Abstract
Background: Congenital cytomegalovirus (cCMV) infection is frequent and potentially severe. The immunobiology of cCMV infection is poorly understood, involving cytokines that could be carried within or on the surface of extracellular vesicles (EV). We investigated intra-amniotic cytokines, mediated or not by EV, in cCMV infection. Methods: Forty infected fetuses following early maternal primary infection and forty negative controls were included. Infected fetuses were classified according to severity at birth: asymptomatic, moderately or severely symptomatic. Following the capture of EV in amniotic fluid (AF), the concentrations of 38 cytokines were quantified. The association with infection and its severity was determined using univariate and multivariate analysis. A prediction analysis based on principal component analysis was conducted. Results: cCMV infection was nominally associated with an increase in six cytokines, mainly soluble (IP-10, IL-18, ITAC, and TRAIL). EV-associated IP-10 was also increased in cases of fetal infection. Severity of fetal infection was nominally associated with an increase in twelve cytokines, including five also associated with fetal infection. A pattern of specific increase in six proteins fitted severely symptomatic infection, including IL-18soluble, TRAILsoluble, CRPsoluble, TRAILsurface, MIGinternal, and RANTESinternal. Conclusion: Fetal infection and its severity are associated with an increase in pro-inflammatory cytokines involved in Th1 immune response.
1. Introduction
Congenital cytomegalovirus (cCMV) infection is the most common congenital infection, affecting 0.5–2% of live newborns worldwide [1]. cCMV infection is characterized by a variable severity, ranging from asymptomatic to severe neurological disabilities or perinatal death [2,3,4,5,6,7]. Sequelae are limited to cases following maternal infection before 14 weeks of gestation [8]. In such cases and despite changes in definitions over time, around 30% of newborns are considered symptomatic at birth, with sensorineural impairment (hearing loss (SNHL) and vestibulitis) in 10–15% and with 10–25% suffering more severe neurological damage including intellectual disability or developmental delay [5,9,10,11,12,13,14,15,16,17,18,19]. The prenatal assessment of infected fetuses is based on prenatal ultrasound, MRI, and biological data, including fetal thrombocytopenia [20,21,22,23,24,25,26,27]. Outside severe cerebral features on prenatal imaging, which are associated with a poor outcome, the prediction of neonatal status is limited, and this uncertainty weighs heavily on prenatal counselling [2]. The identification of new prognostic markers in amniotic fluid that is sampled by amniocentesis for the diagnosis of fetal infection could improve timely prenatal assessment of infected fetuses. Among biological processes involved in the innate immunity, many cytokines are involved in the immune control of cCMV infection in fetuses and immunodeficient adults [28].
The main objective of this study was to investigate all fractions of intra-amniotic cytokines in cCMV infection according to the severity at birth in order to identify suitable candidate biomarkers.
2. Materials and Methods
2.1. Study Population
All subjects were enrolled in our Fetal Medicine Unit at Necker Hospital (AP-HP hospitals of Paris and Paris-Cité University) between December 2011 and December 2017. Patients provided written informed consent, and studies were approved by the local ethics committee and registered in the clinicaltrial.gov website as NCT03090841 (BiocCMV) and NCT01651585 (CYMEVAL2). According to the study protocol, amniotic fluid that was not used for clinical management-related investigations was stored for research purposes. The women included were referred following maternal primary infection (MPI) within 2 months prior to conception or in the first trimester of pregnancy. They underwent amniocentesis to diagnose cCMV infection. For each infected fetus, a negative control with a non-infected and euploid fetus was included and matched accordingly to fetal gender and gestational age at amniocentesis. All women had no relevant medical history, especially no immune disorders or treatment affecting immunity.
2.2. Diagnosis of MPI, Fetal, and Neonatal Infection
The timing of MPI was determined as previously described, using an in-house algorithm based on CMV IgG and IgM antibody concentrations (LIAISON XL CMV IgG II and IgM, Diasorin, Antony, France) and IgG avidity (LIAISON CMV IgG Avidity II and/or VIDAS CMV IgG avidity II, BioMerieux, Marcy L’Etoile, France) [29].
Infected fetuses were defined by a positive CMV DNA PCR on amniotic fluid sampled by amniocentesis after 17 weeks of gestation and at least 8 weeks following maternal primary infection. At birth, neonatal infection was confirmed by CMV DNA PCR in neonatal blood, urine, and saliva. All virological tests were performed in our expert virology laboratory.
2.3. Clinical Classification of Infected Newborns
The pregnancy outcome was determined based upon prenatal ultrasound performed every two weeks from referral to delivery. Magnetic resonance imaging (MRI) of the fetus was performed at 28 and 32 weeks. Cordocentesis for analysis of the fetal blood was performed by ultrasound guided umbilical funipuncture at 20–28 weeks. At birth, infected fetuses were classified into two groups: symptomatic or asymptomatic newborns. Newborns with at least one abnormal neonatal feature and deceased fetuses following termination for severe brain lesions, as confirmed by postmortem examination or spontaneous intrauterine fetal death were considered symptomatic. Two sub-groups of symptomatic neonates were defined: (1) neonates with an abnormal clinical and/or complementary investigation, and (2) neonates with more severe neurological impairment as well as stillbirth or terminated fetuses with confirmed severity of brain lesions postmortem. The protocol of neonatal assessment is detailed in Supplementary Materials.
2.4. Preparation of Extracellular Vesicle Fractions
Amniotic fluid samples stored in the virology laboratory of Necker University Hospital at −80 °C, were transported in a temperature-controlled device (−80 °C on dry ice) to the University of Bethesda. Preparation of extracellular vesicle was made using the methodology previously described by Bhatti et al. using Exoquick-TC™ (System Biosciences, SBI, Palo Alto, CA, USA) to sediment extracellular vesicles according to manufacturer’s instructions [30,31]. The supernatant fluid was collected into a separate tube for subsequent immunoassay later the same day (‘soluble fraction’). The pellet was centrifuged again at 1500× g for 5 min, and the supernatant fluid was aspirated. The pellet was resuspended in 130 μL phosphate-buffered saline (PBS, pH7.4) for subsequent analyte assay of extracellular vesicles (‘surface’ and ‘internal’ fraction). Extracellular vesicles were lysed by Triton™ X-100 at final concentration of 0.5%.
2.5. Cytokines Concentrations Measurement
Inflammatory mediator concentrations were determined using an in-house multiplexed bead-based assay, as previously described, with minor modifications for 38 cytokines (list available in Appendix A) [30,32]. All antibody pairs and protein standards were purchased from R&D Systems (Minneapolis, MN, USA), except those for IFN-β (Abcam, Cambridge, UK). Magnetic beads (Luminex Corporation, Austin, TX, USA) with distinct spectral signatures (regions) were coupled to analyte-specific capture antibodies according to manufacturer’s recommendations in 96-well flat bottom plates (Nunc, ThermoFisher) and incubated at 4 °C overnight. Plates were washed on a magnetic plate washer (405 TS, Biotek Winooski, VT, USA) followed by incubation with polyclonal biotinylated anti-analyte antibodies and streptavidin-phycoerythrin (ThermoFisher Scientific). Beads were resuspended in PBS and read on a Luminex 200 analyzer (Luminex Corporation) with acquisition of 100 beads for each region and analyzed using Bioplex Manager software (Bio-Rad Laboratories, Hercules, CA, USA).
Analyte concentrations (pg/mL) were determined using five parameters regression algorithms and expressed as the mean pg/mL ± S.E. Concentrations were corrected for dilution by ExoQuick-TC™ or Triton™ X-100. Extracellular vesicle luminal content (‘internal fraction’) was calculated as [analyte content of lysed vesicle] − [analyte content of intact vesicles (‘surface fraction’)].
2.6. Statistical Analysis
Considering sample size, we first conducted a data pre-processing, filtering out all proteins with less than 20% non-zero value. A total of 77 out of 114 (67%) proteins remained following this filtering. The distributions of all proteins, defined by their variance and proportion of non-zero values, are described in Supplementary Figure S1. To provide an overview of the association profile, we then conducted a systematic univariate marginal association analysis between all available variables, including proteins, but also other measured biomarkers and clinical factors, and the two outcomes, infection and severity, using logistic and linear regressions, respectively.
Because of the large number of proteins as compared to the sample size, prediction of clinical outcomes was performed after a data dimension reduction based on principal component analysis (PCA). Note that principal components were derived based on the covariance matrix of proteins, therefore giving higher weight to proteins with higher variance. The cumulative variance explained by principal components applied to all proteins and by cellular classes are provided in Supplementary Figure S2. For all sets considered, 10 PCs or less were necessary to explain 80% of the total phenotypic variance.
For infection, the prediction model was derived from multiple logistic regressions using five PCs, and prediction accuracy measured using the area under the roc curve (AUC). Given the limited sample size, the AUC was derived as the average over 50 rounds of cross-validation to limit overfitting. For each round, a training set including 80% of the data was randomly chosen to estimate regression coefficients, and the remaining 20% was used as a test set.
For severity, the prediction models were derived from multiple linear regressions, and prediction accuracy was measured as the adjusted R-squared. We investigated models including 1–40 PCs, using PCA derived in all proteins or within cellular classes. We further assessed the significance of the adjusted R-squared through permutations, where multiple regression was derived over 200 replicates after shuffling the severity values.
All analyses were performed using R. p-values were considered statistically significant if below the Bonferroni correction threshold for a baseline alpha value of 0.05.
3. Results
3.1. Description of the Cohort
We enrolled 80 pairs of women and fetuses/newborns in this study, including 40 infected fetuses and 40 negative controls. Clinical characteristics were similar between cases and controls (Table 1). Among infected cases, 9 (12.5%) were asymptomatic and 31 (77.5%) were symptomatic, including 13 and 18 with non-severe and severe infections. Neonatal assessment of infected fetuses is detailed in Supplementary Table S1.

Table 1.
Population characteristics.
3.2. Cytokines Profiling in Amniotic Fluid of Infected Fetuses
Concentrations of relevant cytokines and comparison between infected and noninfected fetuses are summarized in Table 2 (full in Supplementary Table S2).

Table 2.
Concentrations of relevant cytokines according to fetal infection (median and interquartile).
Univariate analysis identified a nominally significant difference for six proteins in case of cCMV infection. Most of them were soluble in the amniotic fluid: IP-10, IL-18, ITAC, and TRAIL. In addition, we identified a nominally significant difference for the extracellular vesicle-associated IP-10, both in the internal compartment and in the surface. IP-10 further reached the very stringent Bonferroni-corrected p-value threshold for both infection and severity (p < 0.0003). For each protein previously listed, cCMV infection was associated with an increased concentration in amniotic fluid (Figure 1).
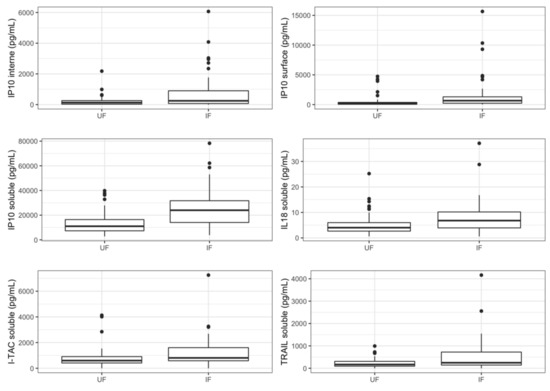
Figure 1.
Concentrations of relevant cytokines according to fetal infection. Boxplots represent variations in cytokines’ concentrations (median). UF: uninfected fetuses, IF: infected fetuses.
3.3. Cytokines Profiling in Amniotic Fluid of Symptomatic Newborns
Concentrations of relevant cytokines and comparison between symptomatic and asymptomatic newborns are summarized in Table 3 (full dataset in Supplementary Table S2).

Table 3.
Concentrations of relevant cytokines in amniotic fluid according to symptomatic status at birth and severity (median and interquartile).
Twelve proteins were significantly correlated to a symptomatic status at birth in the univariate analysis: four were soluble proteins (IP-10, IL-18, TRAIL, and CRP) and eight were associated with EV, mostly located within the EV (IP-10, IL-6, MCP1, MIG, and RANTES). Five cytokines were previously associated with fetal infection (IP-10internal, IP-10surface, IP-10soluble, IL-18soluble, and TRAILsoluble) (Figure 2). A pattern with a specific increase in cases of severe symptomatic infection was identified for six proteins (IL-18soluble, TRAILsoluble, CRPsoluble, TRAILsurface, MIGinternal and RANTESinternal).
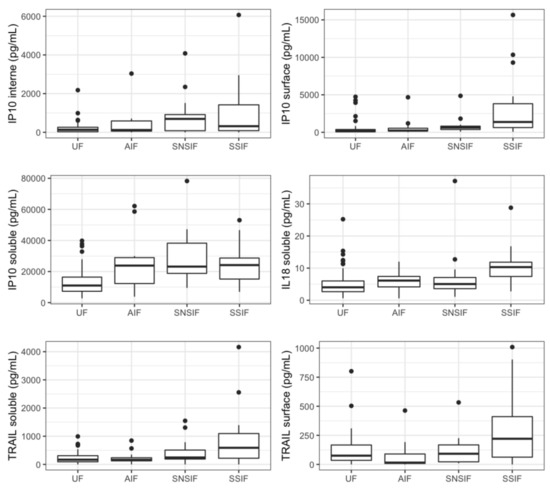
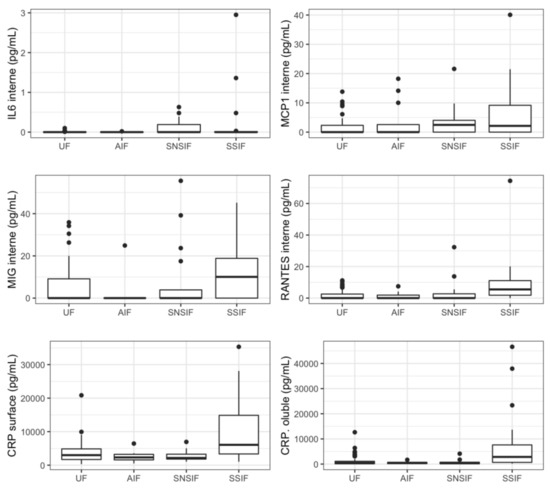
Figure 2.
Concentrations of relevant cytokines according to symptomatic state at birth and severity. Boxplots representing represent variations in cytokines’ concentrations (median). UF: unifected fetuses, AIF: asymptomatic infected fetuses, SNSIF: symptomatic and non-severe infected fetuses, SSIF: symptomatic and severe infected fetuses.
Severe fetal infection was also associated with fetal thrombocytopenia (p < 0.001). The amniotic CMV viral load and fetal liver tests were not associated with fetal symptoms in this series.
3.4. Prediction Analysis
We first assessed the prediction accuracy of fetal infection using a model based on five principal components (PCs) derived from all proteins (Figure 3a). The average AUC (area under the ROC curve) across all cross-validation equals 0.72 (SD = 0.090). In comparison, the AUC derived from a null model equals 0.50 (SD = 0.123), confirming the validity of our estimation. Using this null model, we derived a one-sided Z-score test for the observed AUC, which suggests a nominally significant prediction (p-value = 0.037). We then derived the prediction accuracy of various models for the severity of cCMV using 1–40 PCs derived from either intern, surface, soluble, or all proteins jointly (Figure 3b and Supplementary Table S3). The soluble-protein based and all-proteins models performed the best, explaining up to 41% of the total variance of severity. We compared these results against a null model using randomized severity status, which again, confirmed the calibration of our estimation. Together, those two analyses confirm a strong potential for using cytokines to predict both infection and severity.
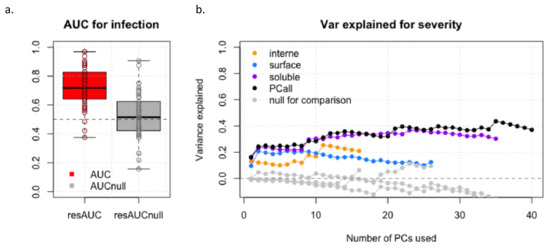
Figure 3.
Prediction accuracy of fetal infection (a) and of severity (b).
4. Discussion
The natural history of cCMV infection is complex, and the pathophysiology of fetal injury is incompletely understood [28]. Immune response to cCMV involves both innate and adaptative immunity in the mother, placenta, and fetus at each step of the vertical transmission [33]. Among biological processes involved in the innate immunity, many cytokines appear essential to control cCMV infection as well as human CMV (hCMV) in immunodeficient adults. Only one previous study investigated cytokines profiling in amniotic fluid collected in eight infected fetuses at midtrimester and reported higher interferon gamma-induced protein 10 (IP-10) concentrations in infected cases [34].
Extracellular vesicles (EVs) include a wide spectrum of lipidic cell-derived membranous structures [35]. EVs are involved in many physiological and pathological processes, especially to mediate paracrine inter-cellular communication by carrying various types of molecules including nucleic acids, proteins, lipids, and other metabolites [35,36,37,38,39]. EVs are present in many biological fluids, including amniotic fluid [40,41], because they are released by fetal epithelial cells from skin, urine, and lungs [42,43,44,45,46,47,48]. Recent data suggested that intraamniotic EV could contain biomarkers relating to a wide spectrum of fetal disorders including bacterial intra-amniotic infection [30,40,45,49,50,51,52].
Our data suggest that cCMV infection and related symptoms at birth are associated with changes in the immunological signature in the amniotic fluid. Four soluble pro-inflammatory mediators (IP-10, IL-18, ITAC, and TRAIL) and one mediated by EV (IP-10) were increased in case of cCMV infection. Among these proteins, five were related to symptoms at birth (IP-10internal, IP-10surface, IP-10soluble, IL-18 soluble, and TRAIL soluble). Seven other cytokines, not related to cCMV infection, were significantly associated with symptomatic status at birth; therefore, a pattern for severe infection can be drawn with a specific increase in the presence and concentration of six mediators (IL-18soluble, TRAILsoluble, CRPsoluble, TRAILsurface, MIGinternal, and RANTESinternal).
Most of these relevant cytokines were previously reported in immunodeficient adults with hCMV infection but not within the immunological process involved in cCMV infection based on animal models or in in vitro studies using cell lines cultures [53]. However, the viral host specificity was not considered in these studies, leading to major limitations. TNF-related apoptosis-inducing ligand (TRAIL) induces apoptosis by binding and cross-linking death-domain receptors and activation of caspases [54,55]. In vitro, human CMV-infected fibroblasts co-up-regulated secretion of TRAIL and expression of TRAIL-DR [56]. Independently from CMV, the secretion of TRAIL is inducible by IFN-γ and TNF-α. TRAIL is also secreted by natural killer (NK) cells, an important effector of innate immunity, of which a subset expressing the NKG2C activating receptor is a preferential target for hCMV [57,58,59]. RANTES stands for regulated on activation, normal T cell expressed, and secreted; it is a chemoattractant for T lymphocytes, monocytes, macrophages, and eosinophiles [60]. In vitro, RANTES is released by HCMV-infected fibroblasts [61]. RANTES promotes TNF-α excretion from macrophages, proliferation of NK cells, and T lymphocytes co-activation with MCP-1 [62]. IL-18 is produced by inflammasome, a multimeric protein complex assembled in the cytosol of cells belonging to the innate immune system, especially macrophages [63]. It follows the recognition of pathogen-associated molecular patterns (PAMPs) or damage-associated molecular patterns (DAMPs) [64]. In a murine model, inflammasome activation in the cochlea has been involved in SNHL [65]. IP-10 is a chemokine released by several subset of cells following IFN-γ induction and implicated in regulation of NK cells, monocytes, and lymphocytes [66,67]. IP-10 recruits Th1 cells, which produce IFN-γ, leading to increasing IP-10 concentration. In addition, IP-10 downregulates Th2 cytokine production [68]. MCP-1, monocyte chemoattractant protein-1, is a chemokine involved in attraction and activation of granulocytes, T cells, and monocytes [69]. hCMV infection is associated with high levels of MCP-1 both in vivo and in vitro [61,70,71]. Low levels of MCP-1 seem related to virus survival and chronic infection [72]. Interferon-inducible T-cell alpha chemoattractant (I-TAC) is a chemokine secreted by infected fibroblasts and induced by IFN-γ [73].
Data concerning c reactive protein (CRP) and monokine induced by gamma interferon (MIG) are very limited and restricted to hCMV infection in transplant recipients [74,75]. All relevant cytokines are inducible by type-1 cytokine response, including TNF-α and IFN-γ, suggesting a Th-1 cell polarization of the immune response [68]. Our data contribute to the controversy on the respective importance of Th-1 cell polarization over Th-2 cell polarization involved in viral infection immunity [33,61].
hCMV has developed various mechanisms to evade the immune response, including modulation of cytokine response [28,69,76,77]. The main one uses a CMV homolog of IL-10 to reduce the immune response [76]. CMV also encodes homologs of cytokine receptors, binding RANTES, e.g., which contribute to anti-inflammatory evasion [78]. hCMV reduces the expression of TRAIL-DR, leading to a restriction of the TRAIL/TRAIL-DR pathways and viral proliferation [56]. Figure 4 summarizes the previous discussion, especially interactions between relevant cytokines and immune system cells.
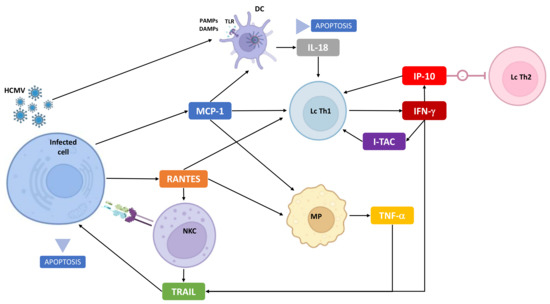
Figure 4.
Immunobiology of cCMV: focus on cytokines identified in this study and interactions with infected and immune cells. NKC: natural-killer cell, MP: macrophage, Lc Th1: T-cell involved in Th1 immune response, Lc Th2: T-cell involved in Th2 immune response, DC: dendritic cell, TLR: Toll-like receptor, PAMPS/DAMPs: pathogen-associated molecular patterns/damage-associated molecular patterns.
Few studies have investigated prognostic markers associated with cCMV infection in utero. Multi-OMICS approaches offer new perspectives to identify relevant biomarkers. In two studies, proteomic analysis of the amniotic fluid using liquid chromatography–mass spectrometry, LC-MS, or capillary electrophoresis–mass spectrometry, CE-MS, identified a set of potential biomarkers associated with severe fetal infection [79,80]. Unfortunately, there was no recurrent protein between those series. Vorontsov et al. reported a statistical association between severe fetal infection and CRP (fold change: 2.5, p = 0.005). We also found an association between CRP-soluble and the severity of fetal infection, suggesting that CRP is a potential prognostic biomarker. One study investigated transcriptomic changes in the case of fetal cCMV infection. Whole transcriptomic analysis using RNA-Seq was performed on 26 samples (13 infected and 13 matched controls) collected between 18 and 23 weeks. Among the 12 most relevant up-regulated genes, there was no recurrent genes with proteins previously identified.
In our study, amniocentesis was performed at a median GA of 22 WG. Recent evidence suggested that an earlier invasive sampling may now be offered to diagnose fetal infection, including amniocentesis from 17 WG and 8 weeks following maternal infection. Our findings should be replicated in a larger prospective cohort, particularly at 17 WG, to be implemented in clinical practice. In addition, a subsequent analysis should be conducted to investigate association between candidate biomarkers and long-term endpoints, including delayed SNHL.
In utero therapy using valaciclovir (8 g/day, 2 g four time a day) was progressively extended from curative (in case of infected fetuses) to preventive treatment (risk of fetal infection following PMI) [81,82]. Our group has recently implemented the diagnosis of fetal infection using CMV-PCR on trophoblast samples obtained by chorionic villus sampling (CVS) at 13–14 weeks [83]. Profiling of inflammatory mediators on infected trophoblast samples could provide additional data on placental immunity and on the pathophysiology of vertical transmission.
The strength of our study is the size of the series and the number of cytokines investigated although the unbalanced ratio of cytokines over cases could be considered a limitation. In our study, cytokines concentrations were assessed using ELISA, the most common technology for measuring proteins concentrations. Most EV cytokines were undetectable or excluded by the pre-processing filtering, possibly because of a lack of sensitivity of ELISA. Considering trafficking of EV, EV cytokines are promising biomarkers, especially for non-invasive tests using maternal blood. New tools based upon single molecule counting technology, such as SiMoA, could be used to detect ultra-low cytokines concentrations to characterize further the profile of EV-cytokines [84].
5. Conclusions
Our data suggest that cCMV infection and its severity are associated with differential cytokines expression in amniotic fluid at mid-gestation. These proteins, mainly soluble in amniotic fluid, could be considered as candidate biomarkers of severity in case of fetal infection diagnosed by CMV-PCR.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/v14102145/s1. Figure S1. Pre-processing. (A) Distributions of all proteins (variance). (B) Proportion of non-zero values. Figure S2. Data dimension reduction based on principal component analysis (PCA): cumulative variance explained by cellular classes are provided. (A) Internal cytokines. (B) Surface-linked cytokines. (C) Soluble cytokines. (D) Overall cytokines. Table S1: Summary of neonatal assessment of infected neonates. Table S2: Concentrations of all cytokines according to fetal infection, symptomatic status at birth, and severity (median and interquartile). Table S3: Prediction analysis based on principal component analysis. Contribution of each PC for infection and severity.
Author Contributions
Conceptualization, R.R., L.M., Y.V. and M.L.-V.; methodology, W.F., R.R., Y.V., L.M. and M.L.-V.; formal analysis, N.B., W.F., H.A., J.S. and L.M.; investigation, W.F., T.G., R.R. and L.M.; resources, R.R. and L.M.; data curation, N.B. and W.F.; writing—original draft preparation, N.B.; writing—review and editing, W.F., H.A., J.-F.M., J.S., R.R., Y.V., L.M. and M.L.-V.; supervision, R.R., Y.V., L.M. and M.L.-V.; funding acquisition, R.R. and L.M. All authors have read and agreed to the published version of the manuscript.
Funding
The work of W.F. and L.M. was supported by the NICHD Intramural Program. This work was also supported by grants from NIH founded by R. Romero.
Institutional Review Board Statement
This study was conducted in accordance with the Declaration of Helsinki, approved by the Ethics Committee, and registered in the clinicaltrial.gov website as NCT03090841 (BiocCMV) and NCT01651585 (CYMEVAL2).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The data presented in this study are available from the corresponding author upon request.
Acknowledgments
We thank the families for taking part in the study.
Conflicts of Interest
The authors declare no conflict of interest.
Appendix A. Cytokines Studied
IL-1α: IL-1β, IL-2, IL-4, IL-6, IL-8, IL-10, IL-13, IL-15, IL-16, IL-18, IL-33, Calgranulin A (S100A8), Calgranulin C (S100A12), C-reactive protein (CRP), CXCL6 (granulocyte chemotactic protein 2), CXCL13 (B lymphocyte chemoattractant), Eotaxin (CCL11), granulocyte-macrophage colony-stimulating factor (GM-CSF), growth-regulated alpha (GRO-α or CXCL1), HMGB1 (high mobility group box 1), interferon-β (IFN-β), interferon-γ (IFN-γ), interferon-γ-induced protein (IP-10 or CXCL10), interferon-inducible T-cell alpha chemoattractant (I-TAC or CXCL11), macrophage colony-stimulating factor (M-CSF), monocyte chemoattractant protein-1 (MCP-1 or CCL2), macrophage migration inhibitory factor (MIF), monokine induced by IFN-γ (MIG or CXCL9), macrophage inflammatory protein-1α (MIP-1α or CCL3), MIP-1β (CCL4), MIP-3α (CCL20), regulated on activation normally T-cell expressed and secreted (RANTES or CCL5), transforming growth factor-β (TGF-β), tumor necrosis factor-α (TNF-α), and TNF-related apoptosis inducing ligand (TRAIL).
Appendix B. Neonatal Assessment of Infected Fetuses
In the case of continuation of pregnancy, a standardized neonatal check-up of all infected and alive newborns was performed. Neonatal assessment included physical examination, blood tests (full blood count (FBC) and liver function test), hearing tests (otoacoustic emissions (OAE) and/or automated auditory brainstem response (AABR) in case of abnormal OAE), fundoscopic examination (FE), and neonatal transcranial US examination (TUS). In the case of abnormal TUS and clinical examination, a brain MRI was offered. Neonates were asymptomatic if there is no growth restriction (z-score < 1.28 using the intergrowth standards); no abnormal clinical features; no biological abnormalities (thrombocytopenia, hepatic cytolysis, or mixed hyperbilirubinemia); no abnormality on OAE/AABR, FE, or TUS. Fetuses with isolated unilateral minor cerebral features (subependymal cysts and/or calcifications of lenticulostriate vasculopathy) were considered asymptomatic. The others were considered symptomatic. Fetuses harboring at least one severe cerebral feature (cortical abnormalities, ventriculomegaly >15 mm, enlarged percicerebral spaces, or microcephaly) independently of the pregnancy outcome (termination of pregnancy, intrauterine fetal demise, perinatal death, or live birth) were considered severe symptomatic fetuses. In case of termination of pregnancy, intrauterine fetal demise, or perinatal death, a postmortem examination was systematically offered. In all lethal cases, postmortem examination confirmed a severe infection with focal necrosis.
References
- Kenneson, A.; Cannon, M.J. Review and Meta-Analysis of the Epidemiology of Congenital Cytomegalovirus (CMV) Infection. Rev. Med. Virol. 2007, 17, 253–276. [Google Scholar] [CrossRef] [PubMed]
- Leruez-Ville, M.; Foulon, I.; Pass, R.; Ville, Y. Cytomegalovirus Infection during Pregnancy: State of the Science. Am. J. Obstet. Gynecol. 2020, 223, 330–349. [Google Scholar] [CrossRef] [PubMed]
- Smithers-Sheedy, H.; Raynes-Greenow, C.; Badawi, N.; Fernandez, M.A.; Kesson, A.; McIntyre, S.; Leung, K.-C.; Jones, C.A. Congenital Cytomegalovirus among Children with Cerebral Palsy. J. Pediatr. 2017, 181, 267–271.e1. [Google Scholar] [CrossRef] [PubMed]
- Korver, A.M.H.; de Vries, J.J.C.; Konings, S.; de Jong, J.W.; Dekker, F.W.; Vossen, A.C.T.M.; Frijns, J.H.M.; Oudesluys-Murphy, A.M.; DECIBEL collaborative study group. DECIBEL Study: Congenital Cytomegalovirus Infection in Young Children with Permanent Bilateral Hearing Impairment in the Netherlands. J. Clin. Virol. 2009, 46 (Suppl. 4), S27–S31. [Google Scholar] [CrossRef] [PubMed]
- Goderis, J.; De Leenheer, E.; Smets, K.; Van Hoecke, H.; Keymeulen, A.; Dhooge, I. Hearing Loss and Congenital CMV Infection: A Systematic Review. Pediatrics 2014, 134, 972–982. [Google Scholar] [CrossRef]
- Avettand-Fenoël, V.; Marlin, S.; Vauloup-Fellous, C.; Loundon, N.; François, M.; Couloigner, V.; Rouillon, I.; Drouin-Garraud, V.; Laccourreye, L.; Denoyelle, F.; et al. Congenital Cytomegalovirus Is the Second Most Frequent Cause of Bilateral Hearing Loss in Young French Children. J. Pediatr. 2013, 162, 593–599. [Google Scholar] [CrossRef]
- Nance, W.E.; Lim, B.G.; Dodson, K.M. Importance of Congenital Cytomegalovirus Infections as a Cause for Pre-Lingual Hearing Loss. J. Clin. Virol. 2006, 35, 221–225. [Google Scholar] [CrossRef]
- Faure-Bardon, V.; Millischer, A.-E.; Deloison, B.; Sonigo, P.; Grévent, D.; Salomon, L.; Stirnemann, J.; Nicloux, M.; Magny, J.-F.; Leruez-Ville, M.; et al. Refining the Prognosis of Fetuses Infected with Cytomegalovirus in the First Trimester of Pregnancy by Serial Prenatal Assessment: A Single-Centre Retrospective Study. BJOG Int. J. Obstet. Gynaecol. 2020, 127, 355–362. [Google Scholar] [CrossRef]
- Rawlinson, W.D.; Boppana, S.B.; Fowler, K.B.; Kimberlin, D.W.; Lazzarotto, T.; Alain, S.; Daly, K.; Doutré, S.; Gibson, L.; Giles, M.L.; et al. Congenital Cytomegalovirus Infection in Pregnancy and the Neonate: Consensus Recommendations for Prevention, Diagnosis, and Therapy. Lancet Infect. Dis. 2017, 17, e177–e188. [Google Scholar] [CrossRef]
- Dollard, S.C.; Grosse, S.D.; Ross, D.S. New Estimates of the Prevalence of Neurological and Sensory Sequelae and Mortality Associated with Congenital Cytomegalovirus Infection. Rev. Med. Virol. 2007, 17, 355–363. [Google Scholar] [CrossRef]
- Goderis, J.; Keymeulen, A.; Smets, K.; Van Hoecke, H.; De Leenheer, E.; Boudewyns, A.; Desloovere, C.; Kuhweide, R.; Muylle, M.; Royackers, L.; et al. Hearing in Children with Congenital Cytomegalovirus Infection: Results of a Longitudinal Study. J. Pediatr. 2016, 172, 110–115.e2. [Google Scholar] [CrossRef] [PubMed]
- Foulon, I.; Naessens, A.; Foulon, W.; Casteels, A.; Gordts, F. A 10-Year Prospective Study of Sensorineural Hearing Loss in Children with Congenital Cytomegalovirus Infection. J. Pediatr. 2008, 153, 84–88. [Google Scholar] [CrossRef] [PubMed]
- Foulon, I.; De Brucker, Y.; Buyl, R.; Lichtert, E.; Verbruggen, K.; Piérard, D.; Camfferman, F.A.; Gucciardo, L.; Gordts, F. Hearing Loss With Congenital Cytomegalovirus Infection. Pediatrics 2019, 144, e20183095. [Google Scholar] [CrossRef] [PubMed]
- Grosse, S.D.; Ross, D.S.; Dollard, S.C. Congenital Cytomegalovirus (CMV) Infection as a Cause of Permanent Bilateral Hearing Loss: A Quantitative Assessment. J. Clin. Virol. 2008, 41, 57–62. [Google Scholar] [CrossRef]
- Fowler, K.B.; McCollister, F.P.; Dahle, A.J.; Boppana, S.; Britt, W.J.; Pass, R.F. Progressive and Fluctuating Sensorineural Hearing Loss in Children with Asymptomatic Congenital Cytomegalovirus Infection. J. Pediatr. 1997, 130, 624–630. [Google Scholar] [CrossRef]
- Dahle, A.J.; Fowler, K.B.; Wright, J.D.; Boppana, S.B.; Britt, W.J.; Pass, R.F. Longitudinal Investigation of Hearing Disorders in Children with Congenital Cytomegalovirus. J. Am. Acad. Audiol. 2000, 11, 283–290. [Google Scholar] [CrossRef]
- Ross, S.A.; Fowler, K.B.; Ashrith, G.; Stagno, S.; Britt, W.J.; Pass, R.F.; Boppana, S.B. Hearing Loss in Children with Congenital Cytomegalovirus Infection Born to Mothers with Preexisting Immunity. J. Pediatr. 2006, 148, 332–336. [Google Scholar] [CrossRef]
- Leruez-Ville, M.; Magny, J.-F.; Couderc, S.; Pichon, C.; Parodi, M.; Bussières, L.; Guilleminot, T.; Ghout, I.; Ville, Y. Risk Factors for Congenital Cytomegalovirus Infection Following Primary and Nonprimary Maternal Infection: A Prospective Neonatal Screening Study Using Polymerase Chain Reaction in Saliva. Clin. Infect. Dis. 2017, 65, 398–404. [Google Scholar] [CrossRef]
- Mussi-Pinhata, M.M.; Yamamoto, A.Y.; Brito, R.M.M.; de Lima Isaac, M.; de Carvalhoe Oliveira, P.F.; Boppana, S.; Britt, W.J. Birth Prevalence and Natural History of Congenital Cytomegalovirus Infection in a Highly Seroimmune Population. Clin. Infect. Dis. 2009, 49, 522–528. [Google Scholar] [CrossRef]
- Benoist, G.; Salomon, L.; Jacquemard, F.; Daffos, F.; Ville, Y. The Prognostic Value of Ultrasound Abnormalities and Biological Parameters in Blood of Fetuses Infected with Cytomegalovirus. BJOG Int. J. Obstet. Gynaecol. 2008, 115, 823–829. [Google Scholar] [CrossRef]
- Cannie, M.M.; Devlieger, R.; Leyder, M.; Claus, F.; Leus, A.; De Catte, L.; Cossey, V.; Foulon, I.; Van der valk, E.; Foulon, W.; et al. Congenital Cytomegalovirus Infection: Contribution and Best Timing of Prenatal MR Imaging. Eur. Radiol. 2016, 26, 3760–3769. [Google Scholar] [CrossRef]
- Picone, O.; Simon, I.; Benachi, A.; Brunelle, F.; Sonigo, P. Comparison between Ultrasound and Magnetic Resonance Imaging in Assessment of Fetal Cytomegalovirus Infection. Prenat. Diagn. 2008, 28, 753–758. [Google Scholar] [CrossRef] [PubMed]
- Lipitz, S.; Hoffmann, C.; Feldman, B.; Tepperberg-Dikawa, M.; Schiff, E.; Weisz, B. Value of Prenatal Ultrasound and Magnetic Resonance Imaging in Assessment of Congenital Primary Cytomegalovirus Infection. Ultrasound Obstet. Gynecol. 2010, 36, 709–717. [Google Scholar] [CrossRef] [PubMed]
- Leruez-Ville, M.; Stirnemann, J.; Sellier, Y.; Guilleminot, T.; Dejean, A.; Magny, J.-F.; Couderc, S.; Jacquemard, F.; Ville, Y. Feasibility of Predicting the Outcome of Fetal Infection with Cytomegalovirus at the Time of Prenatal Diagnosis. Am. J. Obstet. Gynecol. 2016, 215, 342.e1–342.e9. [Google Scholar] [CrossRef] [PubMed]
- Guerra, B.; Simonazzi, G.; Puccetti, C.; Lanari, M.; Farina, A.; Lazzarotto, T.; Rizzo, N. Ultrasound Prediction of Symptomatic Congenital Cytomegalovirus Infection. Am. J. Obstet. Gynecol. 2008, 198, 380.e1–380.e7. [Google Scholar] [CrossRef] [PubMed]
- Leyder, M.; Vorsselmans, A.; Done, E.; Berkel, K.V.; Faron, G.; Foulon, I.; Naessens, A.; Jansen, A.; Foulon, W.; Gucciardo, L. Primary Maternal Cytomegalovirus Infections: Accuracy of Fetal Ultrasound for Predicting Sequelae in Offspring. Am. J. Obstet. Gynecol. 2016, 215, 638.e1–638.e8. [Google Scholar] [CrossRef]
- Fabbri, E.; Revello, M.G.; Furione, M.; Zavattoni, M.; Lilleri, D.; Tassis, B.; Quarenghi, A.; Rustico, M.; Nicolini, U.; Ferrazzi, E.; et al. Prognostic Markers of Symptomatic Congenital Human Cytomegalovirus Infection in Fetal Blood. BJOG Int. J. Obstet. Gynaecol. 2011, 118, 448–456. [Google Scholar] [CrossRef]
- Griffiths, P.; Reeves, M. Pathogenesis of Human Cytomegalovirus in the Immunocompromised Host. Nat. Rev. Microbiol. 2021, 19, 759–773. [Google Scholar] [CrossRef]
- Faure-Bardon, V.; Magny, J.-F.; Parodi, M.; Couderc, S.; Garcia, P.; Maillotte, A.-M.; Benard, M.; Pinquier, D.; Astruc, D.; Patural, H.; et al. Sequelae of Congenital Cytomegalovirus Following Maternal Primary Infections Are Limited to Those Acquired in the First Trimester of Pregnancy. Clin. Infect. Dis. 2019, 69, 1526–1532. [Google Scholar] [CrossRef] [PubMed]
- Bhatti, G.; Romero, R.; Rice, G.E.; Fitzgerald, W.; Pacora, P.; Gomez-Lopez, N.; Kavdia, M.; Tarca, A.L.; Margolis, L. Compartmentalized Profiling of Amniotic Fluid Cytokines in Women with Preterm Labor. PLoS ONE 2020, 15, e0227881. [Google Scholar] [CrossRef] [PubMed]
- Romero, R.; Grivel, J.-C.; Tarca, A.L.; Chaemsaithong, P.; Xu, Z.; Fitzgerald, W.; Hassan, S.S.; Chaiworapongsa, T.; Margolis, L. Evidence of Perturbations of the Cytokine Network in Preterm Labor. Am. J. Obstet. Gynecol. 2015, 213, 836.e1–836.e18. [Google Scholar] [CrossRef] [PubMed]
- Fitzgerald, W.; Freeman, M.L.; Lederman, M.M.; Vasilieva, E.; Romero, R.; Margolis, L. A System of Cytokines Encapsulated in ExtraCellular Vesicles. Sci. Rep. 2018, 8, 8973. [Google Scholar] [CrossRef] [PubMed]
- Schleiss, M.R. Cytomegalovirus in the Neonate: Immune Correlates of Infection and Protection. Clin. Dev. Immunol. 2013, 2013, 501801. [Google Scholar] [CrossRef] [PubMed]
- Scott, G.M.; Chow, S.S.W.; Craig, M.E.; Pang, C.N.I.; Hall, B.; Wilkins, M.R.; Jones, C.A.; Lloyd, A.R.; Rawlinson, W.D. Cytomegalovirus Infection during Pregnancy with Maternofetal Transmission Induces a Proinflammatory Cytokine Bias in Placenta and Amniotic Fluid. J. Infect. Dis. 2012, 205, 1305–1310. [Google Scholar] [CrossRef] [PubMed]
- Van Niel, G.; D’Angelo, G.; Raposo, G. Shedding Light on the Cell Biology of Extracellular Vesicles. Nat. Rev. Mol. Cell Biol. 2018, 19, 213–228. [Google Scholar] [CrossRef]
- Tkach, M.; Théry, C. Communication by Extracellular Vesicles: Where We Are and Where We Need to Go. Cell 2016, 164, 1226–1232. [Google Scholar] [CrossRef]
- Tetta, C.; Ghigo, E.; Silengo, L.; Deregibus, M.C.; Camussi, G. Extracellular Vesicles as an Emerging Mechanism of Cell-to-Cell Communication. Endocrine 2013, 44, 11–19. [Google Scholar] [CrossRef]
- Keller, S.; Sanderson, M.P.; Stoeck, A.; Altevogt, P. Exosomes: From Biogenesis and Secretion to Biological Function. Immunol. Lett. 2006, 107, 102–108. [Google Scholar] [CrossRef]
- Costa, A.; Quarto, R.; Bollini, S. Small Extracellular Vesicles from Human Amniotic Fluid Samples as Promising Theranostics. Int. J. Mol. Sci. 2022, 23, 590. [Google Scholar] [CrossRef]
- Sheller-Miller, S.; Menon, R. Chapter Ten—Isolation and Characterization of Human Amniotic Fluid-Derived Exosomes. In Methods in Enzymology; Extracellular Vesicles; Spada, S., Galluzzi, L., Eds.; Academic Press: Cambridge, MA, USA, 2020; Volume 645, pp. 181–194. [Google Scholar]
- Sidhom, K.; Obi, P.O.; Saleem, A. A Review of Exosomal Isolation Methods: Is Size Exclusion Chromatography the Best Option? Int. J. Mol. Sci. 2020, 21, 6466. [Google Scholar] [CrossRef]
- Zhao, B.; Zhang, Y.; Han, S.; Zhang, W.; Zhou, Q.; Guan, H.; Liu, J.; Shi, J.; Su, L.; Hu, D. Exosomes Derived from Human Amniotic Epithelial Cells Accelerate Wound Healing and Inhibit Scar Formation. J. Mol. Histol. 2017, 48, 121–132. [Google Scholar] [CrossRef] [PubMed]
- Farhadihosseinabadi, B.; Farahani, M.; Tayebi, T.; Jafari, A.; Biniazan, F.; Modaresifar, K.; Moravvej, H.; Bahrami, S.; Redl, H.; Tayebi, L.; et al. Amniotic Membrane and Its Epithelial and Mesenchymal Stem Cells as an Appropriate Source for Skin Tissue Engineering and Regenerative Medicine. Artif. Cells Nanomed. Biotechnol. 2018, 46, 431–440. [Google Scholar] [CrossRef] [PubMed]
- Tan, J.L.; Lau, S.N.; Leaw, B.; Nguyen, H.P.T.; Salamonsen, L.A.; Saad, M.I.; Chan, S.T.; Zhu, D.; Krause, M.; Kim, C.; et al. Amnion Epithelial Cell-Derived Exosomes Restrict Lung Injury and Enhance Endogenous Lung Repair. Stem Cells Transl. Med. 2018, 7, 180–196. [Google Scholar] [CrossRef] [PubMed]
- Sheller, S.; Papaconstantinou, J.; Urrabaz-Garza, R.; Richardson, L.; Saade, G.; Salomon, C.; Menon, R. Amnion-Epithelial-Cell-Derived Exosomes Demonstrate Physiologic State of Cell under Oxidative Stress. PLoS ONE 2016, 11, e0157614. [Google Scholar] [CrossRef]
- Keller, S.; Rupp, C.; Stoeck, A.; Runz, S.; Fogel, M.; Lugert, S.; Hager, H.-D.; Abdel-Bakky, M.S.; Gutwein, P.; Altevogt, P. CD24 Is a Marker of Exosomes Secreted into Urine and Amniotic Fluid. Kidney Int. 2007, 72, 1095–1102. [Google Scholar] [CrossRef]
- Asea, A.; Jean-Pierre, C.; Kaur, P.; Rao, P.; Linhares, I.M.; Skupski, D.; Witkin, S.S. Heat Shock Protein-Containing Exosomes in Mid-Trimester Amniotic Fluids. J. Reprod. Immunol. 2008, 79, 12–17. [Google Scholar] [CrossRef]
- Mellows, B.; Mitchell, R.; Antonioli, M.; Kretz, O.; Chambers, D.; Zeuner, M.-T.; Denecke, B.; Musante, L.; Ramachandra, D.L.; Debacq-Chainiaux, F.; et al. Protein and Molecular Characterization of a Clinically Compliant Amniotic Fluid Stem Cell-Derived Extracellular Vesicle Fraction Capable of Accelerating Muscle Regeneration Through Enhancement of Angiogenesis. Stem Cells Dev. 2017, 26, 1316–1333. [Google Scholar] [CrossRef]
- Bellio, M.A.; Young, K.C.; Milberg, J.; Santos, I.; Abdullah, Z.; Stewart, D.; Arango, A.; Chen, P.; Huang, J.; Williams, K.; et al. Amniotic Fluid-Derived Extracellular Vesicles: Characterization and Therapeutic Efficacy in an Experimental Model of Bronchopulmonary Dysplasia. Cytotherapy 2021, 23, 1097–1107. [Google Scholar] [CrossRef]
- Tavanasefat, H.; Li, F.; Koyano, K.; Gourtani, B.K.; Marty, V.; Mulpuri, Y.; Lee, S.H.; Shin, K.-H.; Wong, D.T.W.; Xiao, X.; et al. Molecular Consequences of Fetal Alcohol Exposure on Amniotic Exosomal MiRNAs with Functional Implications for Stem Cell Potency and Differentiation. PLoS ONE 2020, 15, e0242276. [Google Scholar] [CrossRef]
- Dixon, C.L.; Sheller-Miller, S.; Saade, G.R.; Fortunato, S.J.; Lai, A.; Palma, C.; Guanzon, D.; Salomon, C.; Menon, R. Amniotic Fluid Exosome Proteomic Profile Exhibits Unique Pathways of Term and Preterm Labor. Endocrinology 2018, 159, 2229–2240. [Google Scholar] [CrossRef]
- Fabietti, I.; Nardi, T.; Favero, C.; Dioni, L.; Cantone, L.; Pergoli, L.; Hoxha, M.; Pinatel, E.; Mosca, F.; Bollati, V.; et al. Extracellular Vesicles and Their MiRNA Content in Amniotic and Tracheal Fluids of Fetuses with Severe Congenital Diaphragmatic Hernia Undergoing Fetal Intervention. Cells 2021, 10, 1493. [Google Scholar] [CrossRef] [PubMed]
- Clement, M.; Humphreys, I.R. Cytokine-Mediated Induction and Regulation of Tissue Damage During Cytomegalovirus Infection. Front. Immunol. 2019, 10, 78. [Google Scholar] [CrossRef] [PubMed]
- Pitti, R.M.; Marsters, S.A.; Ruppert, S.; Donahue, C.J.; Moore, A.; Ashkenazi, A. Induction of Apoptosis by Apo-2 Ligand, a New Member of the Tumor Necrosis Factor Cytokine Family. J. Biol. Chem. 1996, 271, 12687–12690. [Google Scholar] [CrossRef]
- Sheridan, J.; Marsters, S.; Pitti, R.; Gurney, A.; Skubatch, M.; Baldwin, D.; Ramakrishnan, L.; Gray, C.; Baker, K.; Wood, W.; et al. Control of TRAIL-Induced Apoptosis by a Family of Signaling and Decoy Receptors. Science 1997, 277, 818–821. [Google Scholar] [CrossRef]
- Verma, S.; Loewendorf, A.; Wang, Q.; McDonald, B.; Redwood, A.; Benedict, C.A. Inhibition of the TRAIL Death Receptor by CMV Reveals Its Importance in NK Cell-Mediated Antiviral Defense. PLoS Pathog. 2014, 10, e1004268. [Google Scholar] [CrossRef]
- Sedger, L.M.; Shows, D.M.; Blanton, R.A.; Peschon, J.J.; Goodwin, R.G.; Cosman, D.; Wiley, S.R. IFN-Gamma Mediates a Novel Antiviral Activity through Dynamic Modulation of TRAIL and TRAIL Receptor Expression. J. Immunol. Baltim. 1999, 163, 920–926. [Google Scholar]
- Wu, Z.; Sinzger, C.; Frascaroli, G.; Reichel, J.; Bayer, C.; Wang, L.; Schirmbeck, R.; Mertens, T. Human Cytomegalovirus-Induced NKG2Chi CD57hi Natural Killer Cells Are Effectors Dependent on Humoral Antiviral Immunity. J. Virol. 2013, 87, 7717–7725. [Google Scholar] [CrossRef]
- Lopez-Vergès, S.; Milush, J.M.; Schwartz, B.S.; Pando, M.J.; Jarjoura, J.; York, V.A.; Houchins, J.P.; Miller, S.; Kang, S.-M.; Norris, P.J.; et al. Expansion of a Unique CD57+NKG2Chi Natural Killer Cell Subset during Acute Human Cytomegalovirus Infection. Proc. Natl. Acad. Sci. USA 2011, 108, 14725–14732. [Google Scholar] [CrossRef]
- Wang, D.; Bresnahan, W.; Shenk, T. Human Cytomegalovirus Encodes a Highly Specific RANTES Decoy Receptor. Proc. Natl. Acad. Sci. USA 2004, 101, 16642–16647. [Google Scholar] [CrossRef]
- Mansfield, A.S.; Nevala, W.K.; Dronca, R.S.; Leontovich, A.A.; Shuster, L.; Markovic, S.N. Normal Ageing Is Associated with an Increase in Th2 Cells, MCP-1 (CCL1) and RANTES (CCL5), with Differences in SCD40L and PDGF-AA between Sexes. Clin. Exp. Immunol. 2012, 170, 186–193. [Google Scholar] [CrossRef]
- Bernasconi, S.; Cinque, P.; Peri, G.; Sozzani, S.; Crociati, A.; Torri, W.; Vicenzi, E.; Vago, L.; Lazzarin, A.; Poli, G.; et al. Selective Elevation of Monocyte Chemotactic Protein-1 in the Cerebrospinal Fluid of AIDS Patients with Cytomegalovirus Encephalitis. J. Infect. Dis. 1996, 174, 1098–1101. [Google Scholar] [CrossRef] [PubMed]
- Van de Veerdonk, F.L.; Netea, M.G.; Dinarello, C.A.; Joosten, L.A.B. Inflammasome Activation and IL-1β and IL-18 Processing during Infection. Trends Immunol. 2011, 32, 110–116. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.; Callaway, J.B.; Ting, J.P.-Y. Inflammasomes: Mechanism of Action, Role in Disease, and Therapeutics. Nat. Med. 2015, 21, 677–687. [Google Scholar] [CrossRef]
- Shi, X.; Dong, Y.; Li, Y.; Zhao, Z.; Li, H.; Qiu, S.; Li, Y.; Guo, W.; Qiao, Y. Inflammasome Activation in Mouse Inner Ear in Response to MCMV Induced Hearing Loss. J. Otol. 2015, 10, 143–149. [Google Scholar] [CrossRef] [PubMed]
- Hokeness, K.L.; Deweerd, E.S.; Munks, M.W.; Lewis, C.A.; Gladue, R.P.; Salazar-Mather, T.P. CXCR3-Dependent Recruitment of Antigen-Specific T Lymphocytes to the Liver during Murine Cytomegalovirus Infection. J. Virol. 2007, 81, 1241–1250. [Google Scholar] [CrossRef]
- Basílio-Queirós, D.; Venturini, L.; Luther-Wolf, S.; Dammann, E.; Ganser, A.; Stadler, M.; Falk, C.S.; Weissinger, E.M. Adaptive NK Cells Undergo a Dynamic Modulation in Response to Human Cytomegalovirus and Recruit T Cells in in Vitro Migration Assays. Bone Marrow Transplant. 2022, 57, 712–720. [Google Scholar] [CrossRef]
- Van de Berg, P.J.; Heutinck, K.M.; Raabe, R.; Minnee, R.C.; Young, S.L.; van Donselaar-van der Pant, K.A.; Bemelman, F.J.; van Lier, R.A.; ten Berge, I.J. Human Cytomegalovirus Induces Systemic Immune Activation Characterized by a Type 1 Cytokine Signature. J. Infect. Dis. 2010, 202, 690–699. [Google Scholar] [CrossRef]
- Cheeran, M.C.; Hu, S.; Yager, S.L.; Gekker, G.; Peterson, P.K.; Lokensgard, J.R. Cytomegalovirus Induces Cytokine and Chemokine Production Differentially in Microglia and Astrocytes: Antiviral Implications. J. Neurovirol. 2001, 7, 135–147. [Google Scholar] [CrossRef]
- Lee, J.; Choi, J.A.; Ju, H.-H.; Kim, J.-E.; Paik, S.-Y.; Rao, P.V. Role of MCP-1 and IL-8 in Viral Anterior Uveitis, and Contractility and Fibrogenic Activity of Trabecular Meshwork Cells. Sci. Rep. 2021, 11, 14950. [Google Scholar] [CrossRef]
- Rott, D.; Zhu, J.; Zhou, Y.F.; Burnett, M.S.; Zalles-Ganley, A.; Epstein, S.E. IL-6 Is Produced by Splenocytes Derived from CMV-Infected Mice in Response to CMV Antigens, and Induces MCP-1 Production by Endothelial Cells: A New Mechanistic Paradigm for Infection-Induced Atherogenesis. Atherosclerosis 2003, 170, 223–228. [Google Scholar] [CrossRef]
- Froberg, M.K.; Dannen, D.; Adams, A.; Parker-Thornburg, J.; Kolattukudy, P. Murine Cytomegalovirus Infection Markedly Reduces Serum MCP-1 Levels in MCP-1 Transgenic Mice. Ann. Clin. Lab. Sci. 2006, 36, 179–184. [Google Scholar] [PubMed]
- Alcendor, D.J.; Charest, A.M.; Zhu, W.Q.; Vigil, H.E.; Knobel, S.M. Infection and Upregulation of Proinflammatory Cytokines in Human Brain Vascular Pericytes by Human Cytomegalovirus. J. Neuroinflamm. 2012, 9, 95. [Google Scholar] [CrossRef] [PubMed]
- Talaya, A.; Giménez, E.; Vinuesa, V.; Pérez, A.; Amat, P.; Piñana, J.L.; Albert, E.; Hernández-Boluda, J.C.; Solano, C.; Navarro, D. Kinetics of Inflammatory Biomarkers in Plasma Predict the Occurrence and Features of Cytomegalovirus DNAemia Episodes in Allogeneic Hematopoietic Stem Cell Transplant Recipients. Med. Microbiol. Immunol. 2019, 208, 405–414. [Google Scholar] [CrossRef] [PubMed]
- Karason, K.; Jernås, M.; Hägg, D.A.; Svensson, P.-A. Evaluation of CXCL9 and CXCL10 as Circulating Biomarkers of Human Cardiac Allograft Rejection. BMC Cardiovasc. Disord. 2006, 6, 29. [Google Scholar] [CrossRef][Green Version]
- Poole, E.; Neves, T.C.; Oliveira, M.T.; Sinclair, J.; da Silva, M.C.C. Human Cytomegalovirus Interleukin 10 Homologs: Facing the Immune System. Front. Cell. Infect. Microbiol. 2020, 10, 245. [Google Scholar] [CrossRef]
- Lučin, P.; Mahmutefendić, H.; Blagojević Zagorac, G.; Ilić Tomaš, M. Cytomegalovirus Immune Evasion by Perturbation of Endosomal Trafficking. Cell. Mol. Immunol. 2015, 12, 154–169. [Google Scholar] [CrossRef]
- Billstrom, M.A.; Lehman, L.A.; Scott Worthen, G. Depletion of Extracellular RANTES during Human Cytomegalovirus Infection of Endothelial Cells. Am. J. Respir. Cell Mol. Biol. 1999, 21, 163–167. [Google Scholar] [CrossRef]
- Desveaux, C.; Klein, J.; Leruez-Ville, M.; Ramirez-Torres, A.; Lacroix, C.; Breuil, B.; Froment, C.; Bascands, J.-L.; Schanstra, J.P.; Ville, Y. Identification of Symptomatic Fetuses Infected with Cytomegalovirus Using Amniotic Fluid Peptide Biomarkers. PLOS Pathog. 2016, 12, e1005395. [Google Scholar] [CrossRef]
- Vorontsov, O.; Levitt, L.; Lilleri, D.; Vainer, G.W.; Kaplan, O.; Schreiber, L.; Arossa, A.; Spinillo, A.; Furione, M.; Alfi, O.; et al. Amniotic Fluid Biomarkers Predict the Severity of Congenital Cytomegalovirus Infection. J. Clin. Investig. 2022, 132, e157415. [Google Scholar] [CrossRef]
- Jacquemard, F.; Yamamoto, M.; Costa, J.-M.; Romand, S.; Jaqz-Aigrain, E.; Dejean, A.; Daffos, F.; Ville, Y. Maternal Administration of Valaciclovir in Symptomatic Intrauterine Cytomegalovirus Infection. BJOG Int. J. Obstet. Gynaecol. 2007, 114, 1113–1121. [Google Scholar] [CrossRef]
- Shahar-Nissan, K.; Pardo, J.; Peled, O.; Krause, I.; Bilavsky, E.; Wiznitzer, A.; Hadar, E.; Amir, J. Valaciclovir to Prevent Vertical Transmission of Cytomegalovirus after Maternal Primary Infection during Pregnancy: A Randomised, Double-Blind, Placebo-Controlled Trial. Lancet 2020, 396, 779–785. [Google Scholar] [CrossRef]
- Faure-Bardon, V.; Fourgeaud, J.; Guilleminot, T.; Magny, J.-F.; Salomon, L.J.; Bernard, J.-P.; Leruez-Ville, M.; Ville, Y. First-Trimester Diagnosis of Congenital Cytomegalovirus Infection after Maternal Primary Infection in Early Pregnancy: Feasibility Study of Viral Genome Amplification by PCR on Chorionic Villi Obtained by CVS. Ultrasound Obstet. Gynecol. 2021, 57, 568–572. [Google Scholar] [CrossRef] [PubMed]
- Schubert, S.M.; Arendt, L.M.; Zhou, W.; Baig, S.; Walter, S.R.; Buchsbaum, R.J.; Kuperwasser, C.; Walt, D.R. Ultra-Sensitive Protein Detection via Single Molecule Arrays towards Early Stage Cancer Monitoring. Sci. Rep. 2015, 5, 11034. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).