Reduced Replication of Highly Pathogenic Avian Influenza Virus in Duck Endothelial Cells Compared to Chicken Endothelial Cells Is Associated with Stronger Antiviral Responses
Abstract
1. Introduction
2. Material and Methods
2.1. Cell Culture
2.2. Virus Origin and Propagation
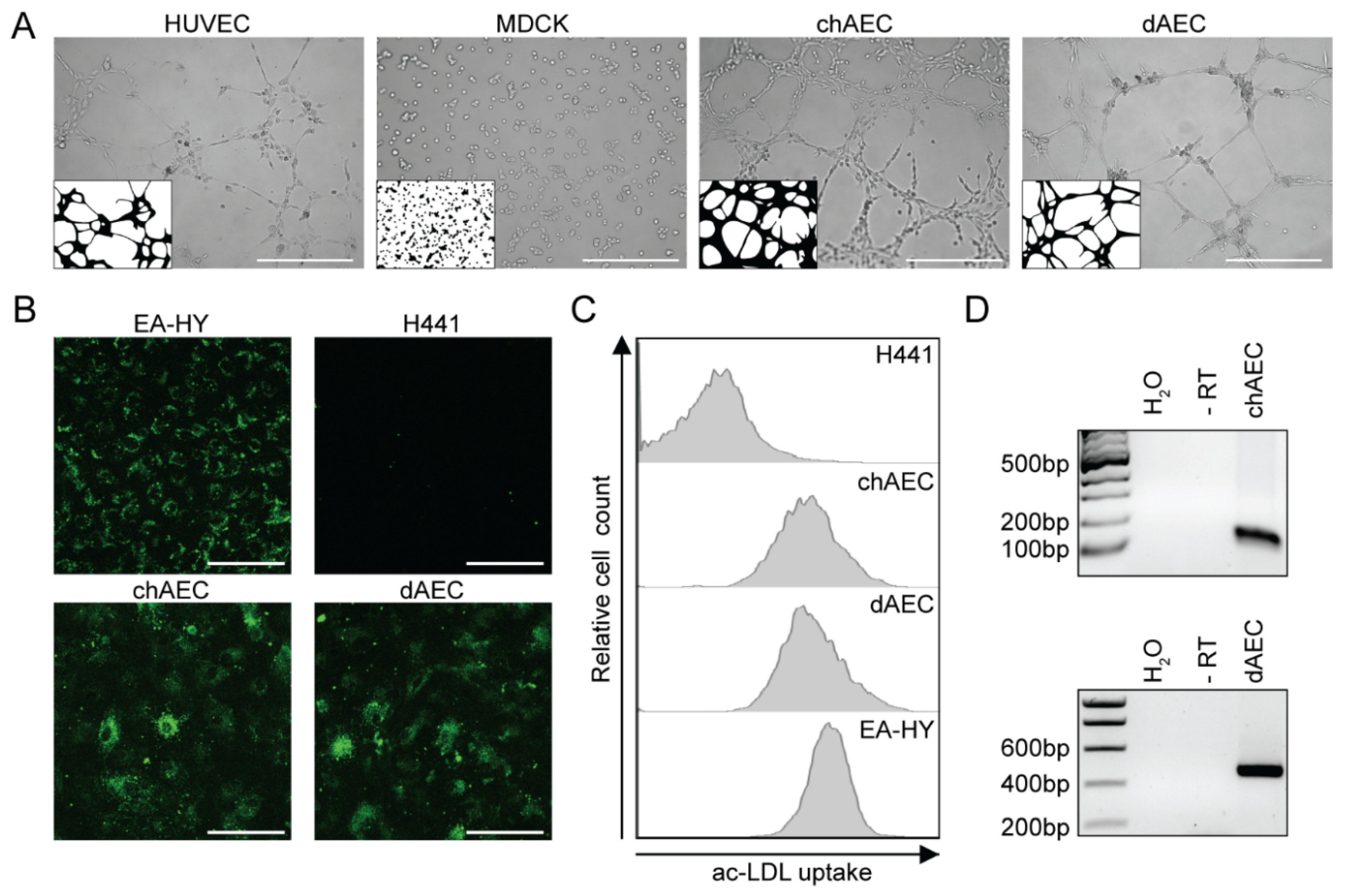
2.3. Tube Formation Assay
2.4. Acetylated Low-Density Lipoproteins Uptake
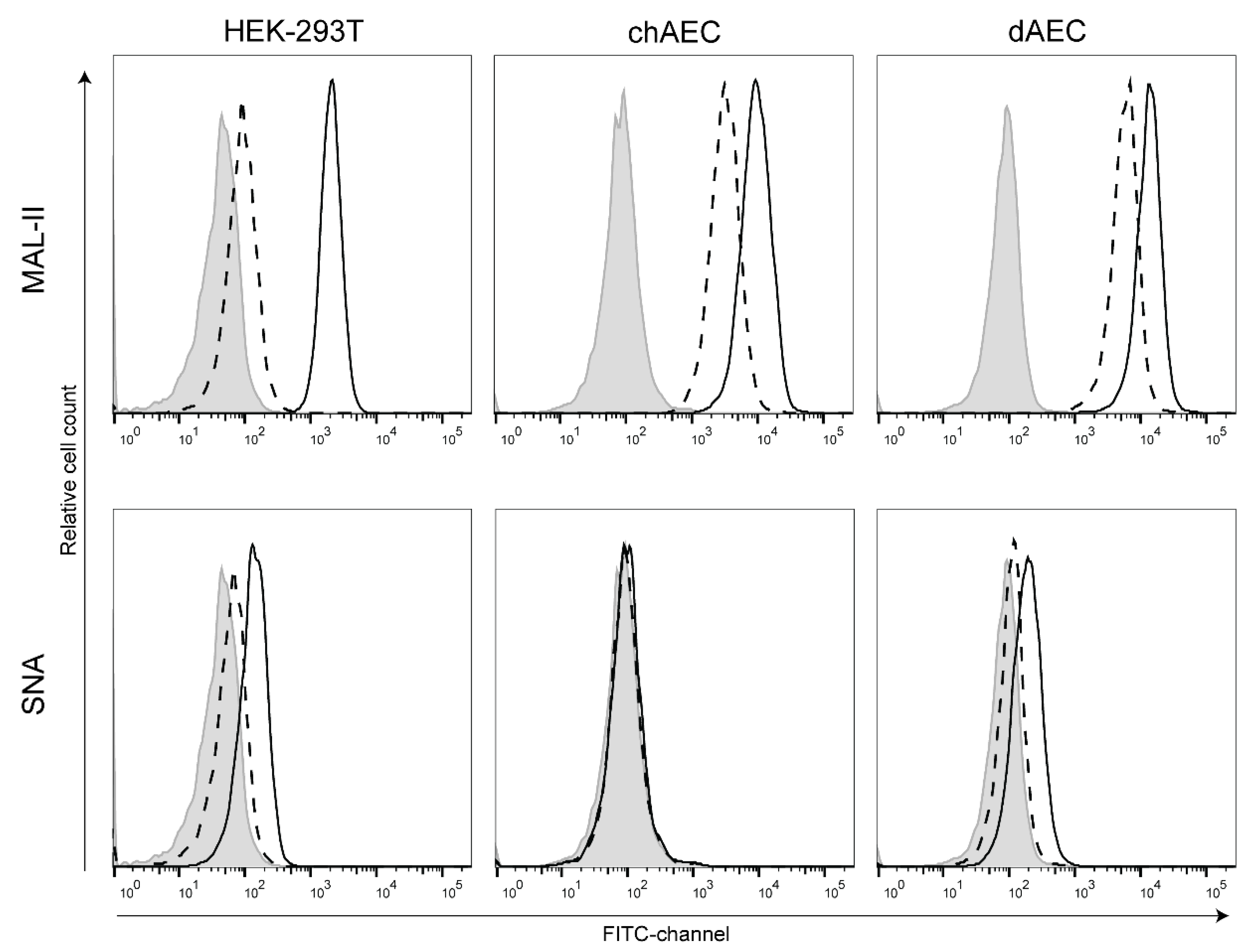
2.5. Lectin Staining
2.6. Detection of the Nucleoprotein by Flow Cytometry
2.7. Viral Replication Kinetics
2.8. RT-qPCR
2.9. Detection of Von Willebrand Factor mRNA by RT-PCR
2.10. In Ovo Inoculation and Immunohistochemical Analysis
2.11. Statistical Analysis
3. Results
3.1. Primary Avian Aortic Endothelial Cells Have Endothelial Cell Characteristics
3.2. Duck AEC Mainly Express α2,3-Linked Sialic Acid Moieties
3.3. Productive Infection of HPAIV H5N1 in dAEC
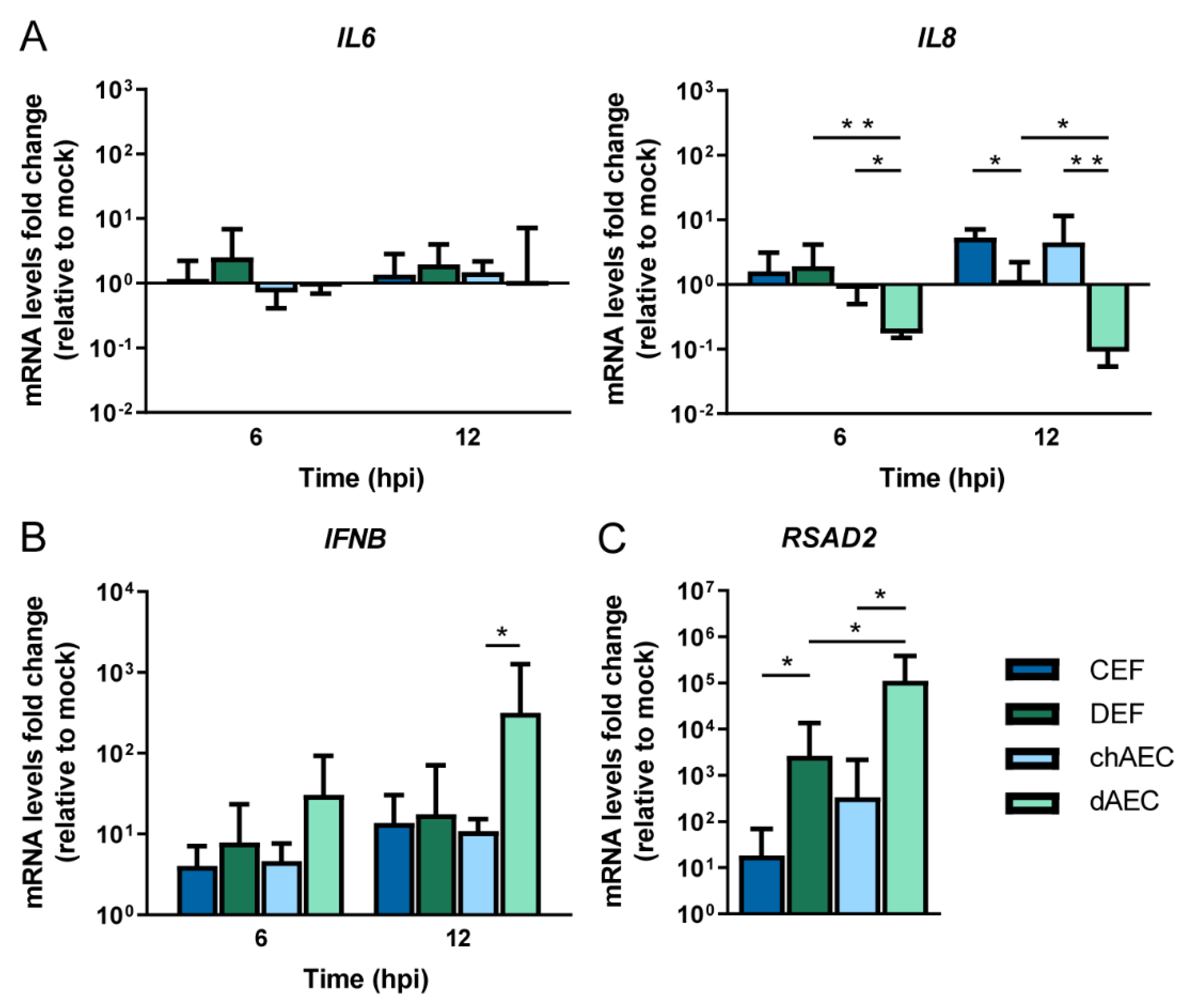
3.4. Differential Host Responses in dAEC upon HPAIV Inoculation Compared to chAEC and DEF
3.5. Endothelial Cells Are a Target of HPAIV Infection in Duck Embryonated Eggs
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Stallknecht, D.E.; Shane, S.M. Host range of avian influenza virus in free-living birds. Vet. Res. Commun. 1988, 12, 125–141. [Google Scholar] [CrossRef]
- Olsen, B.; Munster, V.J.; Wallensten, A.; Waldenström, J.; Osterhaus, A.D.M.E.; Fouchier, R.A.M. Global patterns of influenza A virus in wild birds. Science 2006, 312, 384–388. [Google Scholar] [CrossRef]
- Daoust, P.Y.; Kibenge, F.S.B.; Fouchier, R.A.M.; van de Bildt, M.W.G.; Van Riel, D.; Kuiken, T. Replication of low pathogenic avian influenza virus in naturally infected mallard ducks (Anas platyrhynchos) causes no morphologic lesions. J. Wildl. Dis. 2011, 47, 401–409. [Google Scholar] [CrossRef]
- Kuiken, T. Is low pathogenic avian influenza virus virulent for wild waterbirds? Proc. R. Soc. B Biol. Sci. 2013, 280. [Google Scholar] [CrossRef] [PubMed]
- Webster, R.G.; Yakhno, M.; Hinshaw, V.S.; Bean, W.J.; Copal Murti, K. Intestinal influenza: Replication and characterization of influenza viruses in ducks. Virology 1978, 84, 268–278. [Google Scholar] [CrossRef]
- Alexander, D.J. An overview of the epidemiology of avian influenza. Vaccine 2007, 25, 5637–5644. [Google Scholar] [CrossRef]
- França, M.S.; Brown, J.D. Influenza pathobiology and pathogenesis in avian species. Curr. Top. Microbiol. Immunol. 2014, 385, 221–242. [Google Scholar] [CrossRef]
- Bosch, F.X.; Orlich, M.; Klenk, H.D.; Rott, R. The structure of the hemagglutinin, a determinant for the pathogenicity of influenza viruses. Virology 1979, 95, 197–207. [Google Scholar] [CrossRef]
- Whitel, J.; Kartenbeck, J.; Heleniuse, A. Membrane fusion activity of influenza virus. EMIBO J. 1982, 1, 217–222. [Google Scholar] [CrossRef]
- Huang, R.T.C.; Wahn, K.; Klenk, H.D.; Rott, R. Fusion between cell membrane and liposomes containing the glycoproteins of influenza virus. Virology 1980, 104, 294–302. [Google Scholar] [CrossRef]
- Garten, W.; Braden, C.; Arendt, A.; Peitsch, C.; Baron, J.; Lu, Y.; Pawletko, K.; Hardes, K.; Steinmetzer, T.; Böttcher-Friebertshäuser, E. Influenza virus activating host proteases: Identification, localization and inhibitors as potential therapeutics. Eur. J. Cell Biol. 2015, 94, 375–383. [Google Scholar] [CrossRef]
- Horimoto, T.; Nakayama, K.; Smeekens, S.P.; Kawaoka, Y. Proprotein-processing endoproteases PC6 and furin both activate hemagglutinin of virulent avian influenza viruses. J. Virol. 1994, 68, 6074–6078. [Google Scholar] [CrossRef]
- Stieneke-Gröber, A.; Vey, M.; Angliker, H.; Shaw, E.; Thomas, G.; Roberts, C.; Klenk, H.D.; Garten, W. Influenza virus hemagglutinin with multibasic cleavage site is activated by furin, a subtilisin-like endoprotease. EMBO J. 1992, 11, 2407–2414. [Google Scholar] [CrossRef]
- Horimoto, T.; Kawaoka, Y. Reverse genetics provides direct evidence for a correlation of hemagglutinin cleavability and virulence of an avian influenza A virus. J. Virol. 1994, 68, 3120–3128. [Google Scholar] [CrossRef]
- Wibawa, H.; Bingham, J.; Nuradji, H.; Lowther, S.; Payne, J.; Harper, J.; Wong, F.; Lunt, R.; Junaidi, A.; Middleton, D.; et al. The pathobiology of two Indonesian H5N1 avian influenza viruses representing different clade 2.1 sublineages in chickens and ducks. Comp. Immunol. Microbiol. Infect. Dis. 2013, 36, 175–191. [Google Scholar] [CrossRef] [PubMed]
- Van Riel, D.; van den Brand, J.M.A.; Munster, V.J.; Besteboer, T.M.; Fouchier, R.A.M.; Osterhaus, A.D.M.E.; Kuiken, T. Pathology and virus distribution in chickens naturally infected with highly pathogenic avian influenza a virus (H7N7) during the 2003 outbreak in The Netherlands. Vet. Pathol. 2009, 46, 971–976. [Google Scholar] [CrossRef] [PubMed]
- Suarez, D.L.; Perdue, M.L.; Cox, N.; Rowe, T.; Bender, C.; Huang, J.; Swayne, D.E. Comparisons of Highly Virulent H5N1 Influenza A Viruses Isolated from Humans and Chickens from Hong Kong. J. Virol. 1998, 72, 6678–6688. [Google Scholar] [CrossRef] [PubMed]
- Jones, Y.L.; Swayne, D.E. Comparative pathobiology of low and high pathogenicity H7N3 Chilean avian influenza viruses in chickens. Avian Dis. 2004, 48, 119–128. [Google Scholar] [CrossRef]
- Brown, C.C.; Olander, H.J.; Senne, D.A. A pathogenesis study of highly pathogenic avian influenza virus H5N2 in chickens, using immunohistochemistry. J. Comp. Pathol. 1992, 107, 341–348. [Google Scholar] [CrossRef]
- Scheibner, D.; Ulrich, R.; Fatola, O.I.; Graaf, A.; Gischke, M.; Salaheldin, A.H.; Harder, T.C.; Veits, J.; Mettenleiter, T.C.; Abdelwhab, E.M. Variable impact of the hemagglutinin polybasic cleavage site on virulence and pathogenesis of avian influenza H7N7 virus in chickens, turkeys and ducks. Sci. Rep. 2019, 9, 11556. [Google Scholar] [CrossRef]
- Schat, K.A.; Bingham, J.; Butler, J.M.; Chen, L.-M.; Lowther, S.; Crowley, T.M.; Moore, R.J.; Donis, R.O.; Lowenthal, J.W. Role of Position 627 of PB2 and the Multibasic Cleavage Site of the Hemagglutinin in the Virulence of H5N1 Avian Influenza Virus in Chickens and Ducks. PLoS ONE 2012, 7, e30960. [Google Scholar] [CrossRef]
- Bogs, J.; Veits, J.; Gohrbandt, S.; Hundt, J.; Stech, O.; Breithaupt, A.; Teifke, J.P.; Mettenleiter, T.C.; Stech, J. Highly pathogenic H5N1 influenza viruses carry virulence determinants beyond the polybasic hemagglutinin cleavage site. PLoS ONE 2010, 5, 11826. [Google Scholar] [CrossRef] [PubMed]
- Gohrbandt, S.; Veits, J.; Hundt, J.; Bogs, J.; Breithaupt, A.; Teifke, J.P.; Weber, S.; Mettenleiter, T.C.; Stech, J. Amino acids adjacent to the haemagglutinin cleavage site are relevant for virulence of avian influenza viruses of subtype H5. J. Gen. Virol. 2011, 92, 51–59. [Google Scholar] [CrossRef] [PubMed]
- Michiels, C. Endothelial cell functions. J. Cell. Physiol. 2003, 196, 430–443. [Google Scholar] [CrossRef]
- Mackow, E.R.; Gorbunova, E.E.; Gavrilovskaya, I.N. Endothelial cell dysfunction in viral hemorrhage and edema. Front. Microbiol. 2015, 6, 733. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, Y.; Horimoto, T.; Kawaoka, Y.; Alexander, D.J.; Itakura, C. Pathological studies of chickens experimentally infected with two highly pathogenic avian influenza viruses. Avian Pathol. 1996, 25, 285–304. [Google Scholar] [CrossRef] [PubMed]
- Muramoto, Y.; Ozaki, H.; Takada, A.; Park, C.H.; Sunden, Y.; Umemura, T.; Kawaoka, Y.; Matsuda, H.; Kida, H. Highly pathogenic H5N1 influenza virus causes coagulopathy in chickens. Microbiol. Immunol. 2006, 50, 73–81. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, K.; Okada, H.; Itoh, T.; Tada, T.; Mase, M.; Nakamura, K.; Kubo, M.; Tsukamoto, K. Association of Increased Pathogenicity of Asian H5N1 Highly Pathogenic Avian Influenza Viruses in Chickens with Highly Efficient Viral Replication Accompanied by Early Destruction of Innate Immune Responses. J. Virol. 2009, 83, 7475–7486. [Google Scholar] [CrossRef]
- Burggraaf, S.; Karpala, A.J.; Bingham, J.; Lowther, S.; Selleck, P.; Kimpton, W.; Bean, A.G.D. H5N1 infection causes rapid mortality and high cytokine levels in chickens compared to ducks. Virus Res. 2014, 185, 23–31. [Google Scholar] [CrossRef] [PubMed]
- Kuribayashi, S.; Sakoda, Y.; Kawasaki, T.; Tanaka, T.; Yamamoto, N.; Okamatsu, M.; Isoda, N.; Tsuda, Y.; Sunden, Y.; Umemura, T.; et al. Excessive Cytokine Response to Rapid Proliferation of Highly Pathogenic Avian Influenza Viruses Leads to Fatal Systemic Capillary Leakage in Chickens. PLoS ONE 2013, 8, e68375. [Google Scholar] [CrossRef]
- Teijaro, J.R.; Walsh, K.B.; Cahalan, S.; Fremgen, D.M.; Roberts, E.; Scott, F.; Martinborough, E.; Peach, R.; Oldstone, M.B.A.; Rosen, H. Endothelial cells are central orchestrators of cytokine amplification during influenza virus infection. Cell 2011, 146, 980–991. [Google Scholar] [CrossRef]
- Verhagen, J.H.; Fouchier, R.A.M.; Lewis, N. Highly Pathogenic Avian Influenza Viruses at the Wild–Domestic Bird Interface in Europe: Future Directions for Research and Surveillance. Viruses 2021, 13, 212. [Google Scholar] [CrossRef] [PubMed]
- Becker, W.B. The isolation and classification of Tern virus: Influenza Virus A/Tern/South Africa/1961. J. Hyg. 1966, 64, 309–320. [Google Scholar] [CrossRef]
- Gaidet, N.; Cattoli, G.; Hammoumi, S.; Newman, S.H.; Hagemeijer, W.; Takekawa, J.Y.; Cappelle, J.; Dodman, T.; Joannis, T.; Gil, P.; et al. Evidence of infection by H5N2 highly pathogenic avian influenza viruses in healthy wild waterfowl. PLoS Pathog. 2008, 4, e1000127. [Google Scholar] [CrossRef] [PubMed]
- OIE.; Highly Pathogenic Influenza A Viruses (Inf. with)(Non-Poultry Including Wild Birds)(2017-), Lithuania. Available online: https://wahis.oie.int/#/report-info?reportId=37130 (accessed on 8 November 2021).
- OIE.; Highly Pathogenic Influenza A Viruses (Inf. with)(Non-Poultry Including Wild Birds)(2017-), France. Available online: https://wahis.oie.int/#/report-info?reportId=41817 (accessed on 8 November 2021).
- OIE.; Highly Pathogenic Avian Influenza, Algeria. Available online: https://wahis.oie.int/#/report-info?reportId=8691 (accessed on 8 November 2021).
- Pantin-Jackwood, M.J.; Costa-Hurtado, M.; Shepherd, E.; DeJesus, E.; Smith, D.; Spackman, E.; Kapczynski, D.R.; Suarez, D.L.; Stallknecht, D.E.; Swayne, D.E. Pathogenicity and Transmission of H5 and H7 Highly Pathogenic Avian Influenza Viruses in Mallards. J. Virol. 2016, 90, 9967–9982. [Google Scholar] [CrossRef] [PubMed]
- Nakayama, M.; Uchida, Y.; Shibata, A.; Kobayashi, Y.; Mine, J.; Takemae, N.; Tsunekuni, R.; Tanikawa, T.; Harada, R.; Osaka, H.; et al. A novel H7N3 reassortant originating from the zoonotic H7N9 highly pathogenic avian influenza viruses that has adapted to ducks. Transbound. Emerg. Dis. 2019, 66, 2342–2352. [Google Scholar] [CrossRef]
- Cornelissen, J.B.W.J.; Vervelde, L.; Post, J.; Rebel, J.M.J. Differences in highly pathogenic avian influenza viral pathogenesis and associated early inflammatory response in chickens and ducks. Avian Pathol. 2013, 42, 347–364. [Google Scholar] [CrossRef]
- Alexander, D.J.; Parsons, G.; Manvell, R.J. Experimental Assessment Of The Pathogenicity Of Eight Avian Influenza A Viruses Of H5 Subtype For Chickens, Turkeys, Ducks And Quail. Avian Pathol. 1986, 15, 647–662. [Google Scholar] [CrossRef]
- Pantin-Jackwood, M.J.; Stephens, C.B.; Bertran, K.; Swayne, D.E.; Spackman, E. The pathogenesis of H7N8 low and highly pathogenic avian influenza viruses from the United States 2016 outbreak in chickens, turkeys and mallards. PLoS ONE 2017, 12, e0177265. [Google Scholar] [CrossRef] [PubMed]
- Sturm-Ramirez, K.M.; Hulse-Post, D.J.; Govorkova, E.A.; Humberd, J.; Seiler, P.; Puthavathana, P.; Buranathai, C.; Nguyen, T.D.; Chaisingh, A.; Long, H.T.; et al. Are Ducks Contributing to the Endemicity of Highly Pathogenic H5N1 Influenza Virus in Asia? J. Virol. 2005, 79, 11269–11279. [Google Scholar] [CrossRef] [PubMed]
- Keawcharoen, J.; Van Riel, D.; Van Amerongen, G.; Bestebroer, T.; Beyer, W.E.; Van Lavieren, R.; Osterhaus, A.D.M.E.; Fouchier, R.A.M.; Kuiken, T. Wild ducks as long-distance vectors of highly pathogenic avian influenza virus (H5N1). Emerg. Infect. Dis. 2008, 14, 600–607. [Google Scholar] [CrossRef]
- Pantin-Jackwood, M.J.; Suarez, D.L.; Spackman, E.; Swayne, D.E. Age at infection affects the pathogenicity of Asian highly pathogenic avian influenza H5N1 viruses in ducks. Virus Res. 2007, 130, 151–161. [Google Scholar] [CrossRef]
- Short, K.R.; Veldhuis Kroeze, E.J.B.; Reperant, L.A.; Richard, M.; Kuiken, T. Influenza virus and endothelial cells: A species specific relationship. Front. Microbiol. 2014, 5, 653. [Google Scholar] [CrossRef]
- Brown, J.D.; Stallknecht, D.E.; Swayne, D.E. Experimental infection of swans and geese with highly pathogenic avian influenza virus (H5N1) of Asian lineage. Emerg. Infect. Dis. 2008, 14, 136–142. [Google Scholar] [CrossRef] [PubMed]
- Caliendo, V.; Leijten, L.; Begeman, L.; Poen, M.J.; Fouchier, R.A.M.; Beerens, N.; Kuiken, T. Enterotropism of highly pathogenic avian influenza virus H5N8 from the 2016/2017 epidemic in some wild bird species. Vet. Res. 2020, 51, 117. [Google Scholar] [CrossRef] [PubMed]
- Blaurock, C.; Breithaupt, A.; Scheibner, D.; Bagato, O.; Karger, A.; Mettenleiter, T.C.; Abdelwhab, E.M. Preferential Selection and Contribution of Non-Structural Protein 1 (NS1) to the Efficient Transmission of Panzootic Avian Influenza H5N8 Virus Clades 2.3.4.4A and B in Chickens and Ducks. J. Virol. 2021, 95. [Google Scholar] [CrossRef] [PubMed]
- Van Campen, H.; Davis, M.R. Isolation and characterization of chicken aortic endothelial cells. J. Tissue Cult. Methods 1993, 15, 171–175. [Google Scholar] [CrossRef]
- Twal, W.O.; Leach, R.M. Isolation and characterization of microvascular endothelial cells from chicken fat pads. Vitr. Cell. Dev. Biol. Anim. 1996, 32, 403–408. [Google Scholar] [CrossRef]
- Bai, C.; Hou, L.; Zhang, M.; Pu, Y.; Guan, W.; Ma, Y. Characterization of vascular endothelial progenitor cells from chicken bone marrow. BMC Vet. Res. 2012, 8, 54. [Google Scholar] [CrossRef]
- Lion, A.; Esnault, E.; Kut, E.; Guillory, V.; Trapp-Fragnet, L.; Soubies, S.M.; Chanteloup, N.; Niepceron, A.; Guabiraba, R.; Marc, D.; et al. Chicken endothelial cells are highly responsive to viral innate immune stimuli and are susceptible to infections with various avian pathogens. Avian Pathol. 2019, 48, 121–134. [Google Scholar] [CrossRef]
- Lion, A.; Richard, M.; Esnault, E.; Kut, E.; Soubieux, D.; Guillory, V.; Germond, M.; Blondeau, C.; Guabiraba, R.; Short, K.R.; et al. Productive replication of avian influenza viruses in chicken endothelial cells is determined by hemagglutinin cleavability and is related to innate immune escape. Virology 2018, 513, 29–42. [Google Scholar] [CrossRef] [PubMed]
- Tong, Z.W.M.; Karawita, A.C.; Kern, C.; Zhou, H.; Sinclair, J.E.; Yan, L.; Chew, K.Y.; Lowther, S.; Trinidad, L.; Challagulla, A.; et al. Primary chicken and duck endothelial cells display a differential response to infection with highly pathogenic avian influenza virus. Genes 2021, 12, 901. [Google Scholar] [CrossRef] [PubMed]
- Davis, R.L.; Choi, G.; Kuiken, T.; Quéré, P.; Trapp, S.; Short, K.R.; Richard, M. The culture of primary duck endothelial cells for the study of avian influenza. BMC Microbiol. 2018, 18, 138. [Google Scholar] [CrossRef]
- Hernandez, R.; Brown, D.T. Growth and maintenance of chick embryo fibroblasts (CEF). Curr. Protoc. Microbiol. 2010, 4. [Google Scholar] [CrossRef] [PubMed]
- De Wit, E.; Spronken, M.I.J.; Bestebroer, T.M.; Rimmelzwaan, G.F.; Osterhaus, A.D.M.E.; Fouchier, R.A.M. Efficient generation and growth of influenza virus A/PR/8/34 from eight cDNA fragments. Virus Res. 2004, 103, 155–161. [Google Scholar] [CrossRef]
- Herfst, S.; Mok, C.K.P.; van den Brand, J.M.A.; van der Vliet, S.; Rosu, M.E.; Spronken, M.I.; Yang, Z.; de Meulder, D.; Lexmond, P.; Bestebroer, T.M.; et al. Human Clade 2.3.4.4 A/H5N6 Influenza Virus Lacks Mammalian Adaptation Markers and Does Not Transmit via the Airborne Route between Ferrets. mSphere 2018, 3, e00405-17. [Google Scholar] [CrossRef]
- Kihara, A.H.; Paschon, V.; Akamine, P.S.; Saito, K.C.; Leonelli, M.; Jiang, J.X.; Hamassaki, D.E.; Britto, L.R.G. Differential expression of connexins during histogenesis of the chick retina. Dev. Neurobiol. 2008, 68, 1287–1302. [Google Scholar] [CrossRef]
- Jiang, H.; Yang, H.; Kapczynski, D.R. Chicken interferon alpha pretreatment reduces virus replication of pandemic H1N1 and H5N9 avian influenza viruses in lung cell cultures from different avian species. Virol. J. 2011, 8, 447. [Google Scholar] [CrossRef]
- Cornelissen, J.B.W.J.; Post, J.; Peeters, B.; Vervelde, L.; Rebel, J.M.J. Differential innate responses of chickens and ducks to low-pathogenic avian influenza. Avian Pathol. 2012, 41, 519–529. [Google Scholar] [CrossRef]
- Cui, Z.; Hu, J.; He, L.; Li, Q.; Gu, M.; Wang, X.; Hu, S.; Liu, H.; Liu, W.; Liu, X.; et al. Differential immune response of mallard duck peripheral blood mononuclear cells to two highly pathogenic avian influenza H5N1 viruses with distinct pathogenicity in mallard ducks. Arch. Virol. 2014, 159, 339–343. [Google Scholar] [CrossRef]
- Zhong, Z.; Ji, Y.; Fu, Y.; Liu, B.; Zhu, Q. Molecular characterization and expression analysis of the duck viperin gene. Gene 2015, 570, 100–107. [Google Scholar] [CrossRef]
- Saito, L.B.; Diaz-Satizabal, L.; Evseev, D.; Fleming-Canepa, X.; Mao, S.; Webster, R.G.; Magor, K.E. IFN and cytokine responses in ducks to genetically similar H5N1 influenza a viruses of varying pathogenicity. J. Gen. Virol. 2018, 99, 464–474. [Google Scholar] [CrossRef] [PubMed]
- Voyta, J.C.; Via, D.P.; Butterfield, C.E.; Zetter, B.R. Identification and isolation of endothelial cells based on their increased uptake of acetylated-low density lipoprotein. J. Cell Biol. 1984, 99, 2034–2040. [Google Scholar] [CrossRef]
- Rogers, G.N.; Paulson, J.C. Receptor determinants of human and animal influenza virus isolates: Differences in receptor specificity of the H3 hemagglutinin based on species of origin. Virology 1983, 127, 361–373. [Google Scholar] [CrossRef]
- Nobusawa, E.; Aoyama, T.; Kato, H.; Suzuki, Y.; Tateno, Y.; Nakajima, K. Comparison of complete amino acid sequences and receptor-binding properties among 13 serotypes of hemagglutinins of influenza A viruses. Virology 1991, 182, 475–485. [Google Scholar] [CrossRef]
- Gambaryan, A.S.; Tuzikov, A.B.; Piskarev, V.E.; Yamnikova, S.S.; Lvov, D.K.; Robertson, J.S.; Bovin, N.V.; Matrosovich, M.N. Specification of receptor-binding phenotypes of influenza virus isolates from different hosts using synthetic sialylglycopolymers: Non-egg-adapted human H1 and H3 influenza A and influenza B viruses share a common high binding affinity for 6′-sialyl(N-acetyllactosamine). Virology 1997, 232, 345–350. [Google Scholar] [CrossRef]
- Matrosovich, M.; Tuzikov, A.; Bovin, N.; Gambaryan, A.; Klimov, A.; Castrucci, M.R.; Donatelli, I.; Kawaoka, Y. Early Alterations of the Receptor-Binding Properties of H1, H2, and H3 Avian Influenza Virus Hemagglutinins after Their Introduction into Mammals. J. Virol. 2000, 74, 8502–8512. [Google Scholar] [CrossRef]
- Evseev, D.; Magor, K.E. Innate immune responses to avian influenza viruses in ducks and chickens. Vet. Sci. 2019, 6, 5. [Google Scholar] [CrossRef]
- Campbell, L.K.; Magor, K.E. Pattern Recognition Receptor Signaling and Innate Responses to Influenza A Viruses in the Mallard Duck, Compared to Humans and Chickens. Front. Cell. Infect. Microbiol. 2020, 10, 209. [Google Scholar] [CrossRef] [PubMed]
- Kuchipudi, S.V.; Tellabati, M.; Sebastian, S.; Londt, B.Z.; Jansen, C.; Vervelde, L.; Brookes, S.M.; Brown, I.H.; Dunham, S.P.; Chang, K.C. Highly pathogenic avian influenza virus infection in chickens but not ducks is associated with elevated host immune and pro-inflammatory responses. Vet. Res. 2014, 45, 118. [Google Scholar] [CrossRef] [PubMed]
- Feldmann, A.; Schäfer, M.K.-H.; Garten, W.; Klenk, H.-D. Targeted Infection of Endothelial Cells by Avian Influenza Virus A/FPV/Rostock/34 (H7N1) in Chicken Embryos. J. Virol. 2000, 74, 8018–8027. [Google Scholar] [CrossRef] [PubMed]
- Pantin-Jackwood, M.J.; Swayne, D.E. Pathobiology of Asian highly pathogenic avian influenza H5N1 virus infections in ducks. Avian dis. 2007, 51, 250–259. [Google Scholar] [CrossRef] [PubMed]
- Löndt, B.Z.; Nunez, A.; Banks, J.; Nili, H.; Johnson, L.K.; Alexander, D.J. Pathogenesis of highly pathogenic avian influenza A/turkey/Turkey/1/2005 H5N1 in Pekin ducks (Anas platyrhynchos) infected experimentally. Avian Pathol. 2008, 37, 619–627. [Google Scholar] [CrossRef] [PubMed]
- Kuchipudi, S.V.; Nelli, R.; White, G.A. Differences in influenza virus receptors in chickens and ducks: Implications for interspecies transmission. J. Mol. Genet. Med. 2009, 3, 143. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.E.; Yoon, H.; Lee, H.J.; Lee, J.H.; Chang, B.J.; Song, C.S.; Nahm, S.S. Expression patterns of influenza virus receptors in the respiratory tracts of four species of poultry. J. Vet. Sci. 2011, 12, 7–13. [Google Scholar] [CrossRef] [PubMed][Green Version]
- França, M.; Stallknecht, D.E.; Howerth, E.W. Expression and distribution of sialic acid influenza virus receptors in wild birds. Avian Pathol. 2013, 42, 60–71. [Google Scholar] [CrossRef]
- Short, K.R.; Kasper, J.; Van Der Aa, S.; Andeweg, A.C.; Zaaraoui-Boutahar, F.; Goeijenbier, M.; Richard, M.; Herold, S.; Becker, C.; Scott, D.P.; et al. Influenza virus damages the alveolar barrier by disrupting epithelial cell tight junctions. Eur. Respir. J. 2016, 47, 954–966. [Google Scholar] [CrossRef]
- Yamamoto, Y.; Nakamura, K.; Yamada, M.; Mase, M. Comparative pathology of chickens and domestic ducks experimentally infected with highly pathogenic avian influenza viruses (H5N1) isolated in Japan in 2007 and 2008. Jpn. Agric. Res. Q. 2010, 44, 73–80. [Google Scholar] [CrossRef][Green Version]
- Morahan, P.S.; Grossberg, S.E. Age-related cellular resistance of the chicken embryo to viral infections. I. Interferon and natural resistance to myxoviruses and vesicular stomatitis virus. J. Infect. Dis. 1970, 121, 615–623. [Google Scholar] [CrossRef]
- Alkie, T.N.; Yitbarek, A.; Hodgins, D.C.; Kulkarni, R.R.; Taha-Abdelaziz, K.; Sharif, S. Development of innate immunity in chicken embryos and newly hatched chicks: A disease control perspective. Avian Pathol. 2019, 48, 288–310. [Google Scholar] [CrossRef]
- Rebel, J.M.; Peeters, B.; Fijten, H.; Post, J.; Cornelissen, J.; Vervelde, L. Highly pathogenic or low pathogenic avian influenza virus subtype H7N1 infection in chicken lungs: Small differences in general acute responses. Vet. Res. 2011, 42, 10. [Google Scholar] [CrossRef]
- Smith, J.; Smith, N.; Yu, L.; Paton, I.R.; Gutowska, M.W.; Forrest, H.L.; Danner, A.F.; Patrick Seiler, J.; Digard, P.; Webster, R.G.; et al. A comparative analysis of host responses to avian influenza infection in ducks and chickens highlights a role for the interferon-induced transmembrane proteins in viral resistance. BMC Genom. 2011, 16, 574. [Google Scholar] [CrossRef]
- Pantin-Jackwood, M.J.; Smith, D.M.; Wasilenko, J.L.; Cagle, C.; Shepherd, E.; Sarmento, L.; Kapczynski, D.R.; Afonso, C.L. Effect of age on the pathogenesis and innate immune responses in Pekin ducks infected with different H5N1 highly pathogenic avian influenza viruses. Virus Res. 2012, 167, 196–206. [Google Scholar] [CrossRef]
- Vanderven, H.A.; Petkau, K.; Ryan-Jean, K.E.E.; Aldridge, J.R.; Webster, R.G.; Magor, K.E. Avian influenza rapidly induces antiviral genes in duck lung and intestine. Mol. Immunol. 2012, 51, 316. [Google Scholar] [CrossRef]
- Liang, Q.L.; Luo, J.; Zhou, K.; Dong, J.X.; He, H. xuan Immune-related gene expression in response to H5N1 avian influenza virus infection in chicken and duck embryonic fibroblasts. Mol. Immunol. 2011, 48, 924–930. [Google Scholar] [CrossRef] [PubMed]
- Mcmanus, C.J.; Duff, M.O.; Eipper-Mains, J.; Graveley, B.R.; Barber, M.R.W.; Aldridge, J.R.; Webster, R.G.; Magor, K.E.; Shankarappa, S.A.; Tsui, J.H.; et al. Association of RIG-I with innate immunity of ducks to influenza. Proc. Natl. Acad. Sci. USA 2010, 107, 5913–5918. [Google Scholar] [CrossRef]
- Karpala, A.J.; Stewart, C.; McKay, J.; Lowenthal, J.W.; Bean, A.G.D. Characterization of chicken Mda5 activity: Regulation of IFN-β in the absence of RIG-I functionality. J. Immunol. 2011, 186, 5397–5405. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, T.; Watanabe, C.; Suzuki, Y.; Tanikawa, T.; Uchida, Y.; Saito, T. Chicken MDA5 Senses Short Double-Stranded RNA with Implications for Antiviral Response against Avian Influenza Viruses in Chicken. J. Innate Immun. 2014, 6, 58–71. [Google Scholar] [CrossRef] [PubMed]
- Liniger, M.; Summerfield, A.; Zimmer, G.; McCullough, K.C.; Ruggli, N. Chicken Cells Sense Influenza A Virus Infection through MDA5 and CARDIF Signaling Involving LGP2. J. Virol. 2012, 86, 705. [Google Scholar] [CrossRef] [PubMed]
| (Nucleic Acid Dye-Based) RT-(q)PCR a | |||||
|---|---|---|---|---|---|
| Target | Host | Accession Number | Oligo | Sequence (5′ to 3′) | Reference |
| GAPDH | Chicken | AF047874 | Forward | GGTGCTAAGCGTGTTATCATCTCA | [60] |
| Reverse | CATGGTTGACACCCATCACAA | ||||
| Duck | XM_027449739.1 | Forward | GCCTCTTGCACCACCAACT | This study | |
| Reverse | GGCATGGACAGTGGTCATAA | ||||
| IL6 | Chicken | HM179640.1 | Forward | GCGAGAACAGCATGGAGATG | [61] |
| Reverse | GTAGGTCTGAAAGGCGAACAG | ||||
| Duck | AB191038 | Forward | GCAACGACGATAAGGCAGATG | [62] | |
| Reverse | TCTTATCCGATTTCAGCTTTGTGA | ||||
| IL8 | Chicken | NM_205498.1 | Forward | CTGCGGTGCCAGTGCATTAG | [54] |
| Reverse | AGCACACCTCTCTTCCATCC | ||||
| Duck | NM_205498 | Forward | AGGACAACAGAGAGGTGTGCTTG | [63] | |
| Reverse | GCCTTTACGATCCGCTGTACC | ||||
| RSAD2 | Chicken | NM_001318443.1 | Forward | GCCGAGATTATGCTGTTGCTT | This study |
| Reverse | TGATTAGGCACTGGAACACCT | ||||
| Duck | KP198582.1 | Forward | GCCGAGAGTATGCTGTTGCTT | [64] | |
| Reverse | AATGAGCAGGCACTGGAACAC | ||||
| VWF | Chicken | BK007988.1 | Forward | GTGCTAAAACCTGCCAGAGC | This study |
| Reverse | CACAACGTTCACCATCAAGG | ||||
| Duck | XM_005012640.3 | Forward | ACCACATGTTAGTGAGGAAC | [56] | |
| Reverse | CTTGGTAGGGTATGCTTCTC | ||||
| Primer/probe RT-qPCR | |||||
| GAPDH | Chicken/Duck | Ch: NM_204305.1 Du: XM_027449739.1 | Forward | AGTCGGAGTCAACGGATTTG | This study |
| Reverse | GGCYACCACTTGGACTTTGC | ||||
| Probe | 6-FAM CTGGTGACCAGGCGSCCAATAC BHQ1 | ||||
| IFNB | Chicken | NM_001024836.1 | N.A. b | Gg03344129_s1 (Thermo Fisher Scientific) | N.A. |
| Duck | KM035791.2 | Forward | CTTTTGGACACCGACAAC | [65] | |
| Reverse | AGGATGTTGAAGAGGTGTTG | ||||
| Probe | 6-FAM CAAGCCGCACACACCGCC BHQ1 | ||||
| Matrix gene segment | Influenza A virus | N.A. | Forward | CTTCTRACCGAGGTCGAAACGTA | This study |
| Reverse | TCTTGTCTTTAGCCAYTCCATGAG | ||||
| Probe | 6-FAM TCAGGCCCCCTCAAAGCCGARA BHQ1 | ||||
| Species | Age at Inoculation (Days) | Age at Harvest (Days) | CAM a | Lungs | Heart | Liver | Intestine | Kidney |
|---|---|---|---|---|---|---|---|---|
| Chicken | 14 | 15 | +/+ b | +/+ | +/+ | +/+ | +/+ | +/+ |
| Chicken | 17 | 19 | +/+ | +/+ | +/+ | +/† c | +/+ | N.D. d |
| Duck | 18 | 19 | +/+ | +/+ | +/+ | +/+ | +/+ | +/+ |
| Duck | 21 | 23 | +/+ | +/+ | +/+ | +/† | +/+ | N.D. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
de Bruin, A.C.M.; Spronken, M.I.; Bestebroer, T.M.; Fouchier, R.A.M.; Richard, M. Reduced Replication of Highly Pathogenic Avian Influenza Virus in Duck Endothelial Cells Compared to Chicken Endothelial Cells Is Associated with Stronger Antiviral Responses. Viruses 2022, 14, 165. https://doi.org/10.3390/v14010165
de Bruin ACM, Spronken MI, Bestebroer TM, Fouchier RAM, Richard M. Reduced Replication of Highly Pathogenic Avian Influenza Virus in Duck Endothelial Cells Compared to Chicken Endothelial Cells Is Associated with Stronger Antiviral Responses. Viruses. 2022; 14(1):165. https://doi.org/10.3390/v14010165
Chicago/Turabian Stylede Bruin, Anja C. M., Monique I. Spronken, Theo M. Bestebroer, Ron A. M. Fouchier, and Mathilde Richard. 2022. "Reduced Replication of Highly Pathogenic Avian Influenza Virus in Duck Endothelial Cells Compared to Chicken Endothelial Cells Is Associated with Stronger Antiviral Responses" Viruses 14, no. 1: 165. https://doi.org/10.3390/v14010165
APA Stylede Bruin, A. C. M., Spronken, M. I., Bestebroer, T. M., Fouchier, R. A. M., & Richard, M. (2022). Reduced Replication of Highly Pathogenic Avian Influenza Virus in Duck Endothelial Cells Compared to Chicken Endothelial Cells Is Associated with Stronger Antiviral Responses. Viruses, 14(1), 165. https://doi.org/10.3390/v14010165






