Are There Still Difficult-to-Treat Patients with Chronic Hepatitis C in the Era of Direct-Acting Antivirals?
Abstract
:1. Introduction
2. Materials and Methods
Statistical Analysis
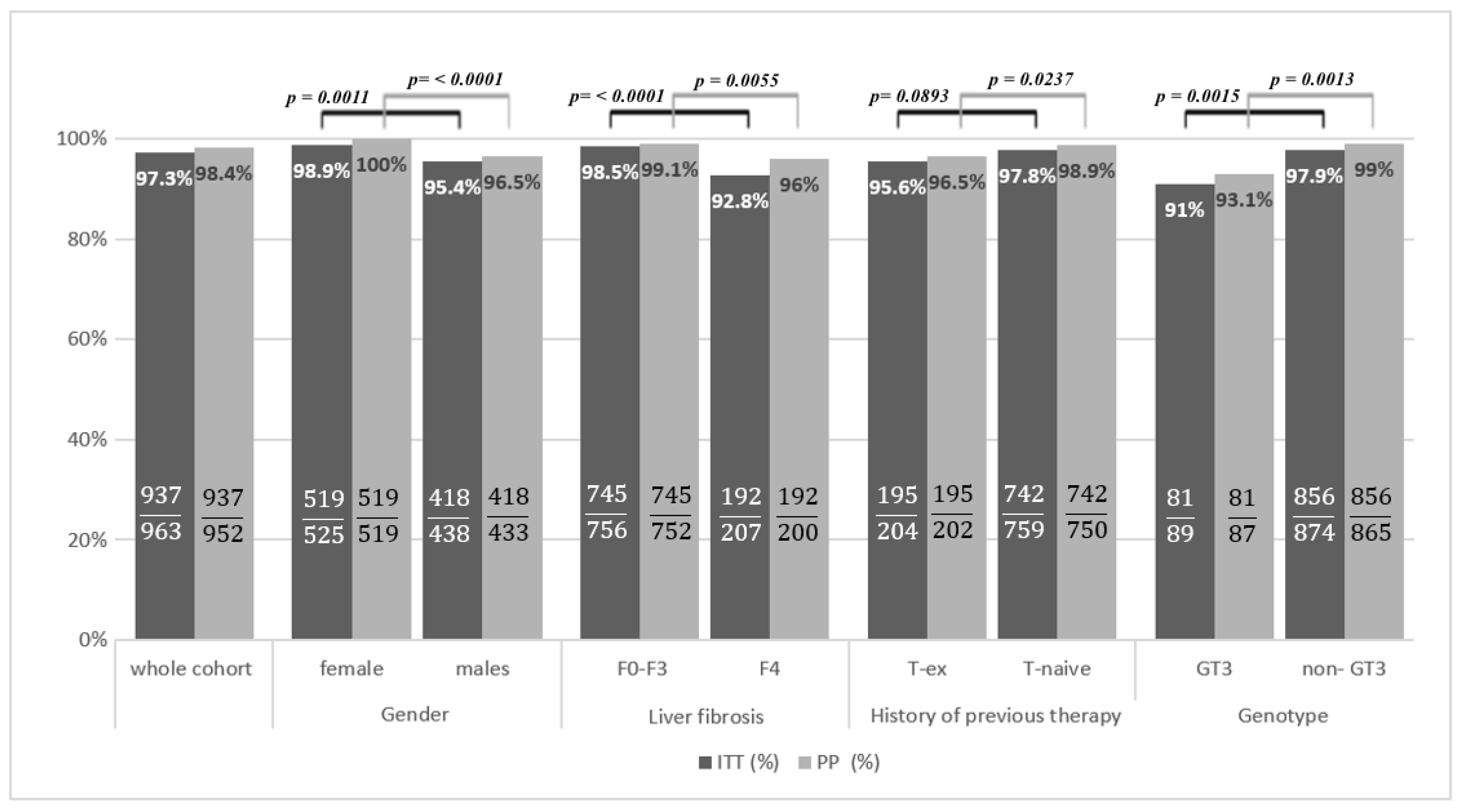
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Boerekamps, A.; Vanwolleghem, T.; Valk, M.; van der Berk, G.E.; van den Kasteren, M.; van Posthouwer, D.; Dofferhoff, A.S.M.; Hoek, B.; van Ramsoekh, D.; Koopsen, J.; et al. 8 Weeks of Sofosbuvir/Ledipasvir Is Effective in DAA-Naive Non-Cirrhotic HCV Genotype 4 Infected Patients (HEPNED-001 Study). J. Hepatol. 2019, 70, 554–557. [Google Scholar] [CrossRef] [Green Version]
- Karlsen, T.H.; Sheron, N.; Zelber-Sagi, S.; Carrieri, P.; Dusheiko, G.; Bugianesi, E.; Pryke, R.; Hutchinson, S.J.; Sangro, B.; Martin, N.K.; et al. The EASL-Lancet Liver Commission: Protecting the next Generation of Europeans against Liver Disease Complications and Premature Mortality. Lancet 2021, 399, 61–116. [Google Scholar] [CrossRef]
- Flisiak, R.; Zarębska-Michaluk, D.; Janczewska, E.; Łapiński, T.; Rogalska, M.; Karpińska, E.; Mikuła, T.; Bolewska, B.; Białkowska, J.; Flejscher-Stępniewska, K.; et al. Five-Year Follow-Up of Cured HCV Patients under Real-World Interferon-Free Therapy. Cancers 2021, 13, 3694. [Google Scholar] [CrossRef] [PubMed]
- Polaris Observatory HCV Collaborators Global Prevalence and Genotype Distribution of Hepatitis C Virus Infection in 2015: A Modelling Study. Lancet Gastroenterol. Hepatol. 2017, 2, 161–176. [CrossRef] [Green Version]
- Manns, M.P.; McHutchison, J.G.; Gordon, S.C.; Rustgi, V.K.; Shiffman, M.; Reindollar, R.; Goodman, Z.D.; Koury, K.; Ling, M.; Albrecht, J.K. Peginterferon Alfa-2b plus Ribavirin Compared with Interferon Alfa-2b plus Ribavirin for Initial Treatment of Chronic Hepatitis C: A Randomised Trial. Lancet 2001, 358, 958–965. [Google Scholar] [CrossRef]
- Fried, M.W.; Shiffman, M.L.; Reddy, K.R.; Smith, C.; Marinos, G.; Gonçales, F.L.; Häussinger, D.; Diago, M.; Carosi, G.; Dhumeaux, D.; et al. Peginterferon Alfa-2a plus Ribavirin for Chronic Hepatitis C Virus Infection. N. Engl. J. Med. 2002, 347, 975–982. [Google Scholar] [CrossRef] [Green Version]
- Hadziyannis, S.J.; Sette, H.; Morgan, T.R.; Balan, V.; Diago, M.; Marcellin, P.; Ramadori, G.; Bodenheimer, H.; Bernstein, D.; Rizzetto, M.; et al. Peginterferon-Alpha2a and Ribavirin Combination Therapy in Chronic Hepatitis C: A Randomized Study of Treatment Duration and Ribavirin Dose. Ann. Intern. Med. 2004, 140, 346–355. [Google Scholar] [CrossRef] [PubMed]
- Flisiak, R.; Pogorzelska, J.; Berak, H.; Horban, A.; Orłowska, I.; Simon, K.; Tuchendler, E.; Madej, G.; Piekarska, A.; Jabłkowski, M.; et al. Efficacy of HCV Treatment in Poland at the Turn of the Interferon Era—The EpiTer Study. Clin. Exp. Hepatol. 2016, 2, 138–143. [Google Scholar] [CrossRef] [Green Version]
- Sulkowski, M.S.; Cooper, C.; Hunyady, B.; Jia, J.; Ogurtsov, P.; Peck-Radosavljevic, M.; Shiffman, M.L.; Yurdaydin, C.; Dalgard, O. Management of Adverse Effects of Peg-IFN and Ribavirin Therapy for Hepatitis C. Nat. Rev. Gastroenterol. Hepatol. 2011, 8, 212–223. [Google Scholar] [CrossRef]
- Grebely, J.; Oser, M.; Taylor, L.E.; Dore, G.J. Breaking down the Barriers to Hepatitis C Virus (HCV) Treatment among Individuals with HCV/HIV Coinfection: Action Required at the System, Provider, and Patient Levels. J. Infect. Dis. 2013, 207 (Suppl. S1), S19–S25. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hézode, C.; Fontaine, H.; Dorival, C.; Larrey, D.; Zoulim, F.; Canva, V.; de Ledinghen, V.; Poynard, T.; Samuel, D.; Bourlière, M.; et al. Triple Therapy in Treatment-Experienced Patients with HCV-Cirrhosis in a Multicentre Cohort of the French Early Access Programme (ANRS CO20-CUPIC)-NCT01514890. J. Hepatol. 2013, 59, 434–441. [Google Scholar] [CrossRef] [PubMed]
- Janczewska, E.; Flisiak, R.; Zarebska-Michaluk, D.; Kozielewicz, D.; Berak, H.; Dobracka, B.; Librant-Suska, M.; Lojewski, W.; Jurczyk, K.; Musialik, J.; et al. Effect of Peginterferon or Ribavirin Dosing on Efficacy of Therapy with Telaprevir in Treatment-Experienced Patients With Chronic Hepatitis C and Advanced Liver Fibrosis: A Multicenter Cohort Study. Medicine 2015, 94, e1411. [Google Scholar] [CrossRef] [PubMed]
- Jacobson, I.; Zeuzem, S.; Flisiak, R.; Knysz, B.; Lueth, S.; Zarebska-Michaluk, D.; Janczewska, E.; Ferenci, P.; Diago, M.; Zignego, A.L.; et al. Daclatasvir vs Telaprevir plus Peginterferon Alfa/Ribavirin for Hepatitis C Virus Genotype 1. World J. Gastroenterol. 2016, 22, 3418–3431. [Google Scholar] [CrossRef] [PubMed]
- Cui, X.; Kong, Y.; Jia, J. Efficacy and Safety of Simeprevir in Combination with Peginterferon and Ribavirin for Patients with Hepatitis C Genotype 1 Infection: A Meta-Analysis of Randomized Trials. Rev. Esp. Enferm. Dig. 2015, 107, 591–597. [Google Scholar] [CrossRef] [Green Version]
- Asselah, T.; Marcellin, P. Second-Wave IFN-Based Triple Therapy for HCV Genotype 1 Infection: Simeprevir, Faldaprevir and Sofosbuvir. Liver Int. 2014, 34 (Suppl. S1), 60–68. [Google Scholar] [CrossRef]
- Zarębska-Michaluk, D.; Jaroszewicz, J.; Pabjan, P.; Łapiński, T.W.; Mazur, W.; Krygier, R.; Dybowska, D.; Halota, W.; Pawłowska, M.; Janczewska, E.; et al. Is an 8-Week Regimen of Glecaprevir/Pibrentasvir Sufficient for All Hepatitis C Virus Infected Patients in the Real-World Experience? J. Gastroenterol. Hepatol. 2021, 36, 1944–1952. [Google Scholar] [CrossRef] [PubMed]
- Halota, W.; Flisiak, R.; Boroń-Kaczmarska, A.; Juszczyk, J.; Pawłowska, M.; Simon, K.; Tomasiewicz, K.; Małkowski, P.; Polish Group of HCV Experts. Recommendations for the Treatment of Hepatitis C Polish Group of HCV Experts—2015. Przegl Epidemiol. 2015, 69, 515–521, 625–631. [Google Scholar] [PubMed]
- Polish Group of Experts for HCV; Halota, W.; Flisiak, R.; Juszczyk, J.; Małkowski, P.; Pawłowska, M.; Simon, K.; Tomasiewicz, K. Recommendations for the Treatment of Hepatitis C in 2017. Clin. Exp. Hepatol. 2017, 3, 47–55. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Halota, W.; Flisiak, R.; Juszczyk, J.; Małkowski, P.; Pawłowska, M.; Simon, K.; Tomasiewicz, K. Recommendations of the Polish Group of Experts for HCV for the Treatment of Hepatitis C in 2020. Clin. Exp. Hepatol. 2020, 6, 163–169. [Google Scholar] [CrossRef] [PubMed]
- European Association for the Study of the Liver. EASL Recommendations on Treatment of Hepatitis C: Final Update of the Series☆. J. Hepatol. 2020, 73, 1170–1218. [Google Scholar] [CrossRef]
- Hézode, C. Treatment of Hepatitis C: Results in Real Life. Liver Int. 2018, 38 (Suppl. S1), 21–27. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Janczewska, E.; Kołek, M.F.; Lorenc, B.; Klapaczyński, J.; Tudrujek-Zdunek, M.; Sitko, M.; Mazur, W.; Zarębska-Michaluk, D.; Buczyńska, I.; Dybowska, D.; et al. Factors Influencing the Failure of Interferon-Free Therapy for Chronic Hepatitis C: Data from the Polish EpiTer-2 Cohort Study. WJG 2021, 27, 2177–2192. [Google Scholar] [CrossRef] [PubMed]
- Lawitz, E.; Flisiak, R.; Abunimeh, M.; Sise, M.E.; Park, J.Y.; Kaskas, M.; Bruchfeld, A.; Wörns, M.-A.; Aglitti, A.; Zamor, P.J.; et al. Efficacy and Safety of Glecaprevir/Pibrentasvir in Renally Impaired Patients with Chronic HCV Infection. Liver Int. 2020, 40, 1032–1041. [Google Scholar] [CrossRef] [PubMed]
- Tronina, O.; Durlik, M.; Orłowska, I.; Lorenc, B.; Łapiński, T.W.; Garlicki, A.; Dybowska, D.; Zarębska-Michaluk, D.; Tudrujek-Zdunek, M.; Citko, J.; et al. Real-World Direct-Acting Antiviral Treatment in Kidney Transplant and Hemodialysis Patients: The EpiTer-2 Multicenter Observational Study. Ann. Gastroenterol. 2021, 34, 438–446. [Google Scholar] [CrossRef]
- Wiegand, J.; Buggisch, P.; Mauss, S.; Boeker, K.H.W.; Klinker, H.; Müller, T.; Günther, R.; Serfert, Y.; Manns, M.P.; Zeuzem, S.; et al. Hepatitis C Therapy with Direct Antiviral Agents in Patients with Advanced Chronic Kidney Disease: Real-World Experience of the German Hepatitis C-Registry (Deutsches Hepatitis C-Register). Eur. J. Gastroenterol. Hepatol. 2019, 31, 1424–1431. [Google Scholar] [CrossRef]
- Zarębska-Michaluk, D.; Jaroszewicz, J.; Buczyńska, I.; Simon, K.; Lorenc, B.; Tudrujek-Zdunek, M.; Tomasiewicz, K.; Sitko, M.; Garlicki, A.; Janczewska, E.; et al. Real-World Experience with Grazoprevir/Elbasvir in the Treatment of Previously “Difficult to Treat” Patients Infected with Hepatitis C Virus Genotype 1 and 4. J. Gastroenterol. Hepatol. 2020, 35, 1238–1246. [Google Scholar] [CrossRef]
- Fabrizi, F.; Cerutti, R.; Dixit, V.; Ridruejo, E. Sofosbuvir-based regimens for HCV in stage 4–stage 5 chronic kidney disease. A systematic review with meta-analysis. Nefrología 2021, 41, 578–589. [Google Scholar] [CrossRef]
- Piekarska, A.; Jabłonowska, E.; Garlicki, A.; Sitko, M.; Mazur, W.; Jaroszewicz, J.; Czauz-Andrzejuk, A.; Buczyńska, I.; Simon, K.; Lorenc, B.; et al. Real Life Results of Direct Acting Antiviral Therapy for HCV Infection in HIV–HCV-Coinfected Patients: Epi-Ter2 Study. AIDS Care 2020, 32, 762–769. [Google Scholar] [CrossRef]
- Patel, S.V.; Jayaweera, D.T.; Althoff, K.N.; Eron, J.J.; Radtchenko, J.; Mills, A.; Moyle, G.; Santiago, S.; Sax, P.E.; Gillman, J.; et al. Real-World Efficacy of Direct Acting Antiviral Therapies in Patients with HIV/HCV. PLoS ONE 2020, 15, e0228847. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, H.; Abushouk, A.I.; Menshawy, A.; Attia, A.; Mohamed, A.; Negida, A.; Abdel-Daim, M.M. Meta-Analysis of Grazoprevir plus Elbasvir for Treatment of Hepatitis C Virus Genotype 1 Infection. Ann. Hepatol. 2018, 17, 18–32. [Google Scholar] [CrossRef] [PubMed]
- Poordad, F.; Hezode, C.; Trinh, R.; Kowdley, K.V.; Zeuzem, S.; Agarwal, K.; Shiffman, M.L.; Wedemeyer, H.; Berg, T.; Yoshida, E.M.; et al. ABT-450/r-Ombitasvir and Dasabuvir with Ribavirin for Hepatitis C with Cirrhosis. N. Engl. J. Med. 2014, 370, 1973–1982. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Feld, J.J.; Moreno, C.; Trinh, R.; Tam, E.; Bourgeois, S.; Horsmans, Y.; Elkhashab, M.; Bernstein, D.E.; Younes, Z.; Reindollar, R.W.; et al. Sustained Virologic Response of 100% in HCV Genotype 1b Patients with Cirrhosis Receiving Ombitasvir/Paritaprevir/r and Dasabuvir for 12weeks. J. Hepatol. 2016, 64, 301–307. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reddy, K.R.; Bourlière, M.; Sulkowski, M.; Omata, M.; Zeuzem, S.; Feld, J.J.; Lawitz, E.; Marcellin, P.; Welzel, T.M.; Hyland, R.; et al. Ledipasvir and Sofosbuvir in Patients with Genotype 1 Hepatitis C Virus Infection and Compensated Cirrhosis: An Integrated Safety and Efficacy Analysis. Hepatology 2015, 62, 79–86. [Google Scholar] [CrossRef] [PubMed]
- Gane, E.; Poordad, F.; Zadeikis, N.; Valdes, J.; Lin, C.-W.; Liu, W.; Asatryan, A.; Wang, S.; Stedman, C.; Greenbloom, S.; et al. Safety and Pharmacokinetics of Glecaprevir/Pibrentasvir in Adults With Chronic Genotype 1–6 Hepatitis C Virus Infections and Compensated Liver Disease. Clin. Infect. Dis. 2019, 69, 1657–1664. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brown, R.S.; Buti, M.; Rodrigues, L.; Chulanov, V.; Chuang, W.-L.; Aguilar, H.; Horváth, G.; Zuckerman, E.; Carrion, B.R.; Rodriguez-Perez, F.; et al. Glecaprevir/Pibrentasvir for 8 weeks in Treatment-Naïve Patients with Chronic HCV Genotypes 1–6 and Compensated Cirrhosis: The EXPEDITION-8 Trial. J. Hepatol. 2020, 72, 441–449. [Google Scholar] [CrossRef] [Green Version]
- Feld, J.J.; Jacobson, I.M.; Hézode, C.; Asselah, T.; Ruane, P.J.; Gruener, N.; Abergel, A.; Mangia, A.; Lai, C.-L.; Chan, H.L.Y.; et al. Sofosbuvir and Velpatasvir for HCV Genotype 1, 2, 4, 5, and 6 Infection. N. Engl. J. Med. 2015, 373, 2599–2607. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Foster, G.R.; Afdhal, N.; Roberts, S.K.; Bräu, N.; Gane, E.J.; Pianko, S.; Lawitz, E.; Thompson, A.; Shiffman, M.L.; Cooper, C.; et al. Sofosbuvir and Velpatasvir for HCV Genotype 2 and 3 Infection. N. Engl. J. Med. 2015, 373, 2608–2617. [Google Scholar] [CrossRef] [Green Version]
- Flisiak, R.; Janczewska, E.; Wawrzynowicz-Syczewska, M.; Jaroszewicz, J.; Zarębska-Michaluk, D.; Nazzal, K.; Bolewska, B.; Bialkowska, J.; Berak, H.; Fleischer-Stępniewska, K.; et al. Real-World Effectiveness and Safety of Ombitasvir/Paritaprevir/Ritonavir ± Dasabuvir ± Ribavirin in Hepatitis C: AMBER Study. Aliment. Pharmacol. Ther. 2016, 44, 946–956. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xia, H.; Lu, C.; Wang, Y.; Zaongo, S.D.; Hu, Y.; Wu, Y.; Yan, Z.; Ma, P. Efficacy and Safety of Direct-Acting Antiviral Therapy in Patients With Chronic Hepatitis C Virus Infection: A Real-World Single-Center Experience in Tianjin, China. Front. Pharmacol. 2020, 11, 710. [Google Scholar] [CrossRef]
- Naguib, G.G.; Farid, A.; Hassan, M.; Elshafie, A.; Shazly, Y.E.; Shaker, M.K.; Ezzat, H.; Safwat, E.; Ahmed, O.A.; Dabbous, H.; et al. Direct-Acting Antiviral Regimens in Egyptian Patients with Chronic Hepatitis C Virus Infection: A Real-World Single-Center Experience. Arab J. Gastroenterol. 2021, 22, 285–291. [Google Scholar] [CrossRef]
- Curry, M.P.; O’Leary, J.G.; Bzowej, N.; Muir, A.J.; Korenblat, K.M.; Fenkel, J.M.; Reddy, K.R.; Lawitz, E.; Flamm, S.L.; Schiano, T.; et al. Sofosbuvir and Velpatasvir for HCV in Patients with Decompensated Cirrhosis. N. Engl. J. Med. 2015, 373, 2618–2628. [Google Scholar] [CrossRef]
- Yek, C.; de la Flor, C.; Marshall, J.; Zoellner, C.; Thompson, G.; Quirk, L.; Mayorga, C.; Turner, B.J.; Singal, A.G.; Jain, M.K. Effectiveness of Direct-Acting Antiviral Therapy for Hepatitis C in Difficult-to-Treat Patients in a Safety-Net Health System: A Retrospective Cohort Study. BMC Med. 2017, 15, 204. [Google Scholar] [CrossRef] [PubMed]
- Reiberger, T.; Rutter, K.; Ferlitsch, A.; Payer, B.A.; Hofer, H.; Beinhardt, S.; Kundi, M.; Ferenci, P.; Gangl, A.; Trauner, M.; et al. Portal pressure predicts outcome and safety of antiviral therapy in cirrhotic patients with hepatitis C virus infection. Clin. Gastroenterol. Hepatol. 2011, 9, 602–608.e1. [Google Scholar] [CrossRef] [PubMed]
- Dietz, J.; Spengler, U.; Müllhaupt, B.; Wiesch, J.S.Z.; Piecha, F.; Mauss, S.; Seegers, B.; Hinrichsen, H.; Antoni, C.; Wietzke-Braun, P.; et al. Efficacy of Retreatment After Failed Direct-Acting Antiviral Therapy in Patients with HCV Genotype 1–3 Infections. Clin. Gastroenterol. Hepatol. 2021, 19, 195–198.e2. [Google Scholar] [CrossRef] [PubMed]
- Zarębska-Michaluk, D.; Buczyńska, I.; Simon, K.; Tudrujek-Zdunek, M.; Janczewska, E.; Dybowska, D.; Sitko, M.; Dobracka, B.; Jaroszewicz, J.; Pabjan, P.; et al. Real World Experience of Chronic Hepatitis C Retreatment with Genotype Specific Regimens in Nonresponders to Previous Interferon-Free Therapy. Can. J. Gastroenterol. Hepatol. 2019, 2019, 4029541. [Google Scholar] [CrossRef] [PubMed]
- Schmitt, A.; Günther, R.; Mauss, S.; Boeker, K.H.W.; Buggisch, P.; Hillenbrand, H.; John, C.; Klinker, H.; Pathil, A.; Simon, K.-G.; et al. Treatment-Failure to Direct Antiviral HCV Regimens in Real World: Frequency, Patient Characteristics and Rescue Therapy—Data from the German Hepatitis C Registry (DHC-R). Z. Gastroenterol. 2020, 58, 341–351. [Google Scholar] [CrossRef] [PubMed]
- Sarrazin, C. Treatment Failure with DAA Therapy: Importance of Resistance. J. Hepatol. 2021, 74, 1472–1482. [Google Scholar] [CrossRef] [PubMed]
- Di Maio, V.C.; Cento, V.; Lenci, I.; Aragri, M.; Rossi, P.; Barbaliscia, S.; Melis, M.; Verucchi, G.; Magni, C.F.; Teti, E.; et al. Multiclass HCV Resistance to Direct-Acting Antiviral Failure in Real-Life Patients Advocates for Tailored Second-Line Therapies. Liver Int. 2017, 37, 514–528. [Google Scholar] [CrossRef] [PubMed]
- Di Maio, V.C.; Barbaliscia, S.; Teti, E.; Fiorentino, G.; Milana, M.; Paolucci, S.; Pollicino, T.; Morsica, G.; Starace, M.; Bruzzone, B.; et al. Resistance Analysis and Treatment Outcomes in Hepatitis C Virus Genotype 3-Infected Patients within the Italian Network VIRONET-C. Liver Int. 2021, 41, 1802–1814. [Google Scholar] [CrossRef] [PubMed]
- Mawatari, S.; Oda, K.; Kumagai, K.; Tabu, K.; Ijuin, S.; Fujisaki, K.; Inada, Y.; Uto, H.; Saisyoji, A.; Hiramine, Y.; et al. Viral and Host Factors Are Associated with Retreatment Failure in Hepatitis C Patients Receiving All-Oral Direct Antiviral Therapy. Hepatol. Res. 2020, 50, 453–465. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Mao, X.; Yu, K.; Suo, C.; Jin, L.; Zhang, T.; Chen, X. Prevalence of HCV Resistance-Associated Substitutions among Treatment-Failure Patients Receiving Direct-Acting Antiviral Agents. J. Viral Hepat. 2020, 27, 585–592. [Google Scholar] [CrossRef]
- Degasperi, E.; Spinetti, A.; Lombardi, A.; Landonio, S.; Rossi, M.C.; Pasulo, L.; Pozzoni, P.; Giorgini, A.; Fabris, P.; Romano, A.; et al. Real-Life Effectiveness and Safety of Sofosbuvir/Velpatasvir/Voxilaprevir in Hepatitis C Patients with Previous DAA Failure. J. Hepatol. 2019, 71, 1106–1115. [Google Scholar] [CrossRef] [PubMed]
- Belperio, P.S.; Shahoumian, T.A.; Loomis, T.P.; Backus, L.I. Real-World Effectiveness of Sofosbuvir/Velpatasvir/Voxilaprevir in 573 Direct-Acting Antiviral Experienced Hepatitis C Patients. J. Viral Hepat. 2019, 26, 980–990. [Google Scholar] [CrossRef] [PubMed]
- Bourlière, M.; Gordon, S.C.; Flamm, S.L.; Cooper, C.L.; Ramji, A.; Tong, M.; Ravendhran, N.; Vierling, J.M.; Tran, T.T.; Pianko, S.; et al. POLARIS-1 and POLARIS-4 Investigators. Sofosbuvir, Velpatasvir, and Voxilaprevir for Previously Treated HCV Infection. N. Engl. J. Med. 2017, 376, 2134–2146. [Google Scholar] [CrossRef] [PubMed]
- Foster, G.R.; Pianko, S.; Brown, A.; Forton, D.; Nahass, R.G.; George, J.; Barnes, E.; Brainard, D.M.; Massetto, B.; Lin, M.; et al. Efficacy of Sofosbuvir plus Ribavirin with or without Peginterferon-Alfa in Patients with Hepatitis C Virus Genotype 3 Infection and Treatment-Experienced Patients with Cirrhosis and Hepatitis C Virus Genotype 2 Infection. Gastroenterology 2015, 149, 1462–1470. [Google Scholar] [CrossRef] [Green Version]
- Flisiak, R.; Zarębska-Michaluk, D.; Janczewska, E.; Staniaszek, A.; Gietka, A.; Mazur, W.; Tudrujek, M.; Tomasiewicz, K.; Belica-Wdowik, T.; Baka-Ćwierz, B.; et al. Treatment of HCV Infection in Poland at the Beginning of the Interferon-Free Era-the EpiTer-2 Study. J. Viral Hepat. 2018, 25, 661–669. [Google Scholar] [CrossRef]
- Esteban, R.; Pineda, J.A.; Calleja, J.L.; Casado, M.; Rodríguez, M.; Turnes, J.; Morano Amado, L.E.; Morillas, R.M.; Forns, X.; Pascasio Acevedo, J.M.; et al. Efficacy of Sofosbuvir and Velpatasvir, with and without Ribavirin, in Patients With Hepatitis C Virus Genotype 3 Infection and Cirrhosis. Gastroenterology 2018, 155, 1120–1127.e4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zarębska-Michaluk, D.; Jaroszewicz, J.; Parfieniuk-Kowerda, A.; Janczewska, E.; Dybowska, D.; Pawłowska, M.; Halota, W.; Mazur, W.; Lorenc, B.; Janocha-Litwin, J.; et al. Effectiveness and Safety of Pangenotypic Regimens in the Most Difficult to Treat Population of Genotype 3 HCV Infected Cirrhotics. J. Clin. Med. 2021, 10, 3280. [Google Scholar] [CrossRef]
- Drazilova, S.; Gazda, J.; Janicko, M.; Jarcuska, P. Chronic Hepatitis C Association with Diabetes Mellitus and Cardiovascular Risk in the Era of DAA Therapy. Can. J. Gastroenterol. Hepatol. 2018, 2018, 6150861. [Google Scholar] [CrossRef]
- Romero-Gómez, M.; Fernández-Rodríguez, C.M.; Andrade, R.J.; Diago, M.; Alonso, S.; Planas, R.; Solá, R.; Pons, J.A.; Salmerón, J.; Barcena, R.; et al. Effect of sustained virological response to treatment on the incidence of abnormal glucose values in chronic hepatitis C. J. Hepatol. 2008, 48, 721–727. [Google Scholar] [CrossRef]
| Parameter | All Patients, n = 963 |
|---|---|
| Gender, females/males, n (%) | 525 (54.5)/438 (45.5) |
| Age [years] mean ± SD; min.–max. | 50.4 ± 15.9; 19–89 |
| Median (Q1, Q3) | 50.0 (36.0, 63.0) |
| Females, age [years] mean ± SD; min.–max. | 52.1 ± 16.3; 19–88 |
| Median (Q1, Q3) | 55.0 (36.0, 65.0) |
| Males, age [years] mean ± SD; min.–max. | 48.4 ± 15.1; 19–89 |
| Median (Q1, Q3) | 45.0 (36.0, 60.0) |
| BMI [kg/m2] mean ± SD; min.–max. | 25.9 ± 4.5; 15.6–45 |
| Median (Q1, Q3) | 25.4 (22.6, 28.5) |
| GT, n (%) | |
| 1 | 34 (3.5) |
| 1a | 12 (1.3) |
| 1b | 797 (82.8) |
| 2 | 0 |
| 3 | 89 (9.2) |
| 4 | 29 (3) |
| 5 | 0 |
| 6 | 2 (0.2) |
| Comorbidities, n (%) | |
| Any comorbidity | 752 (78.1) |
| Hypertension | 337 (35) |
| Diabetes | 117 (12.1) |
| Renal disease | 82 (8.5) |
| Kidney failure–eGRF < 30 mL/min, 30–60 mL/min | 28 (2.9), 9 (0.9) |
| Dialysis | 6 (0.6) |
| Autoimmune diseases | 68 (7.1) |
| Non-HCC tumors | 52 (5.4) |
| Other | 663 (68.8) |
| Concomitant medications, n (%) | 621 (64.5) |
| Liver fibrosis, n (%) | |
| F0 | 43 (4.5) |
| F1 | 472 (49) |
| F2 | 139 (14.4) |
| F3 | 102 (10.6) |
| F4 | 207 (21.5) |
| HCC history, n (%) | 10 (1) |
| OLTx history, n (%) | 4 (0.4) |
| HBV coinfection (HBsAg+), n (%) | 7 (0.7) |
| HIV coinfection, n (%) | 2 (0.2) |
| Extrahepatic manifestations of HCV, n (%) | |
| Cryoglobulinemia | 446 (46.3) |
| Thyroid abnormalities with presence of anti-thyroid antibodies | 86 (8.9) |
| Thrombocytopenia in noncirrhotics | 38 (3.9) |
| Other | 17 (1.8) |
| History of previous therapy, n (%) | |
| Treatment-naive | 759 (78.8) |
| Non-responder | 79 (8.2) |
| Relapser | 78 (8.1) |
| Discontinuation due to safety reason | 47 (4.9) |
| ALT IU/L, mean ± SD | 72.4 ± 57.1 |
| Median (Q1, Q3) | 55.0 (35.0, 91.0) |
| Bilirubin mg/dL, mean ± SD | 0.9 ± 1 |
| Median (Q1, Q3) | 0.8 (0.6, 1.0) |
| Albumin g/dL, mean ± SD | 4 ± 0.4 |
| Median (Q1, Q3) | 4.1 (3.8, 4.3) |
| Creatinine mg/dL, mean ± SD | 1 ± 0.6 |
| Median (Q1, Q3) | 0.9 (0.8, 1.0) |
| Hemoglobin g/dL, mean ± SD | 14.3 ± 1.6 |
| Median (Q1, Q3) | 14.3 (13.3, 15.4) |
| Platelets, ×1000/µL, mean ± SD | 184.8 ± 71.4 |
| Median (Q1, Q3) | 186.0 (140.0, 228.5) |
| HCV RNA × 106 IU/mL, mean ± SD | 2.8 ± 8.6 |
| Median (Q1, Q3) | 1.0 (0.3, 2.8) |
| Parameter | Patients with Liver Cirrhosis, n = 207 |
|---|---|
| Gender, females/males, n (%) | 97 (46.9)/110 (53.1) |
| Age [years] mean ± SD; min.–max. | 59 ± 13.7; 21–89 |
| Median (Q1, Q3) | 60.0 (50.5, 69.0) |
| Females, age [years] mean ± SD; min.–max. | 62.4 ± 12.7; 21–89 |
| Median (Q1, Q3) | 63.0 (56.0, 71.0) |
| Males, age [years] mean ± SD; min.–max. | 56 ± 13.9; 21–89 |
| Median (Q1, Q3) | 57.0 (46.0, 65.8) |
| BMI [kg/m2] mean ± SD; min.–max. | 27 ± 5.1; 17.5–44.9 |
| Median (Q1, Q3) | 26.6 (23.6, 29.7) |
| GT, n (%) | |
| 1 | 2 (1) |
| 1a | 1 (0.5) |
| 1b | 169 (81.6) |
| 2 | 0 |
| 3 | 32 (15.4) |
| 4 | 2 (1) |
| 5 | 0 |
| 6 | 1 (0.5) |
| Comorbidities, n (%) | |
| Any comorbidity | 197 (95.2) |
| Hypertension | 102 (49.3) |
| Diabetes | 53 (25.6) |
| Renal disease | 23 (11.1) |
| Autoimmune diseases | 11 (5.3) |
| Non-HCC tumors | 11 (5.3) |
| Other | 185 (89.4) |
| Concomitant medications, n (%) | 188 (90.8) |
| Diuretics, n (%) | 92 (44.4) |
| History of hepatic decompensation, n (%) | |
| Ascites | 28 (13.5) |
| Encephalopathy | 11 (5.3) |
| Documented esophageal varices, n (%) | 89 (43) |
| Hepatic decompensation at baseline, n (%) | |
| Moderate ascites–responded to diuretics | 20 (9.7) |
| Tense ascites–not responded to diuretics | 4 (1.9) |
| Encephalopathy | 6 (2.9) |
| HCC history, n (%) | 8 (3.9) |
| OLTx history, n (%) | 0 |
| Child-Pugh, n (%) | |
| A | 171 (82.6) |
| B | 31 (15) |
| C | 5 (2.4) |
| MELD, n (%) | |
| <15 | 189 (91.3) |
| 15–18 | 17 (8.2) |
| 19–20 | 0 |
| >20 | 1 (0.5) |
| HBV coinfection (HBsAg+), n (%) | 3 (1.4) |
| HIV coinfection, n (%) | 0 |
| Extrahepatic manifestations of HCV, n (%) | |
| Cryoglobulinemia | 132 (63.8) |
| Thyroid abnormalities with the presence of anti-thyroid antibodies | 18 (8.7) |
| Other | 2 (1) |
| History of previous therapy, n (%) | |
| Treatment-naive | 133 (64.2) |
| Non-responder | 37 (17.9) |
| Relapser | 24 (11.6) |
| Discontinuation due to safety reason | 13 (6.3) |
| Treatment regimens, n (%) | |
| ASV + DCV | 5 (2.4) |
| LDV/SOF ± RBV | 56 (27.1) |
| OBV/PTV/r ± DSV ± RBV | 51 (24.6) |
| GZR/EBR | 22 (10.6) |
| SOF + SMV ± RBV | 4 (1.9) |
| SOF + RBV | 18 (8.7) |
| SOF + DCV + RBV | 1 (0.5) |
| GLE/PIB | 21 (10.2) |
| SOF/VEL ± RBV | 29 (14) |
| ALT IU/L, mean ± SD | 97.2 ± 68.3 |
| Median (Q1, Q3) | 78.0 (48.5, 127.0) |
| Bilirubin mg/dL, mean ± SD | 1.5 ± 1.8 |
| Median (Q1, Q3) | 1.2 (0.9, 1.7) |
| Albumin g/dL, mean ± SD | 3.6 ± 0.5 |
| Median (Q1, Q3) | 3.6 (3.3, 3.9) |
| Creatinine mg/dL, mean ± SD | 0.9 ± 0.3 |
| Median (Q1, Q3) | 0.9 (0.8, 1.0) |
| Hemoglobin g/dL, mean ± SD | 13.6 ± 1.7 |
| Median (Q1, Q3) | 13.8 (12.4, 14.6) |
| Platelets, ×1000/µL, mean ± SD | 108.7 ± 57.4 |
| Median (Q1, Q3) | 97.0 (71.0, 136.5) |
| HCV RNA × 106 IU/mL, mean ± SD | 1.9 ± 5.2 |
| Median (Q1, Q3) | 0.5 (0.1, 1.5) |
| Parameter | HCV-Infected Patients n = 963 |
|---|---|
| Genotype-specific treatment regimens (n = 626), n (%) | |
| ASV + DCV | 19 (2) |
| LDV/SOF ± RBV | 178 (18.5) |
| OBV/PTV/r ± DSV ± RBV | 233 (24.2) |
| GZR/EBR ± RBV | 156 (16.2) |
| SOF + SMV ± RBV | 4 (0.4) |
| SOF + RBV | 34 (3.5) |
| SOF + DCV ± RBV | 2 (0.2) |
| Pangenotypic regimens (n = 337), n (%) | |
| GLE/PIB | 213 (22.1) |
| SOF/VEL ± RBV | 124 (12.9) |
| Regimen/Efficacy | ASV + DCV | GLE/PIB | LDV/SOF ± RBV | OBV/PTV/r ± DSV ± RBV | GZR/EBR | SOF + SMV ± RBV | SOF + RBV | SOF/VEL ± RBV | SOF + DCV ± RBV |
|---|---|---|---|---|---|---|---|---|---|
| SVR ITT, n (%) | 16/19 (84.2) | 209/213 (98.1) | 173/178 (97.2) | 229/233 (98.3) | 152/156 (97.4) | 4/4 (100) | 31/34 (91.2) | 121/124 (97.6) | 2/2 (100) |
| SVR PP, n (%) | 16/17 (94.1) | 209/213 (98.1) | 173/175 (98.9) | 229/232 (98.7) | 152/153 (99.3) | 4/4 (100) | 31/33 (93.9) | 121/123 (98.4) | 2/2 (100) |
| Parameter | Responders n = 937 | Non-Responders n = 15 | p Value |
|---|---|---|---|
| Gender, females/males, n (%) | 519 (55.4)/418 (44.6) | 0/15 (100) | <0.0001 |
| Age [years] mean ± SD; min.–max. | 50.3 ± 15.9; 19–89 | 49.3 ± 8.2; 31–59 | |
| Median (Q1, Q3) | 50 (36, 63) | 52.0 (46.0, 55.5) | 0.842 |
| Females, age [years] mean ± SD; min.–max. | 51.9 ± 16.4; 19–88 | NA | |
| Median (Q1, Q3) | 55 (36, 65) | NA | |
| Males, age [years] mean ± SD; min.–max. | 48.2 ± 15.2; 19–89 | 49.3 ± 8.2; 31–59 | |
| Median (Q1, Q3) | 45 (36, 60) | 52.0 (46.0, 55.5) | 0.5557 |
| BMI [kg/m2] mean ± SD; min.–max | 25.8 ± 4.5; 15.6–45 | 29.4 ± 3.6; 24.9–36.3 | |
| Median (Q1, Q3) | 25.3 (22.6, 28.4) | 28.4 (27.1, 30.2) | 0.0011 |
| GT, n (%) | 0.0105 | ||
| 1 | 34 (3.6) | 0 | |
| 1a | 12 (1.3) | 0 | |
| 1b | 780 (83.3) | 8 (53.3) | |
| 2 | 0 | 0 | |
| 3 | 81 (8.6) | 6 (40) | |
| 4 | 28 (3) | 1 (6.7) | |
| 5 | 0 | 0 | |
| 6 | 2 (0.2) | 0 | |
| GT 3, n (%) | 81 (8.6) | 6 (37.5) | 0.0013 |
| GT 4, n (%) | 28 (3.0) | 1 (6.7) | 0.3735 |
| Comorbidities, n (%) | |||
| Any comorbidity | 728 (77.7) | 13 (86.5) | 0.5424 |
| Hypertension | 328 (35) | 3 (20) | 0.226 |
| Diabetes | 107 (11.4) | 6 (40) | 0.005 |
| Renal disease | 78 (8.3) | 2 (13.3) | 0.3636 |
| Autoimmune diseases | 67 (7.2) | 0 | 0.6171 |
| Non-HCC tumors | 51 (5.4) | 0 | 1 |
| Other | 641 (68.4) | 12 (80) | 0.4131 |
| Kidney failure, n (%) | 35 (3.7) | 0 | 1 |
| Concomitant medications, n (%) | 598 (63.8) | 12 (80) | 0.1951 |
| Liver fibrosis, n (%) | 0.0776 | ||
| F0 | 43 (4.6) | 0 | |
| F1 | 465 (49.6) | 6 (40) | |
| F2 | 136 (14.5) | 1 (6.7) | |
| F3 | 101 (10.8) | 0 | |
| F4 | 192 (20.5) | 8 (53.3) | |
| Liver fibrosis F4, n (%) | 192 (20.5) | 8 (53.3) | 0.0055 |
| History of previous therapy, n (%) | 0.0237 | ||
| Treatment-naive | 742 (79.2) | 8 (53.3) | |
| Treatment-experienced | 195 (20.8) | 7 (46.7) | |
| DAA-experienced patients, n (%) | 10 (1.1) | 3 (20) | 0.0008 |
| History of hepatic decompensation, n (%) | |||
| Ascites | 27 (2.9) | 1 (6.7) | 0.3631 |
| Encephalopathy | 10 (1.1) | 1 (6.7) | 0.1611 |
| Documented esophageal varices, n (%) | 87 (9.3) | 5 (33.3) | 0.0104 |
| Hepatic decompensation at baseline, n (%) | |||
| Moderate ascites–responded to diuretics | 19 (2) | 0 | 1 |
| Tense ascites–not responded to diuretics | 3 (0.3) | 0 | 1 |
| Encephalopathy | 5 (0.5) | 0 | 1 |
| HCC history, n (%) | 9 (1) | 0 | 1 |
| OLTx history, n (%) | 4 (0.4) | 0 | 1 |
| Child-Pugh B or C, n (%) | 30 (3.2) | 3 (20) | 0.013 |
| HBV coinfection (HBsAg+), n (%) | 7 (0.7) | 0 | 1 |
| HIV coinfection, n (%) | 2 (0.2) | 0 | 1 |
| Extrahepatic manifestations of HCV, n (%) | |||
| Cryoglobulinemia | 431 (46) | 9 (60) | 0.2805 |
| Thyroid abnormalities with presence of anti-thyroid antibodies | 85 (9.1) | 0 | 0.3862 |
| Thrombocytopenia in noncirrhotics | 38 (4.1) | 0 | 1 |
| ALT IU/L, mean ± SD | 71.8 ± 57.2 | 96 ± 34.3 | |
| Median (Q1, Q3) | 53.0 (35.0, 90.0) | 91.0 (70.0, 108.5) | 0.002 |
| Bilirubin mg/dL, mean ± SD | 0.9 ± 1 | 1.2 ± 0.6 | |
| Median (Q1, Q3) | 0.8 (0.6, 1.0) | 1.1 (0.8, 1.3) | 0.0123 |
| Albumin g/dL, mean ± SD | 4 ± 0.4 | 3.8 ± 0.6 | |
| Median (Q1, Q3) | 4.1 (3.8, 4.3) | 3.9 (3.5, 4.0) | 0.0511 |
| Creatinine mg/dL, mean ± SD | 1 ± 0.6 | 1 ± 0.2 | |
| Median (Q1, Q3) | 0.9 (0.8, 1.0) | 0.9 (0.8, 1.0) | 0.5054 |
| Hemoglobin g/dL, mean ± SD | 14.3 ± 1.6 | 14.6 ± 1.4 | |
| Median (Q1, Q3) | 14.3 (13.3, 15.4) | 14.6 (14.4, 15.5) | 0.2688 |
| Platelets, ×1000/µL, mean ± SD | 186.2 ± 71.1 | 132.9 ± 60.6 | |
| Median (Q1, Q3) | 187.0 (142.0, 230.0) | 114.0 (77.0, 193.0) | 0.0068 |
| HCV RNA × 106 IU/mL, mean ± SD | 2.9 ± 8.7 | 2.4 ± 2.7 | |
| Median (Q1, Q3) | 1.0 (0.3, 2.8) | 1.5 (0.5, 3.8) | 0.3051 |
| Parameter | Univariable OR | 95% CI | p Value | |
|---|---|---|---|---|
| Age | 1 | 0.96–1.03 | 0.8187 | |
| BMI | 1.15 | 1.05–1.25 | 0.0031 | |
| BMI | <25 | Ref. level | ||
| 25 or more | 12.83 | 1.68–97.88 | 0.0139 | |
| Genotype 3 | no | Ref. level | ||
| yes | 7.05 | 2.45–20.29 | 0.0003 | |
| Genotype 4 | no | Ref. level | ||
| yes | 2.32 | 0.29–18.25 | 0.4243 | |
| Any comorbidity | no | Ref. level | ||
| yes | 1.87 | 0.42–8.33 | 0.4139 | |
| Hypertension | no | Ref. level | ||
| yes | 0.46 | 0.13–1.66 | 0.2371 | |
| Diabetes | no | Ref. level | ||
| yes | 5.17 | 1.81–14.81 | 0.0022 | |
| Renal disease | no | Ref. level | ||
| yes | 1.69 | 0.38–7.64 | 0.4928 | |
| Autoimmune diseases | no | Ref. level | ||
| yes | NA (0 in cell) | |||
| Non-HCC tumors | no | Ref. level | ||
| yes | NA (0 in cell) | |||
| Other comorbidity | no | Ref. level | ||
| yes | 1.85 | 0.52–6.59 | 0.3446 | |
| Concomitant medications | no | Ref. level | ||
| yes | 2.27 | 0.64–8.09 | 0.2072 | |
| Liver fibrosis | F0 | Ref. level | ||
| F1 | NA (0 in cell) | |||
| F2 | NA (0 in cell) | |||
| F3 | NA (0 in cell) | |||
| F4 | NA (0 in cell) | |||
| Liver fibrosis, F4 | no | Ref. level | ||
| yes | 4.43 | 1.59–12.38 | 0.0045 | |
| Ascites | no | Ref. level | ||
| yes | 2.41 | 0.31–18.97 | 0.4043 | |
| Encephalopathy | no | Ref. level | ||
| yes | 6.62 | 0.79–55.29 | 0.0809 | |
| Documented esophageal varices | no | Ref. level | ||
| yes | 4.89 | 1.63–14.62 | 0.0046 | |
| Moderate ascites at baseline | no | Ref. level | ||
| yes | NA (0 in cell) | |||
| Tense ascites at baseline | no | Ref. level | ||
| yes | NA (0 in cell) | |||
| Encephalopathy at baseline | no | Ref. level | ||
| yes | NA (0 in cell) | |||
| HCC history | no | Ref. level | ||
| yes | NA (0 in cell) | |||
| OLTx history | no | Ref. level | ||
| yes | NA (0 in cell) | |||
| MELD | 14 or less | Ref. level | ||
| 15–18 | NA (0 in cell) | |||
| 19–20 | NA (0 in cell) | |||
| 21 or more | NA (0 in cell) | |||
| Child Pugh B or C | no | Ref. level | ||
| yes | 7.56 | 2.03–28.19 | 0.0026 | |
| HBV coinfection HBsAg plus | no | Ref. level | ||
| yes | NA (0 in cell) | |||
| HIV coinfection | no | Ref. level | ||
| yes | NA (0 in cell) | |||
| Cryoglobulinemia | no | Ref. level | ||
| yes | 1.76 | 0.62–4.99 | 0.2866 | |
| History of previous therapy | Treatment-naive | Ref. level | ||
| Nonresponder | 2.44 | 0.51–11.7 | 0.2645 | |
| Relapser | 5.01 | 1.47–17.05 | 0.0098 | |
| Discontinuation due to safety reason | 2.06 | 0.25–16.84 | 0.4998 | |
| History of previous therapy | Treatment naive | Ref. level | ||
| Treatment experienced | 3.33 | 1.19–9.29 | 0.0216 | |
| Therapy | AS + DCV | Ref. level | ||
| LDV/SOF +/− RBV | 0.18 | 0.02–2.15 | 0.1778 | |
| OBV/PTV/r +/− DSV +/− RBV | 0.21 | 0.02–2.13 | 0.1867 | |
| GZR/EBR +/− RBV | 0.11 | 0.01–1.76 | 0.1176 | |
| SOF + SMV +/− RBV | NA (0 in cell) | |||
| SOF + DCV +/− RBV | NA (0 in cell) | |||
| SOF + RBV | 1.03 | 0.09–12.27 | 0.9799 | |
| GLE/PIB | 0.31 | 0.03–2.9 | 0.3025 | |
| SOF/VEL +/− RBV | 0.26 | 0.02–3.08 | 0.2886 | |
| ALT | 1 | 1–1.01 | 0.1092 | |
| ALT | <70 | Ref. level | ||
| 70 or more | 4.85 | 1.53–15.35 | 0.0072 | |
| Bilirubin | 1.12 | 0.88–1.43 | 0.3501 | |
| Bilirubin | <0.98 | Ref. level | ||
| 0.98 or more | 5.07 | 1.72–14.98 | 0.0033 | |
| Albumin | 0.31 | 0.12–0.85 | 0.0231 | |
| Creatinine | 0.98 | 0.39–2.42 | 0.9596 | |
| Hemoglobin | 1.14 | 0.82–1.59 | 0.4286 | |
| Platelets | 0.99 | 0.98–1 | 0.0045 | |
| PLT | <115 | Ref. level | ||
| 115 or more | 0.17 | 0.06–0.47 | 0.0007 | |
| HCV RNA | 0.99 | 0.91–1.08 | 0.8547 |
| Patient | Age | GT | F, CP | Regimen | History of Previous Therapy | Baseline HCV RNA × 106 IU/mL | Treatment Course | EOT | Comment (Possible Reason of Failure) |
|---|---|---|---|---|---|---|---|---|---|
| Male 1 | 48 | 1B | 1 | GZR/EBR, 12 wks | treatment-naive | 6.07 | according to schedule | TND | |
| Male 2 | 44 | 1B | 1 | OBV/PTV/r + DSV, 8 wks | treatment-naive | 1.53 | according to schedule | TND | |
| Male 3 | 56 | 1B | 2 | ASV + DCV, 24 wks | treatment-naive | 1.59 | according to schedule | TD | |
| Male 4 | 59 | 1B | 4, CP-A | OBV/PTV/r + DSV + RBV, 12 wks | non-responder (TVR + pegIFN + RBV) | 0.58 | according to schedule | TD | liver cirrhosis, non-response to previous therapy, liver cirrhosis |
| Male 5 | 59 | 1B | 4, CP-A | LDV/SOF + RBV, 12 wks | relapser (OBV/PTV/r + DSV + RBV) | 1.65 | according to schedule | TND | liver cirrhosis, non-response to the previous therapy, liver cirrhosis |
| Male 6 | 54 | 1B | 4, CP-B | LDV/SOF + RBV, 12 wks | treatment-naive | 0.25 | according to schedule | TND | decompensated liver cirrhosis |
| Male 7 | 31 | 1B | 1 | GLE/PIB, 8 wks | treatment-naive | 0.53 | modified | TD | no adherence (irregular use of the drug due to alcohol abuse) |
| Male 8 | 48 | 1B | 4, CP-B | VEL/SOF + RBV, 12 wks | treatment-naive | 0.31 | according to schedule | TND | decompensated liver cirrhosis |
| Male 9 | 54 | 3 | 4, CP-A | GLE/PIB, 16 wks | relapser (SOF + RBV) | 4.03 | according to schedule | TND | liver cirrhosis, non-response to previous therapy |
| Male 10 | 48 | 4 | 1 | OBV/PTV/r + RBV, 12 wks | relapser (SMV + PegIFN + RBV) | 3.6 | according to schedule | TND | liver cirrhosis, non-response to previous therapy, no adherence to the current treatment |
| Male 11 | 55 | 3 | 4, CP-B | SOF + RBV, 24 wks | non-responder (PegIFN + RBV) | 0.43 | according to schedule | TND | decompensated liver cirrhosis, non-response to previous therapy |
| Male 12 | 52 | 3 | 4, CP-A | SOF + RBV, 24 wks | discontinuation due to safety reason (IFN + RBV) | 0.5 | according to schedule | TND | liver cirrhosis |
| Male 13 | 56 | 3 | 4, CP-A | VEL/SOF + RBV, 24 wks | relapser (GLE/PIB) | 1.08 | according to schedule | TND | liver cirrhosis, non-response to the previous therapy |
| Male 14 | 34 | 3 | 1 | GLE/PIB, 8 wks | treatment-naive | 10.0 | according to schedule | TND | |
| Male 15 | 42 | 3 | 1 | GLE/PIB, 8 wks | treatment-naive | 4.57 | according to schedule | TND |
| Parameter | All Patients n = 963 | Non-Cirrhotics n = 756 | Cirrhotics n = 207 | p Value |
|---|---|---|---|---|
| Treatment course, n (%) | ||||
| according to schedule | 937 (97.3) | 745 (98.6) | 192 (92.8) | <0.0001 |
| therapy modification | 15 (1.6) 1 | 7 (0.9) 6 | 8 (3.9) 9 | |
| therapy discontinuation | 11 (1.1) 2 | 4 (0.5) 7 | 7 (3.4) 10 | |
| Patients with at least one AE, n (%) | 152 (15.8) | 75 (9.9) | 77 (37.2) | <0.0001 |
| Serious adverse events, n (%) | 26 (2.7) 3 | 9 (1.2) 8 | 17 (8.2) 11 | <0.0001 |
| AEs leading to treatment discontinuation, n (%) | 3 (0.3) 4 | 0 | 3 (1.4) 12 | 0.01 |
| Most common AEs (≥2%), n (%) | ||||
| weakness/fatigue | 54 (5.6) | 30 (4) | 24 (11.6) | <0.0001 |
| anemia | 33 (3.4) | 8 (1.1) | 25 (12.1) | <0.0001 |
| AEs of particular interest, n (%) | ||||
| Ascites | 8 (0.8) | NA | 8 (3.9) | <0.0001 |
| hepatic encephalopathy | 6 0.6) | NA | 6 (2.9) | <0.0001 |
| gastrointestinal bleeding | 1 (0.1) | NA | 1 (0.5) | 0.215 |
| Death, n (%) | 8 (0.8) 5 | 0 | 8 (3.9) | <0.0001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pabjan, P.; Brzdęk, M.; Chrapek, M.; Dziedzic, K.; Dobrowolska, K.; Paluch, K.; Garbat, A.; Błoniarczyk, P.; Reczko, K.; Stępień, P.; et al. Are There Still Difficult-to-Treat Patients with Chronic Hepatitis C in the Era of Direct-Acting Antivirals? Viruses 2022, 14, 96. https://doi.org/10.3390/v14010096
Pabjan P, Brzdęk M, Chrapek M, Dziedzic K, Dobrowolska K, Paluch K, Garbat A, Błoniarczyk P, Reczko K, Stępień P, et al. Are There Still Difficult-to-Treat Patients with Chronic Hepatitis C in the Era of Direct-Acting Antivirals? Viruses. 2022; 14(1):96. https://doi.org/10.3390/v14010096
Chicago/Turabian StylePabjan, Paweł, Michał Brzdęk, Magdalena Chrapek, Kacper Dziedzic, Krystyna Dobrowolska, Katarzyna Paluch, Anna Garbat, Piotr Błoniarczyk, Katarzyna Reczko, Piotr Stępień, and et al. 2022. "Are There Still Difficult-to-Treat Patients with Chronic Hepatitis C in the Era of Direct-Acting Antivirals?" Viruses 14, no. 1: 96. https://doi.org/10.3390/v14010096
APA StylePabjan, P., Brzdęk, M., Chrapek, M., Dziedzic, K., Dobrowolska, K., Paluch, K., Garbat, A., Błoniarczyk, P., Reczko, K., Stępień, P., & Zarębska-Michaluk, D. (2022). Are There Still Difficult-to-Treat Patients with Chronic Hepatitis C in the Era of Direct-Acting Antivirals? Viruses, 14(1), 96. https://doi.org/10.3390/v14010096








