nalls, A.; Mathiason, C. Milk collection methods for mice and Reeves’ muntjac deer. J. Vis. Exp. 2014, 89, 51007. [Google Scholar] [CrossRef]
- Fouda, G.G.; Yates, N.L.; Pollara, J.; Shen, X.; Overman, G.R.; Mahlokozera, T.; Wilks, A.B.; Kang, H.H.; Salazar-Gonzalez, J.F.; Salazar, M.G.; et al. HIV-specific functional antibody responses in breast milk mirror those in plasma and are primarily mediated by IgG antibodies. J. Virol. 2011, 85, 9555–9567. [Google Scholar] [CrossRef] [PubMed]
- To, A.; Medina, L.O.; Mfuh, K.O.; Lieberman, M.M.; Wong, T.A.S.; Namekar, M.; Nakano, E.; Lai, C.Y.; Kumar, M.; Nerurkar, V.R.; et al. Recombinant Zika Virus Subunits Are Immunogenic and Efficacious in Mice. mSphere 2018, 3, e00576-17. [Google Scholar] [CrossRef]
- Kumar, M.; O’Connell, M.; Namekar, M.; Nerurkar, V.R. Infection with Non-Lethal West Nile Virus Eg101 Strain Induces Immunity that Protects Mice against the Lethal West Nile Virus NY99 Strain. Viruses 2014, 6, 2328–2339. [Google Scholar] [CrossRef]
- Konduru, K.; Shurtleff, A.C.; Bavari, S.; Kaplan, G. High degree of correlation between Ebola virus BSL-4 neutralization assays and pseudotyped VSV BSL-2 fluorescence reduction neutralization test. J. Virol. Methods 2018, 254, 1–7. [Google Scholar] [CrossRef]
- Xiong, H.L.; Wu, Y.T.; Cao, J.L.; Yang, R.; Liu, Y.X.; Ma, J.; Qiao, X.Y.; Yao, X.Y.; Zhang, B.H.; Zhang, Y.L.; et al. Robust neutralization assay based on SARS-CoV-2 S-protein-bearing vesicular stomatitis virus (VSV) pseudovirus and ACE2-overexpressing BHK21 cells. Emerg. Microbes Infect. 2020, 9, 2105–2113. [Google Scholar] [CrossRef]
- Riepler, L.; Rossler, A.; Falch, A.; Volland, A.; Borena, W.; von Laer, D.; Kimpel, J. Comparison of Four SARS-CoV-2 Neutralization Assays. Vaccines 2020, 9, 13. [Google Scholar] [CrossRef]
- Lin, R.; Heeke, D.; Liu, H.; Rao, E.; Marshall, J.D.; Chio, V.; Cataniag, F.; Yu, L.; Zuo, F.; McCarthy, M.P. Development of a robust, higher throughput green fluorescent protein (GFP)-based Epstein-Barr Virus (EBV) micro-neutralization assay. J. Virol. Methods 2017, 247, 15–21. [Google Scholar] [CrossRef]
- Marzi, A.; Ebihara, H.; Callison, J.; Groseth, A.; Williams, K.J.; Geisbert, T.W.; Feldmann, H. Vesicular stomatitis virus-based Ebola vaccines with improved cross-protective efficacy. J. Infect. Dis. 2011, 204 (Suppl. S3), S1066–S1074. [Google Scholar] [CrossRef] [PubMed]
- Emanuel, J.; Callison, J.; Dowd, K.A.; Pierson, T.C.; Feldmann, H.; Marzi, A. A VSV-based Zika virus vaccine protects mice from lethal challenge. Sci. Rep. 2018, 8, 11043. [Google Scholar] [CrossRef] [PubMed]
- Murin, C.D.; Gilchuk, P.; Crowe, J.E., Jr.; Ward, A.B. Structural Biology Illuminates Molecular Determinants of Broad Ebolavirus Neutralization by Human Antibodies for Pan-Ebolavirus Therapeutic Development. Front. Immunol. 2021, 12, 808047. [Google Scholar] [CrossRef] [PubMed]
- van der Lubbe, J.E.M.; Vreugdenhil, J.; Damman, S.; Vaneman, J.; Klap, J.; Goudsmit, J.; Radosevic, K.; Roozendaal, R. Maternal antibodies protect offspring from severe influenza infection and do not lead to detectable interference with subsequent offspring immunization. Virol. J. 2017, 14, 123. [Google Scholar] [CrossRef] [PubMed]
- Palmeira, P.; Quinello, C.; Silveira-Lessa, A.L.; Zago, C.A.; Carneiro-Sampaio, M. IgG placental transfer in healthy and pathological pregnancies. Clin. Dev. Immunol. 2012, 2012, 985646. [Google Scholar] [CrossRef] [PubMed]
- Zheng, W.; Zhao, W.; Wu, M.; Song, X.; Caro, F.; Sun, X.; Gazzaniga, F.; Stefanetti, G.; Oh, S.; Mekalanos, J.J.; et al. Microbiota-targeted maternal antibodies protect neonates from enteric infection. Nature 2020, 577, 543–548. [Google Scholar] [CrossRef]
- Borghi, S.; Bournazos, S.; Thulin, N.K.; Li, C.; Gajewski, A.; Sherwood, R.W.; Zhang, S.; Harris, E.; Jagannathan, P.; Wang, L.X.; et al. FcRn, but not FcgammaRs, drives maternal-fetal transplacental transport of human IgG antibodies. Proc. Natl. Acad. Sci. USA 2020, 117, 12943–12951. [Google Scholar] [CrossRef]
- Collins, A.M. IgG subclass co-expression brings harmony to the quartet model of murine IgG function. Immunol. Cell Biol. 2016, 94, 949–954. [Google Scholar] [CrossRef]
| Pairing | No. of Female SW | No. of Litters | No. of Pups at Week 3 | No. of Pups at Weeks 6 & 9 | Total |
|---|---|---|---|---|---|
| Immediate | 6 | 4 | 18 | 23 | 41 |
| Delayed | 6 | 6 | 30 | 37 | 67 |
| Group | Litter | Week | 50% Inhibitory Titer | n |
|---|---|---|---|---|
| Immediate Pair | CW1 | 3 | 80 | 4 |
| 9 | 80 | 4 | ||
| CW2 | 3 | 40 | 6 | |
| 9 | 20 | 7 | ||
| CW4 | 3 | 80 | 2 | |
| 9 | 80 | 8 | ||
| CW6 | 3 | 160 | 6 | |
| 9 | 80 | 4 | ||
| Delayed pair | CW7 | 3 | 20 | 7 |
| 9 | <20 | 8 | ||
| CW8 | 3 | 20 | 6 | |
| 9 | <20 | 6 | ||
| CW9 | 3 | <20 | 6 | |
| 9 | <20 | 8 | ||
| CW10 | 3 | 80 | 4 | |
| 9 | 20 | 5 | ||
| CW11 | 3 | 80 | 3 | |
| 9 | 20 | 5 | ||
| CW12 | 3 | 20 | 4 | |
| 9 | 20 | 5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Williams, C.A.; Wong, T.A.S.; Ball, A.H.; Lieberman, M.M.; Lehrer, A.T. Maternal Immunization Using a Protein Subunit Vaccine Mediates Passive Immunity against Zaire ebolavirus in a Murine Model. Viruses 2022, 14, 2784. https://doi.org/10.3390/v14122784
Williams CA, Wong TAS, Ball AH, Lieberman MM, Lehrer AT. Maternal Immunization Using a Protein Subunit Vaccine Mediates Passive Immunity against Zaire ebolavirus in a Murine Model. Viruses. 2022; 14(12):2784. https://doi.org/10.3390/v14122784
Chicago/Turabian StyleWilliams, Caitlin A., Teri Ann S. Wong, Aquena H. Ball, Michael M. Lieberman, and Axel T. Lehrer. 2022. "Maternal Immunization Using a Protein Subunit Vaccine Mediates Passive Immunity against Zaire ebolavirus in a Murine Model" Viruses 14, no. 12: 2784. https://doi.org/10.3390/v14122784
APA StyleWilliams, C. A., Wong, T. A. S., Ball, A. H., Lieberman, M. M., & Lehrer, A. T. (2022). Maternal Immunization Using a Protein Subunit Vaccine Mediates Passive Immunity against Zaire ebolavirus in a Murine Model. Viruses, 14(12), 2784. https://doi.org/10.3390/v14122784






