Mutation Rates, Mutation Frequencies, and Proofreading-Repair Activities in RNA Virus Genetics
Abstract
1. Introduction: Mistakes as Hallmark of the Evolution of Life and of Present Day Viruses
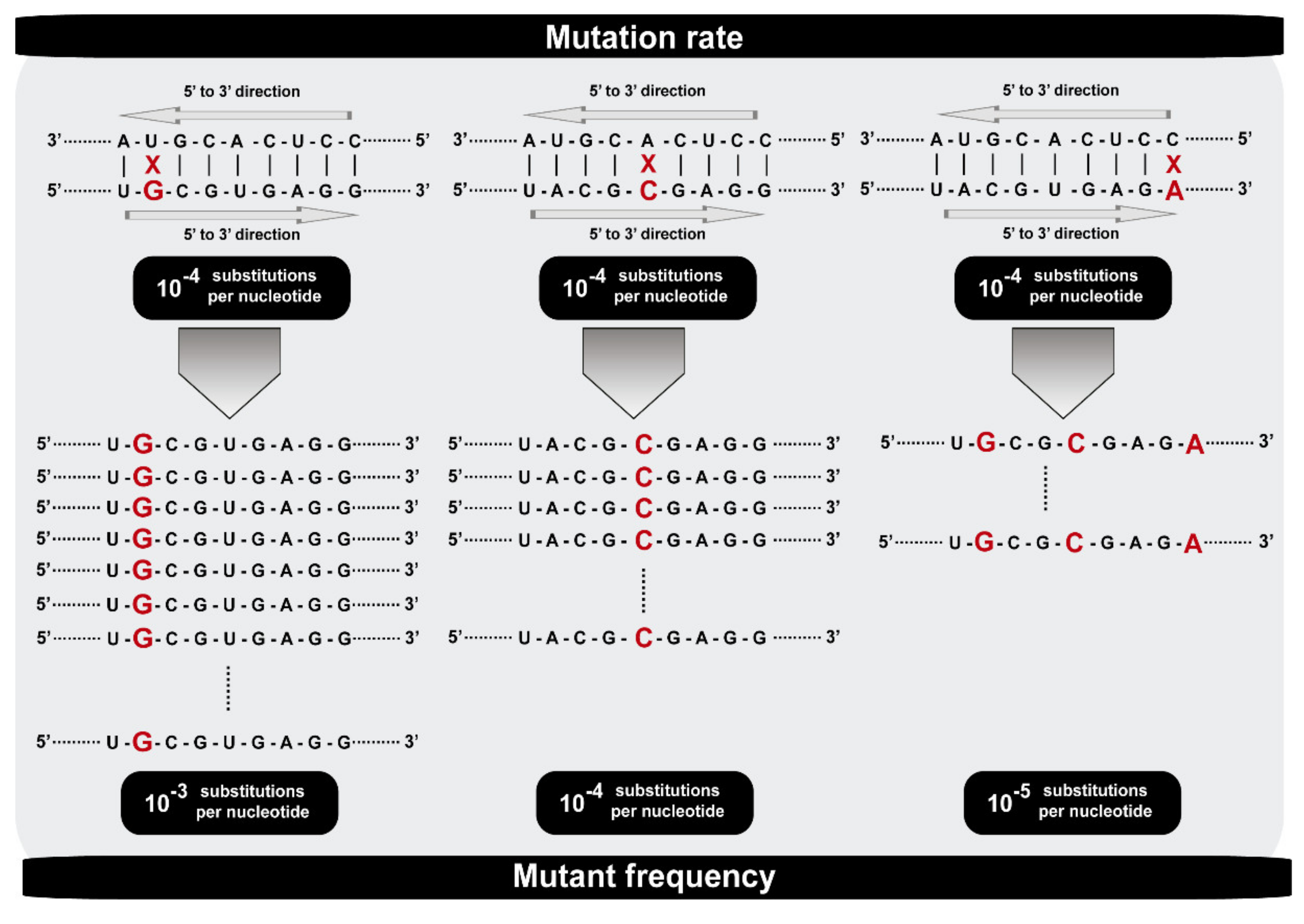
2. Mutation Rates and Frequencies
- The structure of the protein that contains the catalytic domain for nucleotide polymerization because it can directly or indirectly affect the interaction of the catalytic residues (and their neighbors) with template and primer residues, as well as with the incoming nucleotide;
- Other subunits or proteins that functionally interact with the protein that contains the polymerization catalytic site;
- Micro-environment in which template copying takes place (ionic composition, temperature, presence of metabolites);
- The sequence context in the template;
- Presence of functional proofreading-repair activities either as part of the polymerase or in proteins that interact with the polymerase;
- Availability and functionality of post-replicative repair pathways;
- Host-coded editing enzymes that may introduce viral genome mutations, unrelated to attributes of the replication complex.
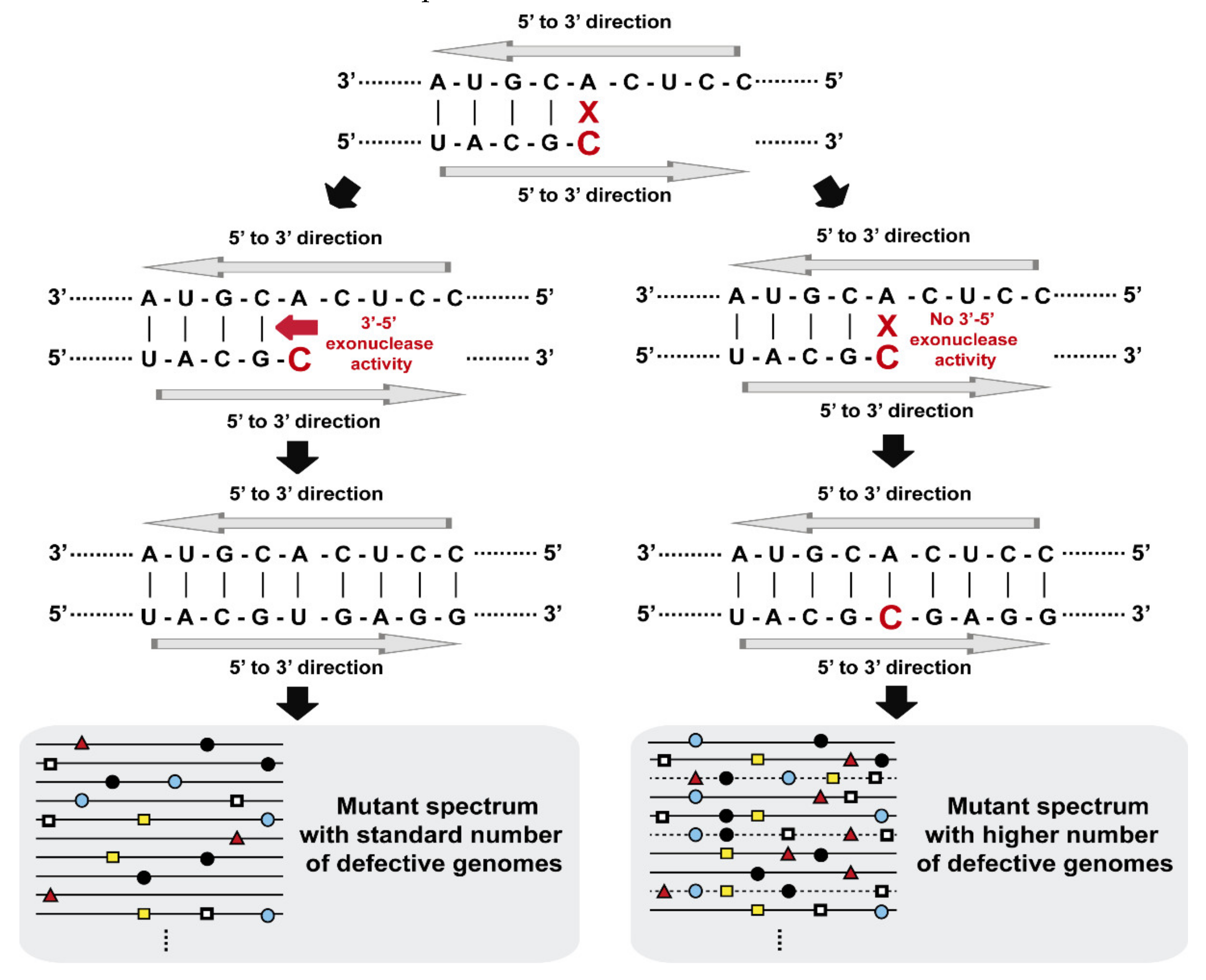
3. Limits to Mutation Rates: The Need of Repair
4. Repair Mechanisms in Viruses
5. The Coronavirus Exonuclease Activity, and Additional Considerations on Repair Evolvability
6. Summary, Conclusions, and Further Comments
Author Contributions
Funding
Conflicts of Interest
References
- Eigen, M.; Schuster, P. Stages of emerging life—five principles of early organization. J. Mol. Evol. 1982, 19, 47–61. [Google Scholar] [CrossRef]
- Eigen, M. Steps towards Life. Oxford University Press: Oxford, UK, 1992. [Google Scholar]
- Kauffman, S.A. The Origins of Order. In Self-Organization and Selection in Evolution; Oxford University Press: New York, NY, USA; Oxford, UK, 1993. [Google Scholar]
- De Duve, C. Life Evolving. Molecules, Mind and Meaning; Oxford University Press: Oxford, UK, 2002. [Google Scholar]
- Domingo, E. Virus as Populations, 2nd ed.; Academic Press, Elsevier: Amsterdam, The Netherlands, 2020. [Google Scholar]
- Szostak, J.W. The Narrow Road to the Deep Past: In Search of the Chemistry of the Origin of Life. Angew. Chem. Int. Ed. Engl. 2017, 56, 11037–11043. [Google Scholar] [CrossRef] [PubMed]
- Jalasvuori, M.; Bamford, J.K. Structural co-evolution of viruses and cells in the primordial world. Orig. Life Evol. Biosph. 2008, 38, 165–181. [Google Scholar] [CrossRef] [PubMed]
- Villarreal, L.P. The Widespread Evolutionary Significance of Viruses. In Origin and Evolution of Viruses, 2nd ed.; Domingo, E., Parrish, C.R., Holland, J.J., Eds.; Elsevier: Oxford, UK, 2008; Volume 393–416, pp. 477–516. [Google Scholar]
- Villarreal, L.P.; Witzany, G. Rethinking quasispecies theory: From fittest type to cooperative consortia. World J. Biol. Chem. 2013, 4, 79–90. [Google Scholar] [CrossRef] [PubMed]
- Domingo, E.; Perales, C. Viral quasispecies. PLoS Genet. 2019, 15, e1008271. [Google Scholar] [CrossRef] [PubMed]
- Domingo, E.; García-Crespo, C.; Perales, C. Historical perspective on the discovery of the quasispecies concept. Annu. Rev. Virol. 2021, in press. [Google Scholar]
- Domingo, E.; Schuster, P. Quasispecies: From Theory to Experimental systems. Current Topics in Microbiology and Immunology; Springer: Berlin, Germany, 2016; Volume 392. [Google Scholar]
- Chumakov, K.; Kew, O. The poliovirus eradication iniciative. In The Picornaviruses; Ehrenfeld, E., Domingo, E., Roos, R.P., Eds.; ASM Press: Washington, DC, USA, 2010; pp. 449–459. [Google Scholar]
- Roberts, L. Polio eradication campaign loses ground. Science 2019, 365, 106–107. [Google Scholar]
- Abraham, P. Polio: The Odyssey of Eradication; C. Hurst & Co. Ltd.: London, UK, 2018. [Google Scholar]
- Estrada, L.D.; Schultz-Cherry, S. Development of a Universal Influenza Vaccine. J. Immunol. 2019, 202, 392–398. [Google Scholar] [CrossRef]
- Richman, D.D.; Wrin, T.; Little, S.J.; Petropoulos, C.J. Rapid evolution of the neutralizing antibody response to HIV type 1 infection. Proc. Natl. Acad. Sci. USA 2003, 100, 4144–4149. [Google Scholar] [CrossRef] [PubMed]
- Lopez Angel, C.J.; Tomaras, G.D. Bringing the path toward an HIV-1 vaccine into focus. PLoS Pathog. 2020, 16, e1008663. [Google Scholar] [CrossRef]
- V’Kovski, P.; Kratzel, A.; Steiner, S.; Stalder, H.; Thiel, V. Coronavirus biology and replication: Implications for SARS-CoV-2. Nat. Rev. Microbiol. 2021, 19, 155–170. [Google Scholar] [CrossRef]
- Badua, C.; Baldo, K.A.T.; Medina, P.M.B. Genomic and proteomic mutation landscapes of SARS-CoV-2. J. Med. Virol. 2021, 93, 1702–1721. [Google Scholar] [CrossRef] [PubMed]
- Harvey, W.T.; Carabelli, A.M.; Jackson, B.; Gupta, R.K.; Thomson, E.C.; Harrison, E.M.; Ludden, C.; Reeve, R.; Rambaut, A.; Consortium, C.-G.U.; et al. SARS-CoV-2 variants, spike mutations and immune escape. Nat. Rev. Microbiol. 2021, 19, 409–424. [Google Scholar] [CrossRef] [PubMed]
- Escarmís, C.; Dávila, M.; Charpentier, N.; Bracho, A.; Moya, A.; Domingo, E. Genetic lesions associated with Muller’s ratchet in an RNA virus. J. Mol. Biol. 1996, 264, 255–267. [Google Scholar] [CrossRef] [PubMed]
- Agol, V.I.; Gmyl, A.P. Emergency Services of Viral RNAs: Repair and Remodeling. Microbiol. Mol. Biol. Rev. 2018, 82, e00067-17. [Google Scholar] [CrossRef]
- Drake, J.W. Rates of spontaneous mutation among RNA viruses. Proc. Natl. Acad. Sci. USA 1993, 90, 4171–4175. [Google Scholar] [CrossRef]
- Mansky, L.M.; Temin, H.M. Lower in vivo mutation rate of human immunodeficiency virus type 1 than that predicted from the fidelity of purified reverse transcriptase. J. Virol. 1995, 69, 5087–5094. [Google Scholar] [CrossRef]
- Drake, J.W.; Charlesworth, B.; Charlesworth, D.; Crow, J.F. Rates of spontaneous mutation. Genetics 1998, 148, 1667–1686. [Google Scholar] [CrossRef]
- Drake, J.W.; Holland, J.J. Mutation rates among RNA viruses. Proc. Natl. Acad. Sci. USA 1999, 96, 13910–13913. [Google Scholar] [CrossRef] [PubMed]
- Sanjuan, R.; Nebot, M.R.; Chirico, N.; Mansky, L.M.; Belshaw, R. Viral mutation rates. J. Virol. 2010, 84, 9733–9748. [Google Scholar] [CrossRef] [PubMed]
- Peck, K.M.; Lauring, A.S. Complexities of Viral Mutation Rates. J. Virol. 2018, 92, e01031-17. [Google Scholar] [CrossRef] [PubMed]
- Ferenci, T. Irregularities in genetic variation and mutation rates with environmental stresses. Environ. Microbiol. 2019, 21, 3979–3988. [Google Scholar] [CrossRef]
- Rosche, W.A.; Foster, P.L. Determining mutation rates in bacterial populations. Methods 2000, 20, 4–17. [Google Scholar] [CrossRef] [PubMed]
- Yeo, J.Y.; Koh, D.W.; Yap, P.; Goh, G.R.; Gan, S.K. Spontaneous Mutations in HIV-1 Gag, Protease, RT p66 in the First Replication Cycle and How They Appear: Insights from an In Vitro Assay on Mutation Rates and Types. Int. J. Mol. Sci. 2020, 22, 370. [Google Scholar] [CrossRef] [PubMed]
- Smith, E.C.; Case, J.B.; Blanc, H.; Isakov, O.; Shomron, N.; Vignuzzi, M.; Denison, M.R. Mutations in coronavirus nonstructural protein 10 decrease virus replication fidelity. J. Virol. 2015, 89, 6418–6426. [Google Scholar] [CrossRef] [PubMed]
- Stapleford, K.A.; Rozen-Gagnon, K.; Das, P.K.; Saul, S.; Poirier, E.Z.; Blanc, H.; Vidalain, P.O.; Merits, A.; Vignuzzi, M. Viral polymerase-helicase complexes regulate replication fidelity to overcome intracellular nucleotide depletion. J. Virol. 2015, 89, 11233–11244. [Google Scholar] [CrossRef] [PubMed]
- Agudo, R.; de la Higuera, I.; Arias, A.; Grande-Perez, A.; Domingo, E. Involvement of a joker mutation in a polymerase-independent lethal mutagenesis escape mechanism. Virology 2016, 494, 257–266. [Google Scholar] [CrossRef]
- Collins, N.D.; Beck, A.S.; Widen, S.G.; Wood, T.G.; Higgs, S.; Barrett, A.D.T. Structural and Nonstructural Genes Contribute to the Genetic Diversity of RNA Viruses. mBio 2018, 9, e01871-18. [Google Scholar] [CrossRef]
- Batschelet, E.; Domingo, E.; Weissmann, C. The proportion of revertant and mutant phage in a growing population, as a function of mutation and growth rate. Gene 1976, 1, 27–32. [Google Scholar] [CrossRef]
- Weissmann, C.; Taniguchi, T.; Domingo, E.; Sabo, D.; Flavell, R.A. Site-directed mutagenesis as a tool in genetics. In Genetic Manipulation as It Affects the Cancer Problem; Schultz, J., Brada, Z., Eds.; Academic Press: New York, NY, USA, 1977; pp. 11–36. [Google Scholar]
- Jones, M.E.; Thomas, S.M.; Rogers, A. Luria-Delbruck fluctuation experiments: Design and analysis. Genetics 1994, 136, 1209–1216. [Google Scholar] [CrossRef] [PubMed]
- Hall, B.M.; Ma, C.X.; Liang, P.; Singh, K.K. Fluctuation analysis CalculatOR: A web tool for the determination of mutation rate using Luria-Delbruck fluctuation analysis. Bioinformatics 2009, 25, 1564–1565. [Google Scholar] [CrossRef]
- Nyinoh, I.W. Spontaneous mutations conferring antibiotic resistance to antitubercular drugs at a range of concentrations in Mycobacterium smegmatis. Drug Dev. Res. 2019, 80, 147–154. [Google Scholar] [CrossRef] [PubMed]
- Sanjuan, R.; Domingo-Calap, P. Mechanisms of viral mutation. Cell. Mol. Life Sci. 2016, 73, 4433–4448. [Google Scholar] [CrossRef]
- Wainberg, M.A.; Drosopoulos, W.C.; Salomon, H.; Hsu, M.; Borkow, G.; Parniak, M.; Gu, Z.; Song, Q.; Manne, J.; Islam, S.; et al. Enhanced fidelity of 3TC-selected mutant HIV-1 reverse transcriptase. Science 1996, 271, 1282–1285. [Google Scholar] [CrossRef] [PubMed]
- Menéndez-Arias, L. Molecular basis of fidelity of DNA synthesis and nucleotide specificity of retroviral reverse transcriptases. Prog. Nucl. Acid Res. Mol. Biol. 2002, 71, 91–147. [Google Scholar]
- Mansky, L.M.; Le Rouzic, E.; Benichou, S.; Gajary, L.C. Influence of reverse transcriptase variants, drugs, and Vpr on human immunodeficiency virus type 1 mutant frequencies. J. Virol. 2003, 77, 2071–2080. [Google Scholar] [CrossRef]
- Pfeiffer, J.K.; Kirkegaard, K. A single mutation in poliovirus RNA-dependent RNA polymerase confers resistance to mutagenic nucleotide analogs via increased fidelity. Proc. Natl. Acad. Sci. USA 2003, 100, 7289–7294. [Google Scholar] [CrossRef] [PubMed]
- Rezende, L.F.; Prasad, V.R. Nucleoside-analog resistance mutations in HIV-1 reverse transcriptase and their influence on polymerase fidelity and viral mutation rates. Int. J. Biochem. Cell Biol. 2004, 36, 1716–1734. [Google Scholar] [CrossRef] [PubMed]
- Vignuzzi, M.; Stone, J.K.; Arnold, J.J.; Cameron, C.E.; Andino, R. Quasispecies diversity determines pathogenesis through cooperative interactions in a viral population. Nature 2006, 439, 344–348. [Google Scholar] [CrossRef] [PubMed]
- Coffey, L.L.; Vignuzzi, M. Host alternation of chikungunya virus increases fitness while restricting population diversity and adaptability to novel selective pressures. J. Virol. 2011, 85, 1025–1035. [Google Scholar] [CrossRef]
- Gnadig, N.F.; Beaucourt, S.; Campagnola, G.; Borderia, A.V.; Sanz-Ramos, M.; Gong, P.; Blanc, H.; Peersen, O.B.; Vignuzzi, M. Coxsackievirus B3 mutator strains are attenuated in vivo. Proc. Natl. Acad. Sci. USA 2012, 109, E2294–E2303. [Google Scholar] [CrossRef]
- Meng, T.; Kwang, J. Attenuation of human enterovirus 71 high-replication-fidelity variants in AG129 mice. J. Virol. 2014, 88, 5803–5815. [Google Scholar] [CrossRef]
- Rozen-Gagnon, K.; Stapleford, K.A.; Mongelli, V.; Blanc, H.; Failloux, A.B.; Saleh, M.C.; Vignuzzi, M. Alphavirus mutator variants present host-specific defects and attenuation in mammalian and insect models. PLoS Pathog. 2014, 10, e1003877. [Google Scholar] [CrossRef]
- Borderia, A.V.; Rozen-Gagnon, K.; Vignuzzi, M. Fidelity Variants and RNA Quasispecies. Curr. Top Microbiol. Immunol. 2016, 392, 303–322. [Google Scholar] [PubMed]
- Lloyd, S.B.; Lichtfuss, M.; Amarasena, T.H.; Alcantara, S.; De Rose, R.; Tachedjian, G.; Alinejad-Rokny, H.; Venturi, V.; Davenport, M.P.; Winnall, W.R.; et al. High fidelity simian immunodeficiency virus reverse transcriptase mutants have impaired replication in vitro and in vivo. Virology 2016, 492, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Kautz, T.F.; Guerbois, M.; Khanipov, K.; Patterson, E.I.; Langsjoen, R.M.; Yun, R.; Warmbrod, K.L.; Fofanov, Y.; Weaver, S.C.; Forrester, N.L. Low-fidelity Venezuelan equine encephalitis virus polymerase mutants to improve live-attenuated vaccine safety and efficacy. Virus Evol. 2018, 4, vey004. [Google Scholar] [CrossRef] [PubMed]
- Patterson, E.I.; Khanipov, K.; Swetnam, D.M.; Walsdorf, S.; Kautz, T.F.; Thangamani, S.; Fofanov, Y.; Forrester, N.L. Measuring Alphavirus Fidelity Using Non-Infectious Virus Particles. Viruses 2020, 12, 546. [Google Scholar] [CrossRef] [PubMed]
- Ward, C.D.; Stokes, M.A.; Flanegan, J.B. Direct measurement of the poliovirus RNA polymerase error frequency in vitro. J. Virol. 1988, 62, 558–562. [Google Scholar] [CrossRef]
- Menendez-Arias, L. Mutation rates and intrinsic fidelity of retroviral reverse transcriptases. Viruses 2009, 1, 1137–1165. [Google Scholar] [CrossRef] [PubMed]
- Cameron, C.E.; Moustafa, I.M.; Arnold, J.J. Fidelity of Nucleotide Incorporation by the RNA-Dependent RNA Polymerase from Poliovirus. Enzymes 2016, 39, 293–323. [Google Scholar]
- Yang, X.; Liu, X.; Musser, D.M.; Moustafa, I.M.; Arnold, J.J.; Cameron, C.E.; Boehr, D.D. Triphosphate Reorientation of the Incoming Nucleotide as a Fidelity Checkpoint in Viral RNA-dependent RNA Polymerases. J. Biol. Chem. 2017, 292, 3810–3826. [Google Scholar] [CrossRef]
- Harris, V.H.; Smith, C.L.; Jonathan Cummins, W.; Hamilton, A.L.; Adams, H.; Dickman, M.; Hornby, D.P.; Williams, D.M. The effect of tautomeric constant on the specificity of nucleotide incorporation during DNA replication: Support for the rare tautomer hypothesis of substitution mutagenesis. J. Mol. Biol. 2003, 326, 1389–1401. [Google Scholar] [CrossRef]
- Friedberg, E.C.; Walker, G.C.; Siede, W.; Wood, R.D.; Schultz, R.A.; Ellenberger, T. DNA Repair and Mutagenesis; American Society for Microbiology: Washington, DC, USA, 2006. [Google Scholar]
- Suarez, P.; Valcarcel, J.; Ortin, J. Heterogeneity of the mutation rates of influenza A viruses: Isolation of mutator mutants. J. Virol. 1992, 66, 2491–2494. [Google Scholar] [CrossRef]
- Earl, D.J.; Deem, M.W. Evolvability is a selectable trait. Proc. Natl. Acad. Sci. USA 2004, 101, 11531–11536. [Google Scholar] [CrossRef] [PubMed]
- Lequime, S.; Fontaine, A.; Ar Gouilh, M.; Moltini-Conclois, I.; Lambrechts, L. Genetic Drift, Purifying Selection and Vector Genotype Shape Dengue Virus Intra-host Genetic Diversity in Mosquitoes. PLoS Genet. 2016, 12, e1006111. [Google Scholar] [CrossRef]
- Kadoya, S.S.; Urayama, S.I.; Nunoura, T.; Hirai, M.; Takaki, Y.; Kitajima, M.; Nakagomi, T.; Nakagomi, O.; Okabe, S.; Nishimura, O.; et al. Bottleneck Size-Dependent Changes in the Genetic Diversity and Specific Growth Rate of a Rotavirus A Strain. J. Virol. 2020, 94, e02083-19. [Google Scholar] [CrossRef] [PubMed]
- Inoue, T.; Orgel, L.E. A nonenzymatic RNA polymerase model. Science 1983, 219, 859–862. [Google Scholar] [CrossRef]
- Kun, A.; Szilagyi, A.; Konnyu, B.; Boza, G.; Zachar, I.; Szathmary, E. The dynamics of the RNA world: Insights and challenges. Ann. N. Y. Acad. Sci. 2015, 1341, 75–95. [Google Scholar] [CrossRef] [PubMed]
- Drake, J.W. Comparative rates of spontaneous mutation. Nature 1969, 221, 1132. [Google Scholar] [CrossRef]
- Drake, J.W. The Molecular Basis of Mutation; Holden-Day: San Francisco, CA, USA, 1970. [Google Scholar]
- Drake, J.W. A constant rate of spontaneous mutation in DNA-based microbes. Proc. Natl. Acad. Sci. USA 1991, 88, 7160–7164. [Google Scholar] [CrossRef] [PubMed]
- Nishimura, I.; Kurokawa, M.; Liu, L.; Ying, B.W. Coordinated Changes in Mutation and Growth Rates Induced by Genome Reduction. mBio 2017, 8, e00676-17. [Google Scholar] [CrossRef]
- Eigen, M.; Schuster, P. The Hypercycle. A Principle of Natural Self-Organization; Springer: Berlin, Germany, 1979. [Google Scholar]
- Swetina, J.; Schuster, P. Self-replication with errors. A model for polynucleotide replication. Biophys. Chem. 1982, 16, 329–345. [Google Scholar] [CrossRef]
- Schuster, P. Quasispecies on fitness landscapes. Curr. Top. Microbiol. Immunol. 2016, 392, 61–120. [Google Scholar]
- Spivak, G. Nucleotide excision repair in humans. DNA Repair 2015, 36, 13–18. [Google Scholar] [CrossRef] [PubMed]
- Mahadevan, J.; Bowerman, S.; Luger, K. Quantitating repair protein accumulation at DNA lesions: Past, present, and future. DNA Repair 2019, 81, 102650. [Google Scholar] [CrossRef]
- Liu, D.; Keijzers, G.; Rasmussen, L.J. DNA mismatch repair and its many roles in eukaryotic cells. Mutat. Res. 2017, 773, 174–187. [Google Scholar] [CrossRef] [PubMed]
- Reha-Krantz, L.J.; Marquez, L.A.; Elisseeva, E.; Baker, R.P.; Bloom, L.B.; Dunford, H.B.; Goodman, M.F. The proofreading pathway of bacteriophage T4 DNA polymerase. J. Biol. Chem. 1998, 273, 22969–22976. [Google Scholar] [CrossRef]
- Reha-Krantz, L.J. Regulation of DNA polymerase exonucleolytic proofreading activity: Studies of bacteriophage T4 “antimutator” DNA polymerases. Genetics 1998, 148, 1551–1557. [Google Scholar] [CrossRef] [PubMed]
- Goodman, M.F.; Fygenson, K.D. DNA polymerase fidelity: From genetics toward a biochemical understanding. Genetics 1998, 148, 1475–1482. [Google Scholar] [CrossRef]
- Knopf, C.W. Evolution of viral DNA-dependent DNA polymerases. Virus Genes 1998, 16, 47–58. [Google Scholar] [CrossRef]
- Gammon, D.B.; Evans, D.H. The 3′-to-5′ exonuclease activity of vaccinia virus DNA polymerase is essential and plays a role in promoting virus genetic recombination. J. Virol. 2009, 83, 4236–4250. [Google Scholar] [CrossRef] [PubMed]
- Czarnecki, M.W.; Traktman, P. The vaccinia virus DNA polymerase and its processivity factor. Virus Res. 2017, 234, 193–206. [Google Scholar] [CrossRef] [PubMed]
- Lawler, J.L.; Mukherjee, P.; Coen, D.M. Herpes Simplex Virus 1 DNA Polymerase RNase H Activity Acts in a 3′-to-5′ Direction and Is Dependent on the 3′-to-5′ Exonuclease Active Site. J. Virol. 2018, 92, e01813-17. [Google Scholar] [CrossRef]
- Ishihama, A.; Mizumoto, K.; Kawakami, K.; Kato, A.; Honda, A. Proofreading function associated with the RNA-dependent RNA polymerase from influenza virus. J. Biol. Chem. 1986, 261, 10417–10421. [Google Scholar] [CrossRef]
- Jin, Z.; Leveque, V.; Ma, H.; Johnson, K.A.; Klumpp, K. NTP-mediated nucleotide excision activity of hepatitis C virus RNA-dependent RNA polymerase. Proc. Natl. Acad. Sci. USA 2013, 110, E348–E357. [Google Scholar] [CrossRef]
- Meyer, P.R.; Matsuura, S.E.; So, A.G.; Scott, W.A. Unblocking of chain-terminated primer by HIV-1 reverse transcriptase through a nucleotide-dependent mechanism. Proc. Natl. Acad. Sci. USA 1998, 95, 13471–13476. [Google Scholar] [CrossRef]
- Kharytonchyk, S.; King, S.R.; Ndongmo, C.B.; Stilger, K.L.; An, W.; Telesnitsky, A. Resolution of Specific Nucleotide Mismatches by Wild-Type and AZT-Resistant Reverse Transcriptases during HIV-1 Replication. J. Mol. Biol. 2016, 428, 2275–2288. [Google Scholar] [CrossRef]
- Nagy, P.D.; Carpenter, C.D.; Simon, A.E. A novel 3′-end repair mechanism in an RNA virus. Proc. Natl. Acad. Sci. USA 1997, 94, 1113–1118. [Google Scholar] [CrossRef]
- Kwon, S.J.; Chaturvedi, S.; Rao, A.L. Repair of the 3′ proximal and internal deletions of a satellite RNA associated with Cucumber mosaic virus is directed toward restoring structural integrity. Virology 2014, 450–451, 222–232. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Denison, M.R.; Graham, R.L.; Donaldson, E.F.; Eckerle, L.D.; Baric, R.S. Coronaviruses: An RNA proofreading machine regulates replication fidelity and diversity. RNA Biol. 2011, 8, 270–279. [Google Scholar] [CrossRef] [PubMed]
- Steinhauer, D.A.; Domingo, E.; Holland, J.J. Lack of evidence for proofreading mechanisms associated with an RNA virus polymerase. Gene 1992, 122, 281–288. [Google Scholar] [CrossRef]
- Minskaia, E.; Hertzig, T.; Gorbalenya, A.E.; Campanacci, V.; Cambillau, C.; Canard, B.; Ziebuhr, J. Discovery of an RNA virus 3’->5’ exoribonuclease that is critically involved in coronavirus RNA synthesis. Proc. Natl. Acad. Sci. USA 2006, 103, 5108–5113. [Google Scholar] [CrossRef]
- Ogando, N.S.; Zevenhoven-Dobbe, J.C.; van der Meer, Y.; Bredenbeek, P.J.; Posthuma, C.C.; Snijder, E.J. The Enzymatic Activity of the nsp14 Exoribonuclease Is Critical for Replication of MERS-CoV and SARS-CoV-2. J. Virol. 2020, 94, e01246-20. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.; Chen, H.; Chen, Z.; Yang, F.; Ye, F.; Zheng, Y.; Yang, J.; Lin, X.; Sun, H.; Wang, L.; et al. Crystal structure of SARS-CoV-2 nsp10 bound to nsp14-ExoN domain reveals an exoribonuclease with both structural and functional integrity. Nucleic Acids Res. 2021, 49, 5382–5392. [Google Scholar] [CrossRef] [PubMed]
- Ma, Z.; Pourfarjam, Y.; Kim, I.K. Reconstitution and functional characterization of SARS-CoV-2 proofreading complex. Protein Expr. Purif. 2021, 185, 105894. [Google Scholar] [CrossRef]
- Scholle, M.D.; Liu, C.; Deval, J.; Gurard-Levin, Z.A. Label-Free Screening of SARS-CoV-2 NSP14 Exonuclease Activity Using SAMDI Mass Spectrometry. SLAS Discov. 2021, 26, 766–774. [Google Scholar]
- Eckerle, L.D.; Becker, M.M.; Halpin, R.A.; Li, K.; Venter, E.; Lu, X.; Scherbakova, S.; Graham, R.L.; Baric, R.S.; Stockwell, T.B.; et al. Infidelity of SARS-CoV Nsp14-exonuclease mutant virus replication is revealed by complete genome sequencing. PLoS Pathog. 2010, 6, e1000896. [Google Scholar] [CrossRef]
- Eckerle, L.D.; Lu, X.; Sperry, S.M.; Choi, L.; Denison, M.R. High fidelity of murine hepatitis virus replication is decreased in nsp14 exoribonuclease mutants. J. Virol. 2007, 81, 12135–12144. [Google Scholar] [CrossRef]
- Graham, R.L.; Becker, M.M.; Eckerle, L.D.; Bolles, M.; Denison, M.R.; Baric, R.S. A live, impaired-fidelity coronavirus vaccine protects in an aged, immunocompromised mouse model of lethal disease. Nat. Med. 2012, 18, 1820–1826. [Google Scholar] [CrossRef] [PubMed]
- Smith, E.C.; Blanc, H.; Vignuzzi, M.; Denison, M.R. Coronaviruses lacking exoribonuclease activity are susceptible to lethal mutagenesis: Evidence for proofreading and potential therapeutics. PLoS Pathog. 2013, 9, e1003565. [Google Scholar] [CrossRef]
- Smith, E.C.; Denison, M.R. Coronaviruses as DNA wannabes: A new model for the regulation of RNA virus replication fidelity. PLoS Pathog. 2013, 9, e1003760. [Google Scholar] [CrossRef]
- Graepel, K.W.; Lu, X.; Case, J.B.; Sexton, N.R.; Smith, E.C.; Denison, M.R. Proofreading-Deficient Coronaviruses Adapt for Increased Fitness over Long-Term Passage without Reversion of Exoribonuclease-Inactivating Mutations. mBio 2017, 8, e01503-17. [Google Scholar] [CrossRef]
- Gribble, J.; Stevens, L.J.; Agostini, M.L.; Anderson-Daniels, J.; Chappell, J.D.; Lu, X.; Pruijssers, A.J.; Routh, A.L.; Denison, M.R. The coronavirus proofreading exoribonuclease mediates extensive viral recombination. PLoS Pathog. 2021, 17, e1009226. [Google Scholar] [CrossRef]
- Mallory, J.D.; Mallory, X.F.; Kolomeisky, A.B.; Igoshin, O.A. Theoretical Analysis Reveals the Cost and Benefit of Proofreading in Coronavirus Genome Replication. J. Phys. Chem. Lett. 2021, 12, 2691–2698. [Google Scholar] [CrossRef] [PubMed]
- Ellefson, J.W.; Gollihar, J.; Shroff, R.; Shivram, H.; Iyer, V.R.; Ellington, A.D. Synthetic evolutionary origin of a proofreading reverse transcriptase. Science 2016, 352, 1590–1593. [Google Scholar] [CrossRef] [PubMed]
- Trypsteen, W.; Van Cleemput, J.; Snippenberg, W.V.; Gerlo, S.; Vandekerckhove, L. On the whereabouts of SARS-CoV-2 in the human body: A systematic review. PLoS Pathog. 2020, 16, e1009037. [Google Scholar] [CrossRef]
- Sender, R.; Bar-On, Y.M.; Gleizer, S.; Bernshtein, B.; Flamholz, A.; Phillips, R.; Milo, R. The total number and mass of SARS-CoV-2 virions. Proc. Natl. Acad. Sci. USA 2021, 118, e2024815118. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, D.A.; Read, A.F. Monitor for COVID-19 vaccine resistance evolution during clinical trials. PLoS Biol. 2020, 18, e3001000. [Google Scholar] [CrossRef]
- Weisblum, Y.; Schmidt, F.; Zhang, F.; DaSilva, J.; Poston, D.; Lorenzi, J.C.; Muecksch, F.; Rutkowska, M.; Hoffmann, H.H.; Michailidis, E.; et al. Escape from neutralizing antibodies by SARS-CoV-2 spike protein variants. eLife 2020, 9, e61312. [Google Scholar] [CrossRef]
- Hacisuleyman, E.; Hale, C.; Saito, Y.; Blachere, N.E.; Bergh, M.; Conlon, E.G.; Schaefer-Babajew, D.J.; DaSilva, J.; Muecksch, F.; Gaebler, C.; et al. Vaccine Breakthrough Infections with SARS-CoV-2 Variants. N. Engl. J. Med. 2021, 384, 2212–2218. [Google Scholar] [CrossRef] [PubMed]
- Rolland, M.; Gilbert, P.B. Sieve analysis to understand how SARS-CoV-2 diversity can impact vaccine protection. PLoS Pathog. 2021, 17, e1009406. [Google Scholar] [CrossRef]
- Domingo, E. RNA virus evolution and the control of viral disease. Prog. Drug Res. 1989, 33, 93–133. [Google Scholar]
- Moreno, E.; Gallego, I.; Gregori, J.; Lucia-Sanz, A.; Soria, M.E.; Castro, V.; Beach, N.M.; Manrubia, S.; Quer, J.; Esteban, J.I.; et al. Internal Disequilibria and Phenotypic Diversification during Replication of Hepatitis C Virus in a Noncoevolving Cellular Environment. J. Virol. 2017, 91, e02505-16. [Google Scholar] [CrossRef] [PubMed]
- Gallego, I.; Soria, M.E.; Garcia-Crespo, C.; Chen, Q.; Martinez-Barragan, P.; Khalfaoui, S.; Martinez-Gonzalez, B.; Sanchez-Martin, I.; Palacios-Blanco, I.; de Avila, A.I.; et al. Broad and Dynamic Diversification of Infectious Hepatitis C Virus in a Cell Culture Environment. J. Virol. 2020, 94, e01856-19. [Google Scholar] [CrossRef]
- Domingo, E.; Soria, M.E.; Gallego, I.; de Avila, A.I.; Garcia-Crespo, C.; Martinez-Gonzalez, B.; Gomez, J.; Briones, C.; Gregori, J.; Quer, J.; et al. A new implication of quasispecies dynamics: Broad virus diversification in absence of external perturbations. Infect. Genet. Evol. 2020, 82, 104278. [Google Scholar] [CrossRef] [PubMed]
- García-Crespo, C.; Gallego, I.; Soria, M.E.; De Ávila, A.I.; Martínez-González, B.; Vázquez-Sirvent, L.; Lobo-Vega, R.; Moreno, E.; Gómez, J.; Briones, C.; et al. Population disequilibrium as promoter of adaptive explorations in hepatitis C virus. Viruses 2021, 13, 616. [Google Scholar] [CrossRef] [PubMed]
- Delgado, S.; Perales, C.; García-Crespo, C.; Soria, M.E.; Gallego, I.; De Ávila, A.I.; Martínez-González, B.; Vázquez-Sirvent, L.; López-Galíndez, C.; Morán, F.; et al. A Two-Level, Dynamic Fitness Landscape of Hepatitis C Virus Revealed by Self-Organized Haplotype Maps. 2021. Available online: https://www.biorxiv.org/content/10.1101/2021.04.22.441053v1 (accessed on 24 April 2021).
- Garcia-Crespo, C.; Soria, M.E.; Gallego, I.; Avila, A.I.; Martinez-Gonzalez, B.; Vazquez-Sirvent, L.; Gomez, J.; Briones, C.; Gregori, J.; Quer, J.; et al. Dissimilar Conservation Pattern in Hepatitis C Virus Mutant Spectra, Consensus Sequences, and Data Banks. J. Clin. Med. 2020, 9, 3450. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Domingo, E.; García-Crespo, C.; Lobo-Vega, R.; Perales, C. Mutation Rates, Mutation Frequencies, and Proofreading-Repair Activities in RNA Virus Genetics. Viruses 2021, 13, 1882. https://doi.org/10.3390/v13091882
Domingo E, García-Crespo C, Lobo-Vega R, Perales C. Mutation Rates, Mutation Frequencies, and Proofreading-Repair Activities in RNA Virus Genetics. Viruses. 2021; 13(9):1882. https://doi.org/10.3390/v13091882
Chicago/Turabian StyleDomingo, Esteban, Carlos García-Crespo, Rebeca Lobo-Vega, and Celia Perales. 2021. "Mutation Rates, Mutation Frequencies, and Proofreading-Repair Activities in RNA Virus Genetics" Viruses 13, no. 9: 1882. https://doi.org/10.3390/v13091882
APA StyleDomingo, E., García-Crespo, C., Lobo-Vega, R., & Perales, C. (2021). Mutation Rates, Mutation Frequencies, and Proofreading-Repair Activities in RNA Virus Genetics. Viruses, 13(9), 1882. https://doi.org/10.3390/v13091882





