Actin Contributes to the Hyperexpression of Baculovirus Polyhedrin (polh) and p10 as a Component of Transcription Initiation Complex (TIC)
Abstract
1. Introduction
2. Materials and Methods
2.1. Cells, Viruses, and Plasmids and Antibodies
2.2. Pull-Down and Liquid Chromatography–Tandem Mass Spectrometry (LC–MS/MS) Analyses
2.3. Drug Treatments
2.4. Quantitative Reverse Transcription PCR (RT-qPCR)
2.5. Expression of FLAG-Tagged and HA-Tagged Proteins
2.6. Immunofluorescence
2.7. Co-IP Assay
2.8. ChIP-Seq and ChIP-qPCR
2.9. RNA-Seq
2.10. Transfection and Measurement of Luciferase Activity in Cell Culture
2.11. Statistical Analyses
3. Results
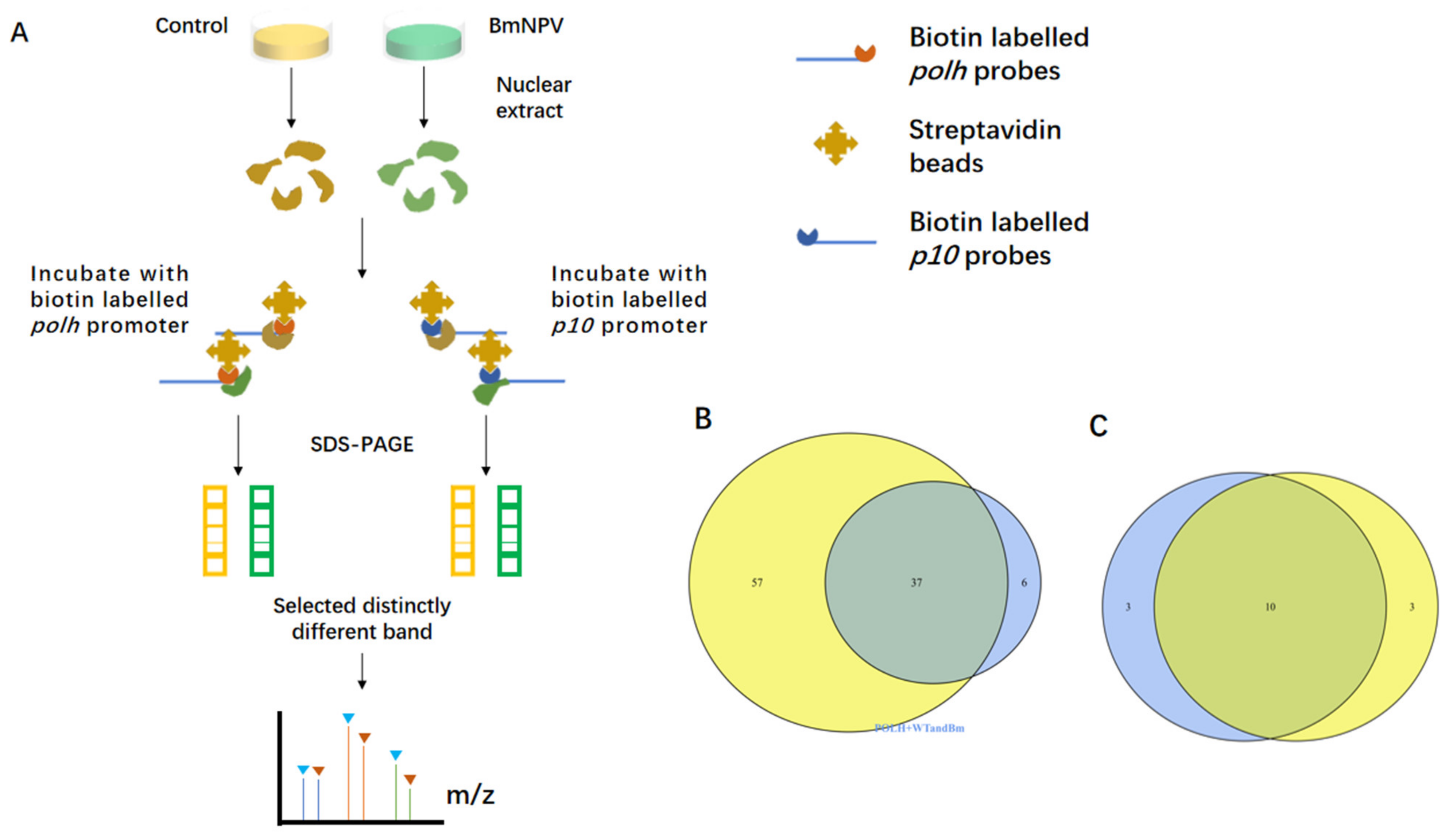
3.1. Screening of the polh and p10 Promoter-Binding Proteins by DNA Pull-Down Assay and Mass Spectrometry
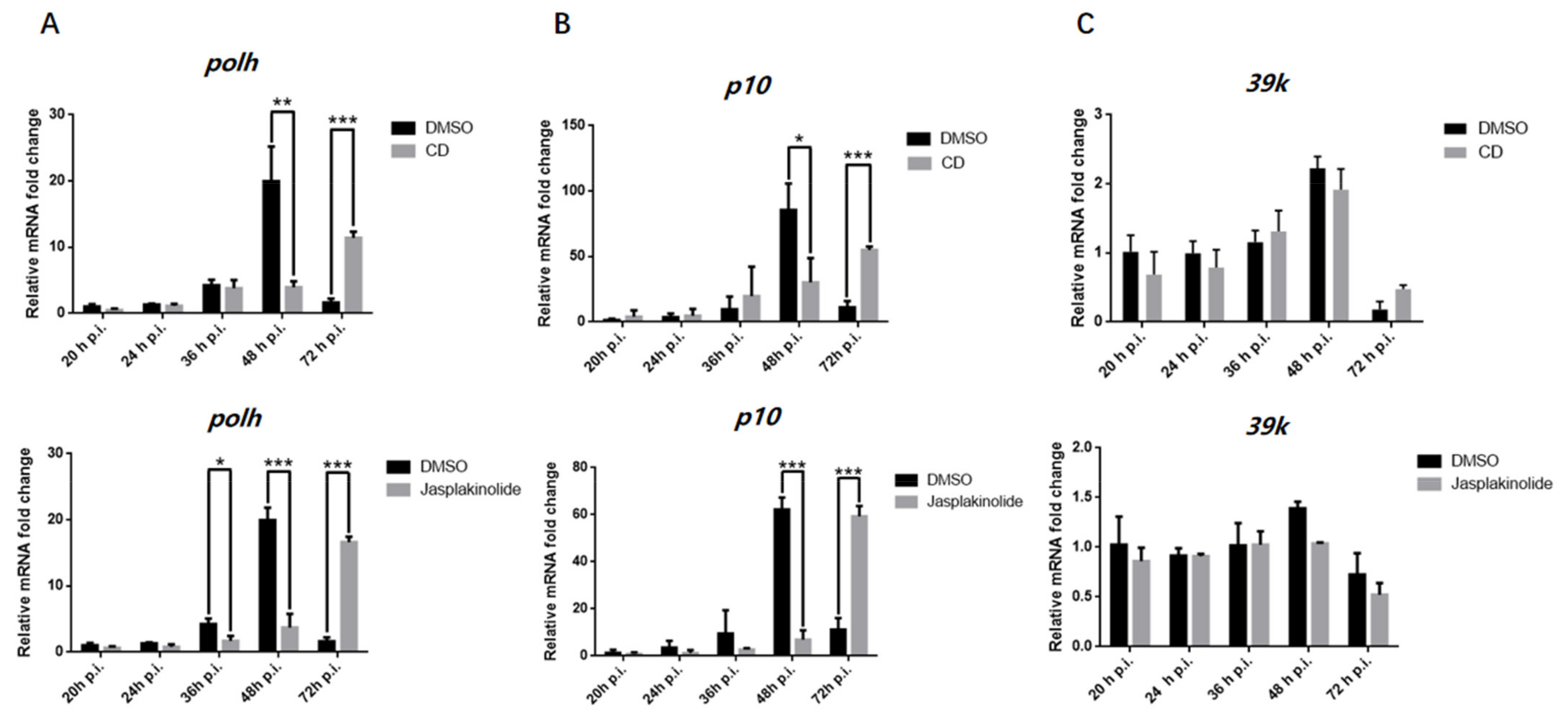
3.2. The Actin Dynamics Was Essential for the Transcription of polh and p10
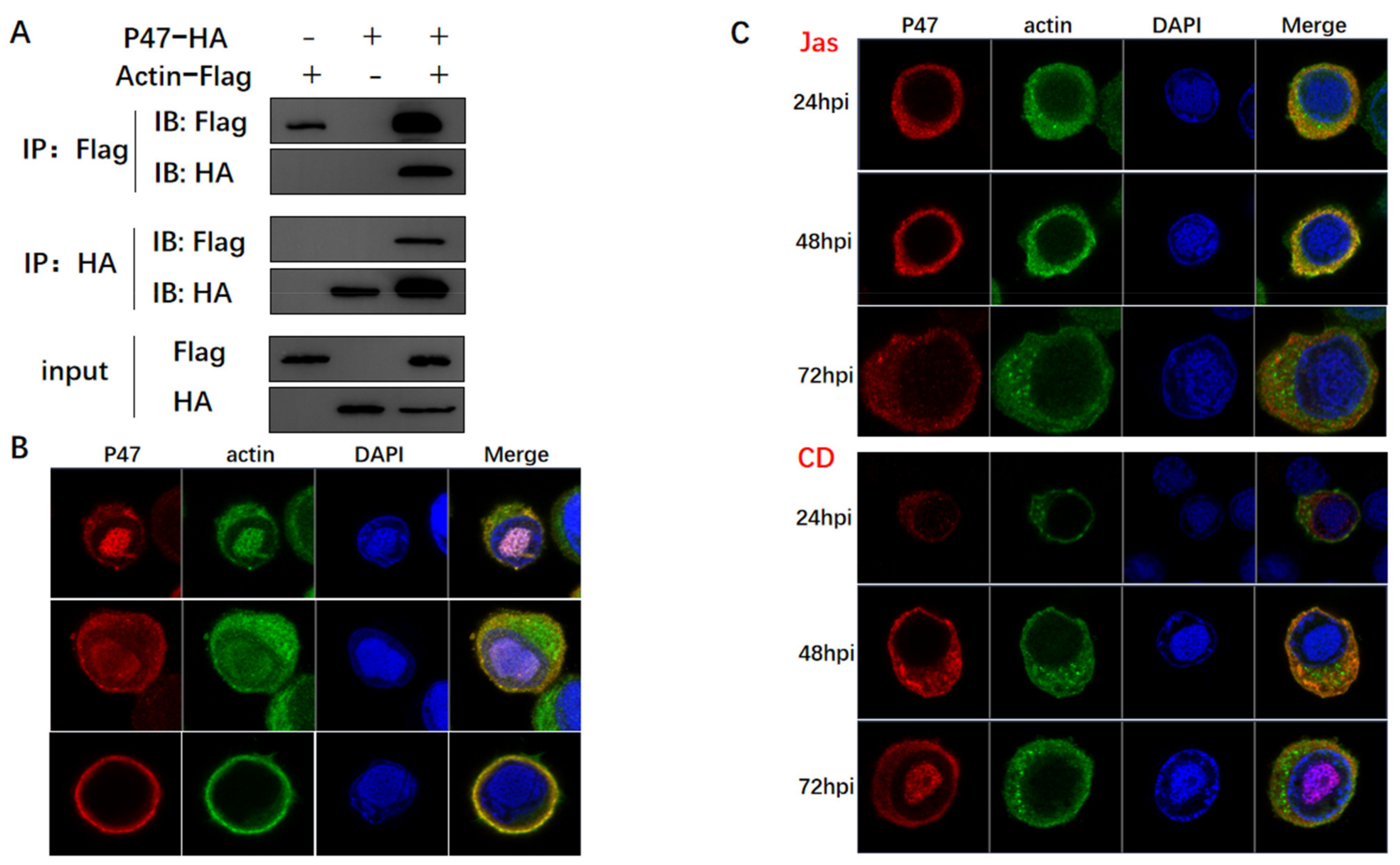
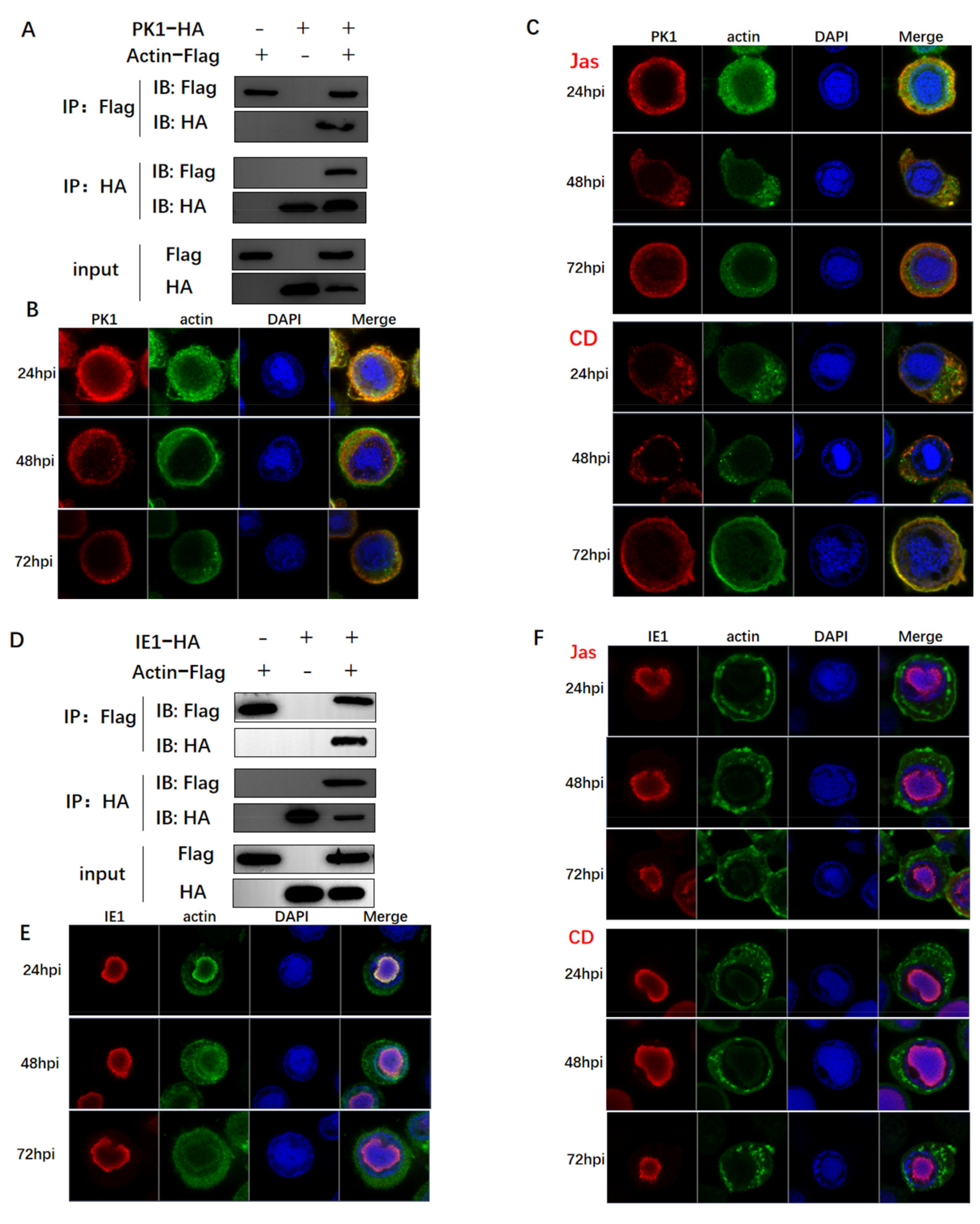
3.3. Actin Was Associated and Colocalized with Viral Polymerase
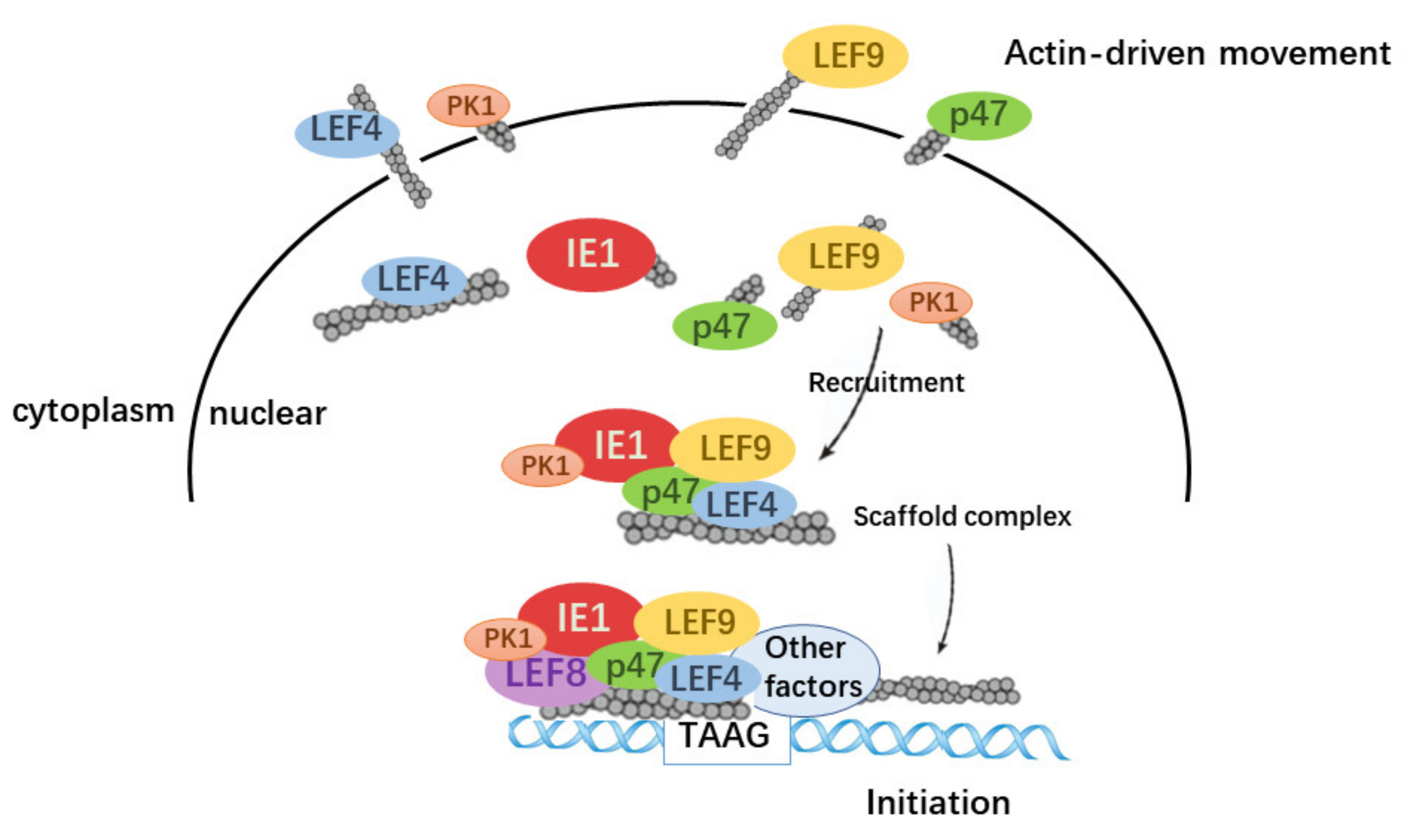
3.4. Actin Was a Component of TIC for polh and p10 Transcription
3.5. Actin Interacted with Transcription Regulators and Might Tether TIC Formation
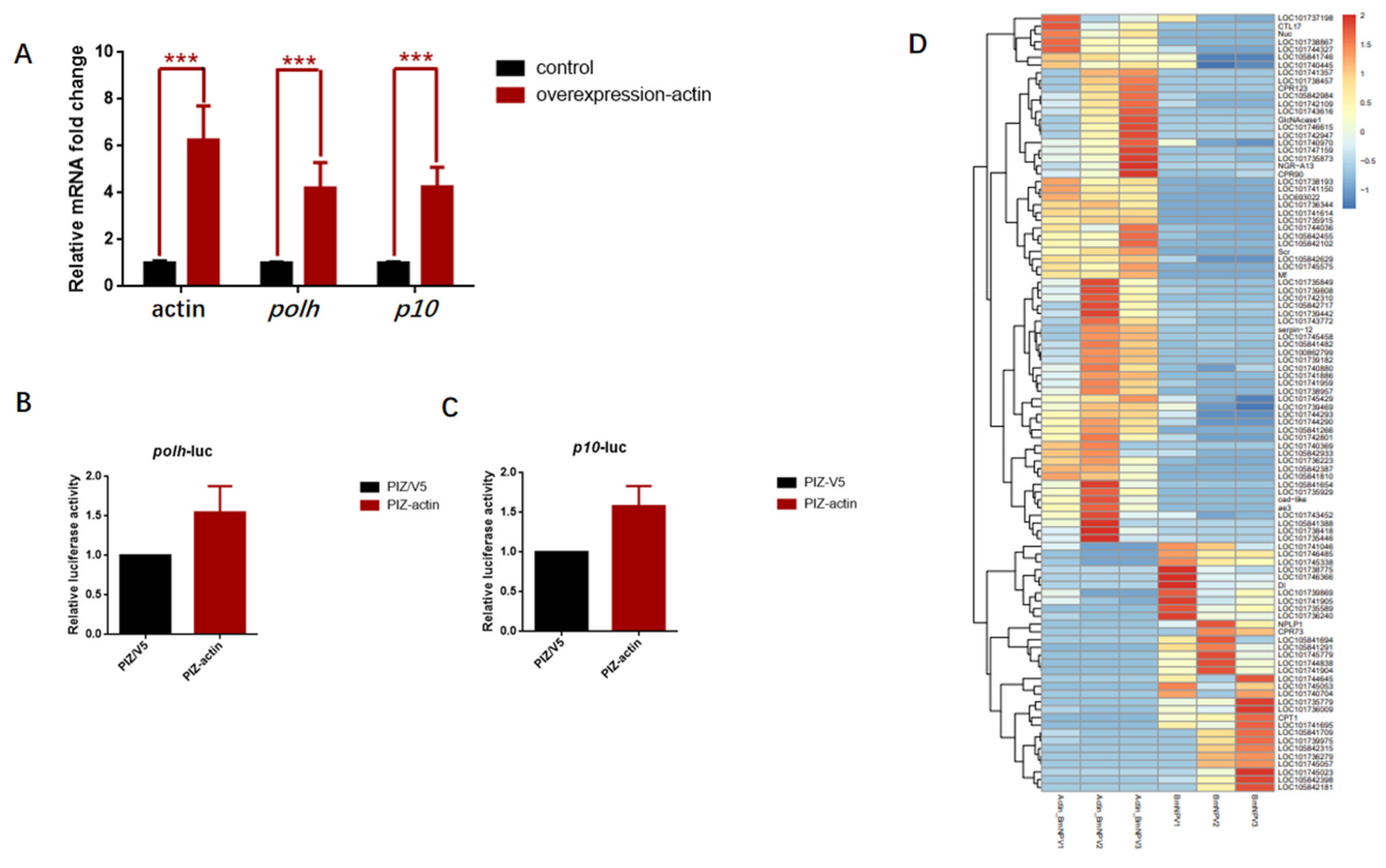
3.6. Overexpression of Actin Stimulated the Transcription of polh and p10 Promoters
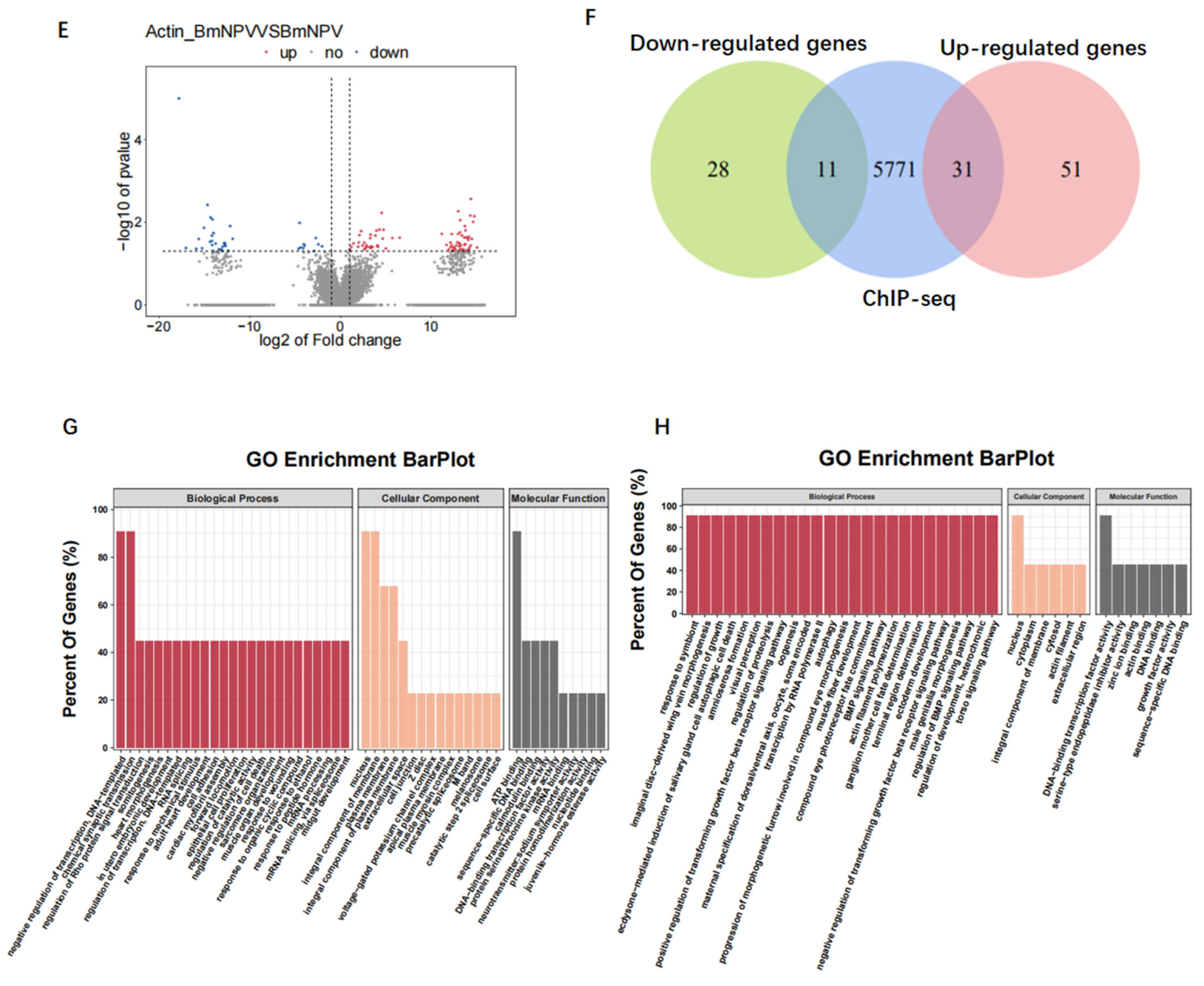
3.7. Baculovirus Might Hijack Actin of the Host Transcription Apparatus
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kost, T.A.; Condreay, J.P.; Jarvis, D.L. Baculovirus as versatile vectors for protein expression in insect and mammalian cells. Nat. Biotechnol. 2005, 23, 567–575. [Google Scholar] [CrossRef]
- Vlak, J.M.; Klinkenberg, A.F.; Zaal, K.J.M.; Usmany, M.; Klinge-Roode, E.C.; Geervliet, J.B.F.; Roosien, J.; Van Lent, J.W.M.; Klinkenberg, F. Functional studies on the p10 gene of Autographa californica nuclear polyhedrosis virus using a recombinant expressing a p10-beta-galactosidase fusion gene. J. Gen. Virol. 1988, 69 Pt 4, 765–776. [Google Scholar] [CrossRef]
- Blissard, G.W.; Rohrmann, G.F. Baculovirus diversity and molecular biology. Annu. Rev. Entomol. 1990, 35, 127–155. [Google Scholar] [CrossRef] [PubMed]
- Felberbaum, R.S. The baculovirus expression vector system: A commercial manufacturing platform for viral vaccines and gene therapy vectors. Biotechnol. J. 2015, 10, 702–714. [Google Scholar] [CrossRef]
- van Oers, M.M.; Pijlman, G.P.; Vlak, J.M. Thirty years of baculovirus-insect cell protein expression: From dark horse to mainstream technology. J. Gen. Virol. 2015, 96 Pt 1, 6–23. [Google Scholar] [CrossRef] [PubMed]
- Ikonomou, L.; Schneider, Y.J.; Agathos, S.N. Insect cell culture for industrial production of recombinant proteins. Appl. Microbiol. Biotechnol. 2003, 62, 1–20. [Google Scholar] [CrossRef]
- Ohkawa, T.; Rowe, A.R.; Volkman, L.E. Identification of Six—Autographa californica—Multicapsid Nucleopolyhedrovirus Early Genes That Mediate Nuclear Localization of G-Actin. J. Virol. 2002, 76, 12281–12289. [Google Scholar] [CrossRef] [PubMed]
- Kyheröinen, S.; Vartiainen, M.K. Nuclear actin dynamics in gene expression and genome organization. Semin. Cell Dev. Biol. 2020, 102, 105–112. [Google Scholar] [CrossRef]
- Goley, E.D.; Ohkawa, T.; Mancuso, J.; Woodruff, J.B.; D’Alessio, J.A.; Cande, W.Z.; Volkman, L.E.; Welch, M.D. Dynamic nuclear actin assembly by Arp2/3 complex and a baculovirus WASP-like protein. Science 2006, 314, 464–467. [Google Scholar] [CrossRef]
- Charlton, C.A.; Volkman, L.E. Sequential rearrangement and nuclear polymerization of actin in baculovirus-infected Spodoptera frugiperda cells. J. Virol. 1991, 65, 1219–1227. [Google Scholar] [CrossRef]
- Charlton, C.A.; Volkman, L.E. Penetration of Autographa californica nuclear polyhedrosis virus nucleocapsids into IPLB Sf 21 cells induces actin cable formation. Virology 1993, 197, 245–254. [Google Scholar] [CrossRef] [PubMed]
- Olson, E.N.; Nordheim, A. Linking actin dynamics and gene transcription to drive cellular motile functions. Nat. Rev. Mol. Cell Biol. 2010, 11, 353–365. [Google Scholar] [CrossRef] [PubMed]
- Burke, E.; Dupuy, L.; Wall, C.; Barik, S. Role of Cellular Actin in the Gene Expression and Morphogenesis of Human Respiratory Syncytial Virus. Virology 1998, 252, 137–148. [Google Scholar] [CrossRef] [PubMed]
- Paoletti, A.; Allouch, A.; Caillet, M.; Saïdi, H.; Subra, F.; Nardacci, R.; Wu, Q.; Muradova, Z.; Voisin, L.; Raza, S.Q.; et al. HIV-1 Envelope Overcomes NLRP3-Mediated Inhibition of F-Actin Polymerization for Viral Entry. Cell Rep. 2019, 28, 3381–3394.e7. [Google Scholar] [CrossRef] [PubMed]
- Ohkawa, T.; Volkman, L.E.; Welch, M.D. Actin-based motility drives baculovirus transit to the nucleus and cell surface. J. Cell Biol. 2010, 190, 187–195. [Google Scholar] [CrossRef] [PubMed]
- Ohkawa, T.; Welch, M.D. Baculovirus Actin-Based Motility Drives Nuclear Envelope Disruption and Nuclear Egress. Curr. Biol. 2018, 28, 2153–2159.e4. [Google Scholar] [CrossRef]
- Sokolova, M.; Moore, H.M.; Prajapati, B.; Dopie, J.; Meriläinen, L.; Honkanen, M.; Matos, R.C.; Poukkula, M.K.; Hietakangas, V.; Vartiainen, M.K. Nuclear Actin Is Required for Transcription during Drosophila Oogenesis. iScience 2018, 9, 63–70. [Google Scholar] [CrossRef]
- Philimonenko, V.V.; Zhao, J.; Iben, S.; Dingová, H.; Kyselá, K.; Kahle, M.; Zentgraf, H.; Hofmann, W.; De Lanerolle, P.; Hozák, P.; et al. Nuclear actin and myosin I are required for RNA polymerase I transcription. Nat. Cell Biol. 2004, 6, 1165–1172. [Google Scholar] [CrossRef]
- Hofmann, W.; Stojiljkovic, L.; Fuchsova, B.; Vargas, G.M.; Mavrommatis, E.; Philimonenko, V.; Kysela, K.; Goodrich, J.A.; Lessard, J.L.; Hope, T.J.; et al. Actin is part of pre-initiation complexes and is necessary for transcription by RNA polymerase II. Nat. Cell Biol. 2004, 6, 1094–1101. [Google Scholar] [CrossRef]
- Hu, P.; Wu, S.; Hernandez, N. A role for beta-actin in RNA polymerase III transcription. Genes Dev. 2004, 18, 3010–3015. [Google Scholar] [CrossRef]
- Huh, N.E.; Weaver, R.F. Identifying the RNA polymerases that synthesize specific transcripts of the Autographa californica nuclear polyhedrosis virus. J. Gen. Virol. 1990, 71 Pt 1, 195–201. [Google Scholar] [CrossRef]
- Emery, V.C. Baculovirus expression vectors : Choice of expression vector. Methods Mol. Biol. 1992, 8, 287–307. [Google Scholar]
- Rohrmann, G.F. Baculovirus Molecular Biology; National Center for Biotechnology Information: Bethesda, MD, USA, 2019.
- Mans, R.M.; Knebel-Mörsdorf, D. In vitro transcription of pe38/polyhedrin hybrid promoters reveals sequences essential for recognition by the baculovirus-induced RNA polymerase and for the strength of very late viral promoters. J. Virol. 1998, 72, 2991–2998. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Morris, T.D.; Miller, L.K. Mutational analysis of a baculovirus major late promoter. Gene 1994, 140, 147–153. [Google Scholar] [CrossRef]
- Ooi, B.G.; Rankin, C.; Miller, L.K. Downstream sequences augment transcription from the essential initiation site of a baculovirus polyhedrin gene. J. Mol. Biol. 1989, 210, 721–736. [Google Scholar] [CrossRef]
- Rankin, C.; Ooi, B.G.; Miller, L.K. Eight base pairs encompassing the transcriptional start point are the major determinant for baculovirus polyhedrin gene expression. Gene 1988, 70, 39–49. [Google Scholar] [CrossRef]
- Singh, B.; Nath, S.K. Identification of Proteins Interacting with Single Nucleotide Polymorphisms (SNPs) by DNA Pull-Down Assay. Methods Mol. Biol. 2019, 1855, 355–362. [Google Scholar] [PubMed]
- Xu, W.; Wang, H.; Liu, H.; Wu, X. Bombyx mori nucleopolyhedrovirus F-like protein Bm14 is a cofactor for GP64-Mediated efficient infection via forming a complex on the envelope of budded virus. Virology 2020, 539, 61–68. [Google Scholar] [CrossRef]
- Marek, M.; Merten, O.-W.; Galibert, L.; Vlak, J.M.; van Oers, M.M. Baculovirus VP80 protein and the F-actin cytoskeleton interact and connect the viral replication factory with the nuclear periphery. J. Virol. 2011, 85, 5350–5362. [Google Scholar] [CrossRef]
- Zhang, J.; Feng, M.; Fan, Y.; Xu, W.; Zheng, Q.; Wu, X. Networks of protein-protein interactions among structural proteins of budded virus of Bombyx mori nucleopolyhedrovirus. Virology 2018, 518, 163–171. [Google Scholar] [CrossRef]
- Wan, Q.-L.; Meng, X.; Dai, W.; Luo, Z.; Wang, C.; Fu, X.; Yang, J.; Ye, Q.; Zhou, Q. N(6)-methyldeoxyadenine and histone methylation mediate transgenerational survival advantages induced by hormetic heat stress. Sci. Adv. 2021, 7, eabc3026. [Google Scholar] [CrossRef]
- Kong, X.; Wei, G.; Chen, N.; Zhao, S.; Shen, Y.; Zhang, J.; Li, Y.; Zeng, X.; Wu, X. Dynamic chromatin accessibility profiling reveals changes in host genome organization in response to baculovirus infection. PLoS Pathog. 2020, 16, e1008633. [Google Scholar] [CrossRef]
- Liu, Y.; Su, H.; Li, R.; Li, X.; Xu, Y.; Dai, X.; Zhou, Y.; Wang, H. Comparative transcriptome analysis of Glyphodes pyloalis Walker (Lepidoptera: Pyralidae) reveals novel insights into heat stress tolerance in insects. BMC Genom. 2017, 18, 974. [Google Scholar] [CrossRef] [PubMed]
- Guarino, L.A.; Xu, B.; Jin, J.; Dong, W. A Virus-Encoded RNA Polymerase Purified from Baculovirus-Infected Cells. J. Virol. 1998, 72, 7985. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Miller, L.K. Activation of Baculovirus Very Late Promoters by Interaction with Very Late Factor 1. J. Virol. 1999, 73, 3404. [Google Scholar] [CrossRef]
- Zhang, J.; Li, Y.; Zhao, S.; Wu, X. Identification of A functional region in Bombyx mori nucleopolyhedrovirus VP39 that is essential for nuclear actin polymerization. Virology 2020, 550, 37–50. [Google Scholar] [CrossRef]
- Wei, N.; Volkman, L.E. Hyperexpression of baculovirus polyhedrin and p10 is inversely correlated with actin synthesis. Virology 1992, 191, 42–48. [Google Scholar] [CrossRef]
- Talhouk, S.N.; Volkman, L.E. Autographa californica M nuclear polyhedrosis virus and cytochalasin D: Antagonists in the regulation of protein synthesis. Virology 1991, 182, 626–634. [Google Scholar] [CrossRef]
- Serebryannyy, L.A.; Parilla, M.; Annibale, P.; Cruz, C.M.; Laster, K.; Gratton, E.; Kudryashov, D.; Kosak, S.T.; Gottardi, C.J.; de Lanerolle, P. Persistent nuclear actin filaments inhibit transcription by RNA polymerase II. J. Cell Sci. 2016, 129, 3412–3425. [Google Scholar] [CrossRef]
- Friesen, P.D.; Miller, L.K. The regulation of baculovirus gene expression. Curr. Top. Microbiol. Immunol. 1986, 131, 31–49. [Google Scholar]
- Liang, C.; Su, X.; Xu, G.; Dai, X.; Zhao, S. Autographa californica multiple nucleopolyhedrovirus PK1 is a factor that regulates high-level expression of very late genes in viral infection. Virology 2017, 512, 56–65. [Google Scholar] [CrossRef] [PubMed]
- Mishra, G.; Chadha, P.; Chaudhury, I.; Das, R.H. Inhibition of Autographa californica nucleopolyhedrovirus (AcNPV) polyhedrin gene expression by DNAzyme knockout of its serine/threonine kinase (pk1) gene. Virus Res. 2008, 135, 197–201. [Google Scholar] [CrossRef]
- Mistretta, T.A.; Guarino, L.A. Transcriptional activity of baculovirus very late factor 1. J. Virol. 2005, 79, 1958–1960. [Google Scholar] [CrossRef] [PubMed]
- Wei, M.; Fan, X.; Ding, M.; Li, R.; Shao, S.; Hou, Y.; Meng, S.; Tang, F.; Li, C.; Sun, Y. Nuclear actin regulates inducible transcription by enhancing RNA polymerase II clustering. Sci. Adv. 2020, 6, eaay6515. [Google Scholar] [CrossRef]
- Hansen, M.D.H.; Kwiatkowski, A.V. Control of actin dynamics by allosteric regulation of actin binding proteins. In International Review of Cell and Molecular Biology; Jeon, K.W., Ed.; Academic Press: Cambridge, MA, USA, 2013; Chapter One; pp. 1–25. [Google Scholar]
- Mishra, G.; Chadha, P.; Das, R.H. Serine/threonine kinase (pk-1) is a component of Autographa californica multiple nucleopolyhedrovirus (AcMNPV) very late gene transcription complex and it phosphorylates a 102 kDa polypeptide of the complex. Virus Res. 2008, 137, 147–149. [Google Scholar] [CrossRef]
- Rosen, G.A.; Baek, I.; Friedman, L.J.; Joo, Y.J.; Buratowski, S.; Gelles, J. Dynamics of RNA polymerase II and elongation factor Spt4/5 recruitment during activator-dependent transcription. Proc. Natl. Acad. Sci. USA 2020, 117, 32348–32357. [Google Scholar] [CrossRef] [PubMed]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, N.; Chen, G.; Kong, X.; Wu, X. Actin Contributes to the Hyperexpression of Baculovirus Polyhedrin (polh) and p10 as a Component of Transcription Initiation Complex (TIC). Viruses 2022, 14, 153. https://doi.org/10.3390/v14010153
Chen N, Chen G, Kong X, Wu X. Actin Contributes to the Hyperexpression of Baculovirus Polyhedrin (polh) and p10 as a Component of Transcription Initiation Complex (TIC). Viruses. 2022; 14(1):153. https://doi.org/10.3390/v14010153
Chicago/Turabian StyleChen, Nan, Guanping Chen, Xiangshuo Kong, and Xiaofeng Wu. 2022. "Actin Contributes to the Hyperexpression of Baculovirus Polyhedrin (polh) and p10 as a Component of Transcription Initiation Complex (TIC)" Viruses 14, no. 1: 153. https://doi.org/10.3390/v14010153
APA StyleChen, N., Chen, G., Kong, X., & Wu, X. (2022). Actin Contributes to the Hyperexpression of Baculovirus Polyhedrin (polh) and p10 as a Component of Transcription Initiation Complex (TIC). Viruses, 14(1), 153. https://doi.org/10.3390/v14010153




