Vaccination against Borna Disease: Overview, Vaccine Virus Characterization and Investigation of Live and Inactivated Vaccines
Abstract
1. Introduction
2. Materials and Methods
2.1. Viruses
2.2. Vaccines
2.2.1. Authorized Live Virus Vaccine
2.2.2. Experimental Batches of Vaccines
Vaccines Based on Strain V
Vaccines Based on Strain “Dessau”
Vaccines for Dose Titration
Inactivation of Viruses and Inactivation Kinetics
2.3. Virus Titration
2.4. Serological Investigations
2.5. Animal Experiments and Vaccinations
2.5.1. Study of Vaccine Virus Virulence in Rats
2.5.2. Titration of BoDV-1 in Rabbits
2.5.3. Dose Titration of Vaccines in Rabbits
2.5.4. Proof of Efficacy of the BD Live Vaccine “Dessau” in Rabbits
2.5.5. Investigation of Experimental Batches of Inactivated Vaccines in Rabbits
2.5.6. Vaccination of Horses with BoDV-1 Inactivated Vaccine “Dessau”
2.5.7. Vaccination of Horses with BoDV-1 Live Vaccine “Dessau”
2.6. PCR, Sequence Analysis and Phylogenetic Investigations
3. Results
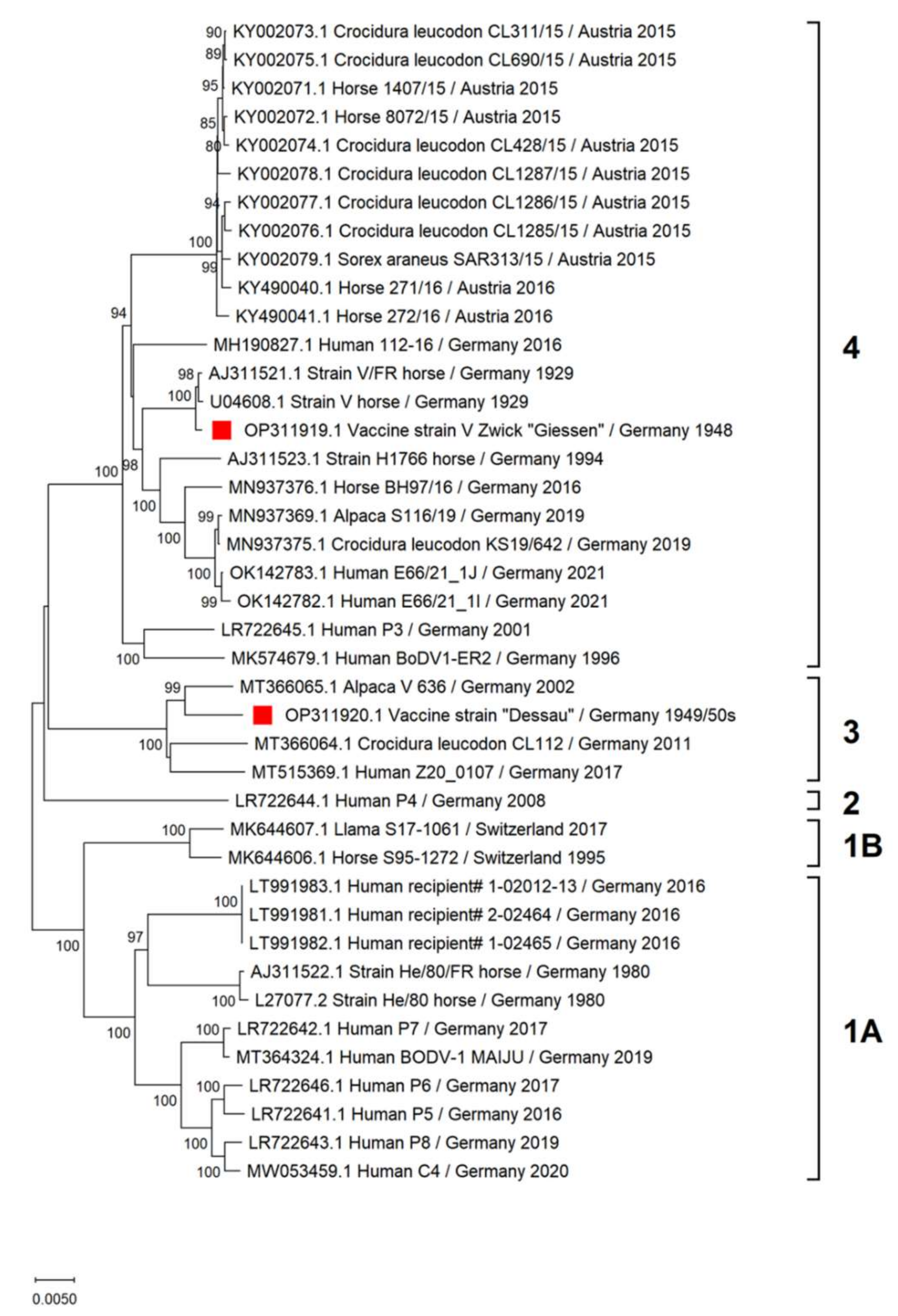
3.1. Genetic Characterization of Vaccine Viruses
3.1.1. Vaccine Virus V Zwick “Giessen”
3.1.2. Vaccine Virus “Dessau”
3.1.3. Virulence of Vaccine Virus “Dessau” in Rats
3.2. Inactivation Kinetics
3.3. Immunological Aspects of Vaccination
3.3.1. High Doses of Cell-Cultured Infectious Virus Protect from Disease
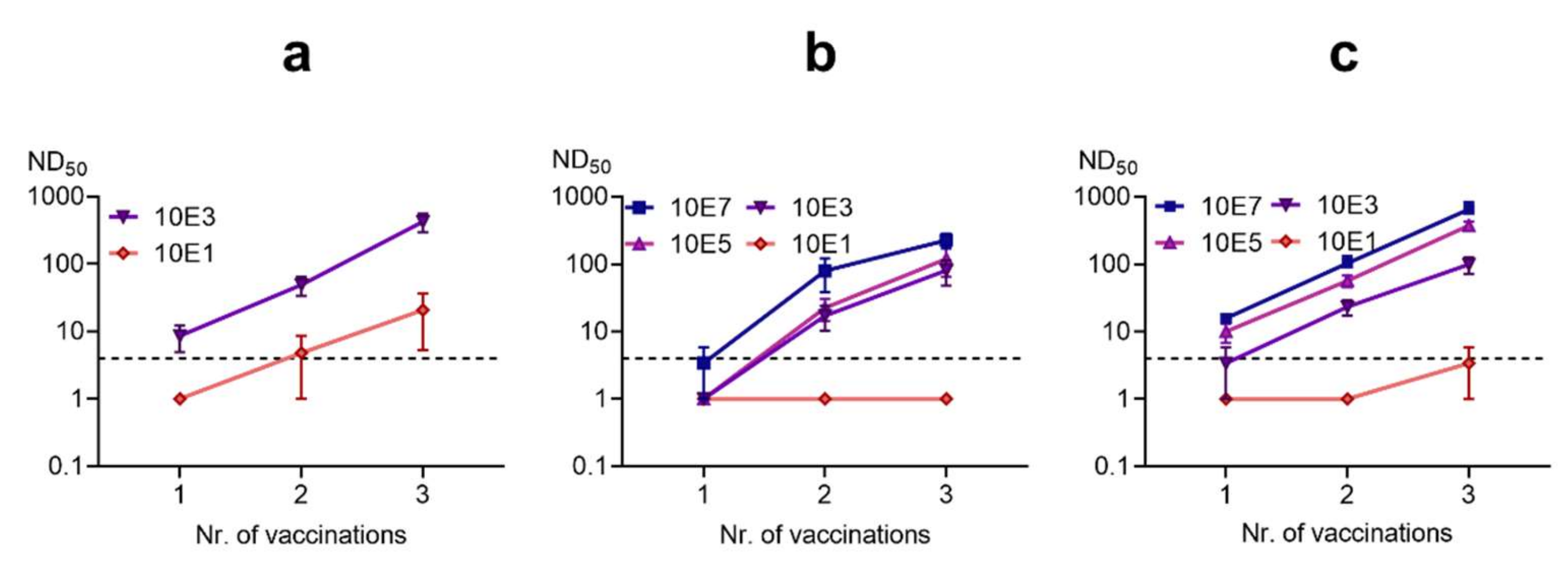
3.3.2. Titration of Vaccine Doses
3.3.3. 90% of Rabbits Vaccinated with BoDV-1 Live Vaccine “Dessau” Were Protected from Disease after Challenge Infection
3.3.4. Inactivated Vaccines Induce Antibodies in Rabbits and Protect from Disease
3.3.5. Inactivated Vaccines Stimulate Antibodies in Horses
3.3.6. The BoDV-1 Live Vaccine “Dessau” Induces Neutralizing Antibodies in Horses
4. Discussion
4.1. Sequence Characterization of BoDV-1 Vaccine Viruses
4.2. Immunogenicity of BoDV-1 Depends on Infection Dose and Virus Source
4.3. Inactivated BoDV-1 Vaccines Are Capable of Inducing Protection against Clinical Disease
4.4. Antibody Kinetics and Immunity after Live Vaccination
4.5. Historic Perspective
4.6. Limitations and Future Perspectives
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ludwig, H.; Bode, L.; Gosztonyi, G. Borna disease: A persistent virus infection of the central nervous system. Prog. Med. Virol. 1988, 35, 107–151. [Google Scholar]
- Rubbenstroth, D.; Briese, T.; Dürrwald, R.; Horie, M.; Hyndman, T.H.; Kuhn, J.H.; Nowotny, N.; Payne, S.; Stenglein, M.D.; Tomonaga, K.; et al. ICTV Virus Taxonomy Profile: Bornaviridae. J. Gen. Virol. 2021, 102, 001613. [Google Scholar] [CrossRef] [PubMed]
- Kuhn, J.H.; Dürrwald, R.; Bào, Y.; Briese, T.; Carbone, K.; Clawson, A.N.; DeRisi, J.L.; Garten, W.; Jahrling, P.B.; Kolodziejek, J.; et al. Taxonomic reorganization of the family Bornaviridae. Arch. Virol. 2015, 160, 621–632. [Google Scholar] [CrossRef] [PubMed]
- Horie, M.; Honda, T.; Suzuki, Y.; Kobayashi, Y.; Daito, T.; Oshida, T.; Ikuta, K.; Jern, P.; Gojobori, T.; Coffin, J.M.; et al. Endogenous non-retroviral RNA virus elements in mammalian genomes. Nature 2010, 463, 84–87. [Google Scholar] [CrossRef] [PubMed]
- Horie, M.; Kobayashi, Y.; Suzuki, Y.; Tomonaga, K. Comprehensive analysis of endogenous bornavirus-like elements in eukaryote genomes. Philos. Trans. R. Soc. B Biol. Sci. 2013, 368, 20120499. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, Y.; Hayashi, Y.; Omori, H.; Honda, T.; Daito, T.; Horie, M.; Ikuta, K.; Fujino, K.; Nakamura, S.; Schneider, U.; et al. Bornavirus Closely Associates and Segregates with Host Chromosomes to Ensure Persistent Intranuclear Infection. Cell Host Microbe 2012, 11, 492–503. [Google Scholar] [CrossRef] [PubMed]
- Schneider, U.; Schwemmle, M.; Staeheli, P. Genome trimming: A unique strategy for replication control employed by Borna disease virus. Proc. Natl. Acad. Sci. USA 2005, 102, 3441–3446. [Google Scholar] [CrossRef]
- Schneider, U.; Martin, A.; Schwemmle, M.; Staeheli, P. Genome trimming by Borna disease viruses: Viral replication control or escape from cellular surveillance? Cell Mol. Life Sci. 2007, 64, 1038–1042. [Google Scholar] [CrossRef]
- Poenisch, M.; Burger, N.; Staeheli, P.; Bauer, G.; Schneider, U. Protein X of Borna Disease Virus Inhibits Apoptosis and Promotes Viral Persistence in the Central Nervous Systems of Newborn-Infected Rats. J. Virol. 2009, 83, 4297–4307. [Google Scholar] [CrossRef][Green Version]
- Poenisch, M.; Wille, S.; Ackermann, A.; Staeheli, P.; Schneider, U. The X Protein of Borna Disease Virus Serves Essential Functions in the Viral Multiplication Cycle. J. Virol. 2007, 81, 7297–7299. [Google Scholar] [CrossRef] [PubMed]
- Werner-Keišs, N.; Garten, W.; Richt, J.A.; Porombka, D.; Algermissen, D.; Herzog, S.; Baumgärtner, W.; Herden, C. Restricted expression of Borna disease virus glycoprotein in brains of experimentally infected Lewis rats. Neuropathol. Appl. Neurobiol. 2008, 34, 590–602. [Google Scholar] [CrossRef] [PubMed]
- Schneemann, A.; Schneider, P.A.; Lamb, R.A.; Lipkin, W. The Remarkable Coding Strategy of Borna Disease Virus: A New Member of the Nonsegmented Negative Strand RNA Viruses. Virology 1995, 210, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Schneider, P.A.; Schneemann, A.; Lipkin, W.I. RNA splicing in Borna disease virus, a nonsegmented, negative-strand RNA virus. J. Virol. 1994, 68, 5007–5012. [Google Scholar] [CrossRef] [PubMed]
- Cubitt, B.; Oldstone, C.; Valcarcel, J.; de la Torre, J.C. RNA splicing contributes to the generation of mature mRNAs of Borna disease virus, a non-segmented negative strand RNA virus. Virus Res. 1994, 34, 69–79. [Google Scholar] [CrossRef] [PubMed]
- Jehle, C.; Lipkin, W.I.; Staeheli, P.; Marion, R.M.; Schwemmle, M. Authentic Borna disease virus transcripts are spliced less efficiently than cDNA-derived viral RNAs. J. Gen. Virol. 2000, 81 Pt 8, 1947–1954. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Tomonaga, K.; Kobayashi, T.; Ikuta, K. Molecular and cellular biology of Borna disease virus infection. Microbes Infect. 2002, 4, 491–500. [Google Scholar] [CrossRef]
- Cubitt, B.; Ly, C.; De La Torre, J.C. Identification and characterization of a new intron in Borna disease virus. J. Gen. Virol. 2001, 82, 641–646. [Google Scholar] [CrossRef]
- Schneider, P.A.; Kim, R.; Lipkin, W.I. Evidence for translation of the Borna disease virus G protein by leaky ribosomal scanning and ribosomal reinitiation. J. Virol. 1997, 71, 5614–5619. [Google Scholar] [CrossRef]
- Walker, M.P.; Jordan, I.; Briese, T.; Fischer, N.; Lipkin, W.I. Expression and Characterization of the Borna Disease Virus Polymerase. J. Virol. 2000, 74, 4425–4428. [Google Scholar] [CrossRef]
- De la Torre, J.C. Molecular biology of Borna disease virus and persistence. Front. Biosci. 2002, 7, d569–d579. [Google Scholar] [CrossRef]
- Richt, J.A.; Fürbringer, T.; Koch, A.; Pfeuffer, I.; Herden, C.; Bause-Niedrig, I.; Garten, W. Processing of the Borna disease virus glycoprotein gp94 by the subtilisin-like endoprotease furin. J. Virol. 1998, 72, 4528–4533. [Google Scholar] [CrossRef]
- Dürrwald, R.; Ludwig, H. Borna disease virus (BDV), a (zoonotic?) worldwide pathogen. A review of the history of the disease and the virus infection with comprehensive bibliography. Zentralblatt fur Veter-Reihe B. J. Veter-Med. Ser. B 1997, 44, 147–184. [Google Scholar]
- Kolodziejek, J.; Dürrwald, R.; Herzog, S.; Ehrensperger, F.; Lussy, H.; Nowotny, N. Genetic clustering of Borna disease virus natural animal isolates, laboratory and vaccine strains strongly reflects their regional geographical origin. J. Gen. Virol. 2005, 86, 385–398. [Google Scholar] [CrossRef] [PubMed]
- Schulze, V.; Große, R.; Fürstenau, J.; Forth, L.F.; Ebinger, A.; Richter, M.T.; Tappe, D.; Mertsch, T.; Klose, K.; Schlottau, K.; et al. Borna disease outbreak with high mortality in an alpaca herd in a previously unreported endemic area in Germany. Transbound. Emerg. Dis. 2020, 67, 2093–2107. [Google Scholar] [CrossRef]
- Autenrieth, C.F. Ueber die hitzige Kopf-Krankheit der Pferde. In Auf Verlangen des Münsinger Vereins zur Beförderung der Pferdezucht auf der Alp, und zunächst für diese Gegend; Heinrich Laupp: Tübingen, Germany, 1823. [Google Scholar]
- Siedamgrotzky, A.; Schlegel, M. Zur Kenntnis der seuchenhaften Cerebrospinalmeningitis der Pferde. Arch. Tierheilk 1896, 22, 287–332. [Google Scholar]
- Zwick, W. Zur Frage der Uebertragbarkeit der seuchenhaften Gehirn- und Rückenmarksentzündung der Pferde (Bornaschen Krankheit) auf kleine Versuchstiere. Berl Tierärztl Wochenschr 1925, 41, 453–455. [Google Scholar]
- Zwick, W.; Seifried, O. Uebertragbarkeit der seuchenhaften Gehirn- und Rückenmarksenrzündung des Pferdes (Borna’schen Krankheit) auf kleine Versuchstiere (Kaninchen). Berl. Tierärztl Wochenschr 1925, 41, 129–132. [Google Scholar]
- Zwick, W.; Seifried, O.; Witte, J. Experimentelle Untersuchungen über die seuchenhafte Gehirn- und Rückenmarksentzündung der Pferde (bornasche Krankheit). Z. Infektionskrh Haustiere 1927, 30, 42–136. [Google Scholar]
- Zwick, W. Bornasche Krankheit und Enzephalomyelitis der Tiere. In Handbuch der Viruskrankheiten; Gildenmeister, E., Haagen, E., Waldmann, O., Eds.; Fischer: Jena, Germany, 1939; Volume 2, pp. 254–354. [Google Scholar]
- Briese, T.; Schneemann, A.; Lewis, A.J.; Park, Y.S.; Kim, S.; Ludwig, H.; Lipkin, W.I. Genomic organization of Borna disease virus. Proc. Natl. Acad. Sci. USA 1994, 91, 4362–4366. [Google Scholar] [CrossRef]
- Danner, K. Borna-Virus und Borna-Infektionen. Vom Miasma zum Modell; Enke: Stuttgart, Germany, 1982. [Google Scholar]
- Dürrwald, R. Die natürliche Borna-Virus-Infektion der Einhufer und Schafe: Untersuchungen zur Epidemiologie, zu neueren diagnostischen Methoden (ELISA, PCR) und zur Antikörperkinetik bei Pferden nach Vakzination mit Lebendimpfstoff; Freie Universität: Berlin, Germany, 1993. [Google Scholar]
- Ernst, W. Neuere Arbeiten über Encephalitiden bei Tieren. Ergeb Hyg. Bakteriol. Immunitätsforsch Exp. Ther. 1931, 12, 1–14. [Google Scholar]
- Fortner, J. Die ansteckende Blutarmut der Einhufer in den Impfstoffwerken. Berl. Münch Tierärztl. Wochenschr. 1948, 61, 49–53. [Google Scholar]
- Furrer, E.; Bilzer, T.; Stitz, L.; Planz, O. High-Dose Borna Disease Virus Infection Induces a Nucleoprotein-Specific Cytotoxic T-Lymphocyte Response and Prevention of Immunopathology. J. Virol. 2001, 75, 11700–11708. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Gminder, A. Versuche über die Immunisierung gegen die infektiöse Gehirnrückenmarksentzündung (Bornasche Krankheit oder Kopfkrankheit) der Pferde in Württemberg. Berl. Tierärztl Wochenschr. 1934, 12, 203–205. [Google Scholar]
- Hameed, S.S.; Guo, J.; Tizard, I.; Shivaprasad, H.; Payne, S. Studies on immunity and immunopathogenesis of parrot bornaviral disease in cockatiels. Virology 2018, 515, 81–91. [Google Scholar] [CrossRef] [PubMed]
- Hausmann, J.; Baur, K.; Engelhardt, K.R.; Fischer, T.; Rziha, H.-J.; Staeheli, P. Vaccine-induced protection against Borna disease in wild-type and perforin-deficient mice. J. Gen. Virol. 2005, 86, 399–403. [Google Scholar] [CrossRef]
- Hausmann, J.; Schamel, K.; Staeheli, P. CD8 + T Lymphocytes Mediate Borna Disease Virus-Induced Immunopathology Independently of Perforin. J. Virol. 2001, 75, 10460–10466. [Google Scholar] [CrossRef]
- Henkel, M.; Planz, O.; Fischer, T.; Stitz, L.; Rziha, H.-J. Prevention of Virus Persistence and Protection against Immunopathology after Borna Disease Virus Infection of the Brain by a Novel Orf Virus Recombinant. J. Virol. 2005, 79, 314–325. [Google Scholar] [CrossRef]
- Lewis, A.J.; Whitton, J.L.; Hatalski, C.G.; Weissenböck, H.; Lipkin, W.I. Effect of immune priming on Borna disease. J. Virol. 1999, 73, 2541–2546. [Google Scholar] [CrossRef]
- Matthias, D. Zur Epidemiologie der Bornaschen Krankheit. Arch. Exp. Veterinärmed. 1955, 9, 824–843. [Google Scholar]
- Matthias, D. Weitere Untersuchungen zur Bornaschen Krankheit der Pferde und Schafe. Arch. Exp. Veterinärmed. 1958, 12, 920–947. [Google Scholar]
- Mayr, A.; Danner, K. Production of Borna Virus in Tissue Culture. Exp. Biol. Med. 1972, 140, 511–515. [Google Scholar] [CrossRef] [PubMed]
- Möhlmann, H.; Maas, A. Wertigkeitsprüfung des Borna-Trockenimpfstoffes “Dessau” bei Pferden unter den Verhältnissen der Praxis. Arch. Exp. Veterinarmed. 1960, 14, 1267–1280. [Google Scholar]
- Nicolau, S.; Galloway, I.A. L’encéphalo-myélte enzootique experimentale (maladie de Borna). Ann. Inst. Pasteur 1930, 45, 457–523. [Google Scholar]
- Oldach, D.; Zink, M.; Pyper, J.; Herzog, S.; Rott, R.; Narayan, O.; Clements, J. Induction of protection against Borna disease by inoculation with high-dose-attenuated Borna disease virus. Virology 1995, 206, 426–434. [Google Scholar] [CrossRef][Green Version]
- Pauli, G.; Ludwig, H. Increase of virus yields and releases of Borna disease virus from persistently infected cells. Virus Res. 1985, 2, 29–33. [Google Scholar] [CrossRef]
- Planz, O.; Bilzer, T.; Stitz, L. Immunopathogenic role of T-cell subsets in Borna disease virus-induced progressive encephalitis. J. Virol. 1995, 69, 896–903. [Google Scholar] [CrossRef]
- Planz, O.; Stitz, L. Borna Disease Virus Nucleoprotein (p40) Is a Major Target for CD8 + -T-Cell-Mediated Immune Response. J. Virol. 1999, 73, 1715–1718. [Google Scholar] [CrossRef]
- Rall, I.; Amann, R.; Malberg, S.; Herden, C.; Rubbenstroth, D. Recombinant Modified Vaccinia Virus Ankara (MVA) Vaccines Efficiently Protect Cockatiels Against Parrot Bornavirus Infection and Proventricular Dilatation Disease. Viruses 2019, 11, 1130. [Google Scholar] [CrossRef]
- Richt, J.; Stitz, L.; Deschl, U.; Frese, K.; Rott, R. Borna disease virus-induced meningoencephalomyelitis caused by a virus-specific CD4+ T cell-mediated immune reaction. J. Gen. Virol. 1990, 71 Pt 11, 2565–2573. [Google Scholar] [CrossRef]
- Richt, J.A.; Stitz, L.; Wekerle, H.; Rott, R. Borna disease, a progressive meningoencephalomyelitis as a model for CD4+ T cell-mediated immunopathology in the brain. J. Exp. Med. 1989, 170, 1045–1050. [Google Scholar] [CrossRef]
- Richter, K.; Baur, K.; Ackermann, A.; Schneider, U.; Hausmann, J.; Staeheli, P. Pathogenic Potential of Borna Disease Virus Lacking the Immunodominant CD8 T-Cell Epitope. J. Virol. 2007, 81, 11187–11194. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Runge, S.; Olbert, M.; Herden, C.; Malberg, S.; Römer-Oberdörfer, A.; Staeheli, P.; Rubbenstroth, D. Viral vector vaccines protect cockatiels from inflammatory lesions after heterologous parrot bornavirus 2 challenge infection. Vaccine 2017, 35, 557–563. [Google Scholar] [CrossRef] [PubMed]
- Schamel, K.; Staeheli, P.; Hausmann, J. Identification of the Immunodominant H-2K k -Restricted Cytotoxic T-Cell Epitope in the Borna Disease Virus Nucleoprotein. J. Virol. 2001, 75, 8579–8588. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Schulz, J.A.; Müller, H.; Lippmann, R. Untersuchungen zur Prophylaxe der Bornaschen Krankheit bei Schafen mittels aktiver Immunisierung. Arch. Exp. Veterinarmed 1968, 22, 571–583. [Google Scholar] [PubMed]
- Steinbrück, Ueber die bornasche Krankheit der Einhufer, ihre Beziehungen zur Viehversicherung und Versuche zu ihrer Bekämpfung im Regierungsbezirk Merseburg. Berl. Tierärztl. Wochenschr. 1931, 47, 793–798.
- Zwick, W. Heutiger Stand der Forschungen über die Bornasche Krankheit. Berl. Tierärztl. Wochenschr. 1931, 47, 797–798. [Google Scholar]
- Zwick, W.; Seifried, O.; Witte, J. Weitere Untersuchungen über die seuchenhafte Gehirn-Rückenmarksentzündung der Pferde (Bornasche Krankheit). Z. Infektionskr. Haustiere 1928, 32, 150–179. [Google Scholar]
- Zwick, W.; Witte, J. Über die Widerstandsfähigkeit des Virus der Bornaschen Krankheit gegen Trocknung und über Schutzimpfungsversuche mit getrockneter virushaltiger Gehirnsubstanz. Berl. Tierärztl. Wochenschr. 1931, 47, 33–35. [Google Scholar]
- Cubitt, B.; Oldstone, C.; De La Torre, J.C. Sequence and genome organization of Borna disease virus. J. Virol. 1994, 68, 1382–1396. [Google Scholar] [CrossRef]
- Dieckhöfer, R. Epidemiologische Untersuchungen zur equinen BDV-Infektion, der Bornaschen Krankheit beim Pferd, der Therapie und die dazugehörige aktuelle Gesetzessituation in Deutschland; Freie Universität: Berlin, Germany, 2006. [Google Scholar]
- Lipkin, W.I.; Travis, G.H.; Carbone, K.M.; Wilson, M.C. Isolation and characterization of Borna disease agent cDNA clones. Proc. Natl. Acad. Sci. USA 1990, 87, 4184–4188. [Google Scholar] [CrossRef]
- Ludwig, H.; Furuya, K.; Bode, L.; Klein, N.; Dürrwald, R.; Lee, D.S. Biology and neurobiology of Borna disease viruses (BDV), defined by antibodies, neutralizability and their pathogenic potential. Arch. Virol. Suppl. 1993, 7, 111–133. [Google Scholar] [CrossRef] [PubMed]
- Möhlmann, H. 10 Jahre Forschungsinstitut für Impfstoffe Dessau. Arch. Exp. Veterinarmed. 1965, 19, 253–260. [Google Scholar] [PubMed]
- Nitzschke, E. Variation beim Virus der Bornaschen Krankheit (infektiöse Encephalomyelitis der Pferde und Schafe) durch Rattenpassagen. In Proceedings of the VIIth International Congress for Microbiology, Stockholm, Sweeden, 4–5 August 1958; pp. 275–276. [Google Scholar]
- Nitzschke, E. Untersuchungen über die experimentelle Bornavirus-Infektion bei der Ratte. Zentralbl Veterinärmed 1963, 10, 470–527. [Google Scholar] [CrossRef]
- Pleschka, S.; Staeheli, P.; Kolodziejek, J.; Richt, J.A.; Nowotny, N.; Schwemmle, M. Conservation of coding potential and terminal sequences in four different isolates of Borna disease virus. J. Gen. Virol. 2001, 82, 2681–2690. [Google Scholar] [CrossRef] [PubMed]
- Zwick, W.; Witte, J.; Schwarzmaier, E. Zur Frage der Pluralität des Bornavirus. Z. Infektionskr. Haustiere 1937, 51, 261–267. [Google Scholar]
- Olbert, M.; Römer-Oberdörfer, A.; Herden, C.; Malberg, S.; Runge, S.; Staeheli, P.; Rubbenstroth, D. Viral vector vaccines expressing nucleoprotein and phosphoprotein genes of avian bornaviruses ameliorate homologous challenge infections in cockatiels and common canaries. Sci. Rep. 2016, 6, 36840. [Google Scholar] [CrossRef]
- Furrer, E.; Bilzer, T.; Stitz, L.; Planz, O. Neutralizing Antibodies in Persistent Borna Disease Virus Infection: Prophylactic Effect of gp94-Specific Monoclonal Antibodies in Preventing Encephalitis. J. Virol. 2001, 75, 943–951. [Google Scholar] [CrossRef]
- Hilbe, M.; Herrsche, R.; Kolodziejek, J.; Nowotny, N.; Zlinszky, K.; Ehrensperger, F. Shrews as Reservoir Hosts of Borna Disease Virus. Emerg. Infect. Dis. 2006, 12, 675–677. [Google Scholar] [CrossRef]
- Kistler, A.L.; Gancz, A.; Clubb, S.; Skewes-Cox, P.; Fischer, K.; Sorber, K.; Chiu, C.Y.; Lublin, A.; Mechani, S.; Farnoushi, Y.; et al. Recovery of divergent avian bornaviruses from cases of proventricular dilatation disease: Identification of a candidate etiologic agent. Virol. J. 2008, 5, 88. [Google Scholar] [CrossRef]
- Hoffmann, B.; Tappe, D.; Höper, D.; Herden, C.; Boldt, A.; Mawrin, C.; Niederstraßer, O.; Müller, T.; Jenckel, M.; van der Grinten, E.; et al. A Variegated Squirrel Bornavirus Associated with Fatal Human Encephalitis. N. Engl. J. Med. 2015, 373, 154–162. [Google Scholar] [CrossRef]
- Cadar, D.; Allendorf, V.; Schulze, V.; Ulrich, R.G.; Schlottau, K.; Ebinger, A.; Hoffmann, B.; Hoffmann, D.; Rubbenstroth, D.; Ismer, G.; et al. Introduction and spread of variegated squirrel bornavirus 1 (VSBV-1) between exotic squirrels and spill-over infections to humans in Germany. Emerg. Microbes Infect. 2021, 10, 602–611. [Google Scholar] [CrossRef]
- Niller, H.H.; Angstwurm, K.; Rubbenstroth, D.; Schlottau, K.; Ebinger, A.; Giese, S.; Wunderlich, S.; Banas, B.; Forth, L.F.; Hoffmann, D.; et al. Zoonotic spillover infections with Borna disease virus 1 leading to fatal human encephalitis, 1999–2019: An epidemiological investigation. Lancet Infect. Dis. 2020, 20, 467–477. [Google Scholar] [CrossRef] [PubMed]
- Schlottau, K.; Forth, L.; Angstwurm, K.; Höper, D.; Zecher, D.; Liesche, F.; Hoffmann, B.; Kegel, V.; Seehofer, D.; Platen, S.; et al. Fatal Encephalitic Borna Disease Virus 1 in Solid-Organ Transplant Recipients. N. Engl. J. Med. 2018, 379, 1377–1379. [Google Scholar] [CrossRef] [PubMed]
- Korn, K.; Coras, R.; Bobinger, T.; Herzog, S.M.; Lücking, H.; Stöhr, R.; Huttner, H.B.; Hartmann, A.; Ensser, A. Fatal Encephalitis Associated with Borna Disease Virus 1. N. Engl. J. Med. 2018, 379, 1375–1377. [Google Scholar] [CrossRef] [PubMed]
- Frank, C.; Wickel, J.; Brämer, D.; Matschke, J.; Ibe, R.; Gazivoda, C.; Günther, A.; Hartmann, C.; Rehn, K.; Cadar, D.; et al. Human Borna disease virus 1 (BoDV-1) encephalitis cases in the north and east of Germany. Emerg. Microbes Infect. 2021, 11, 6–13. [Google Scholar] [CrossRef] [PubMed]
- Coras, R.; Korn, K.; Kuerten, S.; Huttner, H.B.; Ensser, A. Severe bornavirus-encephalitis presenting as Guillain-Barre-syndrome. Acta Neuropathol. 2019, 137, 1017–1019. [Google Scholar] [CrossRef] [PubMed]
- Joest, E. Vergleichend-Anatomische Betrachtungen Über Encephalitis. Klin. Wochenschr. 1926, 5, 209–211. [Google Scholar] [CrossRef]
- Seifried, O.; Spatz, H. Die Ausbreitung der encephalitischen Reaktion bei der Bornaschen Krankheit der Pferde und deren Beziehungen zur Encephalitis epidemica, zur Heine-Medinschen Krankheit und zur Lyssa des Menschen. Eine vergleichend-pathologische Studie. Z. Ges Neurol. Psychiat. 1930, 124, 317–383. [Google Scholar] [CrossRef]
- Rott, R.; Herzog, S.; Fleischer, B.; Winokur, A.; Amsterdam, J.; Dyson, W.; Koprowski, H. Detection of Serum Antibodies to Borna Disease Virus in Patients with Psychiatric Disorders. Science 1985, 228, 755–756. [Google Scholar] [CrossRef]
- Bode, L.; Riegel, S.; Lange, W.; Ludwig, H. Human infections with Borna disease virus: Seroprevalence in patients with chronic diseases and healthy individuals. J. Med. Virol. 1992, 36, 309–315. [Google Scholar] [CrossRef]
- Schwemmle, M. Borna disease virus infection in psychiatric patients: Are we on the right track? Lancet Infect Dis 2001, 1, 46–52. [Google Scholar] [CrossRef] [PubMed]
- Rubbenstroth, D.; Schlottau, K.; Schwemmle, M.; Rissland, J.; Beer, M. Human bornavirus research: Back on track! PLoS Pathog. 2019, 15, e1007873. [Google Scholar] [CrossRef] [PubMed]
- Schlottau, K.; Feldmann, F.; Hanley, P.W.; Lovaglio, J.; Tang-Huau, T.-L.; Meade-White, K.; Callison, J.; Williamson, B.N.; Rosenke, R.; Long, D.; et al. Development of a nonhuman primate model for mammalian bornavirus infection. PNAS Nexus 2022, 1, pgac073. [Google Scholar] [CrossRef]
- Pauli, G.; Grunmach, J.; Ludwig, H. Focus-Immunoassay for Borna Disease Virus-Specific Antigens. Zentralbl. Veterinarmed. B 1984, 31, 552–557. [Google Scholar] [CrossRef] [PubMed]
- Dürrwald, R.; Kolodziejek, J.; Herzog, S.; Nowotny, N. Meta-analysis of putative human bornavirus sequences fails to provide evidence implicating Borna disease virus in mental illness. Rev. Med. Virol. 2007, 17, 181–203. [Google Scholar] [CrossRef] [PubMed]
- Weissenböck, H.; Bagó, Z.; Kolodziejek, J.; Hager, B.; Dürrwald, R.; Nowotny, N. Infections of horses and shrews with Bornaviruses in Upper Austria: A novel endemic area of Borna disease. Emerg. Microbes Infect. 2017, 6, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular Evolutionary Genetics Analysis across Computing Platforms. Mol. Biol. Evol. 2018, 35, 1547–1549. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; He, P.; Sun, L.; Zhang, X.; Xu, X.; Tang, T.; Zhou, W.; Li, Q.; Zou, D.; Bode, L.; et al. Full-length genomic sequencing and characterization of Borna disease virus 1 isolates: Lessons in epidemiology. J. Med. Virol. 2020, 92, 3125–3137. [Google Scholar] [CrossRef]
- Bode, L.; Guo, Y.; Xie, P. Molecular epidemiology of human Borna disease virus 1 infection revisited. Emerg. Microbes Infect. 2022, 11, 1335–1338. [Google Scholar] [CrossRef]
- Dürrwald, R.; Kolodziejek, J.; Weissenböck, H.; Nowotny, N. The Bicolored White-Toothed Shrew Crocidura leucodon (HERMANN 1780) Is an Indigenous Host of Mammalian Borna Disease Virus. PLoS ONE 2014, 9, e93659. [Google Scholar] [CrossRef]
- Malbon, A.J.; Dürrwald, R.; Kolodziejek, J.; Nowotny, N.; Kobera, R.; Pöhle, D.; Muluneh, A.; Dervas, E.; Cebra, C.; Steffen, F.; et al. New World camelids are sentinels for the presence of Borna disease virus. Transbound. Emerg. Dis. 2021, 69, 451–464. [Google Scholar] [CrossRef] [PubMed]
- Zwick, W.; Seifried, O.; Witte, J. Weitere Beiträge zur Erforschung der Bornaschen Krankheit des Pferdes. Arch. Tierheilk 1929, 59, 511–545. [Google Scholar]
- Stitz, L.; Nöske, K.; Planz, O.; Furrer, E.; Lipkin, W.I.; Bilzer, T. A functional role for neutralizing antibodies in Borna disease: Influence on virus tropism outside the central nervous system. J. Virol. 1998, 72, 8884–8892. [Google Scholar] [CrossRef] [PubMed]
- Narayan, O.; Herzog, S.; Frese, K.; Scheefers, H.; Rott, R. Behavioral Disease in Rats Caused by Immunopathological Responses to Persistent Borna Virus in the Brain. Science 1983, 220, 1401–1403. [Google Scholar] [CrossRef]
- Zimmermann, V.; Rinder, M.; Kaspers, B.; Staeheli, P.; Rubbenstroth, D. Impact of antigenic diversity on laboratory diagnosis of Avian bornavirus infections in birds. J. Veter-Diagn. Investig. 2014, 26, 769–777. [Google Scholar] [CrossRef] [PubMed]
- Hatalski, C.G.; Kliche, S.; Stitz, L.; Lipkin, W.I. Neutralizing antibodies in Borna disease virus-infected rats. J. Virol. 1995, 69, 741–747. [Google Scholar] [CrossRef]
- Stoyloff, R.; Bode, L.; Borchers, K.; Ludwig, H. Neutralization of borna disease virus depends upon terminal carbohydrate residues (alpha-D-man, beta-D-GlcNAc) of glycoproteins gp17 and gp94. Intervirology 1998, 41, 135–140. [Google Scholar] [CrossRef] [PubMed]
- Cubitt, B.; De La Torre, J.C. Borna disease virus (BDV), a nonsegmented RNA virus, replicates in the nuclei of infected cells where infectious BDV ribonucleoproteins are present. J. Virol. 1994, 68, 1371–1381. [Google Scholar] [CrossRef]
- Lennartz, F.; Bayer, K.; Czerwonka, N.; Lu, Y.; Kehr, K.; Hirz, M.; Steinmetzer, T.; Garten, W.; Herden, C. Surface glycoprotein of Borna disease virus mediates virus spread from cell to cell. Cell. Microbiol. 2015, 18, 340–354. [Google Scholar] [CrossRef]
- Clemente, R.; de la Torre, J.C. Cell-to-cell spread of Borna disease virus proceeds in the absence of the virus primary receptor and furin-mediated processing of the virus surface glycoprotein. J. Virol. 2007, 81, 5968–5977. [Google Scholar] [CrossRef]
- Compans, R.W.; Melsen, L.R.; de la Torre, J.C. Virus-like particles in MDCK cells persistently infected with Borna disease virus. Virus Res. 1994, 33, 261–268. [Google Scholar] [CrossRef] [PubMed]
- Matthias, D. Beitrag zum Infektionsmodus der Bornaschen Krankheit der Pferde. Exp. Veterinärmed 1953, 7, 313–318. [Google Scholar]
- Fechner, J. Die Komplementbindungsreaktion bei experimentell mit Bornavirus infizierten Pferden. Monatsh Veterinärmed 1955, 10, 553–556. [Google Scholar]
- Heinig, A. Zur experimentellen Infektion von Pferden und Schafen mit dem Virus der Bornaschen Krankheit. Arch. Exp. Veterinärmed 1964, 18, 753–766. [Google Scholar]
- Wagner, K.; Ludwig, H.; Paulsen, J. Fluorescence serological demonstration of Borna virus antigen. Berl Munch Tierarztl Wochenschr 1968, 81, 395–396. [Google Scholar]
- Danner, K.; Heubeck, D.; Mayr, A. In vitro studies on Borna virus. I. The use of cell cultures for the demonstration, titration and production of Borna virus. Arch. Virol. 1978, 57, 63–75. [Google Scholar] [CrossRef]
- Eickmann, M.; Kiermayer, S.; Kraus, I.; Gössl, M.; Richt, J.A.; Garten, W. Maturation of Borna disease virus glycoprotein. FEBS Lett. 2005, 579, 4751–4756. [Google Scholar] [CrossRef]
- Zwick, W.; Witte, J. Zur Frage der Schutzimpfung und Inkubationsfrist bei der Bornaschen Krankheit. Arch. Tierheilk. 1932, 64, 116–124. [Google Scholar]
- Nicolau, S.; Galloway, I.A.; Stroian, N. L’immunité dans l’encéphalo-myélite enzootique (maladie de Borna). Comptes Rendus Soc. Biol. Fil. 1929, 100, 607–610. [Google Scholar]
- Ernst, W.; Hahn, H. Weitere Beiträge zur Borna’schen Krankheit der Pferde und zur Frage der Aetiologie des bösartigen Katarrhalfiebers der Rinder. Münch Tierärztl Wochenschr 1927, 78, 85–89. [Google Scholar]
- Ernst, W. Zur Frage der Encephalitis enzootica infectiosa bei Pferd und Rind. Bornakankheit und bösartiges Katarrhalfieber. Arch. Tierheilk 1931, 63, 38–44. [Google Scholar]
- Lüthgen, K. Untersuchungen über Nachweis, Bildung und Vorkommen von Bornavirus-Antikörpern; Ludwig-Maximilians-Universität München: München, Germany, 1977. [Google Scholar]
- Otta, J. Die Komplementbindungsreaktion bei der Meningo-Encephalomyelitis enzootica equorum (Bornasche Krankheit). Arch. Exp. Veterinärmed. 1957, 11, 235–252. [Google Scholar]
- Wagner, D. Untersuchungen über die Verbreitung der Bornaschen Krankheit in Bayern und über das Vorkommen von Antikörpern im Serum von Pferden, Rindern, Schafen und Schweinen in Bornagebieten; Ludwig-Maximilians-Universität München: München, Germany, 1970. [Google Scholar]
- Friedrichs, E. Betrachtungen über die Bornasche Krankheit der Einhufer. Berl Munch Tierarztl Wochenschr 1951, 64, 89–94. [Google Scholar]
- Achtzehn, W. Bornasche Krankheit, Impfung, Impferfolge und ihre Heilungsaussichten im Land Sachsen-Anhalt. Ein Überblick der Jahre 1900–1950. Exp. Veterinärmed 1953, 5, 19–37. [Google Scholar]
- Rauscher, W. Seuchenkarte Bayerns der Bornaschen Krankheit der Jahre 1949-1958 und Beurteilung des Wertes der Schutzimpfung mit Borna-Vakzine nach Zwick. Monatsh Tierheilk 1959, 11, 221–227. [Google Scholar]
- Dürrwald, R.; Kolodziejek, J.; Muluneh, A.; Herzog, S.; Nowotny, N. Epidemiological pattern of classical Borna disease and regional genetic clustering of Borna disease viruses point towards the existence of to-date unknown endemic reservoir host populations. Microbes Infect. 2006, 8, 917–929. [Google Scholar] [CrossRef]
- Rubbenstroth, D. Avian Bornavirus Research—A Comprehensive Review. Viruses 2022, 14, 1513. [Google Scholar] [CrossRef]
- Kao, M.; Ludwig, H.; Gosztonyi, G. Adaptation of Borna Disease Virus to the Mouse. J. Gen. Virol. 1984, 65, 1845–1849. [Google Scholar] [CrossRef]
- Nanishi, E.; Dowling, D.J.; Levy, O. Toward precision adjuvants: Optimizing science and safety. Curr. Opin. Pediatr. 2020, 32, 125–138. [Google Scholar] [CrossRef]
- Ludwig, H.; Koester, V.; Pauli, G.; Rott, R. The cerebrospinal fluid of rabbits infected with Borna disease virus. Arch. Virol. 1977, 55, 209–223. [Google Scholar] [CrossRef]
- Ludwig, H.; Thein, P. Demonstration of specific antibodies in the central nervous system of horses naturally infected with Borna disease virus. Med. Microbiol. Immunol. 1977, 163, 215–226. [Google Scholar] [CrossRef] [PubMed]
- Hickey, W.F.; Hsu, B.L.; Kimura, H. T-lymphocyte entry into the central nervous system. J. Neurosci. Res. 1991, 28, 254–260. [Google Scholar] [CrossRef] [PubMed]
- Torsteinsdóttir, S.; Georgsson, G.; Gísladóttir, E.; Rafnar, B.; Pálsson, P.A.; Pétursson, G. Pathogenesis of central nervous system lesions in visna: Cell-mediated immunity and lymphocyte subsets in blood, brain and cerebrospinal fluid. J. Neuroimmunol. 1992, 41, 149–158. [Google Scholar] [CrossRef] [PubMed]
- Wekerle, H.; Linington, C.; Lassmann, H.; Meyermann, R. Cellular immun reacrivity within the CNS. Trends Neurosci. 1986, 9, 271–277. [Google Scholar] [CrossRef]
- Bauer, J.; Bradl, M.; Hickey, W.F.; Forss-Petter, S.; Breitschopf, H.; Linington, C.; Wekerle, H.; Lassmann, H. T-Cell Apoptosis in Inflammatory Brain Lesions: Destruction of T Cells Does Not Depend on Antigen Recognition. Am. J. Pathol. 1998, 153, 715–724. [Google Scholar] [CrossRef]
- Alter, A.; Duddy, M.; Hebert, S.; Biernacki, K.; Prat, A.; Antel, J.P.; Yong, V.W.; Nuttall, R.K.; Pennington, C.J.; Edwards, D.R.; et al. Determinants of Human B Cell Migration Across Brain Endothelial Cells. J. Immunol. 2003, 170, 4497–4505. [Google Scholar] [CrossRef]
- Michel, L.; Grasmuck, C.; Charabati, M.; Lécuyer, M.-A.; Zandee, S.; Dhaeze, T.; Alvarez, J.I.; Li, R.; Larouche, S.; Bourbonnière, L.; et al. Activated leukocyte cell adhesion molecule regulates B lymphocyte migration across central nervous system barriers. Sci. Transl. Med. 2019, 11, eaaw0475. [Google Scholar] [CrossRef]
- Marchetti, L.; Engelhardt, B. Immune cell trafficking across the blood-brain barrier in the absence and presence of neuroinflammation. Vasc. Biol. 2020, 2, H1–H18. [Google Scholar] [CrossRef]
- Dietzschold, B.; Kao, M.; Zheng, Y.M.; Chen, Z.Y.; Maul, G.; Fu, Z.F.; Rupprecht, C.E.; Koprowski, H. Delineation of putative mechanisms involved in antibody-mediated clearance of rabies virus from the central nervous system. Proc. Natl. Acad. Sci. USA 1992, 89, 7252–7256. [Google Scholar] [CrossRef]
- Levine, B.; Hardwick, J.M.; Trapp, B.D.; Crawford, T.O.; Bollinger, R.C.; Griffin, D.E. Antibody-mediated clearance of alphavirus infection from neurons. Science 1991, 254, 856–860. [Google Scholar] [CrossRef]
- Pardi, N.; Hogan, M.J.; Porter, F.W.; Weissman, D. mRNA vaccines—A new era in vaccinology. Nat. Rev. Drug Discov. 2018, 17, 261–279. [Google Scholar] [CrossRef] [PubMed]
- Pardi, N.; Hogan, M.; Naradikian, M.S.; Parkhouse, K.; Cain, D.W.; Jones, L.; Moody, M.A.; Verkerke, H.P.; Myles, A.; Willis, E.; et al. Nucleoside-modified mRNA vaccines induce potent T follicular helper and germinal center B cell responses. J. Exp. Med. 2018, 215, 1571–1588. [Google Scholar] [CrossRef]
- Hogan, M.J.; Pardi, N. mRNA Vaccines in the COVID-19 Pandemic and Beyond. Annu. Rev. Med. 2022, 73, 17–39. [Google Scholar] [CrossRef] [PubMed]
- Israel, A.; Shenhar, Y.; Green, I.; Merzon, E.; Golan-Cohen, A.; Schaffer, A.A.; Ruppin, E.; Vinker, S.; Magen, E. Large-Scale Study of Antibody Titer Decay following BNT162b2 mRNA Vaccine or SARS-CoV-2 Infection. Vaccines 2021, 10, 64. [Google Scholar] [CrossRef] [PubMed]
- Guerrera, G.; Picozza, M.; D’Orso, S.; Placido, R.; Pirronello, M.; Verdiani, A.; Termine, A.; Fabrizio, C.; Giannessi, F.; Sambucci, M.; et al. BNT162b2 vaccination induces durable SARS-CoV-2-specific T cells with a stem cell memory phenotype. Sci. Immunol. 2021, 6, eabl5344. [Google Scholar] [CrossRef]
- Reynolds, C.J.; Gibbons, J.M.; Pade, C.; Lin, K.M.; Sandoval, D.M.; Pieper, F.; Butler, D.K.; Liu, S.; Otter, A.D.; Joy, G.; et al. Heterologous infection and vaccination shapes immunity against SARS-CoV-2 variants. Science 2022, 375, 183–192. [Google Scholar] [CrossRef]
- Stamatatos, L.; Czartoski, J.; Wan, Y.H.; Homad, L.J.; Rubin, V.; Glantz, H.; Neradilek, M.; Seydoux, E.; Jennewein, M.F.; MacCamy, A.J.; et al. mRNA vaccination boosts cross-variant neutralizing antibodies elicited by SARS-CoV-2 infection. Science 2021, 372, 1413–1418. [Google Scholar] [CrossRef]
- Kaku, C.I.; Champney, E.R.; Normark, J.; Garcia, M.; Johnson, C.E.; Ahlm, C.; Christ, W.; Sakharkar, M.; Ackerman, M.E.; Klingstrom, J.; et al. Broad anti-SARS-CoV-2 antibody immunity induced by heterologous ChAdOx1/mRNA-1273 vaccination. Science 2022, 375, 1041–1047. [Google Scholar] [CrossRef]
- Doria-Rose, N.; Suthar, M.S.; Makowski, M.; O’Connell, S.; McDermott, A.B.; Flach, B.; Ledgerwood, J.E.; Mascola, J.R.; Graham, B.S.; Lin, B.C.; et al. Antibody Persistence through 6 Months after the Second Dose of mRNA-1273 Vaccine for Covid-19. N. Engl. J. Med. 2021, 384, 2259–2261. [Google Scholar] [CrossRef]
- Barda, N.; Dagan, N.; Cohen, C.; Hernán, M.A.; Lipsitch, M.; Kohane, I.S.; Reis, B.Y.; Balicer, R.D. Effectiveness of a third dose of the BNT162b2 mRNA COVID-19 vaccine for preventing severe outcomes in Israel: An observational study. Lancet 2021, 398, 2093–2100. [Google Scholar] [CrossRef]
- Schmidt, F.; Muecksch, F.; Weisblum, Y.; Da Silva, J.; Bednarski, E.; Cho, A.; Wang, Z.; Gaebler, C.; Caskey, M.; Nussenzweig, M.; et al. Plasma neutralization properties of the SARS-CoV-2 Omicron variant. N. Engl. J. Med. 2021, 386, 599–601. [Google Scholar] [CrossRef] [PubMed]
- Chemaitelly, H.; Ayoub, H.H.; AlMukdad, S.; Tang, P.; Hasan, M.R.; Yassine, H.M.; Khatib, H.A.A.; Smatti, M.K.; Coyle, P.; Kanaani, Z.A.; et al. Duration of protection of BNT162b2 and mRNA-1273 COVID-19 vaccines against symptomatic SARS-CoV-2 Omicron infection in Qatar. medRxiv 2022. [Google Scholar] [CrossRef]
- Tseng, H.F.; Ackerson, B.K.; Luo, Y.; Sy, L.S.; Talarico, C.A.; Tian, Y.; Bruxvoort, K.J.; Tubert, J.E.; Florea, A.; Ku, J.H.; et al. Effectiveness of mRNA-1273 against SARS-CoV-2 Omicron and Delta variants. Nat. Med. 2022, 28, 1063–1071. [Google Scholar] [CrossRef] [PubMed]
- Wilhelm, A.; Widera, M.; Grikscheit, K.; Toptan, T.; Schenk, B.; Pallas, C.; Metzler, M.; Kohmer, N.; Hoehl, S.; Marschalek, R.; et al. Limited neutralisation of the SARS-CoV-2 Omicron subvariants BA.1 and BA.2 by convalescent and vaccine serum and monoclonal antibodies. EBioMedicine 2022, 82, 104158. [Google Scholar] [CrossRef] [PubMed]


| Number of Mutations Protein | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 |
|---|---|---|---|---|---|---|---|---|
| N | - | - | - | - | - | - | - | - |
| P | I34V | - | - | - | - | - | - | - |
| M | E108D | - | - | - | - | - | - | - |
| G | A17V | - | - | - | - | - | - | - |
| L | S134F | T158A | T1430A | I1431V | - | - | - | - |
| Number of Mutations Protein | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 |
|---|---|---|---|---|---|---|---|---|
| N | P30S | - | - | - | - | - | - | - |
| P | - | - | - | - | - | - | - | - |
| M | S71F | V73A | T86I | - | - | - | - | - |
| G | R44K | Q145R | L186V | N192K | Y236H | K245E | T251I | A288V |
| L | V382I | I543V | V1545A | - | - | - | - | - |
| Horse | Weeks Post Vaccination | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 0 | 1 | 2 | 3 | 4 | 5 | 8 | 10 | 13 | 17 | 22 | |
| I | <4 1 | 75 | - 2 | 80 | 20 | - | - | <4 | - | <4 | - |
| II | <4 | 17 | - | 40 | 35 | - | - | 4 | - | 4 | - |
| III | <4 | 10 | - | - | 16 | - | - | <4 | - | <4 | - |
| IV | <4 | 8 | - | - | 32 | - | - | 4 | - | <4 | - |
| V | <4 | 15 | 15 | 8 | - | - | 8 | - | 6 | - | |
| VI | <4 | <4 | - | <4 | <4 | - | - | <4 | - | <4 | - |
| VII | <4 | - | - | 25 | 11 | - | - | <4 | - | <4 | - |
| VIII | <4 | - | - | 30 | 28 | - | - | 4 | - | 6 | - |
| IX | <4 | 80 | - | 40 | - | - | - | 32 | - | 20 | - |
| X | <4 | 16 | - | 80 | - | - | - | - | <4 | - | |
| XI | 8 | 32 | - | 32 | - | - | - | 32 | - | 24 | - |
| XII | <4 | <4 | - | 4 | - | - | - | 4 | - | <4 | - |
| XIII | 16 | 32 | 35 | - | - | - | - | - | - | 16 | - |
| XIV | 16 | 16 | 32 | 20 | - | - | - | - | - | <4 | - |
| XV | <4 | 31 | 18 | 18 | - | - | - | - | - | <4 | - |
| XVI | <4 | <4 | - | - | - | - | - | - | - | - | - |
| XVII | <4 | 30 | 80 | 16 | 18 | - | - | - | - | <4 | - |
| XVIII | <4 | 32 | 85 | 35 | 20 | - | - | - | - | 10 | - |
| XIX | 4 | 80 | 32 | 21 | - | 8 | - | - | - | 8 | - |
| XX | <4 | 15 | 27 | 30 | - | 8 | - | - | - | 8 | - |
| XXI | <4 | 15 | 31 | 17 | - | <4 | - | - | - | <4 | - |
| XXII | <4 | <4 | <4 | - | <4 | - | <4 | - | <4 | - | <4 |
| XXIII | <4 | <4 | <4 | - | <4 | - | <4 | - | <4 | - | <4 |
| XXIV | <4 | <4 | <4 | - | <4 | - | <4 | - | <4 | - | <4 |
| XXV | <4 | <4 | <4 | - | <4 | - | <4 | - | <4 | - | <4 |
| XXVI | <4 | <4 | <4 | - | 10 | - | <4 | - | <4 | - | <4 |
| XXVII | <4 | <4 | <4 | - | <4 | - | <4 | - | <4 | - | <4 |
| XXVIII | 80 | 80 | 80 | - | 300 | - | 520 | - | 640 | - | 640 |
| XXiX | <4 | 80 | 80 | - | 80 | - | 16 | - | 4 | - | <4 |
| XXX | <4 | <4 | <4 | - | <4 | - | <4 | - | <4 | - | <4 |
| XXXI | <4 | 160 | 80 | - | 80 | - | 8 | - | 8 | - | 8 |
| Horse | Weeks Post Vaccination | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 0 | 1 | 2 | 3 | 4 | 5 | 8 | 10 | 13 | 17 | 22 | |
| XXXII | <4 1 | <4 | - 2 | - | - | - | - | <4 | - | <4 | - |
| XXXIII | <4 | <4 | - | <4 | - | 4 | - | <4 | - | <4 | - |
| XXXIV | <4 | <4 | 8 | 60 | - | 40 | - | <4 | - | <4 | - |
| XXXV | <4 | <4 | <4- | <4 | - | 16 | - | <4 | - | <4 | - |
| XXXVI | <4 | <4 | <4 | <4 | - | <4 | - | <4 | - | <4 | - |
| XXXVII | <4 | 4 | 4 | 4 | - | 30 | - | - | - | <4 | - |
| XXXVIII | <4 | <4 | - | 32 | - | - | <4 | - | <4 | - | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dürrwald, R.; Kolodziejek, J.; Oh, D.-Y.; Herzog, S.; Liebermann, H.; Osterrieder, N.; Nowotny, N. Vaccination against Borna Disease: Overview, Vaccine Virus Characterization and Investigation of Live and Inactivated Vaccines. Viruses 2022, 14, 2706. https://doi.org/10.3390/v14122706
Dürrwald R, Kolodziejek J, Oh D-Y, Herzog S, Liebermann H, Osterrieder N, Nowotny N. Vaccination against Borna Disease: Overview, Vaccine Virus Characterization and Investigation of Live and Inactivated Vaccines. Viruses. 2022; 14(12):2706. https://doi.org/10.3390/v14122706
Chicago/Turabian StyleDürrwald, Ralf, Jolanta Kolodziejek, Djin-Ye Oh, Sibylle Herzog, Heinrich Liebermann, Nikolaus Osterrieder, and Norbert Nowotny. 2022. "Vaccination against Borna Disease: Overview, Vaccine Virus Characterization and Investigation of Live and Inactivated Vaccines" Viruses 14, no. 12: 2706. https://doi.org/10.3390/v14122706
APA StyleDürrwald, R., Kolodziejek, J., Oh, D.-Y., Herzog, S., Liebermann, H., Osterrieder, N., & Nowotny, N. (2022). Vaccination against Borna Disease: Overview, Vaccine Virus Characterization and Investigation of Live and Inactivated Vaccines. Viruses, 14(12), 2706. https://doi.org/10.3390/v14122706






