Preparation of Monoclonal Antibodies against the Viral p54 Protein and a Blocking ELISA for Detection of the Antibody against African Swine Fever Virus
Abstract
1. Introduction
2. Materials and Methods
2.1. Cells, Sera and Animals
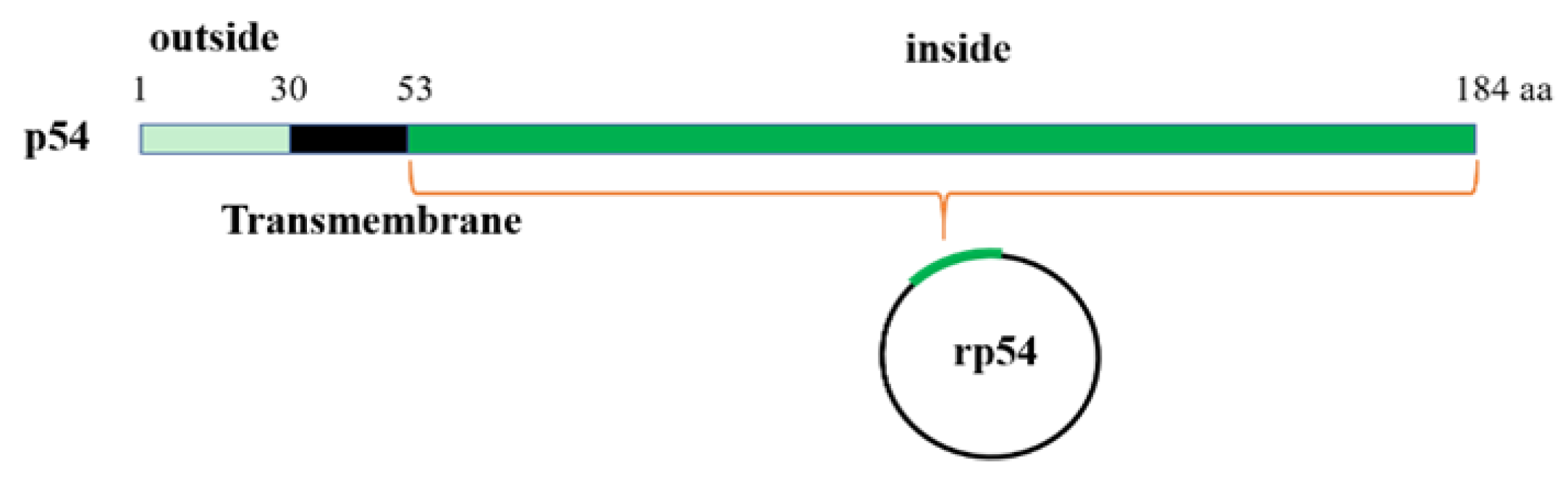
2.2. Construction of p54 Recombinant DNA
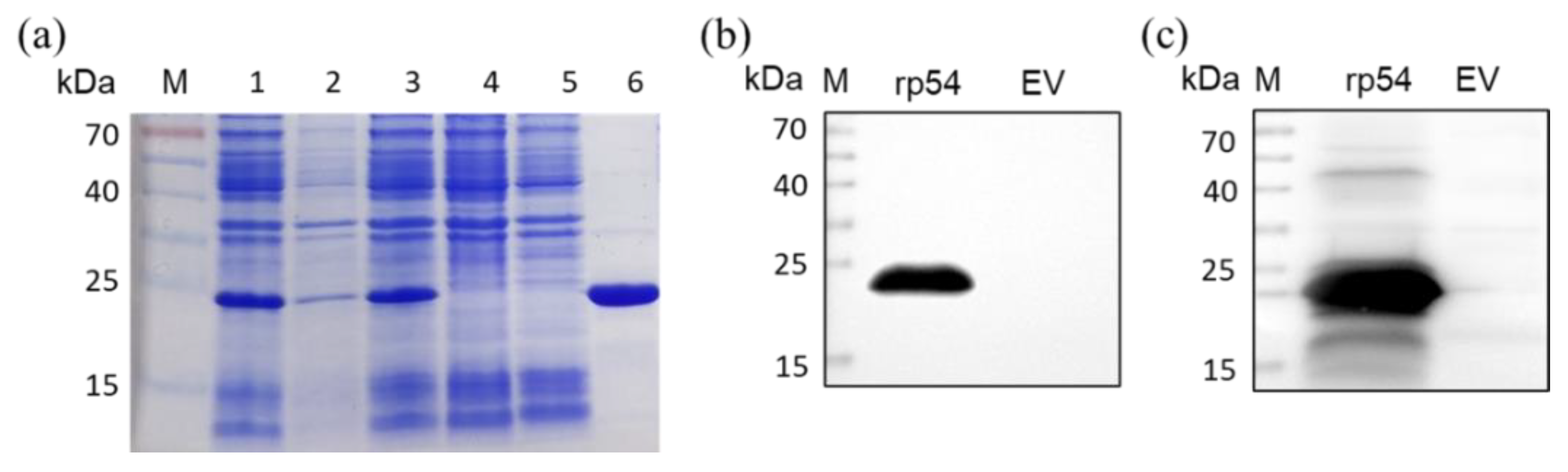
2.3. Expression and Purification of ASFV Recombinant p54 (rp54) Protein
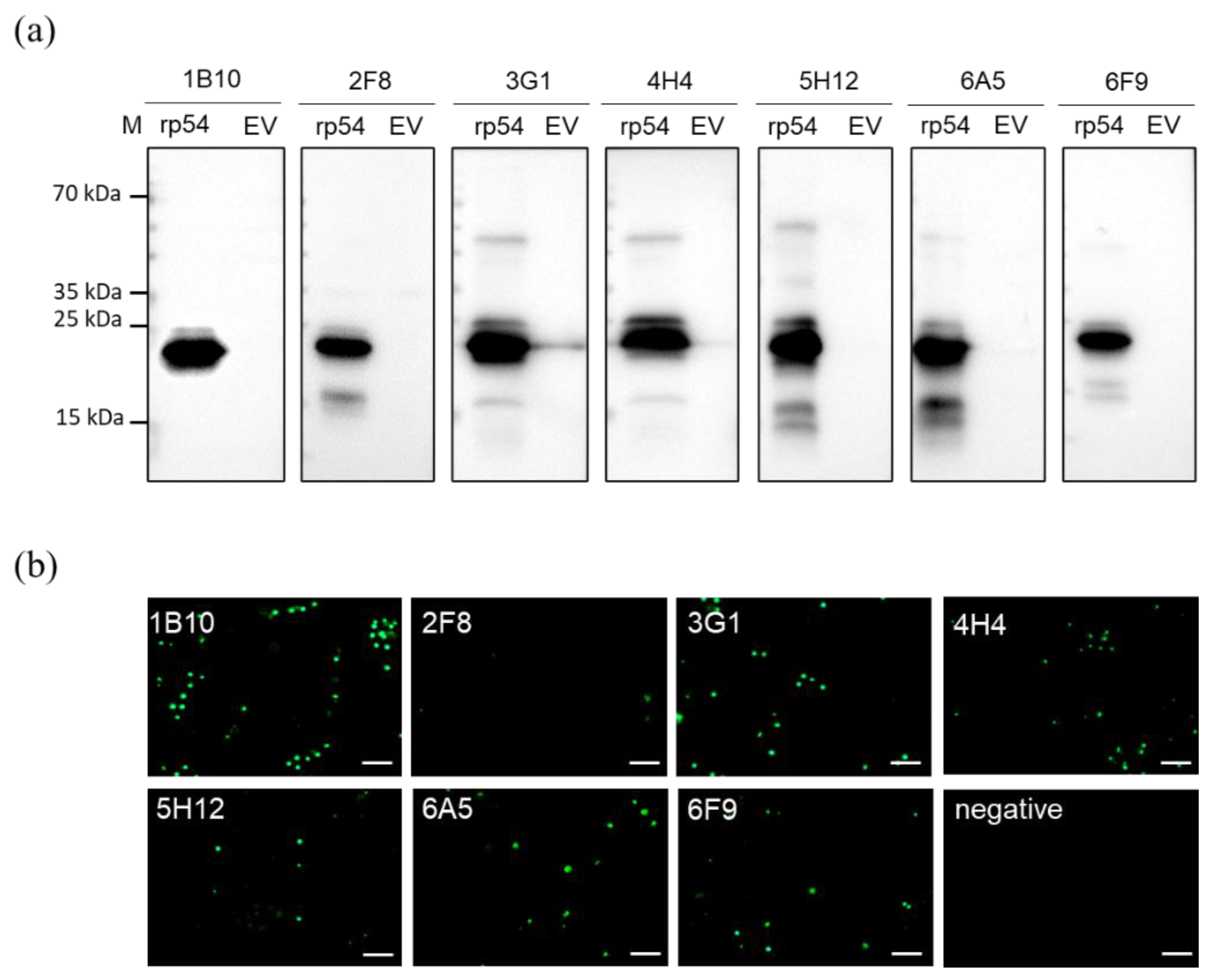
2.4. Rp54 mAbs Production
2.5. Indirect ELISA
2.6. Western Blot Analysis
2.7. Indirect Immunofluorescent Assay
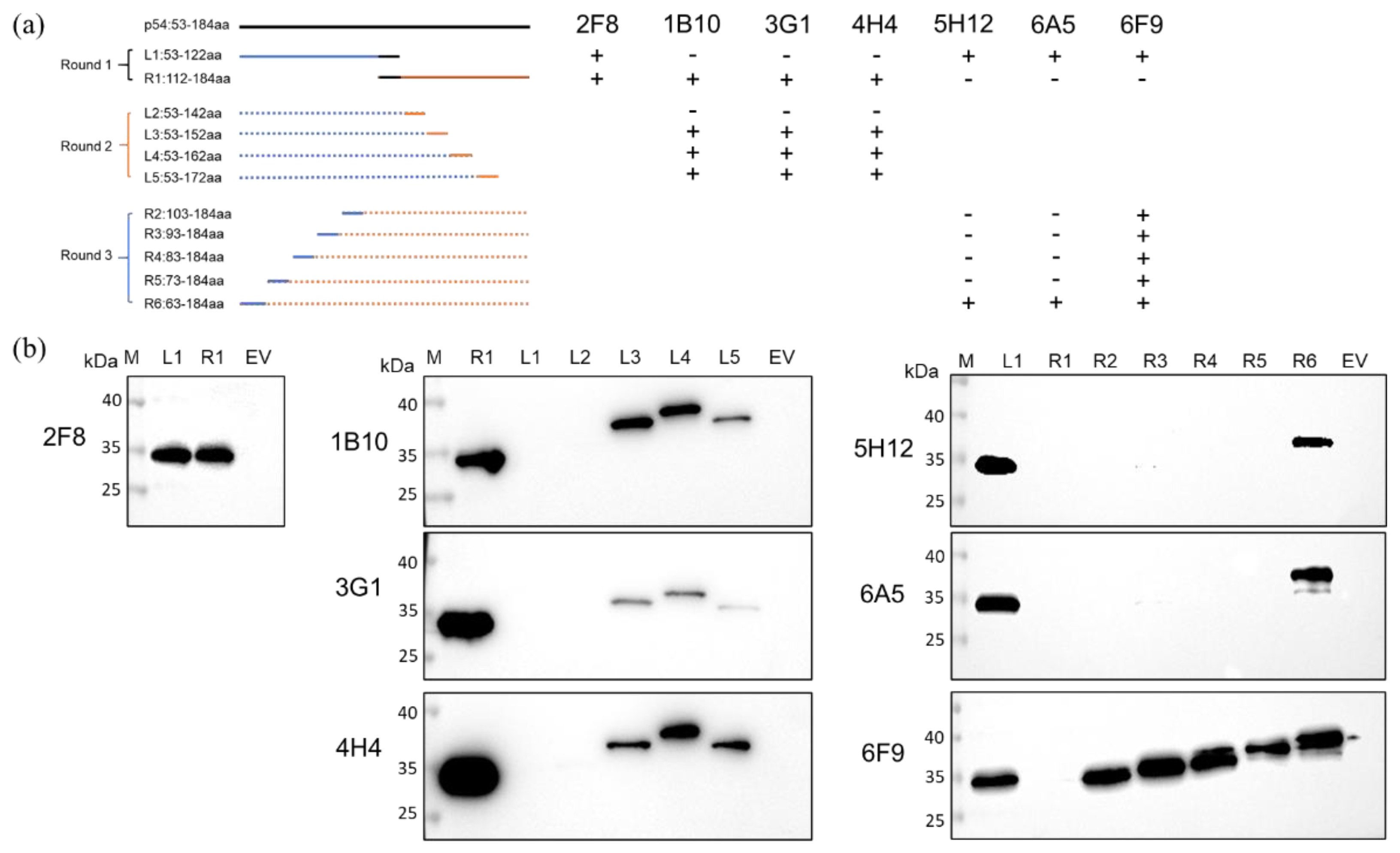
2.8. Antigen Epitope Analysis
2.9. Assessment of the Potential Use of the mAbs for Blocking ELISA
2.10. Development of MAb-Based Blocking ELISA for Detection of African Swine Fever Antibody
2.11. Determination of Cut-Off Value, Diagnostic Sensitivity and Diagnostic Specificity
2.12. Analytical Specificity and Analytical Sensitivity of Blocking ELISA
2.13. Assessment of Blocking ELISA Repeatability and Reproducibility
2.14. Detection of ASFV Antibodies in Pig Sera
3. Results
3.1. Antigen Preparation
3.2. Generation of MAbs against ASFV rp54
3.3. Identification and Conservation Analysis of the rp54 Epitopes
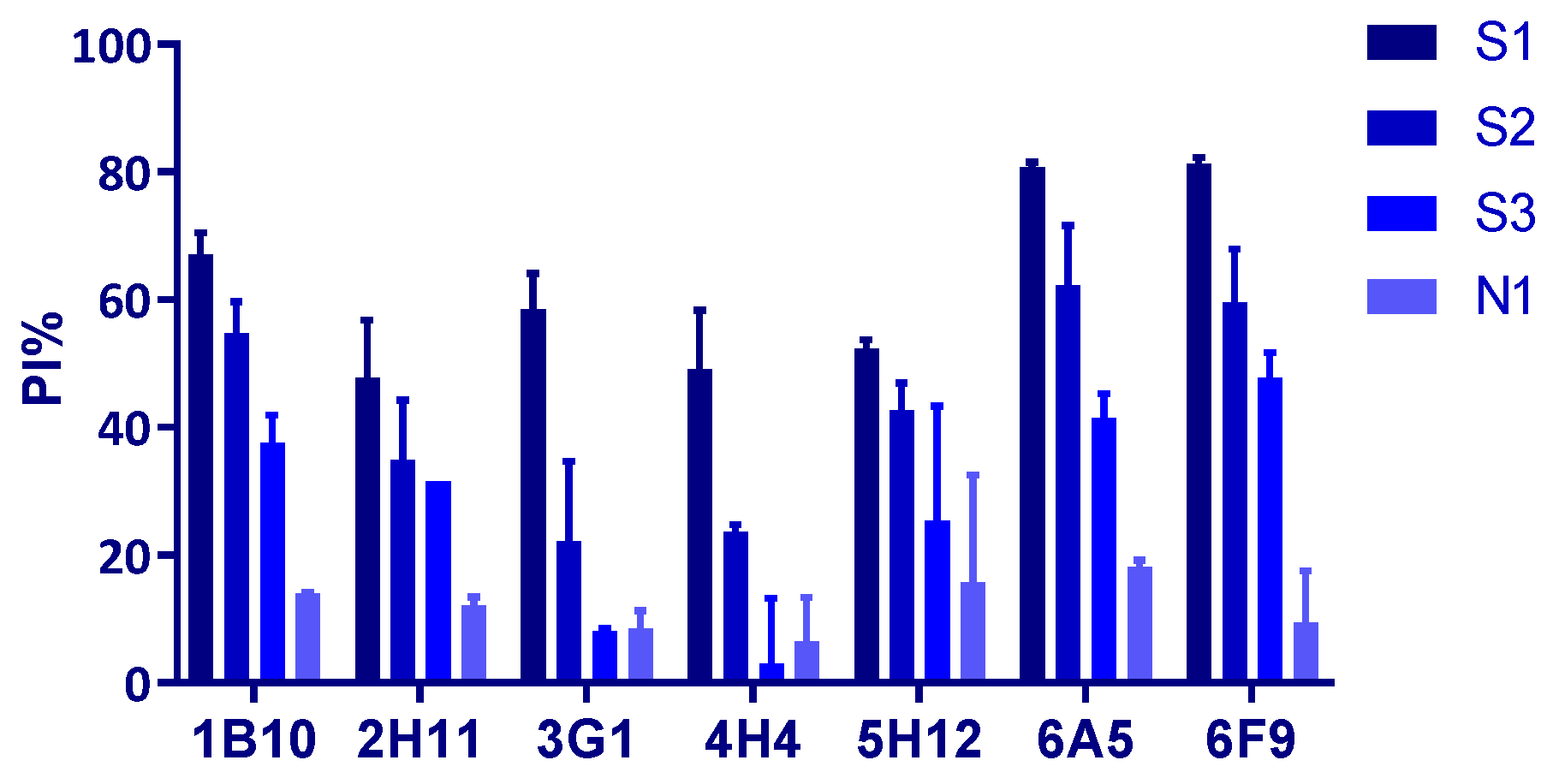
3.4. Assessment of Potential Use of the Rp54 MAbs for Blocking ELISA
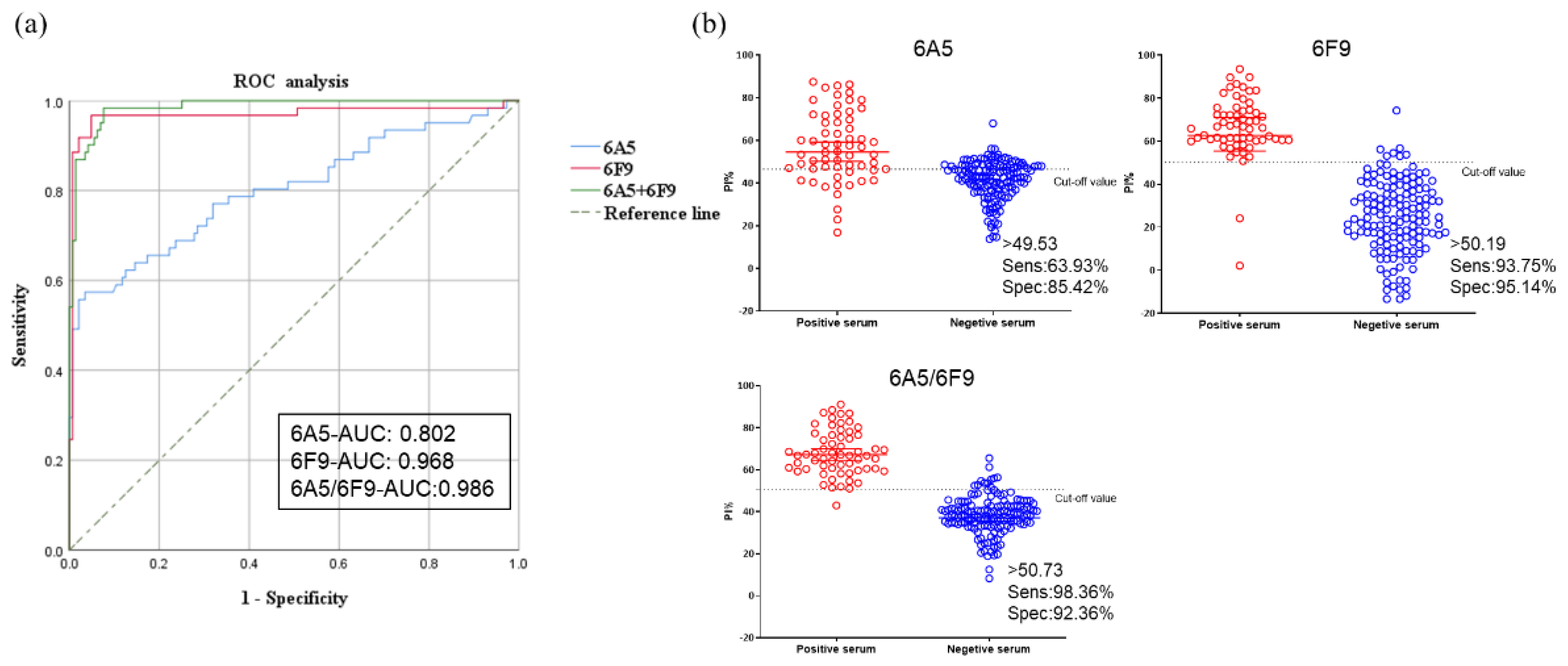
3.5. Standardization and Determining the Cut-Off Value for Blocking ELISA
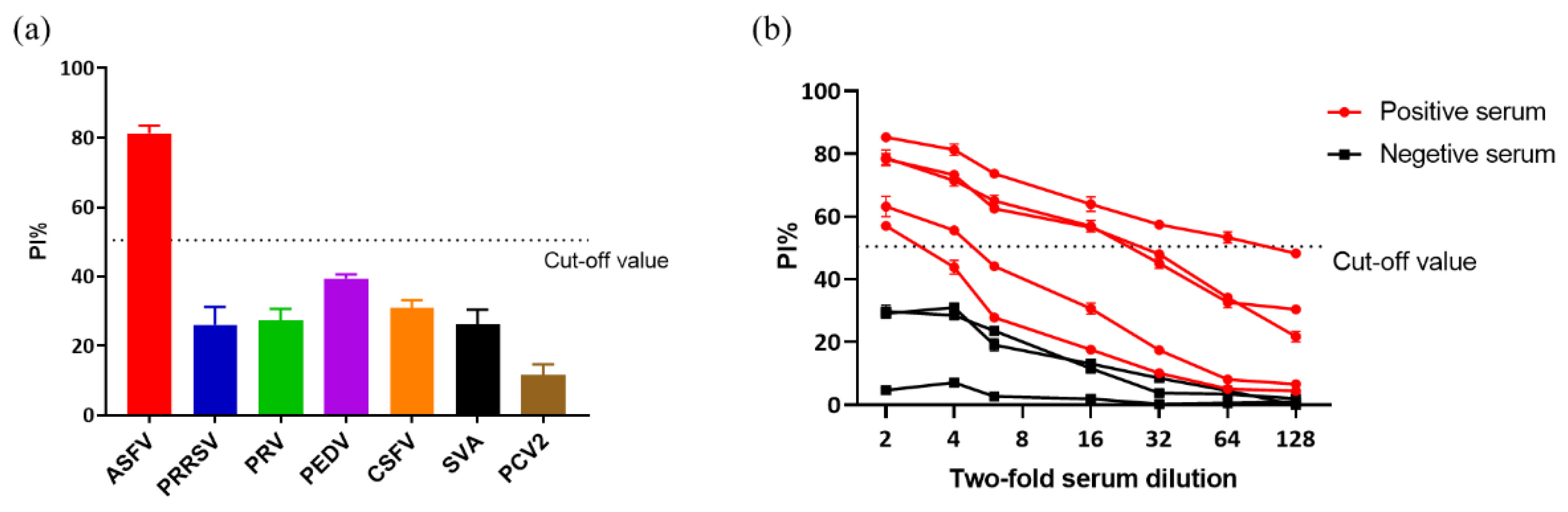
3.6. Analytical Specificity and Analytical Sensitivity of Blocking ELISA
3.7. Assessment of Blocking ELISA Repeatability and Reproductivity
3.8. Presence of ASFV Antibodies in Pig Sera
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Conflicts of Interest
References
- Simões, M.; Freitas, F.B.; Leitao, A.; Martins, C.; Ferreira, F. African swine fever virus replication events and cell nucleus: New insights and perspectives. Virus Res. 2019, 270, 197667. [Google Scholar] [CrossRef] [PubMed]
- Galindo, I.; Alonso, C. African Swine Fever Virus: A Review. Viruses 2017, 9, 103. [Google Scholar] [CrossRef] [PubMed]
- Karger, A.; Pérez-Núñez, D.; Urquiza, J.; Hinojar, P.; Alonso, C.; Freitas, F.B.; Revilla, Y.; Le Potier, M.F.; Montoya, M. An Update on African Swine Fever Virology. Viruses 2019, 11, 864. [Google Scholar] [CrossRef]
- VanderWaal, K.; Deen, J. Global trends in infectious diseases of swine. Proc. Natl. Acad. Sci. USA 2018, 115, 11495–11500. [Google Scholar] [CrossRef]
- Mulumba-Mfumu, L.K.; Saegerman, C.; Dixon, L.K.; Madimba, K.C.; Kazadi, E.; Mukalakata, N.T.; Oura, C.A.; Chenais, E.; Masembe, C.; Ståhl, K.; et al. African swine fever: Update on Eastern, Central and Southern Africa. Transbound. Emerg. Dis. 2019, 66, 1462–1480. [Google Scholar] [CrossRef] [PubMed]
- Ge, S.; Li, J.; Fan, X.; Liu, F.; Li, L.; Wang, Q.; Ren, W.; Bao, J.; Liu, C.; Wang, H.; et al. Molecular Characterization of African Swine Fever Virus, China, 2018. Emerg. Infect. Dis. 2018, 24, 2131–2133. [Google Scholar] [CrossRef] [PubMed]
- Njau, E.P.; Machuka, E.M.; Cleaveland, S.; Shirima, G.M.; Kusiluka, L.J.; Okoth, E.A.; Pelle, R. African Swine Fever Virus (ASFV): Biology, Genomics and Genotypes Circulating in Sub-Saharan Africa. Viruses 2021, 13, 2285. [Google Scholar] [CrossRef]
- Gaudreault, N.N.; Madden, D.W.; Wilson, W.C.; Trujillo, J.D.; Richt, J.A. African Swine Fever Virus: An Emerging DNA Arbovirus. Front. Vet. Sci. 2020, 7, 215. [Google Scholar] [CrossRef]
- Qiu, Z.; Li, Z.; Yan, Q.; Li, Y.; Xiong, W.; Wu, K.; Li, X.; Fan, S.; Zhao, M.; Ding, H.; et al. Development of Diagnostic Tests Provides Technical Support for the Control of African Swine Fever. Vaccines 2021, 9, 343. [Google Scholar] [CrossRef]
- Yu, X.; Zhu, X.; Chen, X.; Li, D.; Xu, Q.; Yao, L.; Sun, Q.; Ghonaim, A.H.; Ku, X.; Fan, S.; et al. Establishment of a Blocking ELISA Detection Method for Against African Swine Fever Virus p30 Antibody. Front. Vet. Sci. 2021, 8, 781373. [Google Scholar] [CrossRef]
- Cubillos, C.; Gómez-Sebastian, S.; Moreno, N.; Nuñez, M.C.; Mulumba-Mfumu, L.K.; Quembo, C.J.; Heath, L.; Etter, E.M.; Jori, F.; Escribano, J.M.; et al. African swine fever virus serodiagnosis: A general review with a focus on the analyses of African serum samples. Virus Res. 2013, 173, 159–167. [Google Scholar] [CrossRef] [PubMed]
- Yuan, F.; Petrovan, V.; Gimenez-Lirola, L.G.; Zimmerman, J.J.; Rowland, R.R.; Fang, Y. Development of a Blocking Enzyme-Linked Immunosorbent Assay for Detection of Antibodies against African Swine Fever Virus. Pathogens 2021, 10, 760. [Google Scholar] [CrossRef] [PubMed]
- Indrabalan, U.B.; Suresh, K.P.; Shivamallu, C.; Patil, S.S. An extensive evaluation of codon usage pattern and bias of structural proteins p30, p54 and, p72 of the African swine fever virus (ASFV). Virusdisease 2021, 32, 810–822. [Google Scholar] [CrossRef]
- Tesfagaber, W.; Wang, L.; Tsegay, G.; Hagoss, Y.T.; Zhang, Z.; Zhang, J.; Huangfu, H.; Xi, F.; Li, F.; Sun, E.; et al. Characterization of Anti-p54 Monoclonal Antibodies and Their Potential Use for African Swine Fever Virus Diagnosis. Pathogens 2021, 10, 178. [Google Scholar] [CrossRef]
- Netherton, C.L.; Goatley, L.C.; Reis, A.L.; Portugal, R.; Nash, R.H.; Morgan, S.B.; Gault, L.; Nieto, R.; Norlin, V.; Gallardo, C.; et al. Identification and Immunogenicity of African Swine Fever Virus Antigens. Front. Immunol. 2019, 10, 1318. [Google Scholar] [CrossRef] [PubMed]
- Gallardo, C.; Reis, A.L.; Kalema-Zikusoka, G.; Malta, J.; Soler, A.; Blanco, E.; Parkhouse, R.M.E.; Leitao, A. Recombinant antigen targets for serodiagnosis of African swine fever. Clin. Vaccine Immunol. 2009, 16, 1012–1020. [Google Scholar] [CrossRef]
- Barderas, M.G.; Rodriguez, F.; Gomez-Puertas, P.; Aviles, M.; Beitia, F.; Alonso, C.; Escribano, J.M. Antigenic and immunogenic properties of a chimera of two immunodominant African swine fever virus proteins. Arch. Virol. 2001, 146, 1681–1691. [Google Scholar] [CrossRef]
- Kollnberger, S.D.; Gutierrez-Castañeda, B.; Foster-Cuevas, M.; Corteyn, A.; Parkhouse, R.M.E. Identification of the principal serological immunodeterminants of African swine fever virus by screening a virus cDNA library with antibody. J. Gen. Virol. 2002, 83, 1331–1342. [Google Scholar] [CrossRef]
- Reis, A.L.; Parkhouse, R.M.E.; Penedos, A.R.; Martins, C.; Leitao, A. Systematic analysis of longitudinal serological responses of pigs infected experimentally with African swine fever virus. J. Gen. Virol. 2007, 88, 2426–2434. [Google Scholar] [CrossRef]
- Zhao, D.; Liu, R.; Zhang, X.; Li, F.; Wang, J.; Zhang, J.; Liu, X.; Wang, L.; Zhang, J.; Wu, X.; et al. Replication and virulence in pigs of the first African swine fever virus isolated in China. Emerg. Microbes Infect. 2019, 8, 438–447. [Google Scholar] [CrossRef]
- Groth, S.F.D.S.; Scheidegger, D. Production of monoclonal antibodies: Strategy and tactics. J. Immunol. Methods 1980, 35, 1–21. [Google Scholar] [CrossRef]
- Wang, L.; Mi, S.; Madera, R.; Ganges, L.; Borca, M.V.; Ren, J.; Cunningham, C.; Cino-Ozuna, A.G.; Li, H.; Tu, C.; et al. A neutralizing monoclonal antibody-based competitive ELISA for classical swine fever C-strain post-vaccination monitoring. BMC Vet. Res. 2020, 16, 14. [Google Scholar] [CrossRef]
- Gaudreault, N.N.; Richt, J.A. Subunit Vaccine Approaches for African Swine Fever Virus. Vaccines 2019, 7, 56. [Google Scholar] [CrossRef] [PubMed]
- Dixon, L.K.; Chapman, D.A.; Netherton, C.L.; Upton, C. African swine fever virus replication and genomics. Virus Res. 2013, 173, 3–14. [Google Scholar] [CrossRef] [PubMed]
- Qu, H.; Ge, S.; Zhang, Y.; Wu, X.; Wang, Z. A systematic review of genotypes and serogroups of African swine fever virus. Virus Genes 2022, 58, 77–87. [Google Scholar] [CrossRef] [PubMed]
- Sun, E.; Huang, L.; Zhang, X.; Zhang, J.; Shen, D.; Zhang, Z.; Wang, Z.; Huo, H.; Wang, W.; Huangfu, H.; et al. Genotype I African swine fever viruses emerged in domestic pigs in China and caused chronic infection. Emerg. Microbes Infect. 2021, 10, 2183–2193. [Google Scholar] [CrossRef]
- Sánchez, E.G.; Pérez-Núñez, D.; Revilla, Y. Development of vaccines against African swine fever virus. Virus Res. 2019, 265, 150–155. [Google Scholar] [CrossRef] [PubMed]
- Montaner-Tarbes, S.; Pujol, M.; Jabbar, T.; Hawes, P.; Chapman, D.; Del Portillo, H.; Fraile, L.; Sánchez-Cordón, P.J.; Dixon, L.; Montoya, M. Serum-Derived Extracellular Vesicles from African Swine Fever Virus-Infected Pigs Selectively Recruit Viral and Porcine Proteins. Viruses 2019, 11, 882. [Google Scholar] [CrossRef]
- Izzati, U.Z.; Inanaga, M.; Hoa, N.T.; Nueangphuet, P.; Myint, O.; Truong, Q.L.; Lan, N.T.; Norimine, J.; Hirai, T.; Yamaguchi, R. Pathological investigation and viral antigen distribution of emerging African swine fever in Vietnam. Transbound. Emerg. Dis. 2021, 68, 2039–2050. [Google Scholar] [CrossRef]
- Sánchez-Vizcaíno, J.M.; Mur, L.; Gomez-Villamandos, J.C.; Carrasco, L. An update on the epidemiology and pathology of African swine fever. J. Comp. Pathol. 2015, 152, 9–21. [Google Scholar] [CrossRef]
- Henriques, A.M.; Fagulha, T.; Barros, S.C.; Ramos, F.; Duarte, M.; Luís, T.; Fevereiro, M. Development and validation of a blocking ELISA test for the detection of avian influenza antibodies in poultry species. J. Virol. Methods 2016, 236, 47–53. [Google Scholar] [CrossRef] [PubMed]
- Zhou, D.; Pei, C.; Yang, K.; Ye, J.; Wan, S.; Li, Q.; Zhang, L.; Chen, H.; Cao, S.; Song, Y. Development and application of a monoclonal-antibody-based blocking ELISA for detection of Japanese encephalitis virus NS1 antibodies in swine. Arch. Virol. 2019, 164, 1535–1542. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.P.; Gao, X.; An, Q.; Sun, Z.; Wang, H.B. Ecological niche modeling based on ensemble algorithms to predicting current and future potential distribution of African swine fever virus in China. Sci. Rep. 2022, 12, 15614. [Google Scholar] [CrossRef] [PubMed]

| Blocking ELISA Method | Optimized Conditions | Antigen Coating | Blocking Conditions | Serum to be Tested | HRP Labeled Antibody | TMB Reaction Time |
|---|---|---|---|---|---|---|
| 6A5 | Concentration/dilution | 0.25 μg/mL | 1% BSA | 1:1 | 1:3000 (0.77 μg/mL) | |
| Reaction conditions | 4 °C 12 h | 37 °C 1 h | 37 °C 1 h | 37 °C 30 min | 37 °C 20 min | |
| 6F9 | Concentration/dilution | 0.25 μg/mL | 1% BSA | 1:1 | 1:2000 (0.95 μg/mL) | |
| Reaction conditions | 4 °C 12 h | 37 °C 1 h | 37 °C 1 h | 37 °C 40 min | 37 °C 15 min | |
| 6A5+6F9 | Concentration/dilution | 0.5 μg/mL | 1% BSA | 1:1 | 6A5—1:3000 mix with 6F9—1:2000 | |
| Reaction conditions | 4 °C 12 h | 37 °C 1 h | 37 °C 1 h | 37 °C 30 min | 37 °C 20 min |
| Monoclonal Antibodies | |||||||
|---|---|---|---|---|---|---|---|
| 1B10 | 2F8 | 3G1 | 4H4 | 5H12 | 6A5 | 6F9 | |
| Ig subclass | IgG2a | IgG1 | IgG1 | IgG1 | IgG1 | IgG2a | IgG2a |
| Light chain type | κ | κ | κ | κ | κ | κ | κ |
| Samples | Intra-Assay | Inter-Assay | ||||
|---|---|---|---|---|---|---|
| Mean PI | SD | CV | Mean PI | SD | CV | |
| Positive1 | 71.13% | 0.001 | 0.17% | 72.36% | 0.011 | 1.54% |
| Positive2 | 52.51% | 0.008 | 1.60% | 52.65% | 0.013 | 2.50% |
| Positive3 | 51.40% | 0.004 | 0.78% | 52.11% | 0.008 | 1.56% |
| Positive4 | 59.42% | 0.036 | 6.04% | 60.67% | 0.027 | 4.48% |
| Positive5 | 65.74% | 0.034 | 5.18% | 68.28% | 0.024 | 3.54% |
| Negetive1 | 21.34% | 0.020 | 9.46% | 18.69% | 0.004 | 2.20% |
| Negetive2 | 17.13% | 0.005 | 2.85% | 16.45% | 0.014 | 8.67% |
| Negetive3 | 22.33% | 0.013 | 5.90% | 20.47% | 0.019 | 9.50% |
| Negetive4 | 16.02% | 0.016 | 9.92% | 13.86% | 0.008 | 5.44% |
| Negetive5 | 22.07% | 0.018 | 8.03% | 24.35% | 0.016 | 6.75% |
| Province | Serum Samples | Positive Samples | Negative Samples | Positive Rate% |
|---|---|---|---|---|
| Henan | 92 | 4 | 88 | 4.3 |
| Zhejiang | 32 | 0 | 32 | 0.0 |
| Jiangsu | 106 | 4 | 102 | 3.8 |
| Shandong | 100 | 20 | 80 | 20.0 |
| Total | 330 | 28 | 302 | 8.5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gao, Y.; Xia, T.; Bai, J.; Zhang, L.; Zheng, H.; Jiang, P. Preparation of Monoclonal Antibodies against the Viral p54 Protein and a Blocking ELISA for Detection of the Antibody against African Swine Fever Virus. Viruses 2022, 14, 2335. https://doi.org/10.3390/v14112335
Gao Y, Xia T, Bai J, Zhang L, Zheng H, Jiang P. Preparation of Monoclonal Antibodies against the Viral p54 Protein and a Blocking ELISA for Detection of the Antibody against African Swine Fever Virus. Viruses. 2022; 14(11):2335. https://doi.org/10.3390/v14112335
Chicago/Turabian StyleGao, Yanni, Tingting Xia, Juan Bai, Lujie Zhang, Haixue Zheng, and Ping Jiang. 2022. "Preparation of Monoclonal Antibodies against the Viral p54 Protein and a Blocking ELISA for Detection of the Antibody against African Swine Fever Virus" Viruses 14, no. 11: 2335. https://doi.org/10.3390/v14112335
APA StyleGao, Y., Xia, T., Bai, J., Zhang, L., Zheng, H., & Jiang, P. (2022). Preparation of Monoclonal Antibodies against the Viral p54 Protein and a Blocking ELISA for Detection of the Antibody against African Swine Fever Virus. Viruses, 14(11), 2335. https://doi.org/10.3390/v14112335





