Transfusion-Acquired HIV: History, Evolution of Screening Tests, and Current Challenges of Unreported Antiretroviral Drug Use in Brazil
Abstract
1. Worldwide and Brazilian HIV Prevalence
2. Beginning of the HIV Epidemic and Transfusion-Transmitted HIV
3. Donor HIV Screening Tests and Blood Safety Policies in Brazil
4. Donor HIV Nucleic Acid Testing (NAT)
5. Current Intervention Measures to Ensure Transfusion Safety
6. New Challenges in Transfusion-Transmissible HIV Infection
7. Strategies to Mitigate These New Challenges
Author Contributions
Funding
Conflicts of Interest
References
- UNAIDS. In Danger: UNAIDS Global AIDS Update. 2022. Available online: https://www.unaids.org/en (accessed on 8 August 2022).
- Brasil. Ministério da Saúde. Secretaria de Vigilância em Saúde. Boletim Epidemiológico de HIV AIDS. 2021. Available online: https://www.gov.br/saude/pt-br/centrais-de-conteudo/publicacoes/boletins/epidemiologicos/especiais/2021/boletim-epidemiologico-especial-hiv-aids-2021.pdf (accessed on 8 August 2022).
- Maartens, G.; Celum, C.; Lewin, S.R. HIV infection: Epidemiology, pathogenesis, treatment, and prevention. Lancet 2014, 384, 258–271. [Google Scholar] [CrossRef]
- Brasil. Ministério da Saúde. Secretaria de Vigilância em Saúde. Departamento de Doenças de Condições Crônicas e Infecções Sexualmente Transmissíveis. Relatório de Monitoramento Clínico do HIV. 2020. Available online: https://bvsms.saude.gov.br/bvs/publicacoes/relatorio_monitoramento_clinico_hiv_2020.pdf (accessed on 8 August 2022).
- Brasil. Ministério da Saúde. Departamento de Condições Crônicas e Infecções Sexualmente Transmissíveis. Painel-Prep. 2022. Available online: http://www.aids.gov.br/pt-br/painel-prep (accessed on 8 August 2022).
- Brasil. Ministério da Saúde. Secretaria de Vigilância em Saúde. Departamento de Vigilância, Prevenção e Controle das infecções Sexualmente Transmissíveis, do HIV/Aids e das Hepatites Virais. Protocolo Clínico e diretrizes Terapêuticas para Profilaxia Pré-Exposição (PrEP) de risco à infecção pelo HIV. 2018. Available online: https://bvsms.saude.gov.br/bvs/publicacoes/protocolo_clinico_diretrizes_terapeuticas_profilaxia_pre_exposicao_risco_infeccao_hiv.pdf (accessed on 8 August 2022).
- Alter, H.J.; Klein, H.G. The hazards of blood transfusion in historical perspective. Blood 2008, 112, 2617–2626. [Google Scholar] [CrossRef]
- (CDC) CfDC. Pneumocystis pneumonia—Los Angeles. MMWR Morb. Mortal. Wkly. Rep. 1981, 30, 250–252. [Google Scholar]
- (CDC) CfDC. Update on Kaposi’s sarcoma and opportunistic infections in previously healthy persons—United States. MMWR Morb. Mortal. Wkly. Rep. 1982, 31, 294, 300–301. [Google Scholar]
- (CDC) CfDC. Opportunistic infections and Kaposi’s sarcoma among Haitians in the United States. MMWR Morb. Mortal. Wkly. Rep. 1982, 31, 60–61+353–354. [Google Scholar]
- (CDC) CfDC. Pneumocystis carinii pneumonia among persons with hemophilia A. MMWR Morb. Mortal. Wkly. Rep. 1982, 31, 365–367. [Google Scholar]
- Ammann, A.J.; Cowan, M.J.; Wara, D.W.; Weintrub, P.; Dritz, S.; Goldman, H.; Perkins, H.A. Acquired immunodeficiency in an infant: Possible transmission by means of blood products. Lancet 1983, 1, 956–958. [Google Scholar] [CrossRef]
- Reichert, C.M.; O’Leary, T.J.; Levens, D.L.; Simrell, C.R.; Macher, A.M. Autopsy pathology in the acquired immune deficiency syndrome. Am. J. Pathol. 1983, 112, 357–382. [Google Scholar]
- Gjerset, G.F.; McGrady, G.; Counts, R.B.; Martin, P.J.; Jason, J.; Kennedy, S.; Evatt, B.; Hansen, J.A. Lymphadenopathy-associated virus antibodies and T cells in hemophiliacs treated with cryoprecipitate or concentrate. Blood 1985, 66, 718–720. [Google Scholar] [CrossRef] [PubMed]
- Barré-Sinoussi, F.; Chermann, J.C.; Rey, F.; Nugeyre, M.T.; Chamaret, S.; Gruest, J.; Dauguet, C.; Axler-Blin, C.; Vezinet-Brun, F.; Montagnier, L.; et al. Isolation of a T-lymphotropic retrovirus from a patient at risk for acquired immune deficiency syndrome (AIDS). Science 1983, 220, 868–871. [Google Scholar] [CrossRef] [PubMed]
- Gallo, R.C.; Salahuddin, S.Z.; Popovic, M.; Shearer, G.M.; Kaplan, M.; Haynes, B.F.; Palker, T.J.; Markham, P.D. Frequent detection and isolation of cytopathic retroviruses (HTLV-III) from patients with AIDS and at risk for AIDS. Science 1984, 224, 500–503. [Google Scholar] [CrossRef] [PubMed]
- Pear, R. AIDS Blood Test to Be Available in 2 to 6 Weeks. 1985. Available online: http://www.nytimes.com/1985/03/03/us/aids-blood-test-to-be-available-in-2-to-6-weeks.html (accessed on 26 July 2022).
- Busch, M.P.; Young, M.J.; Samson, S.M.; Mosley, J.W.; Ward, J.W.; Perkins, H.A. Risk of human immunodeficiency virus (HIV) transmission by blood transfusions before the implementation of HIV-1 antibody screening. The Transfusion Safety Study Group. Transfusion 1991, 31, 4–11. [Google Scholar] [CrossRef] [PubMed]
- Busch, M.P.; Bloch, E.M.; Kleinman, S. Prevention of transfusion-transmitted infections. Blood 2019, 133, 1854–1864. [Google Scholar] [CrossRef] [PubMed]
- Brasil. Lei No 7.649, de 25 de Janeiro de 1988. Estabelece a obrigatoriedade do cadastramento dos doadores de sangue bem como a realização de exames laboratoriais no sangue coletado, visando a prevenir a propagação de doenças, e dá outras providências. 1988. Available online: http://www.planalto.gov.br/ccivil_03/Leis/1980-1988/L7649.htm (accessed on 8 August 2022).
- Paulo, S.; São Paulo. Lei N. 5.190, de 20 de Junho de 1986. Available online: http://dobuscadireta.imprensaoficial.com.br/default.aspx?DataPublicacao=19860621&Caderno=Poder%20Executivo&NumeroPagina=1 (accessed on 8 August 2022).
- Salles, N.A.; Nishiya, A.S.; de Almeida-Neto, C.; Ferreira, S.C.; Rocha, V.; Mendrone, A., Jr. Discard Rate of HIV Serological and Molecular Screening Tests from 1990 to 2020 and HIV NAT Yield Rate between 2011 and 2020, Fundação Pró-Sangue Hemocentro de São Paulo: São Paulo, Brazil, 2022. Personal communication 2022.
- Alexander, T.S. Human Immunodeficiency Virus Diagnostic Testing: 30 Years of Evolution. Clin. Vaccine Immunol. 2016, 23, 249–253. [Google Scholar] [CrossRef]
- Salles, N.A.; Sabino, E.C.; Barreto, C.C.; Barreto, A.M.; Otani, M.M.; Chamone, D.F. The discarding of blood units and the prevalence of infectious diseases in donors at the Pro-Blood Foundation/Blood Center of São Paulo, São Paulo, Brazil. Rev. Panam. Salud. Publica 2003, 13, 111–116. [Google Scholar] [CrossRef][Green Version]
- Zeh, C.; Oyaro, B.; Vandenhoudt, H.; Amornkul, P.; Kasembeli, A.; Bondo, P.; Mwaengo, D.; Thomas, T.K.; Hart, C.; Laserson, K.F.; et al. Performance of six commercial enzyme immunoassays and two alternative HIV-testing algorithms for the diagnosis of HIV-1 infection in Kisumu, Western Kenya. J. Virol. Methods 2011, 176, 24–31. [Google Scholar] [CrossRef]
- Louie, B.; Pandori, M.W.; Wong, E.; Klausner, J.D.; Liska, S. Use of an acute seroconversion panel to evaluate a third-generation enzyme-linked immunoassay for detection of human immunodeficiency virus-specific antibodies relative to multiple other assays. J. Clin. Microbiol. 2006, 44, 1856–1858. [Google Scholar] [CrossRef]
- Salmona, M.; Delarue, S.; Delaugerre, C.; Simon, F.; Maylin, S. Clinical evaluation of BioPlex 2200 HIV Ag-Ab, an automated screening method providing discrete detection of HIV-1 p24 antigen, HIV-1 antibody, and HIV-2 antibody. J. Clin. Microbiol. 2014, 52, 103–107. [Google Scholar] [CrossRef]
- Ward, J.W.; Holmberg, S.D.; Allen, J.R.; Cohn, D.L.; Critchley, S.E.; Kleinman, S.H.; Lenes, B.A.; Ravenholt, O.; Davis, J.R.; Quinn, M.G.; et al. Transmission of human immunodeficiency virus (HIV) by blood transfusions screened as negative for HIV antibody. N. Engl. J. Med. 1988, 318, 473–478. [Google Scholar] [CrossRef] [PubMed]
- Buschi, M.P.; Kleinman, S.H.; Nemo, G.J. Current and emerging infectious risks of blood transfusions. JAMA 2003, 289, 959–962. [Google Scholar] [CrossRef]
- Brasil. Ministério da Saúde. Portaria Nº 2.712, de 12 de Novembro de 2013. Redefine o regulamento técnico de procedimentos hemoterápicos. 2013. Available online: https://bvsms.saude.gov.br/bvs/saudelegis/gm/2013/prt2712_12_11_2013.html (accessed on 8 August 2022).
- Salles, N.A.; Nishiya, A.S.; Ferreira, S.C.; Rocha, V.G.; Mendrone, A., Jr. Detection of HIV-1 infections in blood donors during the pre-seroconversion window period in São Paulo, Brazil. Rev. Soc. Bras. Med. Trop. 2019, 52, e20180432. [Google Scholar] [CrossRef] [PubMed]
- Brasil. Ministério da Saúde. Agência Nacional de Vigilância Sanitária—Anvisa. 9o Boletim de produção Hemoterápica, Hemoprod, 2020. Brasília. 2020. Available online: https://app.powerbi.com/view?r=eyJrIjoiMWM4MDQzNDMtYjZjZC00ZTBhLWFkOTctODdiZjE2ODQ4YTJkIiwidCI6ImI2N2FmMjNmLWMzZjMtNGQzNS04MGM3LWI3MDg1ZjVlZGQ4MSJ9 (accessed on 8 August 2022).
- Brasil. Ministério da Saúde. Agência Nacional de Vigilância Sanitária—Anvisa. Guia para inclusão de critérios na triagem clínica e epidemiológica de candidatos a doação de sangue baseados em práticas individuais acrescidas de risco para infecções transmissíveis pelo sangue. Guia n° 34/2020—versão 1. 2020. Available online: https://www.cevs.rs.gov.br/upload/arquivos/202010/19093256-guia-n-34-versao-1.pdf (accessed on 8 August 2022).
- Brasil. Ministério da Saúde. Portaria de Consolidação nº 5, de 28 de Setembro de 2017, anexo IV, 2017. Consolidação das normas sobre as ações e os serviços de saúde do Sistema Único de Saúde. 2017. Available online: http://portalsinan.saude.gov.br/images/documentos/Legislacoes/Portaria_Consolidacao_5_28_SETEMBRO_2017.pdf (accessed on 8 August 2022).
- Barreto, C.C.; Sabino, E.C.; Gonçalez, T.T.; Laycock, M.E.; Pappalardo, B.L.; Salles, N.A.; Wright, D.J.; Chamone, D.F.; Busch, M.P. Prevalence, incidence, and residual risk of human immunodeficiency virus among community and replacement first-time blood donors in São Paulo, Brazil. Transfusion 2005, 45, 1709–1714. [Google Scholar] [CrossRef]
- Goncalez, T.T.; Sabino, E.C.; Murphy, E.L.; Chen, S.; Chamone, D.A.; McFarland, W. Human immunodeficiency virus test-seeking motivation in blood donors, São Paulo, Brazil. Vox Sang. 2006, 90, 170–176. [Google Scholar] [CrossRef] [PubMed]
- Zucoloto, M.L.; Gonçalez, T.T.; McFarland, W.; Custer, B.; Galdino, G.; Martinez, E.Z. Blood donation deferral policies among men who have sex with men in Brazil. Hematol. Transfus. Cell Ther. 2019, 41, 164–168. [Google Scholar] [CrossRef] [PubMed]
- Brasil. Ministério da Saúde. Agência Nacional de Vigilância Sanitária-Anvisa. Resolução de Diretoria Colegiada—RDC nº399, de 7 de julho de 2020. Revoga a alínea "d" do inciso XXX do art. 25 da Resolução de Diretoria Colegiada—RDC nº 34, de 11 de junho de 2014, que dispõe sobre as Boas Práticas no Ciclo do Sangue, em cumprimento à ordem judicial. 2020. Available online: https://bvsms.saude.gov.br/bvs/saudelegis/anvisa/2020/rdc0399_7_07_2020.pdf (accessed on 8 August 2022).
- Salles, N.A.; Levi, J.E.; Barreto, C.C.; Sampaio, L.P.; Romano, C.M.; Sabino, E.C.; Mendrone, A. Human immunodeficiency virus transfusion transmission despite nucleic acid testing. Transfusion 2013, 53, 2593–2595. [Google Scholar] [CrossRef]
- Cappy, P.; Barlet, V.; Lucas, Q.; Tinard, X.; Pillonel, J.; Gross, S.; Tiberghien, P.; Laperche, S. Transfusion of HIV-infected blood products despite highly sensitive nucleic acid testing. Transfusion 2019, 59, 2046–2053. [Google Scholar] [CrossRef]
- Delwart, E.L.; Kalmin, N.D.; Jones, T.S.; Ladd, D.J.; Foley, B.; Tobler, L.H.; Tsui, R.C.P.; Busch, M.P. First report of human immunodeficiency virus transmission via an RNA-screened blood donation. Vox Sang. 2004, 86, 171–177. [Google Scholar] [CrossRef]
- Schmidt, M.; Korn, K.; Nübling, C.M.; Chudy, M.; Kress, J.; Horst, H.A.; Geusendam, G.; Hennig, H.; Sireis, W.; Rabenau, H.F.; et al. First transmission of human immunodeficiency virus Type 1 by a cellular blood product after mandatory nucleic acid screening in Germany. Transfusion 2009, 49, 1836–1844. [Google Scholar] [CrossRef]
- Vermeulen, M.; Lelie, N.; Coleman, C.; Sykes, W.; Jacobs, G.; Swanevelder, R.; Busch, M.; van Zyl, G.; Grebe, E.; Welte, A.; et al. Assessment of HIV transfusion transmission risk in South Africa: A 10-year analysis following implementation of individual donation nucleic acid amplification technology testing and donor demographics eligibility changes. Transfusion 2019, 59, 267–276. [Google Scholar] [CrossRef]
- Álvarez, M.; Luis-Hidalgo, M.; Bracho, M.A.; Blanquer, A.; Larrea, L.; Villalba, J.; Puig, N.; Planelles, D.; Montoro, J.; Roig, R.; et al. Transmission of human immunodeficiency virus Type-1 by fresh-frozen plasma treated with methylene blue and light. Transfusion 2016, 56, 831–836. [Google Scholar] [CrossRef]
- Oliveira Garcia Mateos, S.; Preiss, L.; Gonçalez, T.T.; Di Lorenzo Oliveira, C.; Grebe, E.; Di Germanio, C.; Stone, M.; Filho, L.A.; Belisario, A.R.; Custer, B.; et al. 10-year analysis of human immunodeficiency virus incidence in first-time and repeat donors in Brazil. Vox Sang. 2021, 116, 207–216. [Google Scholar] [CrossRef]
- de Souza, M.S.; Pinyakorn, S.; Akapirat, S.; Pattanachaiwit, S.; Fletcher, J.L.; Chomchey, N.; Kroon, E.D.; Ubolyam, S.; Michael, N.L.; Robb, M.L.; et al. Initiation of Antiretroviral Therapy During Acute HIV-1 Infection Leads to a High Rate of Nonreactive HIV Serology. Clin. Infect. Dis. 2016, 63, 555–561. [Google Scholar] [CrossRef] [PubMed]
- Hare, C.B.; Pappalardo, B.L.; Busch, M.P.; Karlsson, A.C.; Phelps, B.H.; Alexander, S.S.; Bentsen, C.; Ramstead, C.A.; Nixon, D.F.; Levy, J.A.; et al. Seroreversion in subjects receiving antiretroviral therapy during acute/early HIV infection. Clin. Infect. Dis. 2006, 42, 700–708. [Google Scholar] [CrossRef] [PubMed]
- Donnell, D.; Ramos, E.; Celum, C.; Baeten, J.; Dragavon, J.; Tappero, J.; Lingappa, J.R.; Ronald, A.; Fife, K.; Coombs, R.W.; et al. The effect of oral preexposure prophylaxis on the progression of HIV-1 seroconversion. AIDS 2017, 31, 2007–2016. [Google Scholar] [CrossRef]
- Kassutto, S.; Johnston, M.N.; Rosenberg, E.S. Incomplete HIV type 1 antibody evolution and seroreversion in acutely infected individuals treated with early antiretroviral therapy. Clin. Infect. Dis. 2005, 40, 868–873. [Google Scholar] [CrossRef]
- Custer, B.; Quiner, C.; Haaland, R.; Martin, A.; Stone, M.; Reik, R.; Steele, W.R.; Kessler, D.; Williamson, P.C.; Anderston, S.A.; et al. HIV antiretroviral therapy and prevention use in US blood donors: A new blood safety concern. Blood 2020, 136, 1351–1358. [Google Scholar] [CrossRef]
- Sykes, W.; Van den Berg, K.; Jacobs, G.; Jauregui, A.; Roubinian, N.; Wiesner, L.; Maartens, G.; Custer, B.; Busch, M.; Jentsch, U.; et al. Discovery of False Elite Controllers: HIV Antibody-Positive RNA-Negative Blood Donors Found To Be on Antiretroviral Therapy. J. Infect. Dis. 2019, 220, 643–647. [Google Scholar] [CrossRef]
- van den Berg, K.; Vermeulen, M.; Louw, V.J.; Murphy, E.L.; Maartens, G. Undisclosed HIV status and antiretroviral therapy use among South African blood donors. Transfusion 2021, 61, 2392–2400. [Google Scholar] [CrossRef] [PubMed]
- Nishiya, A.S.; Salles, N.A.; de Almeida-Neto, C.; Ferreira, S.C.; Nogueira, F.A.H.; Rocha, V.; Mendrone-Junior, A. Confirmed Use of Antiretroviral Drugs for HIV Therapy in Two Blood Donors with Potential Elite Controller Profile from São Paulo; Fundação Pró-Sangue Hemocentro de São Paulo: São Paulo, Brazil, 2022. [Google Scholar]
- Harvala, H.; Reynolds, C.; Ijaz, S.; Maddox, V.; Penchala, S.D.; Amara, A.; Else, L.; Brailsford, S.; Khoo, S. Evidence of HIV pre-exposure or post-exposure prophylaxis (PrEP/PEP) among blood donors: A pilot study, England June 2018 to July 2019. Sex Transm. Infect. 2021, 98, 132–135. [Google Scholar] [CrossRef]
- Nishiya, A.S.; Salles, N.A.; de Almeida-Neto, C.; Witkin, S.S.; Ferreira, S.C.; Nogueira, F.A.H.; Facincani, T.; Rocha, V.; Mendrone, A. Influence of unreported HIV prophylaxis on the kinetics of post-blood donation HIV seroconversion. Transfusion 2021, 61, 3488–3492. [Google Scholar] [CrossRef]
- Busch, M.P. Four decades of HIV and transfusion safety: Much accomplished but ongoing challenges. Transfusion 2022, 62, 1334–1339. [Google Scholar] [CrossRef]
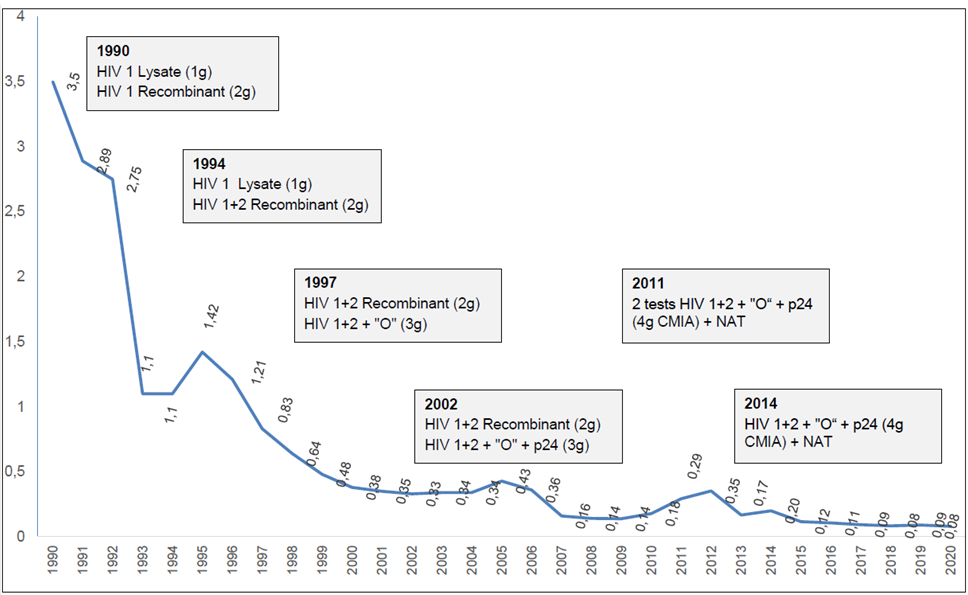
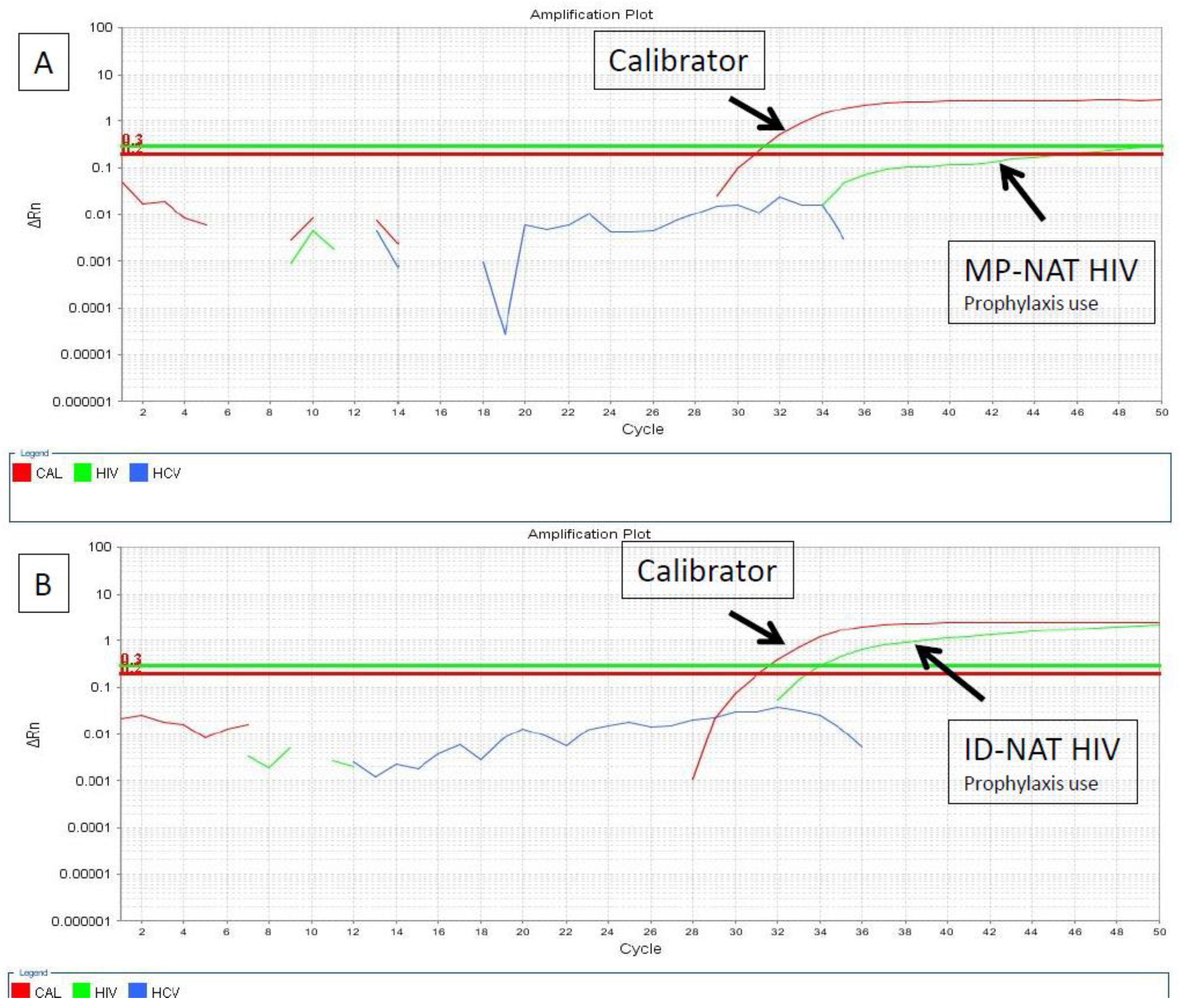
| DATE | Blood Products | HIV Serological Screening | NAT-HIV Screening | NAT-HIV Confirmatory | Pathogen Reduction | Reference |
|---|---|---|---|---|---|---|
| August 2000 | Red blood cells | Non-reactive HIV Ab/Ag Abbott | Negative 24 MP-NAT non-commercial LOD 100 cop/mL | 180 cop/mL NGI Ultraqual™ LOD 30.76 IU/mL | No | [41] |
| November 2005 | Fresh-frozen plasma and red blood cells | Non-reactive Abbott PRISM Ab-HIV-1/2 assay | Negative 44 MP-NAT—COBAS AmpliScreen LOD 78 IU/mL | 135 cop/mL ID-NAT—Procleix Ultrio Assay LOD 20.3 IU/mL | Yes Theraflex | [44] |
| January 2007 | Red blood cells | Non-reactive Abbott PRISM Ab-HIV-1/2 O assay | Negative 96 MP-NAT—COBAS AmpliPrep/TaqMan LOD 4257 IU/mL | 146 IU/mL ID-NAT—COBAS AmpliPrep/TaqMan LOD 44 IU/mL | No | [42] |
| September 2012 | Red blood cells | Non-reactive HIV Ag/Ab Combo Architect Abbott | Negative 6 MP-NAT—Biomanguinhos LOD 240 copies/mL | <LOD (7.5 and 3.7 IU/mL) Procleix Ultrio LOD 34.54 IU/mL and COBAS TaqScreen MPX LOD 49 IU/mL | No | [39] |
| March 2017 | Red blood cells | Non-reactive Abbott PRISM Ab-HIV-1/2/O assay | Negative ID-NAT—Procleix Ultrio assay LOD 39 IU/mL (23 copies/mL) | 34 copies/mL ID-NAT—COBAS TaqMan LOD 17 copies/mL | Yes INTERCEPT | [40] |
| DATE | DONATION | CMIA HIV | MP-NAT-HIV | ID-NAT-HIV | RT-PCR HIV | WBlot HIV |
|---|---|---|---|---|---|---|
| (Cutoff Value of 1.0) | (Copies/mL) | (Positive Bands) | ||||
| 1 July 2020 | index | non-reactive | negative | positive | <DL | not conducted |
| 0.07 | ct 34 | ~0.2 | ||||
| 19 August 2020 | return 48 days | undetermined | not performed | negative | negative | undetermined |
| 0.98 | p24 | |||||
| 10 September 2020 | return 70 days | reactive | not performed | negative | negative | positive |
| 1.13 | p24, gp160 | |||||
| 15 October 2020 | return 105 days | reactive | not performed | positive | 151 | positive |
| 35.4 | ct 27.8 | p24, gp41, p51 | ||||
| p66, gp120, and gp160 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nishiya, A.S.; Ferreira, S.C.; Salles, N.A.; Rocha, V.; Mendrone-Júnior, A. Transfusion-Acquired HIV: History, Evolution of Screening Tests, and Current Challenges of Unreported Antiretroviral Drug Use in Brazil. Viruses 2022, 14, 2214. https://doi.org/10.3390/v14102214
Nishiya AS, Ferreira SC, Salles NA, Rocha V, Mendrone-Júnior A. Transfusion-Acquired HIV: History, Evolution of Screening Tests, and Current Challenges of Unreported Antiretroviral Drug Use in Brazil. Viruses. 2022; 14(10):2214. https://doi.org/10.3390/v14102214
Chicago/Turabian StyleNishiya, Anna S., Suzete C. Ferreira, Nanci A. Salles, Vanderson Rocha, and Alfredo Mendrone-Júnior. 2022. "Transfusion-Acquired HIV: History, Evolution of Screening Tests, and Current Challenges of Unreported Antiretroviral Drug Use in Brazil" Viruses 14, no. 10: 2214. https://doi.org/10.3390/v14102214
APA StyleNishiya, A. S., Ferreira, S. C., Salles, N. A., Rocha, V., & Mendrone-Júnior, A. (2022). Transfusion-Acquired HIV: History, Evolution of Screening Tests, and Current Challenges of Unreported Antiretroviral Drug Use in Brazil. Viruses, 14(10), 2214. https://doi.org/10.3390/v14102214







