Antiviral Activities of Asarones and Rhizomes of Acorus gramineus on Murine Norovirus
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of Cells, MNV, and AGR Extract
2.2. Cell Viability Assay
2.3. Plaque Assay
2.4. Expression and Purification of HuNV GII.4 P domain
2.5. Enzyme-Linked Immunosorbent Assay (ELISA)
2.6. Differential Scanning Fluorimetry (DSF)
2.7. Glutathione (GSH) Assay
2.8. Ferric Reducing Antioxidant Power Assay
2.9. DPPH Radical Scavenging Activity Assay
2.10. Antiviral Effects of AGR Extract, α-Asarone, and β-Asarone under Simulated Digestive Conditions
2.11. HPLC Analysis of AGR Extract
2.12. Data Analysis
3. Results
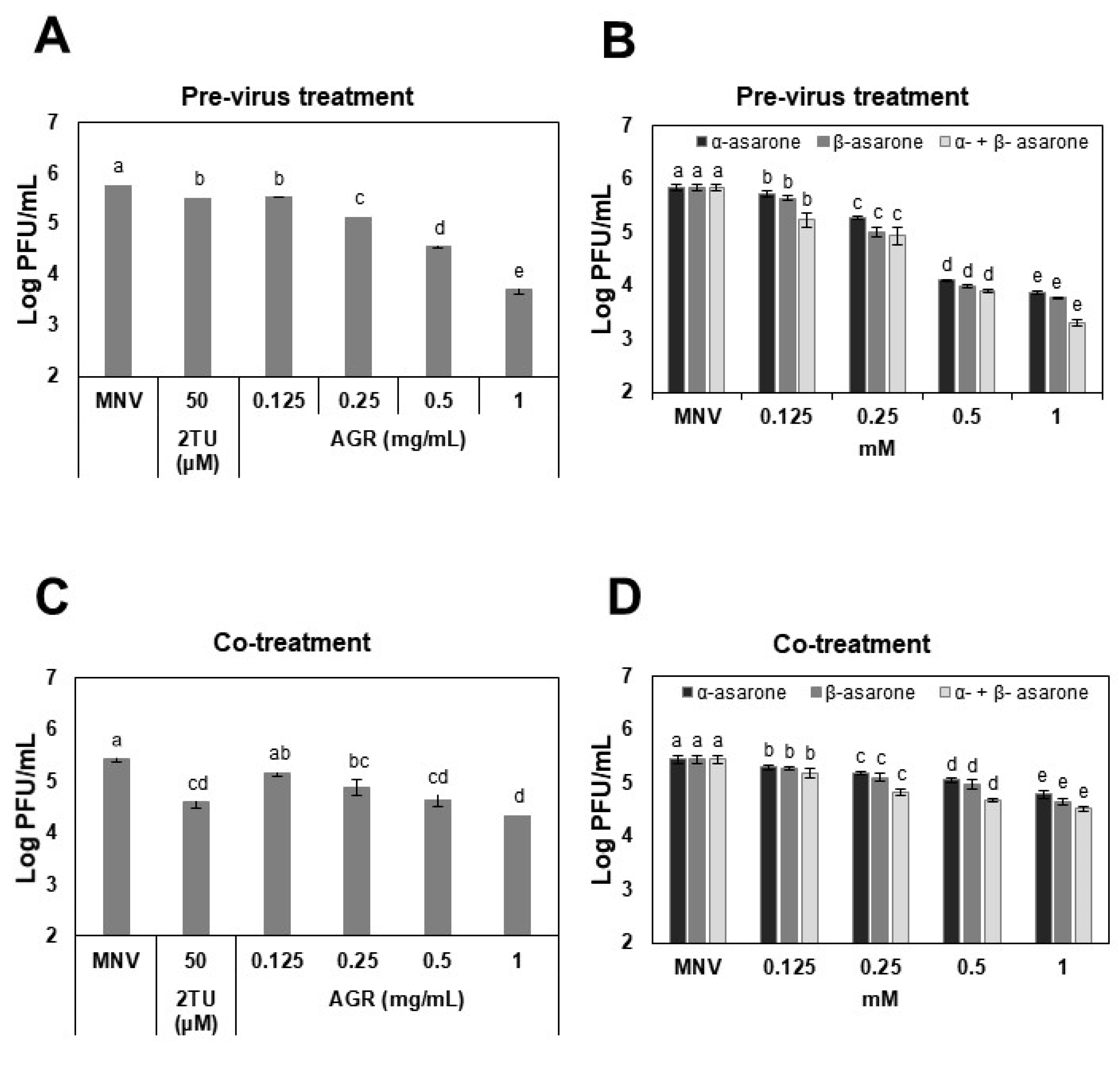
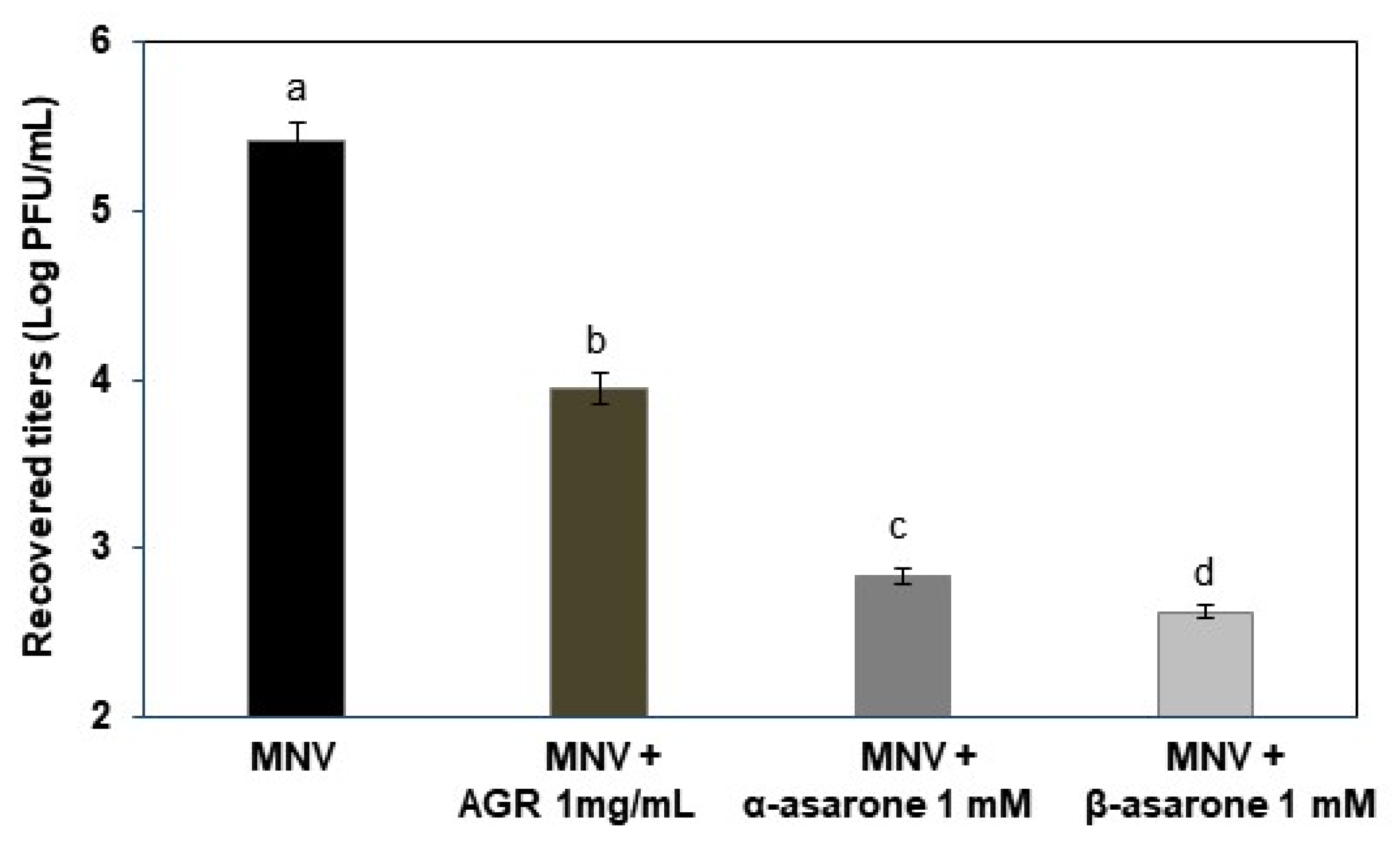
3.1. AGR Extract, α-Asarone, and β-Asarone Show Antiviral Effects against MNV
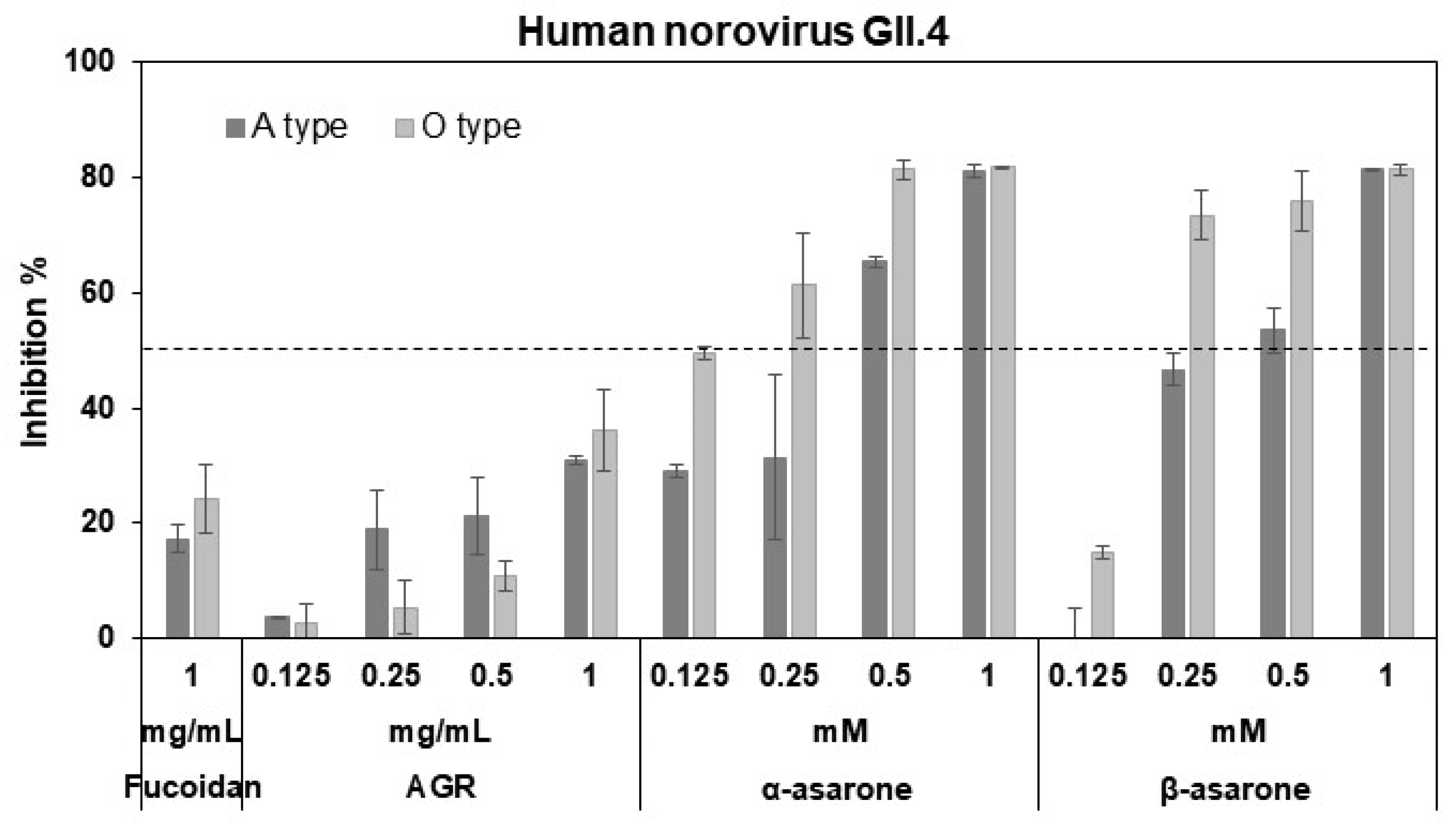
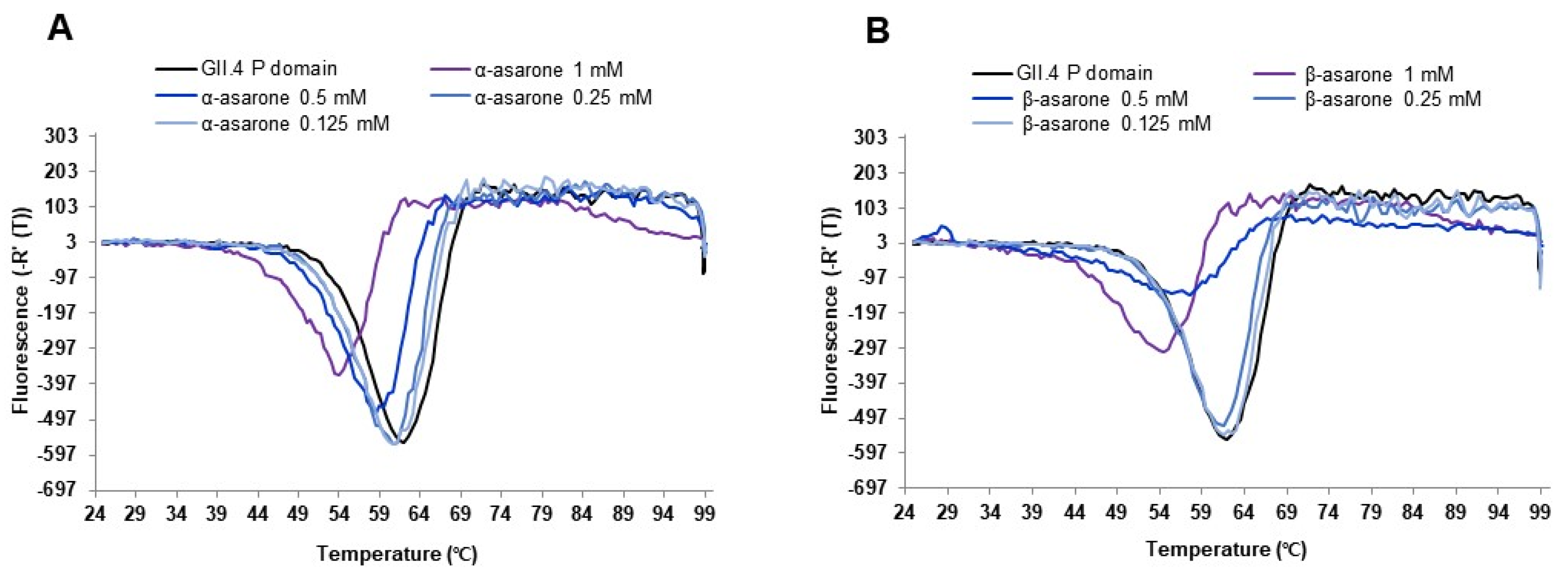
3.2. AGR Extract, α-Asarone, and β-Asarone Inhibit the Binding of HuNV P Domain to a Receptor
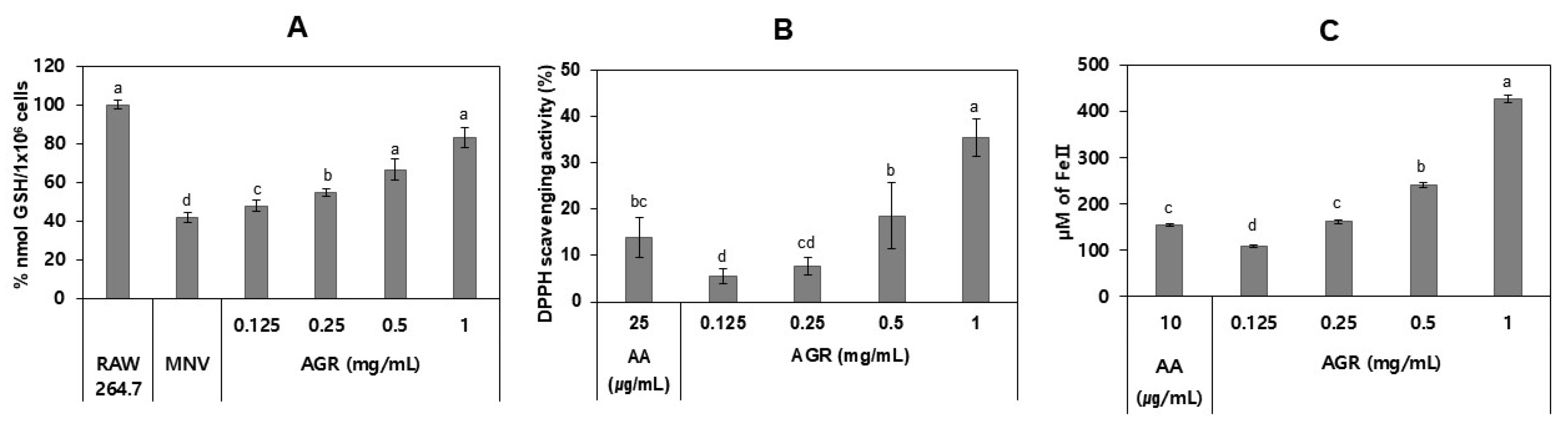
3.3. Antioxidant Effects of AGR Extract in MNV-Infected Cells
3.4. AGR Extract, α-Asarone, and β-Asarone Show Antiviral Effects under Simulated Digestive Conditions
4. Discussion
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Bartsch, S.M.; Lopman, B.A.; Ozawa, S.; Hall, A.J.; Lee, B.Y. Global Economic Burden of Norovirus Gastroenteritis. PLoS ONE 2016, 11, e0151219. [Google Scholar] [CrossRef] [PubMed]
- Bartsch, S.M.; O’Shea, K.J.; Lee, B.Y. The Clinical and Economic Burden of Norovirus Gastroenteritis in the United States. J. Infect. Dis. 2020, 222, 1910–1919. [Google Scholar] [CrossRef] [PubMed]
- Chhabra, P.; de Graaf, M.; Parra, G.I.; Chan, M.C.; Green, K.; Martella, V.; Wang, Q.; White, P.A.; Katayama, K.; Vennema, H.; et al. Updated classification of norovirus genogroups and genotypes. J. Gen. Virol. 2019, 100, 1393–1406. [Google Scholar] [CrossRef]
- Hou, W.; Lv, L.; Wang, Y.; Xing, M.; Guo, Y.; Xie, D.; Wei, X.; Zhang, X.; Liu, H.; Ren, J.; et al. 6-Valent virus-like particle-based vaccine induced potent and sustained immunity against noroviruses in mice. Front Immunol. 2022, 13, 906275. [Google Scholar] [CrossRef] [PubMed]
- Petronella, N.; Ronholm, J.; Suresh, M.; Harlow, J.; Mykytczuk, O.; Corneau, N.; Bidawid, S.; Nasheri, N. Genetic characterization of norovirus GII.4 variants circulati ng in Canada using a metagenomic technique. BMC Infect. Dis. 2018, 18, 521. [Google Scholar] [CrossRef]
- Huang, P.; Farkas, T.; Marionneau, S.; Zhong, W.; Ruvoen-Clouet, N.; Morrow, A.L.; Altaye, M.; Pickering, L.K.; Newburg, D.S.; LePendu, J.; et al. Noroviruses bind to human ABO, Lewis, and secretor histo-blood group antigens: Identification of 4 distinct strain-specific patterns. J. Infect. Dis. 2003, 188, 19–31. [Google Scholar] [CrossRef]
- Thorne, L.G.; Goodfellow, I.G. Norovirus gene expression and replication. J. Gen. Virol. 2014, 95, 278–291. [Google Scholar] [CrossRef]
- Conley, M.J.; McElwee, M.; Azmi, L.; Gabrielsen, M.; Byron, O.; Goodfellow, I.G.; Bhella, D. Calicivirus VP2 forms a portal-like assembly following receptor engagement. Nature 2019, 565, 377–381. [Google Scholar] [CrossRef]
- Marionneau, S.; Cailleau-Thomas, A.; Rocher, J.; Le Moullac-Vaidye, B.; Ruvoen, N.; Clement, M.; Le Pendu, J. ABH and Lewis histo-blood group antigens, a model for the meaning of oligosaccharide diversity in the face of a changing world. Biochimie 2001, 83, 565–573. [Google Scholar] [CrossRef]
- Jones, M.K.; Watanabe, M.; Zhu, S.; Graves, C.L.; Keyes, L.R.; Grau, K.R.; Gonzalez-Hernandez, M.B.; Iovine, N.M.; Wobus, C.E.; Vinje, J.; et al. Enteric bacteria promote human and mouse norovirus infection of B cells. Science 2014, 346, 755–759. [Google Scholar] [CrossRef]
- Ettayebi, K.; Crawford, S.E.; Murakami, K.; Broughman, J.R.; Karandikar, U.; Tenge, V.R.; Neill, F.H.; Blutt, S.E.; Zeng, X.L.; Qu, L.; et al. Replication of human noroviruses in stem cell-derived human enteroids. Science 2016, 353, 1387–1393. [Google Scholar] [CrossRef] [PubMed]
- Belliot, G.; Lavaux, A.; Souihel, D.; Agnello, D.; Pothier, P. Use of murine norovirus as a surrogate to evaluate resistance of human norovirus to disinfectants. Appl. Environ. Microbiol. 2008, 74, 3315–3318. [Google Scholar] [CrossRef] [PubMed]
- Liao, J.F.; Huang, S.Y.; Jan, Y.M.; Yu, L.L.; Chen, C.F. Central inhibitory effects of water extract of Acori graminei rhizoma in mice. J. Ethnopharmacol. 1998, 61, 185. [Google Scholar] [CrossRef]
- Park, C.H.; Kim, K.H.; Lee, I.K.; Lee, S.Y.; Choi, S.U.; Lee, J.H.; Lee, K.R. Phenolic constituents of Acorus gramineus. Arch. Pharm. Res. 2011, 34, 1289–1296. [Google Scholar] [CrossRef] [PubMed]
- Ryuk, J.A.; Kim, Y.S.; Lee, H.W.; Ko, B.S. Identification of Acorus gramineus, A. calamus, and A. tatarinowii using sequence characterized amplified regions (SCAR) primers for monitoring of Acori graminei rhizoma in Korean markets. Int. J. Clin. Exp. Med. 2014, 7, 2488–2496. [Google Scholar]
- Lee, J.Y.; Lee, J.Y.; Yun, B.S.; Hwang, B.K. Antifungal activity of beta-asarone from rhizomes of Acorus gramineus. J. Agric. Food Chem. 2004, 52, 776–780. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Gao, X.; Liu, Q.; Zeng, L.; Zhang, K.; Mu, K.; Zhang, D.; Zou, H.; Wu, N.; Ou, J.; et al. Alpha-asarone improves cognitive function of aged rats by alleviating neuronal excitotoxicity via GABAA receptors. Neuropharmacology 2020, 162, 107843. [Google Scholar] [CrossRef]
- Kim, K.H.; Moon, E.; Kang, K.S.; Kim, S.Y.; Choi, S.U.; Lee, K.R. Alkaloids from Acorus gramineus Rhizomes and their Biological Activity. J. Braz. Chem. Soc. 2015, 26, 3–8. [Google Scholar]
- Han, Y.; Wang, N.; Kang, J.; Fang, Y. beta-Asarone improves learning and memory in Aβ1-42-induced Alzheimer’s disease rats by regulating PINK1-Parkin-mediated mitophagy. Metab. Brain Dis. 2020, 35, 1109–1117. [Google Scholar] [CrossRef]
- Lim, H.; Lee, S.Y.; Lee, K.R.; Kim, Y.S.; Kim, H.P. The Rhizomes of Acorus gramineus and the Constituents Inhibit Allergic Response In vitro and In vivo. Biomol. Ther. 2012, 20, 477–481. [Google Scholar] [CrossRef][Green Version]
- Lim, C.Y.; Kim, H.; Chung, M.S. Mori Cortex Radicis extract inhibits human norovirus surrogate in simulated digestive conditions. Food Sci. Biotechnol. 2021, 30, 1243–1248. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.C.; Liu, X.Q.; Wang, W.; Shen, X.F.; Che, H.L.; Guo, Y.Y.; Zhao, M.G.; Chen, J.Y.; Luo, W.J. Involvement of microglia activation in the lead induced long-term potentiation impairment. PLoS ONE 2012, 7, e43924. [Google Scholar] [CrossRef] [PubMed]
- Bae, G.; Kim, J.; Kim, H.; Sock, J.H.; Lee, D.B.; Kim, K.H.; Chung, M.S. Inactivation of norovirus surrogates by kimchi fermentation in the presence of black raspberry. Food Control 2018, 91, 390–396. [Google Scholar] [CrossRef]
- Kim, H.; Lim, C.Y.; Lee, D.B.; Seok, J.H.; Kim, K.H.; Chung, M.S. Inhibitory effects of Laminaria japonica fucoidans against noroviruses. Viruses 2020, 12, 997. [Google Scholar] [CrossRef] [PubMed]
- Oh, M.; Lee, J.H.; Bae, S.Y.; Seok, J.H.; Kim, S.; Chung, Y.B.; Han, K.R.; Kim, K.H.; Chung, M.S. Protective effects of red wine and resveratrol for foodborne virus surrogates. Food Control 2015, 47, 502–509. [Google Scholar] [CrossRef]
- Rahman, I.; Kode, A.; Biswas, S.K. Assay for quantitative determination of glutathione and glutathione disulfide levels using enzymatic recycling method. Nat. Protoc. 2006, 1, 3159–3165. [Google Scholar] [CrossRef]
- Benzie, I.F.; Strain, J.J. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: The FRAP assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef]
- Shimada, K.; Fujikawa, K.; Yahara, K.; Nakamura, T. Antioxidative properties of xanthan on the autoxidation of soybean oil in cyclodextrin emulsion. J. Agric. Food Chem. 1992, 40, 945–948. [Google Scholar] [CrossRef]
- Minekus, M.; Alminger, M.; Alvito, P.; Balance, S.; Bohn, T.; Bourlieu, C.; Carrière, F.; Boutrou, R.; Corredig, M.; Dupont, D.; et al. A standardised static in vitro digestion method suitable for food—An international consensus. Food Funct. 2014, 5, 1113–1124. [Google Scholar] [CrossRef]
- Eriksen, J.N.; Luu, A.Y.; Dragsted, L.O.; Arrigoni, E. Adaption of an in vitro digestion method to screen carotenoid liberation and in vitro accessibility from differently processed spinach preparations. Food Chem. 2017, 224, 407–413. [Google Scholar] [CrossRef]
- National Institute of Food and Drug Safety Evaluation, South Korea. Discrimination of Acori graminei Rhizoma; National Institute of Food and Drug Safety Evaluation, South Korea: Cheongju City, Korea, 2018; pp. 23–24.
- Ahmed, S.M.; Hall, A.J.; Robinson, A.E.; Verhoef, L.; Premkumar, P.; Parashar, U.D.; Koopmans, M.; Lopman, B.A. Global prevalence of norovirus in cases of gastroenteritis: A systematic review and meta-analysis. Lancet Infect. Dis. 2014, 14, 725–730. [Google Scholar] [CrossRef]
- Falcó, I.; Randazzo, W.; Rodríguez-Díaz, J.; Gozalbo-Rovira, R.; Luque, D.; Aznar, R.; Sánchez, G. Antiviral activity of aged green tea extract in model food systems and under gastric conditions. Int. J. Food Microbiol. 2019, 292, 101–106. [Google Scholar] [CrossRef]
- Joshi, S.S.; Howell, A.B.; D’Souza, D.H. Blueberry proanthocyanidins against human norovirus surrogates in model foods and under simulated gastric conditions. Food Microbiol. 2017, 63, 263–267. [Google Scholar] [CrossRef] [PubMed]
- Xue, Z.; Han, Q.; Huang, P.; Jiang, X.; Tan, M.; Zhao, Y.; Li, N.; Zhang, R. Characterization of Functional Components in Bovine Colostrum That Inhibit Norovirus Capsid Protruding Domains Interacting with HBGA Ligands. Pathogens 2021, 10, 857. [Google Scholar] [CrossRef] [PubMed]
- Tan, M.; Hegde, R.S.; Jiang, X. The P Domain of Norovirus Capsid Protein Forms Dimer and Binds to Histo-Blood Group Antigen Receptors. J. Virol. 2004, 78, 6233–6242. [Google Scholar] [CrossRef] [PubMed]
- Carmona-Vicente, N.; Vila-Vicent, S.; Allen, D.; Gozalbo-Rovira, R.; Iturriza-Gómara, M.; Buesa, J.; Rodríguez-Díaz, J. Characterization of a Novel Conformational GII.4 Norovirus Epitope: Implications for Norovirus-Host Interactions. J. Virol. 2016, 90, 7703–7714. [Google Scholar] [CrossRef] [PubMed]
- Haddadin, Z.; Batarseh, E.; Hamdan, L.; Stewart, L.S.; Piya, B.; Rahman, H.; Spieker, A.J.; Chappell, J.; Wikswo, M.E.; Dunn, J.R.; et al. Characteristics of GII.4 Norovirus versus Other Genotypes in Sporadic Pediatric Infections in Davidson County, Tennessee, USA. Clin. Infect. Dis. 2021, 73, e1525–e1531. [Google Scholar] [CrossRef]
- Rosa, N.; Ristic, M.; Seabrook, S.A.; Lovell, D.; Lucent, D.; Newman, J.J. Meltdown: A Tool to Help in the Interpretation of Thermal Melt Curves Acquired by Differential Scanning Fluorimetry. J. Biomol. Screen. 2015, 20, 898–905. [Google Scholar] [CrossRef]
- Ministry of Food and Drug Safety, South Korea. Possible to Distinguish Whether Jujube (Zizyphus jujuba) and Grass-Leaf Sweet Flag (Acorus gramineus) Real or Fake; Ministry of Food and Drug Safety, South Korea: Seoul, Korea, 2020; pp. 1–2.
- Uebel, T.; Hermes, L.; Haupenthal, S.; Müller, L.; Esselen, M. Alpha-Asarone, beta-asarone, and gamma-asarone: Current status of toxicological evaluation. J. Appl. Toxicol. 2021, 41, 1166–1179. [Google Scholar] [CrossRef]
- Wang, N.; Wang, H.; Li, L.; Li, Y.; Zhang, R. Beta-Asarone Inhibits Amyloid-beta by Promoting Autophagy in a Cell Model of Alzheimer’s Disease. Front. Pharmacol. 2020, 10, 1529. [Google Scholar] [CrossRef]
- European-Commission. Opinion of the Scientific Committee on Food on the Presence of β-Asarone in Flavourings and Other Food Ingredients with Flavouring Properties; Scientific Committee on Food: Brussel, Belgium, 2002; pp. 1–15.
- Amatore, D.; Sgarbanti, R.; Aquilano, K.; Baldelli, S.; Limongi, D.; Civitelli, L.; Nencioni, L.; Garaci, E.; Ciriolo, M.R.; Palamara, A.T. Influenza virus replication in lung epithelial cells depends on redox-sensitive pathways activated by NOX4-derived ROS. Cell. Microbiol. 2015, 17, 131–145. [Google Scholar] [CrossRef] [PubMed]
- Di Sotto, A.; Checconi, P.; Celestino, I.; Locatelli, M.; Carissimi, S.; De Angelis, M.; Rossi, V.; Limongi, D.; Toniolo, C.; Martinoli, L.; et al. Antiviral and antioxidant activity of a hydroalcoholic extract from Humulus lupulus L. Oxid. Med. Cell. Longev. 2018, 2018, 5919237. [Google Scholar] [PubMed]
- Kim, H.; Lim, C.; Chung, M.S. Magnolia officinalis and its honokiol and magnolol constituents inhibit human norovirus surrogates. Foodborne Pathog. Dis. 2021, 18, 24–30. [Google Scholar] [CrossRef] [PubMed]
- Sherman, M.B.; Williams, A.N.; Smith, H.Q.; Pettitt, B.M.; Wobus, C.E.; Smith, T.J. Structural Studies on the Shapeshifting Murine Norovirus. Viruses 2021, 13, 2162. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, H.; Maeng, J.Y.; Lee, D.B.; Kim, K.H.; Chung, M.S. Antiviral Activities of Asarones and Rhizomes of Acorus gramineus on Murine Norovirus. Viruses 2022, 14, 2228. https://doi.org/10.3390/v14102228
Kim H, Maeng JY, Lee DB, Kim KH, Chung MS. Antiviral Activities of Asarones and Rhizomes of Acorus gramineus on Murine Norovirus. Viruses. 2022; 14(10):2228. https://doi.org/10.3390/v14102228
Chicago/Turabian StyleKim, Hyojin, Jin Young Maeng, Dan Bi Lee, Kyung Hyun Kim, and Mi Sook Chung. 2022. "Antiviral Activities of Asarones and Rhizomes of Acorus gramineus on Murine Norovirus" Viruses 14, no. 10: 2228. https://doi.org/10.3390/v14102228
APA StyleKim, H., Maeng, J. Y., Lee, D. B., Kim, K. H., & Chung, M. S. (2022). Antiviral Activities of Asarones and Rhizomes of Acorus gramineus on Murine Norovirus. Viruses, 14(10), 2228. https://doi.org/10.3390/v14102228




