Breakthrough Infections with Multiple Lineages of SARS-CoV-2 Variants Reveals Continued Risk of Severe Disease in Immunosuppressed Patients
Abstract
:1. Introduction
2. Materials and Methods
2.1. Specimen Collection and Processing
2.2. Detection of Viral RNA and Cycle Threshold (Ct)
2.3. Sequencing of SARS-CoV-2 Complete Genome
2.4. Evaluating Antibody Response to SARS-CoV-2 Spike Protein
2.5. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Thompson, M.G.; Burgess, J.L.; Naleway, A.L.; Tyner, H.L.; Yoon, S.K.; Meece, J.; Olsho, L.E.W.; Caban-Martinez, A.J.; Fowlkes, A.; Lutrick, K.; et al. Interim Estimates of Vaccine Effectiveness of BNT162b2 and mRNA-1273 COVID-19 Vaccines in Preventing SARS-CoV-2 Infection Among Health Care Personnel, First Responders, and Other Essential and Frontline Workers—Eight, U.S. Locations, December 2020–March. MMWR Morb. Mortal. Wkly. Rep. 2021, 70, 495–500. [Google Scholar] [CrossRef]
- Dagan, N.; Barda, N.; Kepten, E.; Miron, O.; Perchik, S.; Katz, M.A.; Hernán, M.A.; Lipsitch, M.; Reis, B.; Balicer, R.D. BNT162b2 mRNA COVID-19 Vaccine in a Nationwide Mass Vaccination Setting. N. Engl. J. Med. 2021, 384, 1412–1423. [Google Scholar] [CrossRef]
- Benenson, S.; Oster, Y.; Cohen, M.J.; Nir-Paz, R. BNT162b2 mRNA COVID-19 Vaccine Effectiveness among Health Care Workers. N. Engl. J. Med. 2021, 384, 1775–1777. [Google Scholar] [CrossRef]
- Hacisuleyman, E.; Hale, C.; Saito, Y.; Blachere, N.E.; Bergh, M.; Conlon, E.G.; Schaefer-Babajew, D.J.; DaSilva, J.; Muecksch, F.; Gaebler, C.; et al. Vaccine Breakthrough Infections with SARS-CoV-2 Variants. N. Engl. J. Med. 2021, 384, 2212–2218. [Google Scholar] [CrossRef]
- CDC COVID-19 Vaccine Breakthrough Case Investigations Team. COVID-19 Vaccine Breakthrough Infections Reported to CDC—United States, 1 January–30 April 2021. MMWR Morb. Mortal. Wkly. Rep. 2021, 70, 792–793. [Google Scholar]
- Philomina, J.B.; Jolly, B.; John, N.; Bhoyar, R.C.; Majeed, N.; Senthivel, V.; Fairoz, C.P.; Rophina, M.; Vasudevan, B.; Imran, M.; et al. Genomic Survey of SARS-CoV-2 Vaccine Breakthrough Infections in Healthcare Workers from Kerala, India. J. Infect. 2021, 83, 237–279. [Google Scholar] [CrossRef]
- Brosh-Nissimov, T.; Orenbuch-Harroch, E.; Chowers, M.; Elbaz, M.; Nesher, L.; Stein, M.; Maor, Y.; Cohen, R.; Hussein, K.; Weinberger, M.; et al. BNT162b2 Vaccine Breakthrough: Clinical Characteristics of 152 Fully Vaccinated Hospitalized COVID-19 Patients in Israel. Clin. Microbiol. Infect. 2021. [Google Scholar] [CrossRef]
- Health Public Ontario Confirmed Cases of COVID-19 Following Vaccination in Ontario: 14 December 2020 to 7 August 2021. 2021, pp. 1–27. Available online: https://www.publichealthontario.ca/-/media/documents/ncov/epi/covid-19-epi-confirmed-cases-post-vaccination.pdf?la=en (accessed on 29 July 2021).
- Bergwerk, M.; Gonen, T.; Lustig, Y.; Amit, S.; Lipsitch, M.; Cohen, C.; Mandelboim, M.; Gal Levin, E.; Rubin, C.; Indenbaum, V.; et al. COVID-19 Breakthrough Infections in Vaccinated Health Care Workers. N. Engl. J. Med. 2021, NEJMoa2109072. [Google Scholar] [CrossRef]
- Duerr, R.; Dimartino, D.; Marier, C.; Zappile, P.; Wang, G.; Lighter, J.; Elbel, B.; Troxel, A.; Heguy, A. Dominance of Alpha and Iota Variants in SARS-CoV-2 Vaccine Breakthrough Infections in New York City. J. Clin. Investig. 2021. [Google Scholar] [CrossRef]
- Kustin, T.; Harel, N.; Finkel, U.; Perchik, S.; Harari, S.; Tahor, M.; Caspi, I.; Levy, R.; Leshchinsky, M.; Ken Dror, S.; et al. Evidence for Increased Breakthrough Rates of SARS-CoV-2 Variants of Concern in BNT162b2-mRNA-Vaccinated Individuals. Nat. Med. 2021, 27, 1379–1384. [Google Scholar] [CrossRef]
- Brown, C.M.; Vostok, J.; Johnson, H.; Burns, M.; Gharpure, R.; Sami, S. Outbreak of SARS-CoV-2 Infections, Including COVID-19 Vaccine Breakthrough Infections, Associated with Large Public Gatherings—Barnstable County, Massachusetts, July 2021. MMWR Morb. Mortal. Wkly. Rep. 2021, 70, 1059–1062. [Google Scholar] [CrossRef]
- Jacobson, K.B.; Pinsky, B.A.; Montez Rath, M.E.; Wang, H.; Miller, J.A.; Skhiri, M.; Shepard, J.; Mathew, R.; Lee, G.; Bohman, B.; et al. Post-Vaccination Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) Infections and Incidence of the Presumptive, B.1.427/B.1.429 Variant Among Healthcare Personnel at a Northern California Academic Medical Center. Clin. Infect. Dis. 2021, 2, 1–8. [Google Scholar] [CrossRef]
- Olsen, R.J.; Christensen, P.A.; Long, S.W.; Subedi, S.; Hodjat, P.; Olson, R.; Nguyen, M.; Davis, J.J.; Yerramilli, P.; Saavedra, M.O.; et al. Trajectory of Growth of Severe Acute Respiratory (SARS-CoV-2) Syndrome Coronavirus 2 Variants in Houston, Texas, January through May 2021, Based on 12,476 Genome Sequences. Am. J. Pathol. 2021, 1–19. [Google Scholar] [CrossRef]
- Keehner, J.; Horton, L.E.; Pfeffer, M.A.; Longhurst, C.A.; Schooley, R.T.; Currier, J.S.; Abeles, S.R.; Torriani, F.J. SARS-CoV-2 Infection after Vaccination in Health Care Workers in California. N. Engl. J. Med. 2021, 384, 1774–1775. [Google Scholar] [CrossRef]
- Cavanaugh, A.M.; Fortier, S.; Lewis, P.; Arora, V.; Johnson, M.; George, K.; Tobias, J.; Lunn, S.; Miller, T.; Thoroughman, D.; et al. COVID-19 Outbreak Associated with a SARS-CoV-2 R.1 Lineage Variant in a Skilled Nursing Facility After Vaccination Program—Kentucky, March 2021. MMWR. Morb. Mortal. Wkly. Rep. 2021, 70, 639–643. [Google Scholar] [CrossRef]
- Shen, X.; Tang, H.; McDanal, C.; Wagh, K.; Fischer, W.; Theiler, J.; Yoon, H.; Li, D.; Haynes, B.F.; Sanders, K.O.; et al. SARS-CoV-2 Variant, B.1.1.7 Is Susceptible to Neutralizing Antibodies Elicited by Ancestral Spike Vaccines. Cell Host Microbe 2021, 29, 529–539.e3. [Google Scholar] [CrossRef]
- Collier, D.A.; de Marco, A.; Ferreira, I.A.T.M.; Meng, B.; Datir, R.P.; Walls, A.C.; Kemp, S.A.; Bassi, J.; Pinto, D.; Silacci-Fregni, C.; et al. Sensitivity of SARS-CoV-2 B.1.1.7 to mRNA Vaccine-Elicited Antibodies. Nature 2021, 593, 136–141. [Google Scholar] [CrossRef]
- Peled, Y.; Ram, E.; Lavee, J.; Sternik, L.; Segev, A.; Wieder-Finesod, A.; Mandelboim, M.; Indenbaum, V.; Levy, I.; Raanani, E.; et al. BNT162b2 Vaccination in Heart Transplant Recipients: Clinical Experience and Antibody Response. J. Heart Lung Transplant. 2021. [Google Scholar] [CrossRef]
- Marinaki, S.; Adamopoulos, S.; Degiannis, D.; Roussos, S.; Pavlopoulou, I.D.; Hatzakis, A.; Boletis, I.N. Immunogenicity of SARS-CoV-2 BNT162b2 Vaccine in Solid Organ Transplant Recipients. Am. J. Transplant. 2021. [Google Scholar] [CrossRef]
- Grupper, A.; Rabinowich, L.; Schwartz, D.; Schwartz, I.F.; Ben-Yehoyada, M.; Shashar, M.; Katchman, E.; Halperin, T.; Turner, D.; Goykhman, Y.; et al. Reduced Humoral Response to mRNA SARS-CoV-2 BNT162b2 Vaccine in Kidney Transplant Recipients without Prior Exposure to the Virus. Am. J. Transplant. 2021, 21, 2719–2726. [Google Scholar] [CrossRef]
- Rincon-Arevalo, H.; Choi, M.; Stefanski, A.L.; Halleck, F.; Weber, U.; Szelinski, F.; Jahrsdörfer, B.; Schrezenmeier, H.; Ludwig, C.; Sattler, A.; et al. Impaired Humoral Immunity to SARS-CoV-2 BNT162b2 Vaccine in Kidney Transplant Recipients and Dialysis Patients. Sci. Immunol. 2021, 6, eabj1031. [Google Scholar] [CrossRef]
- Boyarsky, B.J.; Werbel, W.A.; Avery, R.K.; Tobian, A.A.R.; Massie, A.B.; Segev, D.L.; Garonzik-Wang, J.M. Antibody Response to 2-Dose Sars-Cov-2 mRNA Vaccine Series in Solid Organ Transplant Recipients. JAMA J. Am. Med. Assoc. 2021, 325, 2204–2206. [Google Scholar] [CrossRef]
- Miele, M.; Busà, R.; Russelli, G.; Sorrentino, M.C.; di Bella, M.; Timoneri, F.; Mularoni, A.; Panarello, G.; Vitulo, P.; Conaldi, P.G.; et al. Impaired Anti-SARS-CoV-2 Humoral and Cellular Immune Response Induced by Pfizer-BioNTech BNT162b2 mRNA Vaccine in Solid Organ Transplanted Patients. Am. J. Transplant. 2021, 21, 2919–2921. [Google Scholar] [CrossRef]
- Tyson, J.R.; James, P.; Stoddart, D.; Sparks, N.; Wickenhagen, A.; Hall, G.; Choi, J.H.; Lapointe, H.; Kamelian, K.; Smith, A.D.; et al. Improvements to the ARTIC Multiplex PCR Method for SARS-CoV-2 Genome Sequencing Using Nanopore. Biorxiv Prepr. Serv. Biol. 2020. [Google Scholar] [CrossRef]
- Grubaugh, N.D.; Gangavarapu, K.; Quick, J.; Matteson, N.L.; De Jesus, J.G.; Main, B.J.; Tan, A.L.; Paul, L.M.; Brackney, D.E.; Grewal, S.; et al. An Amplicon-Based Sequencing Framework for Accurately Measuring Intrahost Virus Diversity Using PrimalSeq and IVar. Genome Biol. 2019, 20, 8. [Google Scholar] [CrossRef] [Green Version]
- Hsieh, C.L.; Goldsmith, J.A.; Schaub, J.M.; DiVenere, A.M.; Kuo, H.C.; Javanmardi, K.; Le, K.C.; Wrapp, D.; Lee, A.G.; Liu, Y.; et al. Structure-Based Design of Prefusion-Stabilized SARS-CoV-2 Spikes. Science 2020, 369, 1501–1505. [Google Scholar] [CrossRef] [PubMed]
- Stadlbauer, D.; Amanat, F.; Chromikova, V.; Jiang, K.; Strohmeier, S.; Arunkumar, G.A.; Tan, J.; Bhavsar, D.; Capuano, C.; Kirkpatrick, E.; et al. SARS-CoV-2 Seroconversion in Humans: A Detailed Protocol for a Serological Assay, Antigen Production, and Test Setup. Curr. Protoc. Microbiol. 2020, 57, e100. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- USA-CDC. CDC COVID Data Tracker. Available online: https://covid.cdc.gov/covid-data-tracker/#variant-proportions (accessed on 25 May 2021).
- Deng, X.; Garcia-Knight, M.A.; Khalid, M.M.; Servellita, V.; Wang, C.; Morris, M.K.; Sotomayor-González, A.; Glasner, D.R.; Reyes, K.R.; Gliwa, A.S.; et al. Transmission, Infectivity, and Antibody Neutralization of an Emerging SARS-CoV-2 Variant in California Carrying a L452R Spike Protein Mutation. Medrxiv Prepr. Serv. Health Sci. 2021. [Google Scholar] [CrossRef]
- Li, Q.; Wu, J.; Nie, J.; Zhang, L.; Hao, H.; Liu, S.; Zhao, C.; Zhang, Q.; Liu, H.; Nie, L.; et al. The Impact of Mutations in SARS-CoV-2 Spike on Viral Infectivity and Antigenicity. Cell 2020, 182, 1284–1294.e9. [Google Scholar] [CrossRef]
- Song, C.C.; Christensen, J.; Kumar, D.; Vissichelli, N.; Morales, M.; Gupta, G. Early Experience with SARs-CoV-2 mRNA Vaccine Breakthrough Among Kidney Transplant Recipients. Transpl. Infect. Dis. 2021, e13654. [Google Scholar] [CrossRef]
- Wadei, H.M.; Gonwa, T.A.; Leoni, J.C.; Shah, S.Z.; Aslam, N.; Speicher, L.L. COVID-19 Infection in Solid Organ Transplant Recipients after SARS-CoV-2 Vaccination. Am. J. Transplant. 2021. [Google Scholar] [CrossRef] [PubMed]
- Qin, C.X.; Moore, L.W.; Anjan, S.; Rahamimov, R.; Sifri, C.D.; Ali, N.M.; Morales, M.K.; Tsapepas, D.S.; Basic-Jukic, N.; Miller, R.A.; et al. Risk of Breakthrough SARS-CoV-2 Infections in Adult Transplant Recipients. Transplantation 2021, 1–9. [Google Scholar] [CrossRef]
- Kamar, N.; Abravanel, F.; Marion, O.; Couat, C.; Izopet, J.; del Bello, A. Three Doses of an mRNA COVID-19 Vaccine in Solid-Organ Transplant Recipients. N. Engl. J. Med. 2021, 385, 661–662. [Google Scholar] [CrossRef]
- Hall, V.; Ferreira, V.; Ku, T.; Ierullo, M.; Majchrzak-Kita, B.; Chaparro, C.; Selzner, N.; Schiff, J.; McDonald, M.; Tomlinson, G.; et al. Randomized Trial of a Third Dose of mRNA-1273 Vaccine in Transplant Recipients. N. Engl. J. Med. 2021. [Google Scholar] [CrossRef]
- Teran, R.A.; Walblay, K.A.; Shane, E.L.; Xydis, S.; Gretsch, S.; Gagner, A.; Samala, U.; Choi, H.; Zelinski, C.; Black, S.R. Postvaccination SARS-CoV-2 Infections Among Skilled Nursing Facility Residents and Staff Members—Chicago, Illinois, December 2020–March 2021. MMWR Morb. Mortal. Wkly. Rep. 2021, 70, 632–638. [Google Scholar] [CrossRef]
- Levine-Tiefenbrun, M.; Yelin, I.; Katz, R.; Herzel, E.; Golan, Z.; Schreiber, L.; Wolf, T.; Nadler, V.; Ben-Tov, A.; Kuint, J.; et al. Initial Report of Decreased SARS-CoV-2 Viral Load after Inoculation with the BNT162b2 Vaccine. Nat. Med. 2021, 27, 790–792. [Google Scholar] [CrossRef]
- Lange, B.; Gerigk, M.; Tenenbaum, T. Breakthrough Infections in BNT162b2-Vaccinated Health Care Workers. N. Engl. J. Med. 2021. [Google Scholar] [CrossRef]
- Plante, J.A.; Liu, Y.; Liu, J.; Xia, H.; Johnson, B.A.; Lokugamage, K.G.; Zhang, X.; Muruato, A.E.; Zou, J.; Fontes-Garfias, C.R.; et al. Spike Mutation D614G Alters SARS-CoV-2 Fitness. Nature 2021, 592, 116–121. [Google Scholar] [CrossRef]
- Resende, P.C.; Bezerra, J.F.; Vasconcelos, R.H.T.; Arantes, I.; Appolinario, L.; Mendoncą, A.C.; Paixao, A.C.; Duarte, A.C.; Silva, T.; Rocha, A.S.; et al. Severe Acute Respiratory Syndrome Coronavirus 2 P.2 Lineage Associated with Reinfection Case, Brazil, June-October 2020. Emerg. Infect. Dis. 2021, 27, 1789–1794. [Google Scholar] [CrossRef]
- Matic, N.; Lowe, C.F.; Ritchie, G.; Stefanovic, A.; Lawson, T.; Jang, W.; Young, M.; Dong, W.; Brumme, Z.L.; Brumme, C.J.; et al. Rapid Detection of SARS-CoV-2 Variants of Concern, Including, B.1.1.28/P.1, British Columbia, Canada. Emerg. Infect. Dis. 2021, 27, 1673–1676. [Google Scholar] [CrossRef]
- Connor, B.A.; Couto-Rodriguez, M.; Barrows, J.E.; Gardner, M.; Rogova, M.; O’Hara, N.B.; Nagy-Szakal, D. Monoclonal Antibody Therapy in a Vaccine Breakthrough SARS-CoV-2 Hospitalized Delta (B.1.617.2) Variant Case. Int. J. Infect. Dis. 2021. [Google Scholar] [CrossRef] [PubMed]
- Farinholt, T.; Doddapaneni, H.; Qin, X.; Menon, V.; Meng, Q.; Metcalf, G.; Chao, H.; Gingras, M.-C.; Farinholt, P.; Agrawal, C.; et al. Transmission Event of SARS-CoV-2 Delta Variant Reveals Multiple Vaccine Breakthrough Infections. Medrxiv Prepr. Serv. Health Sci. 2021. [Google Scholar] [CrossRef]
- Wu, K.; Choi, A.; Koch, M.; Ma, L.; Hill, A.; Nunna, N.; Huang, W.; Oestreicher, J.; Colpitts, T.; Bennett, H.; et al. Preliminary Analysis of Safety and Immunogenicity of a SARS-CoV-2 Variant Vaccine Booster. Medrxiv Prepr. Serv. Health Sci. 2021. [Google Scholar] [CrossRef]
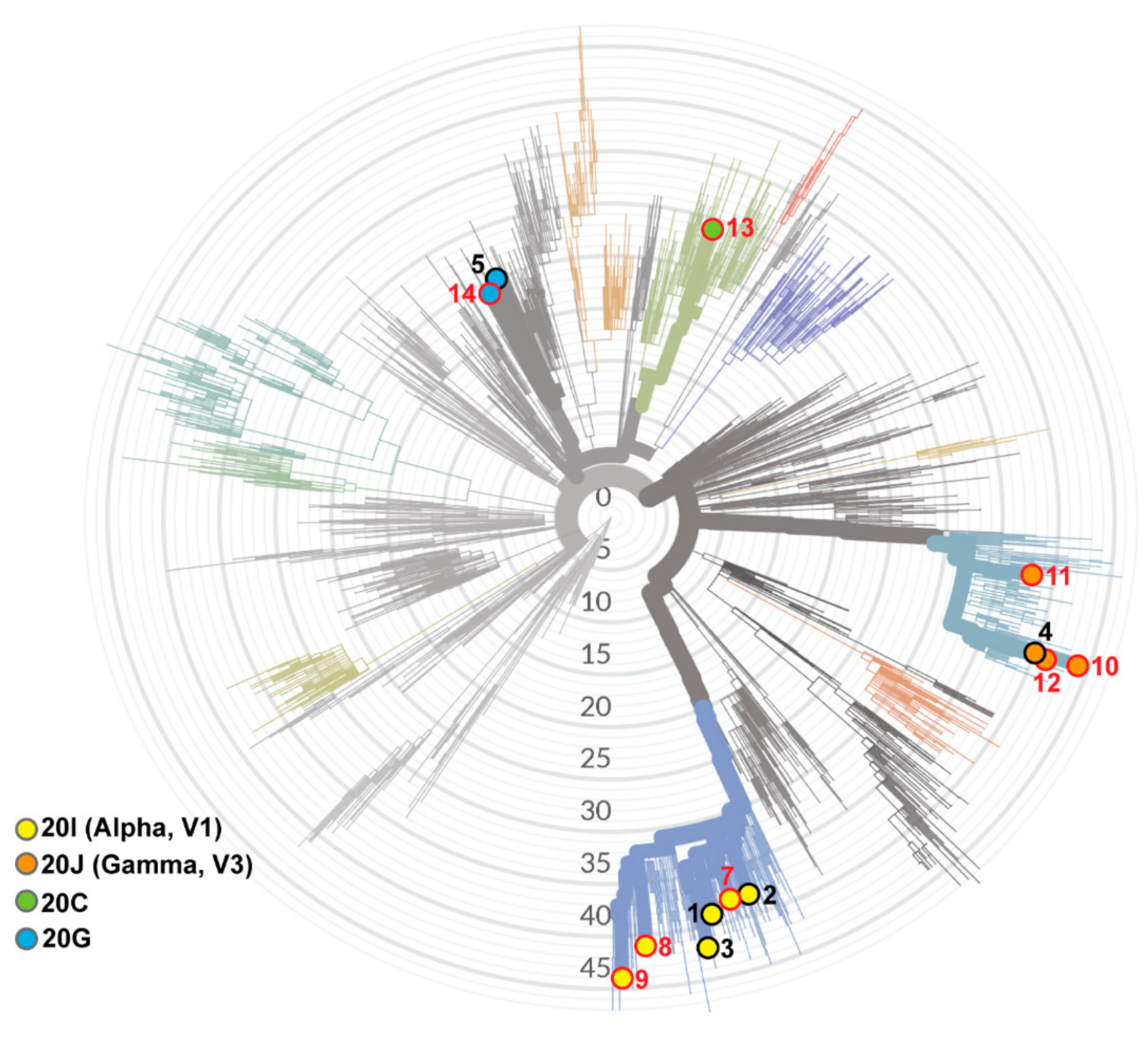
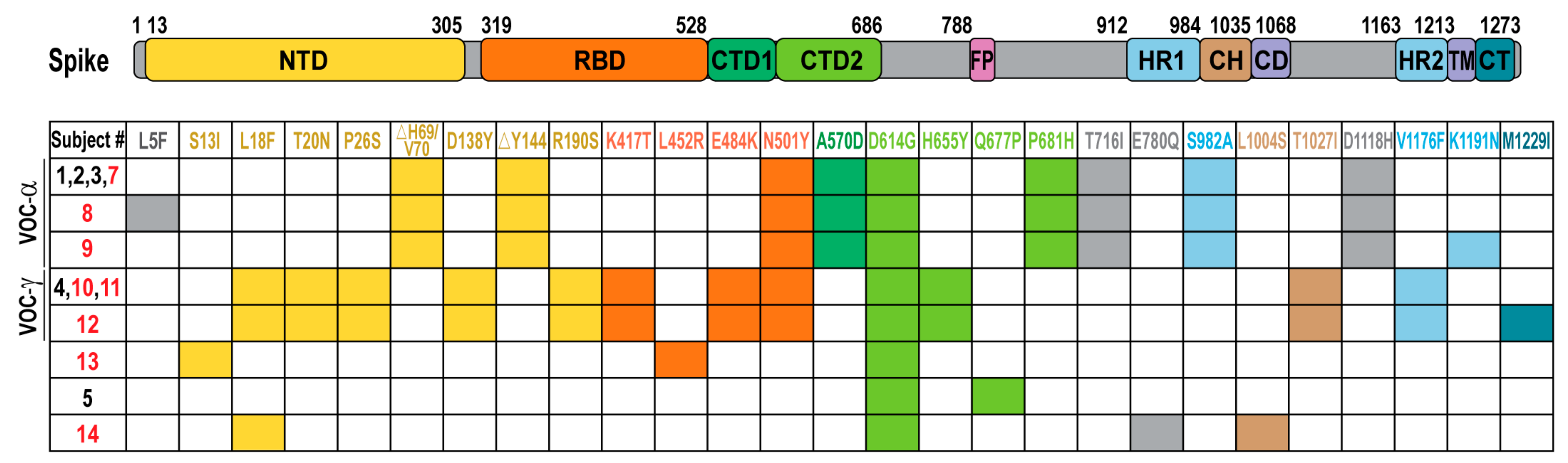
| Subject | Sex | Age Range | Additional Risk Factors | Immunosuppressive Medication | Vaccine Type | Symptoms | Hospitalization | Viral Load (Ct) | NextClade Lineage | |
|---|---|---|---|---|---|---|---|---|---|---|
| Asymptomatic/mild | 1 | F | 60 | HCW | No | Pfizer | Rhinorrhea | No | 18.8 | 20I/S:501Y.V1/VOC-alpha |
| 2 | M | 58 | None | No | Pfizer | Chill, subjective fever | No | 19.1 | 20I/S:501Y.V1/VOC-alpha | |
| 3 | F | 48 | Smoke | No | Pfizer | Weakness, congestion loss of taste/smell, fatique | No | 20.9 | 20I/S:501Y.V1/VOC-alpha | |
| 4 | F | 51 | NASH | Yes | M/P | Headache, cough, rhinorrhea ageusia, anosmia | No | 17.1 | 20J/S:501Y.V3/VOC-gamma | |
| 5 | F | 37 | HCW | No | Moderna | Asymptomatic | No | 19.5 | 20G | |
| 6 | F | 50 | HCW | No | Pfizer | Asymptomatic | No | 34.2 | ND | |
| hospitalized | 7 | F | 81 | Heart disease, CVA | No | J&J | Shortness of breath, cough | Yes | 18.8 | 20I/S:501Y.V1/VOC-alpha |
| 8 | M | 65 | SOT-kidney and heart | Yes | Pfizer | Diarrhea, myalgia, chills, fever, pneumonia | Yes | 20.1 | 20I/S:501Y.V1/VOC-alpha | |
| 9 | M | 55 | SOT-kidney | Yes | Pfizer | Cough, acute hypoxic respiratory failure, sepsis | Yes, ICU, died | 22.3 | 20I/S:501Y.V1/VOC-alpha | |
| 10 | M | 70 | SOT-liver | Yes | Pfizer | Cough, weakness, fever, dyspnea | Yes | 19.6 | 20J/S:501Y.V3/VOC-gamma | |
| 11 | M | 68 | SOT-lung | Yes | Moderna | Acute hypoxia, acute pneumonia, hemoptysis | Yes, ICU | 21.4 | 20J/S:501Y.V3/VOC-gamma | |
| 12 | F | 60 | SOT-lung | Yes | Moderna | Shortness of breath, fever, chills, body aches, hypoxia | Yes, ICU | 15.7 | 20J/S:501Y.V3/VOC-gamma | |
| 13 | M | 65 | SOT-liver | Yes | Pfizer | Diarrhea, nausea, weakness cough, dyspnea | Yes | 22.1 | 20C/epsilon | |
| 14 | F | 76 | None | No | Pfizer | Fever, chills, acute respiratory failure | Yes, ICU | 18.3 | 20G |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Deng, X.; Evdokimova, M.; O’Brien, A.; Rowe, C.L.; Clark, N.M.; Harrington, A.; Reid, G.E.; Uprichard, S.L.; Baker, S.C. Breakthrough Infections with Multiple Lineages of SARS-CoV-2 Variants Reveals Continued Risk of Severe Disease in Immunosuppressed Patients. Viruses 2021, 13, 1743. https://doi.org/10.3390/v13091743
Deng X, Evdokimova M, O’Brien A, Rowe CL, Clark NM, Harrington A, Reid GE, Uprichard SL, Baker SC. Breakthrough Infections with Multiple Lineages of SARS-CoV-2 Variants Reveals Continued Risk of Severe Disease in Immunosuppressed Patients. Viruses. 2021; 13(9):1743. https://doi.org/10.3390/v13091743
Chicago/Turabian StyleDeng, Xufang, Monika Evdokimova, Amornrat O’Brien, Cynthia L. Rowe, Nina M. Clark, Amanda Harrington, Gail E. Reid, Susan L. Uprichard, and Susan C. Baker. 2021. "Breakthrough Infections with Multiple Lineages of SARS-CoV-2 Variants Reveals Continued Risk of Severe Disease in Immunosuppressed Patients" Viruses 13, no. 9: 1743. https://doi.org/10.3390/v13091743
APA StyleDeng, X., Evdokimova, M., O’Brien, A., Rowe, C. L., Clark, N. M., Harrington, A., Reid, G. E., Uprichard, S. L., & Baker, S. C. (2021). Breakthrough Infections with Multiple Lineages of SARS-CoV-2 Variants Reveals Continued Risk of Severe Disease in Immunosuppressed Patients. Viruses, 13(9), 1743. https://doi.org/10.3390/v13091743







