The Association between Symptomatic Rotavirus Infection and Histo-Blood Group Antigens in Young Children with Diarrhea in Pretoria, South Africa
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Population
2.2. Ethical Consideration
2.3. Sample Collection
2.4. Rotavirus Detection and Strain Characterization in Stool
2.5. HBGA Phenotyping in Saliva
2.6. Statistical Analysis
3. Results
3.1. Demographics
3.2. Rotavirus Infection in the Sample Population
3.3. Rotavirus Infection and Circulating Rotavirus Strains
3.4. Distribution of ABO Blood Groups, Lewis Antigens and Secretor Status in Rotavirus Infected Children
3.5. Rotavirus Genotype Association to H1 Blood Group Antigens
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Troeger, C.; Khalil, I.A.; Rao, P.C.; Cao, S.; Blacker, B.F.; Ahmed, T.; Armah, G.; Bines, J.E.; Brewer, T.G.; Colombara, D.V.; et al. Rotavirus Vaccination and the Global Burden of Rotavirus Diarrhea among Children Younger Than 5 Years. JAMA Pediatr. 2018, 172, 958–965. [Google Scholar] [CrossRef] [PubMed]
- WHO. Rotavirus Vaccine Position Paper. Wkly. Epidemiol. Rec. 2021, 28, 301–319. [Google Scholar]
- Greenberg, H.B.; Estes, M.K. Rotaviruses: From Pathogenesis to Vaccination. Gastroenterology 2009, 136, 1939–1951. [Google Scholar] [CrossRef] [PubMed]
- Arias, C.F.; Silva-Ayala, D.; López, S. Rotavirus Entry: A Deep Journey into the Cell with Several Exits. J. Virol. 2015, 89, 890–893. [Google Scholar] [CrossRef]
- López, S.; Arias, C.F. Multistep entry of rotavirus into cells: A Versaillesque dance. Trends Microbiol. 2004, 12, 271–278. [Google Scholar] [CrossRef]
- Sánchez-San Martín, C.; López, T.; Arias, C.F.; López, S. Characterization of Rotavirus Cell Entry. J. Virol. 2004, 78, 2310–2318. [Google Scholar] [CrossRef]
- Dóró, R.; László, B.; Martella, V.; Leshem, E.; Gentsch, J.; Parashar, U.; Bányai, K. Review of global rotavirus strain prevalence data from six years post vaccine licensure surveillance: Is there evidence of strain selection from vaccine pressure? Infect. Genet. Evol. 2014, 28, 446–461. [Google Scholar] [CrossRef]
- Blanchard, H.; Yu, X.; Coulson, B.S.; von Itzstein, M. Insight into Host Cell Carbohydrate-recognition by Human and Porcine Rotavirus from Crystal Structures of the Virion Spike Associated Carbohydrate-binding Domain (VP8*). J. Mol. Biol. 2007, 367, 1215–1226. [Google Scholar] [CrossRef]
- Huang, P.; Xia, M.; Tan, M.; Zhong, W.; Wei, C.; Wang, L.; Morrow, A.; Jiang, X. Spike Protein VP8* of Human Rotavirus Recognizes Histo-Blood Group Antigens in a Type-Specific Manner. J. Virol. 2012, 86, 4833–4843. [Google Scholar] [CrossRef]
- Koda, Y.; Soejima, M.; Kimura, H. The polymorphisms of fucosyltransferases. Leg. Med. 2001, 3, 2–14. [Google Scholar] [CrossRef]
- Ravn, V.; Dabelsteen, E. Tissue distribution of histo-blood group antigens. APMIS 2000, 108, 1–28. [Google Scholar] [CrossRef] [PubMed]
- Dabelsteen, E. ABO blood group antigens in oral mucosa. What is new? J. Oral Pathol. Med. 2002, 31, 65–70. [Google Scholar] [CrossRef] [PubMed]
- Marionneau, S.; Cailleau-Thomas, A.; Rocher, J.; Le Moullac-Vaidye, B.; Ruvoën, N.; Clément, M.; Le Pendu, J. ABH and Lewis histo-blood group antigens, a model for the meaning of oligosaccharide diversity in the face of a changing world. Biochimie 2001, 83, 565–573. [Google Scholar] [CrossRef]
- de Mattos, L.C. Structural diversity and biological importance of ABO, H, Lewis and secretor histo-blood group carbohydrates. Rev. Bras. Hematol. Hemoter. 2016, 38, 331–340. [Google Scholar] [CrossRef] [PubMed]
- Imbert-Marcille, B.-M.; Barbé, L.; Dupé, M.; Le Moullac-Vaidye, B.; Besse, B.; Peltier, C.; Ruvoën-Clouet, N.; Le Pendu, J. A FUT2 Gene Common Polymorphism Determines Resistance to Rotavirus A of the P[8] Genotype. J. Infect. Dis. 2014, 209, 1227–1230. [Google Scholar] [CrossRef]
- Ma, X.; Li DDi Sun, X.M.; Guo, Y.Q.; Xiang, J.Y.; Wang, W.H.; Zhang, L.X.; Gu, Q.J.; Duan, Z.J. Binding patterns of rotavirus genotypes P[4], P[6], and P[8] in China with histo-blood group antigens. PLoS ONE 2015, 10, e0134584. [Google Scholar] [CrossRef]
- Nordgren, J.; Sharma, S.; Bucardo, F.; Nasir, W.; Günaydın, G.; Ouermi, D.; Nitiema, L.W.; Becker-Dreps, S.; Simpore, J.; Hammarström, L.; et al. Both Lewis and Secretor Status Mediate Susceptibility to Rotavirus Infections in a Rotavirus Genotype–Dependent Manner. Clin. Infect. Dis. 2014, 59, 1567–1573. [Google Scholar] [CrossRef]
- Payne, D.C.; Currier, R.L.; Staat, M.A.; Sahni, L.C.; Selvarangan, R.; Halasa, N.B.; Englund, J.A.; Weinberg, G.A.; Boom, J.A.; Szilagyi, P.G.; et al. Epidemiologic association between FUT2 secretor status and severe rotavirus gastroenteritis in children in the United States. JAMA Pediatr. 2015, 169, 1040–1045. [Google Scholar] [CrossRef]
- Sun, X.; Guo, N.; Li, D.; Jin, M.; Zhou, Y.; Xie, G.; Pang, L.; Zhang, Q.; Cao, Y.; Duan, Z.-J. Binding specificity of P[8] VP8* proteins of rotavirus vaccine strains with histo-blood group antigens. Virology 2016, 495, 129–135. [Google Scholar] [CrossRef]
- Zhang, X.; Long, Y.; Tan, M.; Zhang, T.; Huang, Q.; Jiang, X. P[8] and P[4] Rotavirus Infection Associated with Secretor Phenotypes among Children in South China. Sci. Rep. 2016, 6, 34591. [Google Scholar] [CrossRef]
- Ayouni, S.; Sdiri-Loulizi, K.; de Rougemont, A.; Estienney, M.; Ambert-Balay, K.; Aho, S.; Hamami, S.; Aouni, M.; Neji-Guediche, M.; Pothier, P.; et al. Rotavirus P[8] infections in persons with secretor and nonsecretor phenotypes, Tunisia. Emerg. Infect. Dis. 2015, 21, 2055–2058. [Google Scholar] [CrossRef] [PubMed]
- Armah, G.E.; Cortese, M.M.; Dennis, F.E.; Yu, Y.; Morrow, A.L.; McNeal, M.M.; Lewis, K.D.C.; Awuni, D.A.; Armachie, J.; Parashar, U.D. Rotavirus vaccine take in infants is associated with secretor status. J. Infect. Dis. 2019, 219, 746–749. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.; Dickson, D.M.; DeCamp, A.C.; Ross Colgate, E.; Diehl, S.A.; Uddin, M.I.; Sharmin, S.; Islam, S.; Bhuiyan, T.R.; Alam, M.; et al. Histo–Blood group antigen phenotype determines susceptibility to genotype-specific rotavirus infections and impacts measures of rotavirus vaccine efficacy. J. Infect. Dis. 2018, 217, 1399–1407. [Google Scholar] [CrossRef] [PubMed]
- Gozalbo-Rovira, R.; Giges-Tomas, J.R.; Vila-Vicent, S.; Buesa, J.; Santiso-Bellon, C.; Mondedero, V.; Yebra, M.J.; Marina, A.; Rodriguez-Diaz, J. Unraveling the role of the secretor antigen in human rotavirus attachment to the histo-blood group antigens. PLoS Pathog. 2019, 15, e1007866. [Google Scholar] [CrossRef]
- WHO. Manual of Rotavirus Detection and Characterization Methods [WWW Document]. Biologicals. 2009. Available online: https://apps.who.int/iris/handle/10665/70122 (accessed on 20 August 2020).
- Gentsch, J.R.; Glass, R.I.; Woods, P.; Gouvea, V.; Gorziglia, M.; Flores, J.; Das, B.K.; Bhan, M.K. Identification of group A rotavirus gene 4 types by polymerase chain reaction. J. Clin. Microbiol. 1992, 30, 1365–1373. [Google Scholar] [CrossRef]
- Gouvea, V.; Glass, R.I.; Woods, P.; Taniguchi, K.; Clark, H.F.; Forrester, B.; Fang, Z.Y. Polymerase chain reaction amplification and typing of rotavirus nucleic acid from stool specimens. J. Clin. Microbiol. 1990, 28, 276–282. [Google Scholar] [CrossRef] [PubMed]
- Seheri, L.M.; Magagula, N.B.; Peenze, I.; Rakau, K.; Ndadza, A.; Mwenda, J.M.; Weldegebriel, G.; Steele, A.D.; Mphahlele, M.J. Rotavirus strain diversity in Eastern and Southern African countries before and after vaccine introduction. Vaccine 2018, 36, 7222–7230. [Google Scholar] [CrossRef] [PubMed]
- Barbé, L.; Le Moullac-Vaidye, B.; Echasserieau, K.; Bernardeau, K.; Carton, T.; Bovin, N.; Nordgren, J.; Svensson, L.; Ruvoën-Clouet, N.; Le Pendu, J. Histo-blood group antigen-binding specificities of human rotaviruses are associated with gastroenteritis but not with in vitro infection. Sci. Rep. 2018, 8, 12961. [Google Scholar] [CrossRef]
- Sharma, S.; Hagbom, M.; Svensson, L.; Nordgren, J. The Impact of Human Genetic Polymorphisms on Rotavirus Susceptibility, Epidemiology, and Vaccine Take. Viruses 2020, 12, 324. [Google Scholar] [CrossRef]
- Bucardo, F.; Nordgren, J.; Reyes, Y.; Gonzalez, F.; Sharma, S.; Svensson, L. The Lewis A phenotype is a restriction factor for Rotateq and Rotarix vaccine-take in Nicaraguan children. Sci. Rep. 2018, 8, 1502. [Google Scholar] [CrossRef]
- Kazi, A.M.; Cortese, M.M.; Yu, Y.; Lopman, B.; Morrow, A.L.; Fleming, J.A.; McNeal, M.M.; Steele, A.D.; Parashar, U.D.; Zaidi, A.K.M.; et al. Secretor and salivary ABO blood group antigen status predict rotavirus vaccine take in infants. J. Infect. Dis. 2017, 215, 786–789. [Google Scholar] [CrossRef] [PubMed]
- Pollock, L.; Bennett, A.; Jere, K.C.; Dube, Q.; Mandolo, J.; Bar-Zeev, N.; Heyderman, R.S.; Cunliffe, N.A.; Iturriza-Gomara, M. Nonsecretor Histo-blood Group Antigen Phenotype Is Associated with Reduced Risk of Clinical Rotavirus Vaccine Failure in Malawian Infants. Clin. Infect. Dis. 2019, 69, 1313–1319. [Google Scholar] [CrossRef] [PubMed]
- Yang, T.A.; Hou, J.Y.; Huang, Y.C.; Chen, C.J. Genetic Susceptibility to Rotavirus Gastroenteritis and Vaccine Effectiveness in Taiwanese Children. Sci. Rep. 2017, 7, 6412. [Google Scholar] [CrossRef] [PubMed]
- Seheri, L.M.; Page, N.A.; Mawela, M.P.B.; Mphahlele, M.J.; Steele, A.D. Rotavirus vaccination within the South African Expanded Programme on Immunisation. Vaccine 2012, 30, C14–C20. [Google Scholar] [CrossRef] [PubMed]
- Groome, M.J.; Page, N.; Cortese, M.M.; Moyes, J.; Zar, H.J.; Kapongo, C.N.; Mulligan, C.; Diedericks, R.; Cohen, C.; Fleming, J.A.; et al. Effectiveness of monovalent human rotavirus vaccine against admission to hospital for acute rotavirus diarrhea in South African children: A case-control study. Lancet Infect. Dis. 2014, 14, 1096–1104. [Google Scholar] [CrossRef]
- Groome, M.J.; Zell, E.R.; Solomon, F.; Nzenze, S.; Parashar, U.D.; Izu, A.; Madhi, S.A. Temporal Association of Rotavirus Vaccine Introduction and Reduction in All-Cause Childhood Diarrheal Hospitalizations in South Africa. Clin. Infect. Dis. 2016, 62, S188–S195. [Google Scholar] [CrossRef]
- Seheri, L.M.; Page, N.; Dewar, J.B.; Geyer, A.; Nemarude, A.L.; Bos, P.; Esona, M.; Steele, A.D. Characterization and Molecular Epidemiology of Rotavirus Strains Recovered in Northern Pretoria, South Africa during 2003–2006. J. Infect. Dis. 2010, 202, S139–S147. [Google Scholar] [CrossRef]
- Page, N.A.; Seheri, L.M.; Groome, M.J.; Moyes, J.; Walaza, S.; Mphahlele, J.; Kahn, K.; Kapongo, C.N.; Zar, H.J.; Tempia, S.; et al. Temporal association of rotavirus vaccination and genotype circulation in South Africa: Observations from 2002 to 2014. Vaccine 2018, 36, 7231–7237. [Google Scholar] [CrossRef]
- Mukaratirwa, A.; Berejena, C.; Nziramasanga, P.; Ticklay, I.; Gonah, A.; Nathoo, K.; Manangazira, P.; Mangwanya, D.; Marembo, J.; Mwenda, J.M.; et al. Distribution of rotavirus genotypes associated with acute diarrhea in Zimbabwean children less than five years old before and after rotavirus vaccine introduction. Vaccine 2018, 36, 7248–7255. [Google Scholar] [CrossRef]
- Simwaka, J.C.; Mpabalwani, E.M.; Seheri, M.; Peenze, I.; Monze, M.; Matapo, B.; Parashar, U.D.; Mufunda, J.; Mphahlele, J.M.; Tate, J.E.; et al. Diversity of rotavirus strains circulating in children under five years of age who presented with acute gastroenteritis before and after rotavirus vaccine introduction, University Teaching Hospital, Lusaka, Zambia, 2008–2015. Vaccine 2018, 36, 7243–7247. [Google Scholar] [CrossRef]
- Liu, Y.; Huang, P.; Tan, M.; Liu Yiliu Biesiada, J.; Meller, J.; Castello, A.A.; Jiang, B.; Jiang, X. Rotavirus VP8*: Phylogeny, Host Range, and Interaction with Histo-Blood Group Antigens. J. Virol. 2012, 86, 9899–9910. [Google Scholar] [CrossRef]
- MacDonald, J.; Groome, M.J.; Mans, J.; Page, N. FUT2 Secretor Status Influences Susceptibility to VP4 Strain-Specific Rotavirus Infections in South African Children. Pathogens 2020, 9, 795. [Google Scholar] [CrossRef]
- Pérez-Ortín, R.; Vila-Vicent, S.; Carmona-Vicente, N.; Santiso-Bellón, C.; Rodríguez-Díaz, J.; Buesa, J. Histo-blood group antigens in children with symptomatic rotavirus infection. Viruses 2019, 11, 339. [Google Scholar] [CrossRef]
- Lee, S.K.; Oh, S.J.; Choi, S.; Choi, S.H.; Shin, S.H.; Lee, E.J.M.D.; Cho, E.J.; Hyun, J.; Kim, H.S. Relationship between rotavirus P[6] infection in korean neonates and histo-blood group antigen: A single-center study. Ann. Lab. Med. 2020, 41, 181–189. [Google Scholar] [CrossRef]
- Magwira, C.A.; Kgosana, L.P.; Esona, M.D.; Seheri, M.L. Low fecal rotavirus vaccine virus shedding is significantly associated with non-secretor histo-blood group antigen phenotype among infants in northern Pretoria, South Africa. Vaccine 2020, 38, 8260–8263. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Liu, Y.; Tan, M. Histo-blood group antigens as receptors for rotavirus, new understanding on rotavirus epidemiology and vaccine strategy. Emerg. Microbes Infect. 2017, 6, e22. [Google Scholar] [CrossRef] [PubMed]
- Oliveira Corvelo, T.C.; Aguiar, D.C.F.; Sagica, F.E.S. The expression of ABH and Lewis antigens in Brazilian semi-isolated Black Communities. Genet. Mol. Biol. 2002, 25, 260–263. [Google Scholar]
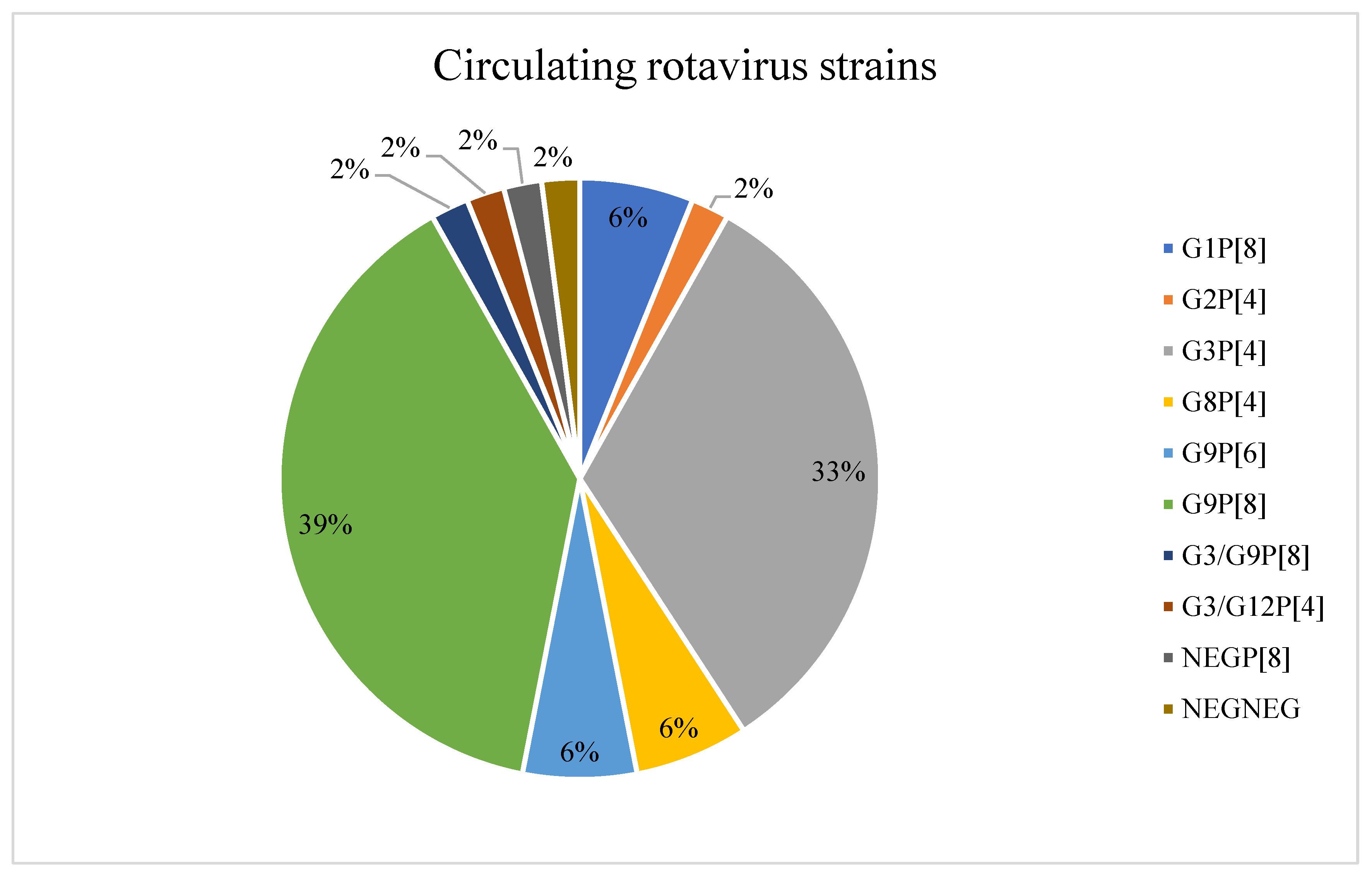
| Total | RV Positive n (%) [95%CI] | RV Negative n (%) [95%CI] | |
|---|---|---|---|
| Patients | 342 (%) | 49 (14.3%) | 293 (85.7%) |
| Sex | p-value = 0.14 1 | ||
| Male | 176 (51.5%) | 30 (61.2%) [46–74%] | 146 (49.8%) [44–56%] |
| Female | 166 (48.5%) | 19 (38.8%) [26–54%] | 147 (50.2%) [44–56%] |
| Age of children in months | p-value = 0.5 1 | ||
| 0–6 | 93 (27.2%) | 10 (20.4%) [11–35%] | 83 (28.3%) [23–34%] |
| 7–12 | 114 (33.3%) | 20 (40.8%) [27–56%] | 94 (32.1%) [27–38%] |
| 13–18 | 62 (18.1%) | 10 (20.4%) [11–35%] | 52 (17.7%) [14–23%] |
| 19–24 | 36 (10.5%) | 6 (12.2%) [5.1–25%] | 30 (10.2%) [7.1–14%] |
| 25–59 | 37 (10.8%) | 3 (6.1%) [1.6–18%] | 34 (11.6%) [8.3–16%] |
| Rotavirus immunization | p-value = 0.5 2 | ||
| 0 dose (unvaccinated) | 4 (1.2%) | 0 (0) [0.00–9.1%] | 4 (1.4%) [0.44–3.7%] |
| 1 dose | 23 (6.7%) | 5 (10.2%) [3.8–23%] | 18 (6.14%) [3.8–9.7%] |
| 2 doses | 283 (82.7%) | 38 (77.6%) [63–88%] | 245 (83.6%) [79–88%] |
| No RTHC 3 | 32 (9.4%) | 6 (12.2%) [5.1–25%] | 26 (8.9%) [6.0–13%] |
| Clinical symptoms 4 | |||
| Fever (n = 334) | p-value = 0.005 1 | ||
| Yes | 87 (26.0%) | 20 (42.5%) [29–58%] | 67 (23.3%) [19–29%] |
| None | 247 (74.0%) | 27 (57.4%) [42–71%] | 220 (76.7%) [71–81%] |
| Vomiting (n = 332) | p-value < 0.001 1 | ||
| Yes | 70 (21.1%) | 21 (44.7%) [30–60%] | 49 (17.2%) [13–22%] |
| None | 262 (78.9%) | 26 (55.3%) [40–70%] | 236 (82.8%) [78–87%] |
| Refusal to eat (n = 332) | p-value = 0.11 1 | ||
| Yes | 108 (32.5%) | 20 (42.5%) [29–58%] | 88 (30.9%) [26–37%] |
| None | 224 (67.5%) | 27 (57.5%) [63–74%] | 197 (69.1%) [42–71%] |
| Duration of diarrhea days | 4.22 | 3.27 | 4.32 |
| Phenotype | Total (%) | RV Positive (%) | RV Negative (%) |
|---|---|---|---|
| Patient sample | 323 | 49 | 274 |
| ABO groups | p-value = 0.002 2 | ||
| A | 84 (26%) | 19 (38.8%) | 65 (23.7%) |
| AB | 7 (2.2%) | 4 (8.2%) | 3 (1.1%) |
| B | 30 (9.3%) | 4 (8.2%) | 26 (9.5%) |
| O | 202 (62.5%) | 22 (44.9%) | 180 (65.7%) |
| Lewis phenotype | p-value = 0.004 1 | ||
| Le(a−b−) | 36 (11.2%) | 10 (20.4%) | 26 (9.5%) |
| Le(a+b+) | 176 (54.5%) | 26 (53.1%) | 150 (54.8%) |
| Le(a−b+) | 66 (20.4%) | 13 (26.5%) | 53 (19.3%) |
| Le(a+b−) | 45 (13.9%) | 0 (0.00%) | 45 (16.4%) |
| Secretor status | p-value = 0.024 1 | ||
| Non-secretor | 56 (17.3%) | 3 (6.1%) | 53 (19.3%) |
| Secretor | 267 (82.7%) | 46 (93.9%) | 221 (80.7%) |
| Combined | p-value < 0.001 2 | ||
| Sec/le-(Le(a−b−)) | 25 (7.7%) | 7 (14.3%) | 18 (6.6%) |
| Sec/Le+ (Le(a−b+); Le(a+b+)) | 242 (74.9%) | 39 (79.6%) | 203 (74.1%) |
| Non-sec/Le+ (Le(a+b−)) | 45 (13.9%) | 0 (0) | 45 (16.4%) |
| Non-sec/le-(Le(a−b−)) | 11 (3.4%) | 3 (6.1%) | 8 (2.9%) |
| Logistic Regression | cOR (95%CI) | p-Value (Wald’s Test) | aOR (95%CI) | p-Value |
|---|---|---|---|---|
| ABO groups vs. RV infection | ||||
| Ref O | ||||
| A | 2.39 [1.21–4.71] | 0.011 | 1.84 [0.89–3.77] | 0.097 |
| AB | 10.9 [2.27–58.49] | 0.003 | 8.99 [1.83–48.96] | 0.006 |
| B | 1.26 [0.35–3.62] | 0.693 | 0.92 [0.25–2.77] | 0.892 |
| Lewis antigen vs. RV infection | ||||
| Ref Le(a+b+) | ||||
| Le(a−b−) | 2.22 [0.93–5.05] | 0.063 | 2.23 [0.78–5.89] | 0.117 |
| Le(a−b+) | 1.42 [0.66–2.91 | 0.355 | 1.36 [0.62–2.87] | 0.423 |
| Le(a+b−) | 0.00 [0.00–infinite] | 0.986 | 0.00 [0.00–infinite] | 0.986 |
| Secretor status vs. RV infection | ||||
| Ref Non-secretor | ||||
| Secretor | 3.68 [1.28–15.55] | 0.034 | 0.77 [0.15–4.61] | 0.762 |
| Rotavirus P-Genotypes | |||
|---|---|---|---|
| Phenotypes | P[8] 24 (%) | P[4] 21 (%) | P[6] 3 (%) |
| ABO groups | |||
| A | 10 (41.7%) | 7 (33.3%) | 1 (33.3%) |
| AB | 1 (4.2%) | 3 (14.3%) | - |
| B | 2 (8.3%) | 1 (4.8%) | 1 (33.3%) |
| O | 11 (45.8%) | 10 (47.6%) | 1 (33.3%) |
| Lewis phenotype | |||
| Le(a+b+) | 13 (54.2%) | 12 (57.1%) | - |
| Le(a−b+) | 5 (20.8%) | 8 (38.1%) | - |
| Le(a−b−) | 6 (25%) | 1 (4.8%) | 3 (100%) |
| Secretor status | |||
| Non-secretor | 2 (8.3%) | - | 1 (33.3%) |
| Secretor | 22 (91.7%) | 21 (100.0%) | 2 (66.7%) |
| Combined | |||
| Sec/Le+ (Le(a−b+); Le(a+b+)) | 18 (75%) | 20 (95.2%) | - |
| Sec/le-(Le(a−b−)) | 4 (16.7%) | 1 (4.8%) | 2 (66.7%) |
| Non-sec/le-(Le(a−b−)) | 2 (8.3%) | 1 (33.3%) | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rakau, K.; Gededzha, M.; Peenze, I.; Huang, P.; Tan, M.; Steele, A.D.; Seheri, L.M. The Association between Symptomatic Rotavirus Infection and Histo-Blood Group Antigens in Young Children with Diarrhea in Pretoria, South Africa. Viruses 2022, 14, 2735. https://doi.org/10.3390/v14122735
Rakau K, Gededzha M, Peenze I, Huang P, Tan M, Steele AD, Seheri LM. The Association between Symptomatic Rotavirus Infection and Histo-Blood Group Antigens in Young Children with Diarrhea in Pretoria, South Africa. Viruses. 2022; 14(12):2735. https://doi.org/10.3390/v14122735
Chicago/Turabian StyleRakau, Kebareng, Maemu Gededzha, Ina Peenze, Pengwei Huang, Ming Tan, Andrew Duncan Steele, and Luyanda Mapaseka Seheri. 2022. "The Association between Symptomatic Rotavirus Infection and Histo-Blood Group Antigens in Young Children with Diarrhea in Pretoria, South Africa" Viruses 14, no. 12: 2735. https://doi.org/10.3390/v14122735
APA StyleRakau, K., Gededzha, M., Peenze, I., Huang, P., Tan, M., Steele, A. D., & Seheri, L. M. (2022). The Association between Symptomatic Rotavirus Infection and Histo-Blood Group Antigens in Young Children with Diarrhea in Pretoria, South Africa. Viruses, 14(12), 2735. https://doi.org/10.3390/v14122735







