Comparative Analysis of In Vitro Models to Study Antibody-Dependent Enhancement of Zika Virus Infection
Abstract
1. Introduction
2. Methods
2.1. Human Subjects
2.2. Cell Lines
2.3. Primary Human Cells
2.4. Virus and Virus Quantification
2.5. ZIKV Infection
2.6. Antibody-Dependent Enhancement Assay
2.7. Flow Cytometry
2.8. Cytokine Detection in Supernatant
2.9. Statistical Analysis
3. Results
3.1. Permissiveness for ZIKV Infection
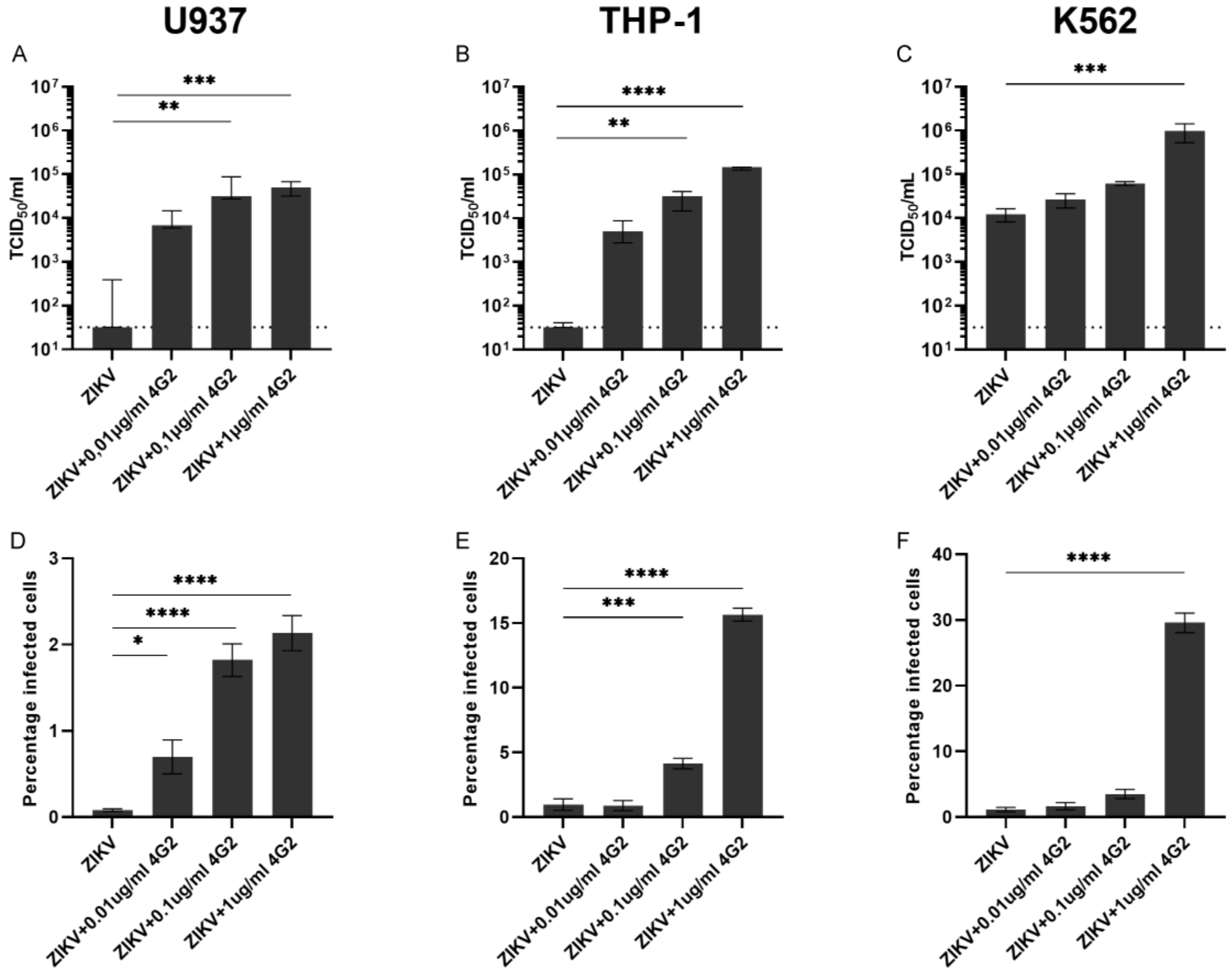
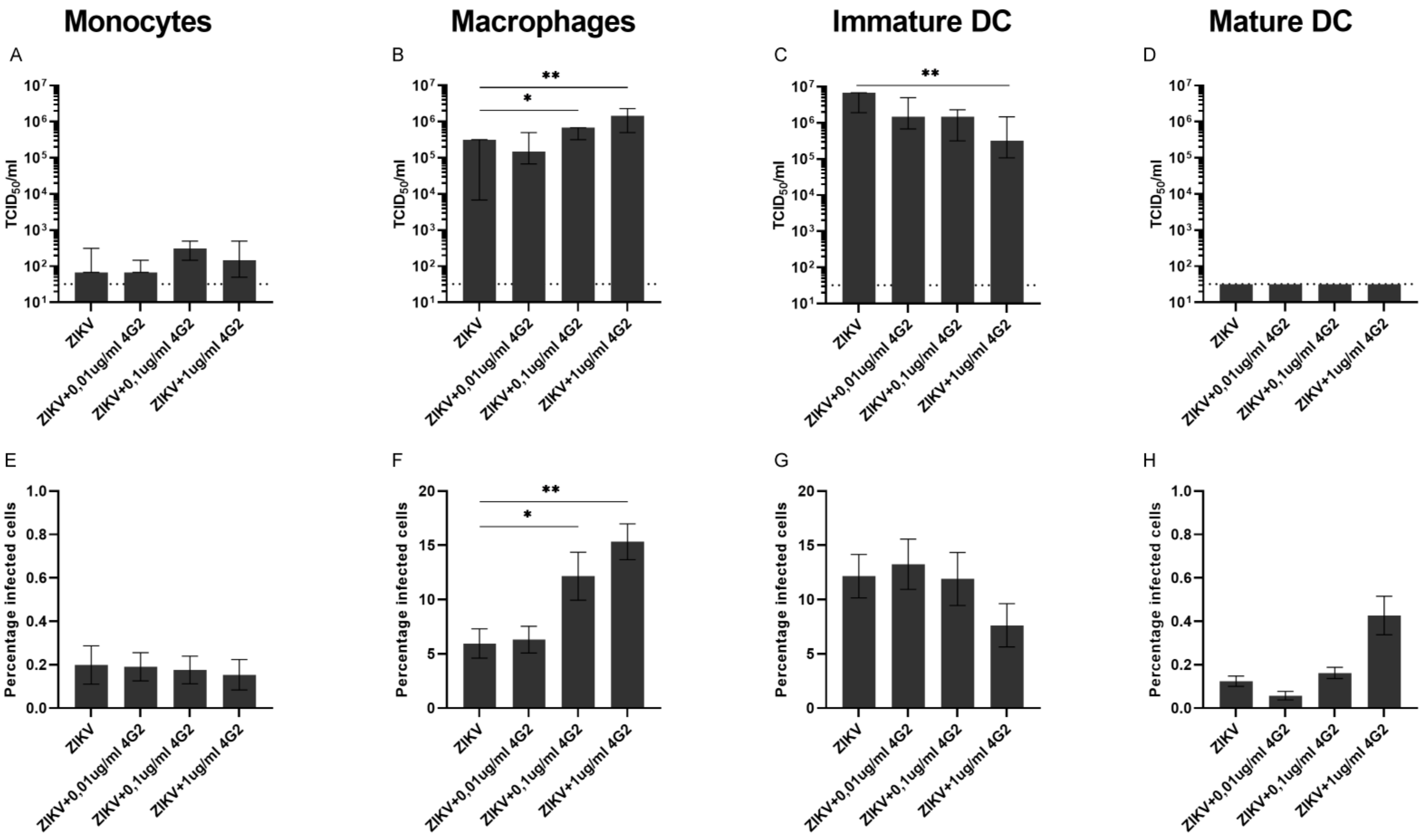
3.2. ADE of ZIKV Infection in Myeloid Cell Lines and Primary Myeloid Cells
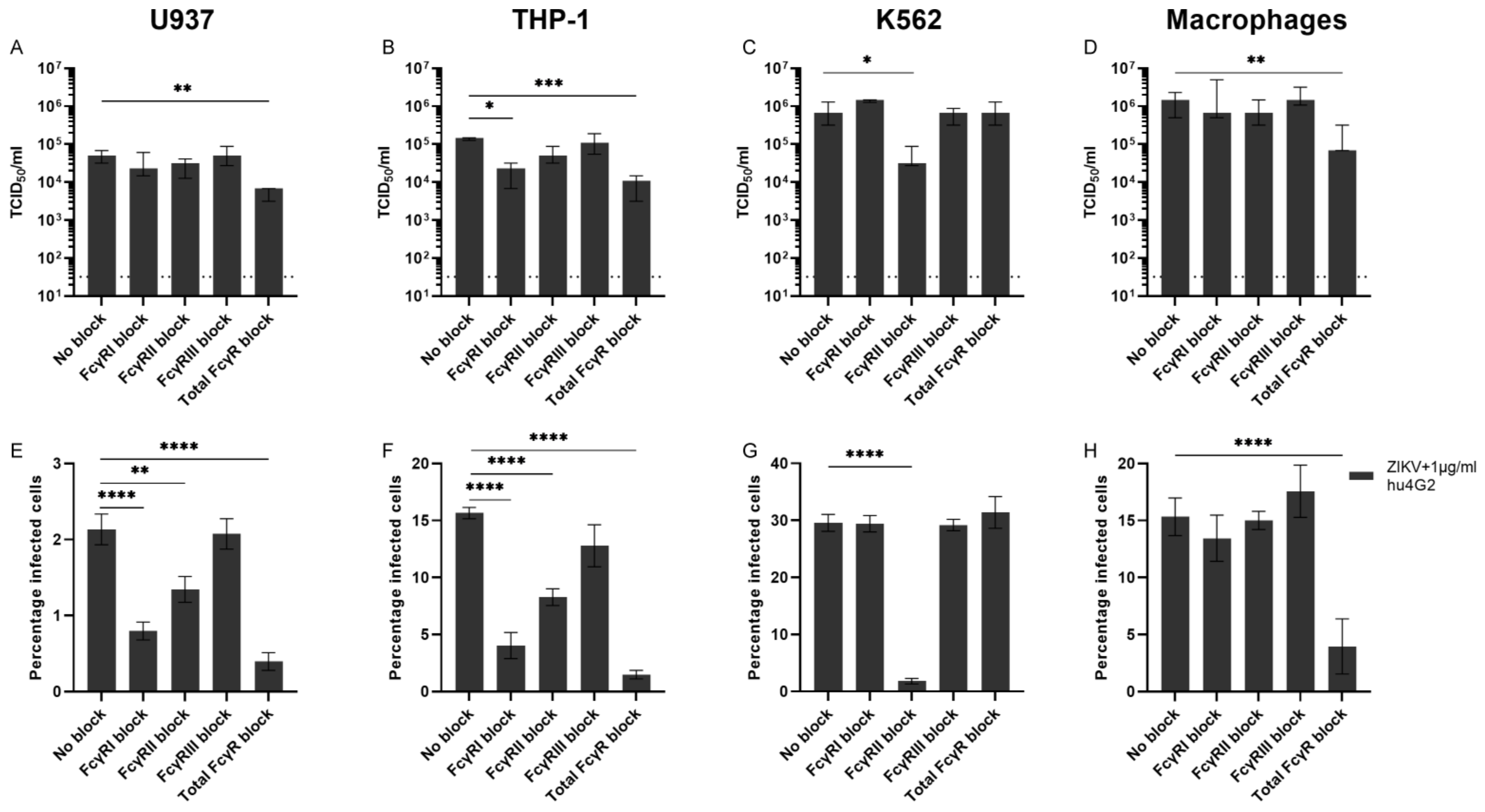
3.3. Fcγ Receptors and ADE of ZIKV Infection
3.4. Cytokine Quantification
3.5. ZIKV Permissiveness in Myeloid Cells from Pregnant Women
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mlakar, J.; Korva, M.; Tul, N.; Popović, M.; Poljšak-Prijatelj, M.; Mraz, J.; Kolenc, M.; Resman Rus, K.; Vesnaver Vipotnik, T.; Fabjan Vodušek, V.; et al. Zika Virus Associated with Microcephaly. N. Engl. J. Med. 2016, 374, 951–958. [Google Scholar] [CrossRef] [PubMed]
- Rasmussen, S.A.; Jamieson, D.J.; Honein, M.A.; Petersen, L.R. Zika Virus and Birth Defects—Reviewing the Evidence for Causality. N. Engl. J. Med. 2016, 374, 1981–1987. [Google Scholar] [CrossRef] [PubMed]
- Cao-Lormeau, V.-M.; Blake, A.; Mons, S.; Lastère, S.; Roche, C.; Vanhomwegen, J.; Dub, T.; Baudouin, L.; Teissier, A.; Larre, P.; et al. Guillain-Barré Syndrome outbreak associated with Zika virus infection in French Polynesia: A case-control study. Lancet 2016, 387, 1531–1539. [Google Scholar] [CrossRef] [PubMed]
- Langerak, T.; van Rooij, I.; Doornekamp, L.; Chandler, F.; Baptista, M.; Yang, H.; Koopmans, M.P.G.; GeurtsvanKessel, C.H.; Jacobs, B.C.; Rockx, B.; et al. Guillain-Barré Syndrome in Suriname; Clinical Presentation and Identification of Preceding Infections. Front. Neurol. 2021, 12, 635753. [Google Scholar] [CrossRef] [PubMed]
- Brasil, P.; Sequeira, P.C.; Freitas, A.D.; Zogbi, H.E.; Calvet, G.A.; de Souza, R.V.; Siqueira, A.M.; de Mendonca, M.C.L.; Nogueira, R.M.R.; de Filippis, A.M.B.; et al. Guillain-Barré syndrome associated with Zika virus infection. Lancet 2016, 387, 1482. [Google Scholar] [CrossRef]
- Guzman, M.G.; Gubler, D.J.; Izquierdo, A.; Martinez, E.; Halstead, S.B. Dengue infection. Nat Rev Dis Primers. 2016, 2, 16055. [Google Scholar] [CrossRef]
- Katzelnick, L.C.; Gresh, L.; Halloran, M.E.; Mercado, J.C.; Kuan, G.; Gordon, A.; Balmaseda, A.; Harris, E. Antibody-dependent enhancement of severe dengue disease in humans. Science 2017, 358, 929–932. [Google Scholar] [CrossRef]
- Halstead, S.B. Dengvaxia sensitizes seronegatives to vaccine enhanced disease regardless of age. Vaccine 2017, 35, 6355–6358. [Google Scholar] [CrossRef]
- Halstead, S.B.; Nimmannitya, S.; Cohen, S.N. Observations related to pathogenesis of dengue hemorrhagic fever. IV. Relation of disease severity to antibody response and virus recovered. Yale J. Biol. Med. 1970, 42, 311–328. [Google Scholar]
- Hawkes, R.A. Enhancement of the Infectivity of Arboviruses by Specific Antisera Produced in Domestic Fowls. Aust. J. Exp. Biol. Med. Sci. 1964, 42, 465–482. [Google Scholar] [CrossRef]
- Flipse, J.; Wilschut, J.; Smit, J.M. Molecular mechanisms involved in antibody-dependent enhancement of dengue virus infection in humans. Traffic 2012, 14, 25–35. [Google Scholar] [CrossRef]
- Halstead, S.B.; Mahalingam, S.; Marovich, M.A.; Ubol, S.; Mosser, D.M. Intrinsic antibody-dependent enhancement of microbial infection in macrophages: Disease regulation by immune complexes. Lancet Infect. Dis. 2010, 10, 712–722. [Google Scholar] [CrossRef]
- Halstead, S.B. Dengue Antibody-Dependent Enhancement: Knowns and Unknowns. Microbiol. Spectr. 2014, 2, 249–271. [Google Scholar] [CrossRef]
- Priyamvada, L.; Quicke, K.M.; Hudson, W.H.; Onlamoon, N.; Sewatanon, J.; Edupuganti, S.; Pattanapanyasat, K.; Chokephaibulkit, K.; Mulligan, M.J.; Wilson, P.C.; et al. Human antibody responses after dengue virus infection are highly cross-reactive to Zika virus. Proc. Natl. Acad. Sci. USA 2016, 113, 7852–7857. [Google Scholar] [CrossRef]
- Langerak, T.; Kasbergen, L.; Chandler, F.; Brinkman, T.; Faerber, Z.; Phalai, K.; Ulbert, S.; Rockstroh, A.; Bruin, E.; Koopmans, M.; et al. Zika Virus Antibody Titers Three Years after Confirmed Infection. Viruses 2021, 13, 1345. [Google Scholar] [CrossRef]
- Dejnirattisai, W.; Supasa, P.; Wongwiwat, W.; Rouvinski, A.; Barba-Spaeth, G.; Duangchinda, T.; Sakuntabhai, A.; Cao-Lormeau, V.-M.; Malasit, P.; Rey, F.A.; et al. Dengue virus sero-cross-reactivity drives antibody-dependent enhancement of infection with zika virus. Nat. Immunol. 2016, 17, 1102–1108. [Google Scholar] [CrossRef]
- Brown, J.A.; Singh, G.; Acklin, J.; Lee, S.; Duehr, J.; Chokola, A.; Frere, J.; Hoffman, K.W.; Foster, G.A.; Krysztof, D.; et al. Dengue Virus Immunity Increases Zika Virus-Induced Damage during Pregnancy. Immunity 2019, 50, 751–762.e5. [Google Scholar] [CrossRef]
- Rathore, A.P.S.; Saron, W.A.A.; Lim, T.; Jahan, N.; St. John, A.L. Maternal immunity and antibodies to dengue virus promote infection and Zika virus–induced microcephaly in fetuses. Sci. Adv. 2019, 5, eaav3208. [Google Scholar] [CrossRef]
- Langerak, T.; Mumtaz, N.; Tolk, V.I.; Van Gorp, E.C.M.; Martina, B.E.; Rockx, B.; Koopmans, M.P.G. The possible role of cross-reactive dengue virus antibodies in Zika virus pathogenesis. PLoS Pathog. 2019, 15, e1007640. [Google Scholar] [CrossRef]
- Katzelnick, L.C.; Narvaez, C.; Arguello, S.; Mercado, B.L.; Collado, D.; Ampie, O.; Elizondo, D.; Miranda, T.; Carillo, F.B.; Mercado, J.C.; et al. Zika virus infection enhances future risk of severe dengue disease. Science 2020, 369, 1123–1128. [Google Scholar] [CrossRef]
- Terzian, A.C.B.; Schanoski, A.S.; Mota, M.T.D.O.; Da Silva, R.A.; Estofolete, C.F.; Colombo, T.E.; Rahal, P.; Hanley, K.A.; Vasilakis, N.; Kalil, J.; et al. Viral Load and Cytokine Response Profile Does Not Support Antibody-Dependent Enhancement in Dengue-Primed Zika Virus–Infected Patients. Clin. Infect. Dis. 2017, 65, 1260–1265. [Google Scholar] [CrossRef]
- Davis, D.; Kaufmann, R.; Moticka, E.J. Nonspecific immunity in pregnancy: Monocyte surface Fcgamma receptor expression and function. J. Reprod. Immunol. 1998, 40, 119–128. [Google Scholar] [CrossRef]
- Jiang, L.; Sun, Q. The Role of Autophagy-Mediated Dengue Virus Antibody-Dependent Enhancement Infection of THP-1 Cells. Intervirology 2020, 63, 57–65. [Google Scholar] [CrossRef]
- Dejnirattisai, W.; Jumnainsong, A.; Onsirisakul, N.; Fitton, P.; Vasanawathana, S.; Limpitikul, W.; Puttikhunt, C.; Edwards, C.; Duangchinda, T.; Supasa, S.; et al. Cross-Reacting Antibodies Enhance Dengue Virus Infection in Humans. Science 2010, 328, 745–748. [Google Scholar] [CrossRef]
- Cui, G.; Si, L.; Wang, Y.; Zhou, J.; Yan, H.; Jiang, L. Antibody-dependent enhancement (ADE) of dengue virus: Identification of the key amino acid that is vital in DENV vaccine research. J. Gene Med. 2020, 23, e3297. [Google Scholar] [CrossRef]
- Castanha, P.M.S.; Nascimento, E.J.M.; Braga, C.; Cordeiro, M.T.; de Carvalho, O.V.; de Mendonca, L.R.; Marques, E.T. Dengue Virus-Specific Antibodies Enhance Brazilian Zika Virus Infection. J. Infect. Dis. 2017, 215, 781–785. [Google Scholar] [CrossRef]
- Londono-Renteria, B.; Troupin, A.; Cardenas, J.C.; Hall, A.; Perez, O.G.; Cardenas, L.; Hartstone-Rose, A.; Halstead, S.B.; Colpitts, T.M. A relevant in vitro human model for the study of Zika virus antibody-dependent enhancement. J. Gen. Virol. 2017, 98, 1702–1712. [Google Scholar] [CrossRef] [PubMed]
- Kärber, G. Beitrag zur kollektiven Behandlung pharmakologischer Reihenversuche. Naunyn-Schmiedebergs Arch. Für Exp. Pathol. Und Pharmakol. 1931, 162, 480–483. [Google Scholar] [CrossRef]
- Michlmayr, D.; Andrade, P.; Gonzalez, K.; Balmaseda, A.; Harris, E. CD14(+)CD16(+) monocytes are the main target of Zika virus infection in peripheral blood mononuclear cells in a paediatric study in Nicaragua. Nat. Microbiol. 2017, 2, 1462–1470. [Google Scholar] [CrossRef] [PubMed]
- Stettler, K.; Beltramello, M.; Espinosa, D.A.; Graham, V.; Cassotta, A.; Bianchi, S.; Vanzetta, F.; Minola, A.; Jaconi, S.; Mele, F.; et al. Specificity, cross-reactivity, and function of antibodies elicited by Zika virus infection. Science 2016, 353, 823–826. [Google Scholar] [CrossRef] [PubMed]
- Charles, A.S.; Christofferson, R.C. Utility of a Dengue-Derived Monoclonal Antibody to Enhance Zika Infection In Vitro. PLoS Curr. 2016, 8, 27660733. [Google Scholar] [CrossRef]
- Watanabe, S.; Tan, N.W.W.; Chan, K.W.K.; Vasudevan, S. Dengue Virus and Zika Virus Serological Cross-reactivity and Their Impact on Pathogenesis in Mice. J. Infect. Dis. 2018, 219, 223–233. [Google Scholar] [CrossRef]
- Li, M.; Zhao, L.; Zhang, C.; Wang, X.; Hong, W.; Sun, J.; Liu, R.; Yu, L.; Wang, J.; Zhang, F.; et al. Dengue immune sera enhance Zika virus infection in human peripheral blood monocytes through Fc gamma receptors. PLoS ONE 2018, 13, e0200478. [Google Scholar] [CrossRef]
- Carlin, A.F.; Vizcarra, E.A.; Branche, E.; Viramontes, K.M.; Suarez-Amaran, L.; Ley, K.; Heinz, S.; Benner, C.; Shresta, S.; Glass, C.K. Deconvolution of pro- and antiviral genomic responses in Zika virus-infected and bystander macrophages. Proc. Natl. Acad. Sci. USA 2018, 115, E9172–E9181. [Google Scholar] [CrossRef]
- Hueston, L.; Ramirez, R.; Mahalingam, S. Enhancement of Zika Infection by Dengue Virus–Specific Antibody Is Associated With Low Levels of Antiviral Factors. J. Infect. Dis. 2017, 216, 612–614. [Google Scholar] [CrossRef]
- Chiofalo, M.S.; Teti, G.; Goust, J.-M.; Trifiletti, R.; La Via, M.F. Subclass specificity of the Fc receptor for human IgG on K562. Cell. Immunol. 1988, 114, 272–281. [Google Scholar] [CrossRef]
- Bournazos, S.; Wang, T.T.; Ravetch, J.V. The Role and Function of Fcgamma Receptors on Myeloid Cells. Microbiol Spectr. 2016, 4. [Google Scholar]
- Junker, F.; Gordon, J.; Qureshi, O. Fc Gamma Receptors and Their Role in Antigen Uptake, Presentation, and T Cell Activation. Front. Immunol. 2020, 11, 1393. [Google Scholar] [CrossRef]
- Guilliams, M.; Bruhns, P.; Saeys, Y.; Hammad, H.; Lambrecht, B.N. The function of Fcgamma receptors in dendritic cells and macrophages. Nat. Rev. Immunol. 2014, 14, 94–108. [Google Scholar] [CrossRef]
- Bournazos, S.; Gupta, A.; Ravetch, J.V. The role of IgG Fc receptors in antibody-dependent enhancement. Nat. Rev. Immunol. 2020, 20, 633–643. [Google Scholar] [CrossRef]
- Rothman, A.L. Immunity to dengue virus: A tale of original antigenic sin and tropical cytokine storms. Nat. Rev. Immunol. 2011, 11, 532–543. [Google Scholar] [CrossRef]
- Foo, S.-S.; Chen, W.; Chan, Y.; Bowman, J.W.; Chang, L.-C.; Choi, Y.; Yoo, J.S.; Ge, J.; Cheng, G.; Bonnin, A.; et al. Asian Zika virus strains target CD14(+) blood monocytes and induce M2-skewed immunosuppression during pregnancy. Nat. Microbiol. 2017, 2, 1558–1570. [Google Scholar] [CrossRef]
- Paul, L.M.; Carlin, E.R.; Jenkins, M.M.; Tan, A.L.; Barcellona, C.M.; Nicholson, C.O.; Michael, S.F.; Isern, S. Dengue virus antibodies enhance Zika virus infection. Clin. Transl. Immunol. 2016, 5, e117. [Google Scholar] [CrossRef]
- Littaua, R.; Kurane, I.; Ennis, F.A. Human IgG Fc receptor II mediates antibody-dependent enhancement of dengue virus infection. J. Immunol. 1990, 144, 3183–3186. [Google Scholar]
- Kontny, U.; Kurane, I.; Ennis, F.A. Gamma interferon augments Fc gamma receptor-mediated dengue virus infection of human monocytic cells. J. Virol. 1988, 62, 3928–3933. [Google Scholar] [CrossRef]
- Ubol, S.; Phuklia, W.; Kalayanarooj, S.; Modhiran, N. Mechanisms of Immune Evasion Induced by a Complex of Dengue Virus and Preexisting Enhancing Antibodies. J. Infect. Dis. 2010, 201, 923–935. [Google Scholar] [CrossRef]
- Boonnak, K.; Slike, B.M.; Burgess, T.H.; Mason, R.M.; Wu, S.-J.; Sun, P.; Porter, K.; Rudiman, I.F.; Yuwono, D.; Puthavathana, P.; et al. Role of Dendritic Cells in Antibody-Dependent Enhancement of Dengue Virus Infection. J. Virol. 2008, 82, 3939–3951. [Google Scholar] [CrossRef] [PubMed]
- Hamel, R.; Dejarnac, O.; Wichit, S.; Ekchariyawat, P.; Neyret, A.; Luplertlop, N.; Perera-Lecoin, M.; Surasombatpattana, P.; Talignani, L.; Thomas, F.; et al. Biology of Zika Virus Infection in Human Skin Cells. J. Virol. 2015, 89, 8880–8896. [Google Scholar] [CrossRef] [PubMed]
- Bilińska-Nigot, B.; Majkowski, J. [Effect of diphenylhydantoin on a developed epileptogenic focus in cats with split cerebral hemispheres] Wplyw dwufenylohydantoiny (DPH) na rozwiniete ognisko padaczkowe u kotow z rozdzielonymi polkulami mozgowymi. Neurol Neurochir Pol. 1976, 10, 231–236. [Google Scholar] [PubMed]
- Yang, D.; Chu, H.; Lu, G.; Shuai, H.; Wang, Y.; Hou, Y.; Zhang, X.; Huang, X.; Hu, B.; Chai, Y.; et al. STAT2-dependent restriction of Zika virus by human macrophages but not dendritic cells. Emerg Microbes Infect. 2021, 10, 1024–1037. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.C.; Wang, S.Y.; King, C.C. Bacterial lipopolysaccharide inhibits dengue virus infection of primary human monocytes/macrophages by blockade of virus entry via a CD14-dependent mechanism. J. Virol. 1999, 73, 2650–2657. [Google Scholar] [CrossRef]
- Khaiboullina, S.; Uppal, T.; Sarkar, R.; Gorzalski, A.; Jeor, S.S.; Verma, S.C. ZIKV infection regulates inflammasomes pathway for replication in monocytes. Sci. Rep. 2017, 7, 16050. [Google Scholar] [CrossRef]
- Strottmann, D.M.; Zanluca, C.; Mosimann, A.L.P.; Koishi, A.C.; Auwerter, N.C.; Faoro, H.; Cataneo, A.H.D.; Kuczera, D.; Wowk, P.F.; Bordignon, J.; et al. Genetic and biological characterisation of Zika virus isolates from different Brazilian regions. Mem Inst Oswaldo Cruz. 2019, 114, e190150. [Google Scholar] [CrossRef]
- Bickel, M. The role of interleukin-8 in inflammation and mechanisms of regulation. J. Periodontol. 1993, 64 (Suppl. S5), 456–460. [Google Scholar]
- Zimmerman, M.G.; Quicke, K.M.; O’Neal, J.T.; Arora, N.; Machiah, D.; Priyamvada, L.; Kauffman, R.C.; Register, E.; Adekunle, O.; Swieboda, D.; et al. Cross-Reactive Dengue Virus Antibodies Augment Zika Virus Infection of Human Placental Macrophages. Cell Host Microbe 2018, 24, 731–742.e6. [Google Scholar] [CrossRef]
- Hemann, E.A.; Gale, M., Jr.; Savan, R. Interferon Lambda Genetics and Biology in Regulation of Viral Control. Front Immunol. 2017, 8, 1707. [Google Scholar] [CrossRef]
- Narayan, R.; Tripathi, S. Intrinsic ADE: The Dark Side of Antibody Dependent Enhancement During Dengue Infection. Front Cell Infect Microbiol. 2020, 10, 580096. [Google Scholar] [CrossRef]
- Martina, B.E.E.; Koraka, P.; Osterhaus, A.D.M.E. Dengue Virus Pathogenesis: An Integrated View. Clin. Microbiol. Rev. 2009, 22, 564–581. [Google Scholar] [CrossRef]
- Azeredo, E.L.; Zagne, S.M.; Santiago, M.A.; Gouvea, A.S.; Santana, A.A.; Neves-Souza, P.C.; Kubelka, C.F. Characterisation of lymphocyte response and cytokine patterns in patients with dengue fever. Immunobiology 2001, 204, 494–507. [Google Scholar] [CrossRef]
- Langerak, T.; Broekhuizen, M.; Unger, P.-P.A.; Tan, L.; Koopmans, M.; van Gorp, E.; Danser, A.H.J.; Rockx, B. Transplacental Zika virus transmission in ex vivo perfused human placentas. PLoS Neglected Trop. Dis. 2022, 16, e0010359. [Google Scholar] [CrossRef]
- Vidarsson, G.; Dekkers, G.; Rispens, T. IgG Subclasses and Allotypes: From Structure to Effector Functions. Front. Immunol. 2014, 5, 520. [Google Scholar] [CrossRef] [PubMed]
- Dekkers, G.; Treffers, L.; Plomp, R.; Bentlage, A.E.H.; De Boer, M.; Koeleman, C.A.M.; Lissenberg-Thunnissen, S.N.; Visser, R.; Brouwer, M.; Mok, J.Y.; et al. Decoding the Human Immunoglobulin G-Glycan Repertoire Reveals a Spectrum of Fc-Receptor- and Complement-Mediated-Effector Activities. Front. Immunol. 2017, 8, 877. [Google Scholar] [CrossRef] [PubMed]
- Hayes, J.M.; Wormald, M.R.; Rudd, P.M.; Davey, G.P. Fc gamma receptors: Glycobiology and therapeutic prospects. J. Inflamm. Res. 2016, 9, 209–219. [Google Scholar] [CrossRef] [PubMed]
- Crooks, C.M.; Weiler, A.M.; Rybarczyk, S.L.; Bliss, M.I.; Jaeger, A.S.; Murphy, M.E.; Simmons, H.A.; Mejia, A.; Fritsch, M.K.; Hayes, J.M.; et al. Previous exposure to dengue virus is associated with increased Zika virus burden at the maternal-fetal interface in rhesus macaques. PLoS Neglected Trop. Dis. 2021, 15, e0009641. [Google Scholar] [CrossRef] [PubMed]
- Zimmerman, M.G.; Wrammert, J.; Suthar, M.S. Cross-Reactive Antibodies during Zika Virus Infection: Protection, Pathogenesis, and Placental Seeding. Cell Host Microbe 2020, 27, 14–24. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Langerak, T.; Mumtaz, N.; Koopmans, M.; Schoenmakers, S.; Rockx, B. Comparative Analysis of In Vitro Models to Study Antibody-Dependent Enhancement of Zika Virus Infection. Viruses 2022, 14, 2776. https://doi.org/10.3390/v14122776
Langerak T, Mumtaz N, Koopmans M, Schoenmakers S, Rockx B. Comparative Analysis of In Vitro Models to Study Antibody-Dependent Enhancement of Zika Virus Infection. Viruses. 2022; 14(12):2776. https://doi.org/10.3390/v14122776
Chicago/Turabian StyleLangerak, Thomas, Noreen Mumtaz, Marion Koopmans, Sam Schoenmakers, and Barry Rockx. 2022. "Comparative Analysis of In Vitro Models to Study Antibody-Dependent Enhancement of Zika Virus Infection" Viruses 14, no. 12: 2776. https://doi.org/10.3390/v14122776
APA StyleLangerak, T., Mumtaz, N., Koopmans, M., Schoenmakers, S., & Rockx, B. (2022). Comparative Analysis of In Vitro Models to Study Antibody-Dependent Enhancement of Zika Virus Infection. Viruses, 14(12), 2776. https://doi.org/10.3390/v14122776







