Viral and Bacterial Co-Infections in the Lungs: Dangerous Liaisons
Abstract
1. Respiratory Tract Infections, Their Etiological Agents, and the Weight of Viral–Bacterial Co-Infections
1.1. Influenza Viruses
1.2. Respiratory Syncytial Virus
1.3. SARS-CoV-2
2. Mechanisms Involved in Co-Infections/Superinfections
2.1. Physiological Mechanisms
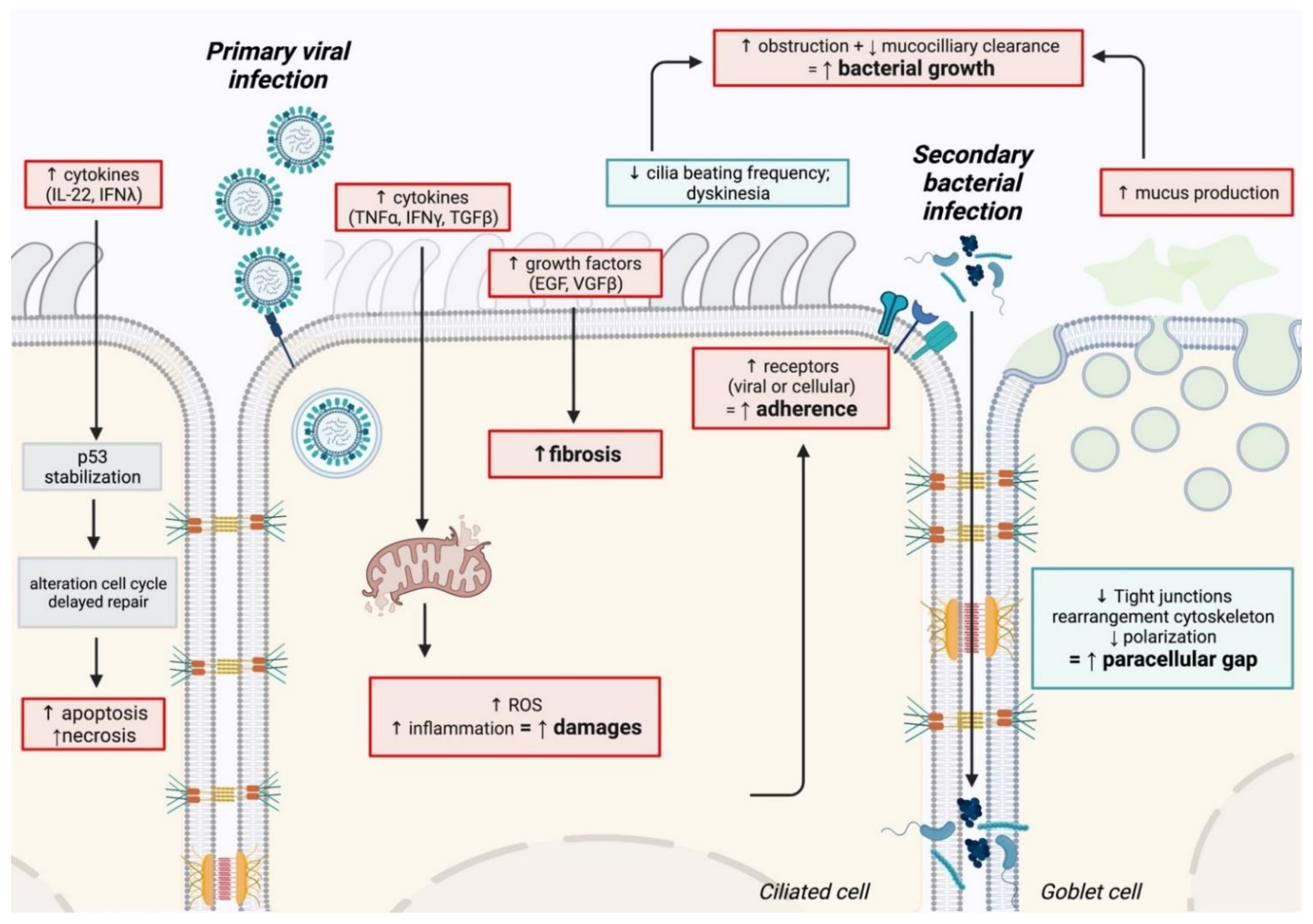
2.1.1. Epithelium Damage
2.1.2. Modification of Airway Function
2.1.3. Enhancement of Bacterial Adhesion after Viral Infection
2.1.4. Repair Delay after Viral Infection
2.2. Immunological Mechanisms
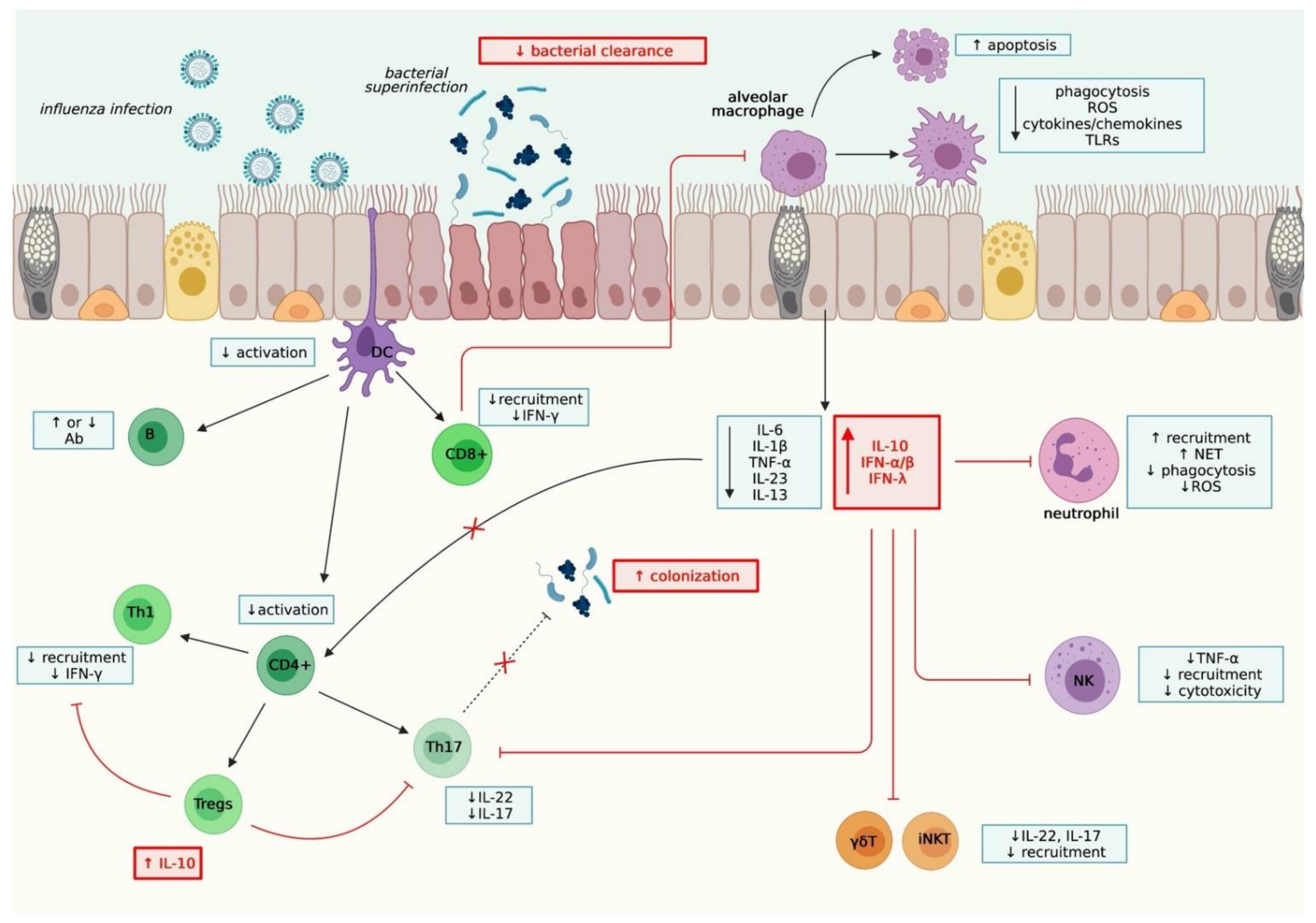
2.2.1. Innate Immunity
2.2.2. Adaptive immunity
2.2.3. Other mechanisms
3. Viral and Bacterial Co-Infections in the Context of Chronic Respiratory Diseases
4. What about the Microbiome?
5. What Are the Best Models for Studying Co-Infections?
5.1. In Vitro Models
5.2. In Vivo Models
5.2.1. Mouse Model
5.2.2. Non-Human Primate Model
5.2.3. Ferret Model
5.2.4. Other Models of Interest
6. Concluding Remarks
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- WHO. Acute Respiratory Infections. Available online: http://apps.who.int/vaccine_research/diseases/ari/en/index.html (accessed on 3 June 2016).
- Schluger, N.W.; Koppaka, R. Lung Disease in a Global Context. A Call for Public Health Action. Ann. Am. Thorac. Soc. 2014, 11, 407–416. [Google Scholar] [CrossRef] [PubMed]
- Roth, G.A.; Abate, D.; Abate, K.H.; Abay, S.M.; Abbafati, C.; Abbasi, N.; Abbastabar, H.; Abd-Allah, F.; Abdela, J.; Abdelalim, A.; et al. Global, regional, and national age-sex-specific mortality for 282 causes of death in 195 countries and territories, 1980–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet 2018, 392, 1736–1788. [Google Scholar] [CrossRef]
- Mandell, L.A. Etiologies of Acute Respiratory Tract Infections. Clin. Infect. Dis. 2005, 41, 503–506. [Google Scholar] [CrossRef] [PubMed]
- Bello, S.; Mincholé, E.; Fandos, S.; Lasierra, A.B.; Ruiz, M.A.; Simon, A.L.; Panadero, C.; Lapresta, C.; Menendez, R.; Torres, A. Inflammatory response in mixed viral-bacterial community-acquired pneumonia. BMC Pulm. Med. 2014, 14, 123. [Google Scholar] [CrossRef]
- Metersky, M.L.; Masterton, R.G.; Lode, H.; File, T.M.; Babinchak, T. Epidemiology, microbiology, and treatment considerations for bacterial pneumonia complicating influenza. Int. J. Infect. Dis. 2012, 16, e321. [Google Scholar] [CrossRef] [PubMed]
- Rice, T.W.; Rubinson, L.; Uyeki, T.M.; Vaughn, F.L.; John, B.B.; Miller, R.R.; Higgs, E.; Randolph, A.; Smoot, B.E.; Thompson, B.T. Critical illness from 2009 pandemic influenza A virus and bacterial coinfection in the United States. Crit. Care Med. 2012, 40, 1487–1498. [Google Scholar] [CrossRef]
- Randolph, A.G.; Vaughn, F.; Sullivan, R.; Rubinson, L.; Thompson, B.T.; Yoon, G.; Smoot, E.; Rice, T.; Loftis, L.L.; Helfaer, M.; et al. Critically Ill Children During the 2009–2010 Influenza Pandemic in the United States. Pediatrics 2011, 128, e1450–e1458. [Google Scholar] [CrossRef] [PubMed]
- Palacios, G.; Hornig, M.; Cisterna, D.; Savji, N.; Bussetti, A.V.; Kapoor, V.; Hui, J.; Tokarz, R.; Briese, T.; Baumeister, E.; et al. Streptococcus pneumoniae Coinfection Is Correlated with the Severity of H1N1 Pandemic Influenza. PLoS ONE 2009, 4, e8540. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, A.; Noymer, A. Influenza Mortality in the United States, 2009 Pandemic: Burden, Timing and Age Distribution. PLoS ONE 2013, 8, e64198. [Google Scholar] [CrossRef] [PubMed]
- Morens, D.M.; Taubenberger, J.K.; Fauci, A.S. Predominant Role of Bacterial Pneumonia as a Cause of Death in Pandemic Influenza: Implications for Pandemic Influenza Preparedness. J. Infect. Dis. 2008, 198, 962–970. [Google Scholar] [CrossRef]
- Blyth, C.C.; Webb, S.A.R.; Kok, J.; Dwyer, D.E.; van Hal, S.; Foo, H.; Ginn, A.N.; Kesson, A.M.; Seppelt, I.; Iredell, J.R.; et al. The impact of bacterial and viral co-infection in severe influenza. Influ. Other Respir. Viruses 2013, 7, 168–176. [Google Scholar] [CrossRef]
- Podewils, L.J.; Liedtke, L.A.; McDonald, L.C.; Hageman, J.C.; Strausbaugh, L.J.; Fischer, T.K.; Jernigan, D.B.; Uyeki, T.M.; Kuehnert, M.J.; Network, T.I.D.S.O.A.E.I. A National Survey of Severe Influenza-Associated Complications among Children and Adults, 2003–2004. Clin. Infect. Dis. 2005, 40, 1693–1696. [Google Scholar] [CrossRef]
- Wong, K.K.; Jain, S.; Blanton, L.; Dhara, R.; Brammer, L.; Fry, A.M.; Finelli, L. Influenza-Associated Pediatric Deaths in the United States, 2004–2012. Pediatrics 2013, 132, 796–804. [Google Scholar] [CrossRef]
- Finelli, L.; Fiore, A.; Dhara, R.; Brammer, L.; Shay, D.; Kamimoto, L.; Fry, A.; Hageman, J.; Gorwitz, R.; Bresee, J.; et al. Influenza-Associated Pediatric Mortality in the United States: Increase of Staphylococcus aureus Coinfection. Pediatrics 2008, 122, 805–811. [Google Scholar] [CrossRef]
- Klein, E.Y.; Monteforte, B.; Gupta, A.; Jiang, W.; May, L.; Hsieh, Y.; Dugas, A. The frequency of influenza and bacterial coinfection: A systematic review and meta-analysis. Influ. Other Respir. Viruses 2016, 10, 394–403. [Google Scholar] [CrossRef]
- Teng, F.; Liu, X.; Guo, S.-B.; Li, Z.; Ji, W.-Q.; Zhang, F.; Zhu, X.-M. Community-acquired bacterial co-infection predicts severity and mortality in influenza-associated pneumonia admitted patients. J. Infect. Chemother. 2019, 25, 129–136. [Google Scholar] [CrossRef] [PubMed]
- Griffiths, C.; Drews, S.J.; Marchant, D.J. Respiratory Syncytial Virus: Infection, Detection, and New Options for Prevention and Treatment. Clin. Microbiol. Rev. 2017, 30, 277–319. [Google Scholar] [CrossRef]
- Pacheco, G.; Gálvez, N.; Soto, J.; Andrade, C.; Kalergis, A. Bacterial and Viral Coinfections with the Human Respiratory Syncytial Virus. Microorganisms 2021, 9, 1293. [Google Scholar] [CrossRef] [PubMed]
- Jiang, W.; Wang, T.; Li, L.; Ji, W.; Wang, Y.; Yan, Y. Impact of bacteria in nasal aspirates on disease severity of bronchiolitis. Infect. Dis. 2016, 48, 82–86. [Google Scholar] [CrossRef] [PubMed]
- Korppi, M.; Leinonen, M.; Koskela, M.; Mäkelä, P.H.; Launiala, K. Bacterial coinfection in children hospitalized with respiratory syncytial virus infections. Pediatr. Infect. Dis. J. 1989, 8, 687–692. [Google Scholar] [CrossRef] [PubMed]
- Randolph, A.; Reder, L.; Englund, J.A. Risk of Bacterial Infection in Previously Healthy Respiratory Syncytial Virus-Infected Young Children Admitted to the Intensive Care Unit. Pediatr. Infect. Dis. J. 2004, 23, 990–994. [Google Scholar] [CrossRef] [PubMed]
- Lee, N.; Lui, C.Y.G.; Wong, K.T.; Li, T.C.M.; Tse, E.C.M.; Chan, J.Y.C.; Yu, J.; Wong, S.S.M.; Choi, K.W.; Wong, R.Y.K.; et al. High Morbidity and Mortality in Adults Hospitalized for Respiratory Syncytial Virus Infections. Clin. Infect. Dis. 2013, 57, 1069–1077. [Google Scholar] [CrossRef]
- WHO. Coronavirus Disease (COVID-19) Dashboard. Available online: https://covid19.who.int/ (accessed on 19 May 2020).
- Russell, C.D.; Fairfield, C.J.; Drake, T.M.; Turtle, L.; Seaton, R.A.; Wootton, D.G.; Sigfrid, L.; Harrison, E.M.; Docherty, A.B.; I de Silva, T.; et al. Co-infections, secondary infections, and antimicrobial use in patients hospitalised with COVID-19 during the first pandemic wave from the ISARIC WHO CCP-UK study: A multicentre, prospective cohort study. Lancet Microbe 2021, 2, e354–e365. [Google Scholar] [CrossRef]
- Karami, Z.; Knoop, B.T.; Dofferhoff, A.S.M.; Blaauw, M.J.T.; Janssen, N.A.; van Apeldoorn, M.; Kerckhoffs, A.P.M.; van de Maat, J.S.; Hoogerwerf, J.J.; Oever, J.T. Few bacterial co-infections but frequent empiric antibiotic use in the early phase of hospitalized patients with COVID-19: Results from a multicentre retrospective cohort study in The Netherlands. Infect. Dis. 2021, 53, 102–110. [Google Scholar] [CrossRef] [PubMed]
- Hughes, S.; Troise, O.; Donaldson, H.; Mughal, N.; Moore, L. Bacterial and fungal coinfection among hospitalized patients with COVID-19: A retrospective cohort study in a UK secondary-care setting. Clin. Microbiol. Infect. 2020, 26, 1395–1399. [Google Scholar] [CrossRef]
- Lansbury, L.; Lim, B.; Baskaran, V.; Lim, W.S. Co-infections in people with COVID-19: A systematic review and meta-analysis. J. Infect. 2020, 81, 266–275. [Google Scholar] [CrossRef] [PubMed]
- Langford, B.J.; So, M.; Raybardhan, S.; Leung, V.; Westwood, D.; MacFadden, D.R.; Soucy, J.-P.R.; Daneman, N. Bacterial co-infection and secondary infection in patients with COVID-19: A living rapid review and meta-analysis. Clin. Microbiol. Infect. 2020, 26, 1622–1629. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Vidal, C.; Sanjuan, G.; Moreno-García, E.; Puerta-Alcalde, P.; Garcia-Pouton, N.; Chumbita, M.; Fernandez-Pittol, M.; Pitart, C.; Inciarte, A.; Bodro, M.; et al. Incidence of co-infections and superinfections in hospitalized patients with COVID-19: A retrospective cohort study. Clin. Microbiol. Infect. 2021, 27, 83–88. [Google Scholar] [CrossRef] [PubMed]
- Chen, N.; Zhou, M.; Dong, X.; Qu, J.; Gong, F.; Han, Y.; Qiu, Y.; Wang, J.; Liu, Y.; Wei, Y.; et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: A descriptive study. Lancet 2020, 395, 507–513. [Google Scholar] [CrossRef]
- Chen, X.; Liao, B.; Cheng, L.; Peng, X.; Xu, X.; Li, Y.; Hu, T.; Li, J.; Zhou, X.; Ren, B. The microbial coinfection in COVID-19. Appl. Microbiol. Biotechnol. 2020, 104, 7777–7785. [Google Scholar] [CrossRef]
- Kojima, T.; Go, M.; Takano, K.-I.; Kurose, M.; Ohkuni, T.; Koizumi, J.-I.; Kamekura, R.; Ogasawara, N.; Masaki, T.; Fuchimoto, J.; et al. Regulation of Tight Junctions in Upper Airway Epithelium. BioMed Res. Int. 2013, 2013, 947072. [Google Scholar] [CrossRef]
- Pizzorno, A.; Padey, B.; Julien, T.; Trouillet-Assant, S.; Traversier, A.; Errazuriz-Cerda, E.; Fouret, J.; Dubois, J.; Gaymard, A.; Lescure, F.-X.; et al. Characterization and Treatment of SARS-CoV-2 in Nasal and Bronchial Human Airway Epithelia. Cell Rep. Med. 2020, 1, 100059. [Google Scholar] [CrossRef]
- De Lamballerie, C.N.; Pizzorno, A.; Dubois, J.; Julien, T.; Padey, B.; Bouveret, M.; Traversier, A.; Legras-Lachuer, C.; Lina, B.; Boivin, G.; et al. Characterization of cellular transcriptomic signatures induced by different respiratory viruses in human reconstituted airway epithelia. Sci. Rep. 2019, 9, 11493. [Google Scholar] [CrossRef]
- Pizzorno, M.A.; Terrier, O.; de Lamballerie, C.N.; Julien, T.; Padey, B.; Traversier, A.; Roche, M.; Hamelin, M.-E.; Rhéaume, C.; Croze, S.; et al. Repurposing of Drugs as Novel Influenza Inhibitors from Clinical Gene Expression Infection Signatures. Front. Immunol. 2019, 10, 60. [Google Scholar] [CrossRef]
- Lu, X.; Tumpey, T.M.; Morken, T.; Zaki, S.R.; Cox, N.J.; Katz, J.M. A Mouse Model for the Evaluation of Pathogenesis and Immunity to Influenza A (H5N1) Viruses Isolated from Humans. J. Virol. 1999, 73, 5903–5911. [Google Scholar] [CrossRef] [PubMed]
- Imai, M.; Iwatsuki-Horimoto, K.; Hatta, M.; Loeber, S.; Halfmann, P.J.; Nakajima, N.; Watanabe, T.; Ujie, M.; Takahashi, K.; Ito, M.; et al. Syrian hamsters as a small animal model for SARS-CoV-2 infection and countermeasure development. Proc. Natl. Acad. Sci. USA 2020, 117, 16587–16595. [Google Scholar] [CrossRef] [PubMed]
- Stittelaar, K.; De Waal, L.; Van Amerongen, G.; Kroeze, E.V.; Fraaij, P.; Van Baalen, C.; Van Kampen, J.; Van Der Vries, E.; Osterhaus, A.; De Swart, R. Ferrets as a Novel Animal Model for Studying Human Respiratory Syncytial Virus Infections in Immunocompetent and Immunocompromised Hosts. Viruses 2016, 8, 168. [Google Scholar] [CrossRef]
- Linfield, D.T.; Raduka, A.; Aghapour, M.; Rezaee, F. Airway tight junctions as targets of viral infections. Tissue Barriers 2021, 9, 1883965. [Google Scholar] [CrossRef] [PubMed]
- Rezaee, F.; DeSando, S.A.; Ivanov, A.I.; Chapman, T.J.; Knowlden, S.A.; Beck, L.A.; Georas, S.N. Sustained Protein Kinase D Activation Mediates Respiratory Syncytial Virus-Induced Airway Barrier Disruption. J. Virol. 2013, 87, 11088–11095. [Google Scholar] [CrossRef]
- Ruan, T.; Sun, J.; Liu, W.; Prinz, R.A.; Peng, D.; Liu, X.; Xu, X. H1N1 Influenza Virus Cross-Activates Gli1 to Disrupt the Intercellular Junctions of Alveolar Epithelial Cells. Cell Rep. 2020, 31, 107801. [Google Scholar] [CrossRef]
- Chertow, D.S.; Kindrachuk, J.; Sheng, Z.-M.; Pujanauski, L.M.; Cooper, K.; Nogee, D.; Claire, M.S.; Solomon, J.; Perry, D.; Sayre, P.; et al. Influenza A and methicillin-resistant Staphylococcus aureus co-infection in rhesus macaques—A model of severe pneumonia. Antivir. Res. 2016, 129, 120–129. [Google Scholar] [CrossRef]
- Berendt, R.F.; Long, G.G.; Walker, J.S. Influenza Alone and in Sequence with Pneumonia Due to Streptococcus pneumoniae in the Squirrel Monkey. J. Infect. Dis. 1975, 132, 689–693. [Google Scholar] [CrossRef]
- Iverson, A.R.; Boyd, K.L.; McAuley, J.; Plano, L.R.; Hart, M.E.; McCullers, J.A. Influenza Virus Primes Mice for Pneumonia from Staphylococcus aureus. J. Infect. Dis. 2011, 203, 880–888. [Google Scholar] [CrossRef]
- Stark, J.M.; Stark, M.A.; Colasurdo, G.N.; Levine, A.M. Decreased bacterial clearance from the lungs of mice following primary respiratory syncytial virus infection. J. Med. Virol. 2006, 78, 829–838. [Google Scholar] [CrossRef]
- Morgene, M.F.; Botelho-Nevers, E.; Grattard, F.; Pillet, S.; Berthelot, P.; Pozzetto, B.; Verhoeven, P.O. Staphylococcus aureus colonization and non-influenza respiratory viruses: Interactions and synergism mechanisms. Virulence 2018, 9, 1354–1363. [Google Scholar] [CrossRef] [PubMed]
- Braun, L.E.; Sutter, D.E.; Eichelberger, M.C.; Pletneva, L.; Kokai-Kun, J.F.; Blanco, J.C.; Prince, G.A.; Ottolini, M.G. Co-infection of the cotton rat (Sigmodon hispidus) with Staphylococcus aureus and influenza A virus results in synergistic disease. Microb. Pathog. 2007, 43, 208–216. [Google Scholar] [CrossRef] [PubMed]
- Schultz-Cherry, S.; Dybdahl-Sissoko, N.; Neumann, G.; Kawaoka, Y.; Hinshaw, V.S. Influenza Virus NS1 Protein Induces Apoptosis in Cultured Cells. J. Virol. 2001, 75, 7875–7881. [Google Scholar] [CrossRef] [PubMed]
- Nailwal, H.; Sharma, S.K.; Mayank, A.K.; Lal, S.K. The nucleoprotein of influenza A virus induces p53 signaling and apoptosis via attenuation of host ubiquitin ligase RNF43. Cell Death Dis. 2015, 6, e1768. [Google Scholar] [CrossRef]
- Kotelkin, A.; Prikhod’Ko, E.A.; Cohen, J.I.; Collins, P.L.; Bukreyev, A. Respiratory Syncytial Virus Infection Sensitizes Cells to Apoptosis Mediated by Tumor Necrosis Factor-Related Apoptosis-Inducing Ligand. J. Virol. 2003, 77, 9156–9172. [Google Scholar] [CrossRef]
- Van Krüchten, A.; Wilden, J.J.; Niemann, S.; Peters, G.; Löffler, B.; Ludwig, S.; Ehrhardt, C. Staphylococcus aureus triggers a shift from influenza virus–induced apoptosis to necrotic cell death. FASEB J. 2018, 32, 2779–2793. [Google Scholar] [CrossRef]
- Zanin, M.; Baviskar, P.; Webster, R.; Webby, R. The Interaction between Respiratory Pathogens and Mucus. Cell Host Microbe 2016, 19, 159–168. [Google Scholar] [CrossRef]
- Kuek, L.E.; Lee, R.J. First contact: The role of respiratory cilia in host-pathogen interactions in the airways. Am. J. Physiol. Cell. Mol. Physiol. 2020, 319, L603–L619. [Google Scholar] [CrossRef] [PubMed]
- McCullers, J.A. Insights into the Interaction between Influenza Virus and Pneumococcus. Clin. Microbiol. Rev. 2006, 19, 571–582. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, K.; Graham, B.S.; Ho, S.B.; Adler, K.B.; Collins, R.D.; Olson, S.J.; Zhou, W.; Suzutani, T.; Jones, P.W.; Goleniewska, K.; et al. Respiratory Syncytial Virus in Allergic Lung Inflammation Increases Muc5ac and Gob-5. Am. J. Respir. Crit. Care Med. 2004, 170, 306–312. [Google Scholar] [CrossRef]
- Vareille, M.; Kieninger, E.; Edwards, M.R.; Regamey, N. The Airway Epithelium: Soldier in the Fight against Respiratory Viruses. Clin. Microbiol. Rev. 2011, 24, 210–229. [Google Scholar] [CrossRef] [PubMed]
- Hament, J.-M.; Aerts, P.C.; Fleer, A.; van Dijk, H.; Harmsen, T.; Kimpen, J.L.L.; Wolfs, T.F.W. Enhanced Adherence of Streptococcus pneumoniae to Human Epithelial Cells Infected with Respiratory Syncytial Virus. Pediatr. Res. 2004, 55, 972–978. [Google Scholar] [CrossRef]
- Hament, J.-M.; Aerts, P.C.; Fleer, A.; van Dijk, H.; Harmsen, T.; Kimpen, J.L.L.; Wolfs, T.F.W. Direct Binding of Respiratory Syncytial Virus to Pneumococci: A Phenomenon that Enhances Both Pneumococcal Adherence to Human Epithelial Cells and Pneumococcal Invasiveness in a Murine Model. Pediatr. Res. 2005, 58, 1198–1203. [Google Scholar] [CrossRef]
- Avadhanula, V.; Wang, Y.; Portner, A.; Adderson, E. Nontypeable Haemophilus influenzae and Streptococcus pneumoniae bind respiratory syncytial virus glycoprotein. J. Med. Microbiol. 2007, 56, 1133–1137. [Google Scholar] [CrossRef]
- Mlacha, S.Z.K.; Peret, T.C.T.; Kumar, N.; Romero-Steiner, S.; Hotopp, J.C.D.; Ishmael, N.; Grinblat-Huse, V.; Riley, D.R.; Erdman, D.D.; Carlone, G.M.; et al. Transcriptional adaptation of pneumococci and human pharyngeal cells in the presence of a virus infection. BMC Genom. 2013, 14, 1–14. [Google Scholar] [CrossRef][Green Version]
- Pittet, L.A.; Hall-Stoodley, L.; Rutkowski, M.R.; Harmsen, A.G. Influenza Virus Infection Decreases Tracheal Mucociliary Velocity and Clearance of Streptococcus pneumoniae. Am. J. Respir. Cell Mol. Biol. 2010, 42, 450–460. [Google Scholar] [CrossRef] [PubMed]
- Plotkowski, M.-C.; Puchelle, E.; Beck, G.; Jacquot, J.; Hannoun, C. Adherence of Type I Streptococcus pneumoniae to Tracheal Epithelium of Mice Infected with Influenza A/PR8 Virus1–3. Am. Rev. Respir. Dis. 1986, 134, 1040–1044. [Google Scholar] [CrossRef]
- McCullers, J.A.; Rehg, J.E. Lethal Synergism between Influenza Virus and Streptococcus pneumoniae: Characterization of a Mouse Model and the Role of Platelet-Activating Factor Receptor. J. Infect. Dis. 2002, 186, 341–350. [Google Scholar] [CrossRef]
- Van Der Sluijs, K.F.; Van Elden, L.; Nijhuis, M.; Schuurman, R.; Florquin, S.; Shimizu, T.; Ishii, S.; Jansen, H.M.; Lutter, R.; Van Der Poll, T. Involvement of the platelet-activating factor receptor in host defense against Streptococcus pneumoniae during postinfluenza pneumonia. Am. J. Physiol. Cell. Mol. Physiol. 2006, 290, L194–L199. [Google Scholar] [CrossRef] [PubMed]
- Avadhanula, V.; Rodriguez, C.A.; DeVincenzo, J.P.; Wang, Y.; Webby, R.J.; Ulett, G.C.; Adderson, E.E. Respiratory Viruses Augment the Adhesion of Bacterial Pathogens to Respiratory Epithelium in a Viral Species- and Cell Type-Dependent Manner. J. Virol. 2006, 80, 1629–1636. [Google Scholar] [CrossRef]
- Sanford, B.A.; Shelokov, A.; Ramsay, M.A. Bacterial Adherence to Virus-Infected Cells: A Cell Culture Model of Bacterial Superinfection. J. Infect. Dis. 1978, 137, 176–181. [Google Scholar] [CrossRef] [PubMed]
- Hafez, M.M.; Abdel-Wahab, K.S.E.; El-Fouhil, D.F.I. Augmented adherence and internalization of group A Streptococcus pyogenes to influenza A virus infected MDCK cells. J. Basic Microbiol. 2010, 50 (Suppl. 1), S46–S57. [Google Scholar] [CrossRef] [PubMed]
- Davison, V.E.; Sanford, B.A. Factors Influencing Adherence of Staphylococcus aureus to Influenza A Virus-Infected Cell Cultures. Infect. Immun. 1982, 37, 946–955. [Google Scholar] [CrossRef] [PubMed]
- Rowe, H.M.; Meliopoulos, V.A.; Iverson, A.; Bomme, P.; Schultz-Cherry, S.; Rosch, J.W. Direct interactions with influenza promote bacterial adherence during respiratory infections. Nat. Microbiol. 2019, 4, 1328–1336. [Google Scholar] [CrossRef]
- Peltola, V.T.; Murti, K.G.; McCullers, J.A. Influenza Virus Neuraminidase Contributes to Secondary Bacterial Pneumonia. J. Infect. Dis. 2005, 192, 249–257. [Google Scholar] [CrossRef] [PubMed]
- McCullers, J.A.; Bartmess, K.C. Role of Neuraminidase in Lethal Synergism between Influenza Virus and Streptococcus pneumoniae. J. Infect. Dis. 2003, 187, 1000–1009. [Google Scholar] [CrossRef]
- Li, N.; Ren, A.; Wang, X.; Fan, X.; Zhao, Y.; Gao, G.F.; Cleary, P.; Wang, B. Influenza viral neuraminidase primes bacterial coinfection through TGF-β–mediated expression of host cell receptors. Proc. Natl. Acad. Sci. USA 2014, 112, 238–243. [Google Scholar] [CrossRef]
- Golda, A.; Malek, N.; Dudek, B.; Zeglen, S.; Wojarski, J.; Ochman, M.; Kucewicz-Czech, E.; Zembala, M.; Potempa, J.; Pyrc, K. Infection with human coronavirus NL63 enhances streptococcal adherence to epithelial cells. J. Gen. Virol. 2011, 92, 1358–1368. [Google Scholar] [CrossRef] [PubMed]
- Snelgrove, R.J.; Godlee, A.; Hussell, T. Airway immune homeostasis and implications for influenza-induced inflammation. Trends Immunol. 2011, 32, 328–334. [Google Scholar] [CrossRef] [PubMed]
- Martin, P.; Leibovich, S.J. Inflammatory cells during wound repair: The good, the bad and the ugly. Trends Cell Biol. 2005, 15, 599–607. [Google Scholar] [CrossRef] [PubMed]
- Pociask, D.A.; Scheller, E.V.; Mandalapu, S.; McHugh, K.J.; Enelow, R.I.; Fattman, C.L.; Kolls, J.K.; Alcorn, J.F. IL-22 Is Essential for Lung Epithelial Repair following Influenza Infection. Am. J. Pathol. 2013, 182, 1286–1296. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Shen, H.-H. Effect of respiratory syncytial virus on the activity of matrix metalloproteinase in mice. Chin. Med. J. 2007, 120, 5–11. [Google Scholar] [CrossRef] [PubMed]
- Kash, J.C.; Walters, K.-A.; Davis, A.S.; Sandouk, A.; Schwartzman, L.M.; Jagger, B.W.; Chertow, D.S.; Qi, L.; Kuestner, R.E.; Ozinsky, A.; et al. Lethal Synergism of 2009 Pandemic H1N1 Influenza Virus and Streptococcus pneumoniae Coinfection Is Associated with Loss of Murine Lung Repair Responses. mBio 2011, 2. [Google Scholar] [CrossRef] [PubMed]
- Major, J.; Crotta, S.; Llorian, M.; McCabe, T.M.; Gad, H.H.; Priestnall, S.L.; Hartmann, R.; Wack, A. Type I and III interferons disrupt lung epithelial repair during recovery from viral infection. Science 2020, 369, 712–717. [Google Scholar] [CrossRef]
- Bellinghausen, C.; Rohde, G.G.U.; Savelkoul, P.H.M.; Wouters, E.F.M.; Stassen, F.R.M. Viral–bacterial interactions in the respiratory tract. J. Gen. Virol. 2016, 97, 3089–3102. [Google Scholar] [CrossRef]
- McCullers, J.A. The co-pathogenesis of influenza viruses with bacteria in the lung. Nat. Rev. Genet. 2014, 12, 252–262. [Google Scholar] [CrossRef]
- Gordon, S.; Read, R. Macrophage defences against respiratory tract infections. Br. Med. Bull. 2002, 61, 45–61. [Google Scholar] [CrossRef]
- Schneider, C.; Nobs, S.P.; Heer, A.K.; Kurrer, M.; Klinke, G.; Van Rooijen, N.; Vogel, J.; Kopf, M. Alveolar Macrophages Are Essential for Protection from Respiratory Failure and Associated Morbidity following Influenza Virus Infection. PLOS Pathog. 2014, 10, e1004053. [Google Scholar] [CrossRef] [PubMed]
- Kaplan, M.J.; Radic, M. Neutrophil Extracellular Traps: Double-Edged Swords of Innate Immunity. J. Immunol. 2012, 189, 2689–2695. [Google Scholar] [CrossRef] [PubMed]
- Subramaniam, R.; Barnes, P.F.; Fletcher, K.; Boggaram, V.; Hillberry, Z.; Neuenschwander, P.; Shams, H. Protecting against Post-influenza Bacterial Pneumonia by Increasing Phagocyte Recruitment and ROS Production. J. Infect. Dis. 2014, 209, 1827–1836. [Google Scholar] [CrossRef]
- Baral, P.; Batra, S.; Zemans, R.L.; Downey, G.; Jeyaseelan, S. Divergent Functions of Toll-like Receptors during Bacterial Lung Infections. Am. J. Respir. Crit. Care Med. 2014, 190, 722–732. [Google Scholar] [CrossRef]
- Eddens, T.; Kolls, J.K. Host defenses against bacterial lower respiratory tract infection. Curr. Opin. Immunol. 2012, 24, 424–430. [Google Scholar] [CrossRef] [PubMed]
- Monroe, K.M.; McWhirter, S.M.; Vance, R.E. Identification of Host Cytosolic Sensors and Bacterial Factors Regulating the Type I Interferon Response to Legionella pneumophila. PLoS Pathog. 2009, 5, e1000665. [Google Scholar] [CrossRef]
- Schmolke, M.; Patel, J.R.; de Castro, E.; Sanchez-Aparicio, M.T.; Uccellini, M.; Miller, J.C.; Manicassamy, B.; Satoh, T.; Kawai, T.; Akira, S.; et al. RIG-I Detects mRNA of Intracellular Salmonella enterica Serovar Typhimurium during Bacterial Infection. mBio 2014, 5, e01006-14. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; Zhao, W. NLRP3 Inflammasome—A Key Player in Antiviral Responses. Front. Immunol. 2020, 11, 211. [Google Scholar] [CrossRef]
- Rodrigues, T.S.; De Sá, K.S.; Ishimoto, A.Y.; Becerra, A.; Oliveira, S.; Almeida, L.; Gonçalves, A.V.; Perucello, D.B.; Andrade, W.A.; Castro, R.; et al. Inflammasomes are activated in response to SARS-CoV-2 infection and are associated with COVID-19 severity in patients. J. Exp. Med. 2021, 218, e20201707. [Google Scholar] [CrossRef]
- Tate, M.D.; Mansell, A. An update on the NLRP3 inflammasome and influenza: The road to redemption or perdition? Curr. Opin. Immunol. 2018, 54, 80–85. [Google Scholar] [CrossRef]
- Iwasaki, A.; Pillai, P.S. Innate immunity to influenza virus infection. Nat. Rev. Immunol. 2014, 14, 315–328. [Google Scholar] [CrossRef]
- Lester, S.N.; Li, K. Toll-Like Receptors in Antiviral Innate Immunity. J. Mol. Biol. 2014, 426, 1246–1264. [Google Scholar] [CrossRef]
- Murawski, M.R.; Bowen, G.N.; Cerny, A.M.; Anderson, L.J.; Haynes, L.M.; Tripp, R.; Kurt-Jones, E.A.; Finberg, R.W. Respiratory Syncytial Virus Activates Innate Immunity through Toll-Like Receptor 2. J. Virol. 2009, 83, 1492–1500. [Google Scholar] [CrossRef]
- Liu, P.; Jamaluddin, M.; Li, K.; Garofalo, R.P.; Casola, A.; Brasier, A.R. Retinoic Acid-Inducible Gene I Mediates Early Antiviral Response and Toll-Like Receptor 3 Expression in Respiratory Syncytial Virus-Infected Airway Epithelial Cells. J. Virol. 2007, 81, 1401–1411. [Google Scholar] [CrossRef]
- Thorne, L.G.; Reuschl, A.; Zuliani-Alvarez, L.; Whelan, M.V.X.; Turner, J.; Noursadeghi, M.; Jolly, C.; Towers, G.J. SARS-CoV-2 sensing by RIG-I and MDA5 links epithelial infection to macrophage inflammation. EMBO J. 2021, 40, e107826. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Chu, H.; Wong, B.H.-Y.; Chiu, M.C.; Wang, D.; Li, C.; Liu, X.; Yang, D.; Poon, V.K.-M.; Cai, J.; et al. Activation of C-Type Lectin Receptor and (RIG)-I-Like Receptors Contributes to Proinflammatory Response in Middle East Respiratory Syndrome Coronavirus-Infected Macrophages. J. Infect. Dis. 2020, 221, 647–659. [Google Scholar] [CrossRef] [PubMed]
- Segovia, J.; Sabbah, A.; Mgbemena, V.; Tsai, S.-Y.; Chang, T.-H.; Berton, M.T.; Morris, I.R.; Allen, I.; Ting, J.P.-Y.; Bose, S. TLR2/MyD88/NF-κB Pathway, Reactive Oxygen Species, Potassium Efflux Activates NLRP3/ASC Inflammasome during Respiratory Syncytial Virus Infection. PLoS ONE 2012, 7, e29695. [Google Scholar] [CrossRef]
- Didierlaurent, A.; Goulding, J.; Patel, S.; Snelgrove, R.; Low, L.; Bebien, M.; Lawrence, T.; Van Rijt, L.S.; Lambrecht, B.N.; Sirard, J.-C.; et al. Sustained desensitization to bacterial Toll-like receptor ligands after resolution of respiratory influenza infection. J. Exp. Med. 2008, 205, 323–329. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Colón, G.J.; Warheit-Niemi, H.; Gurczynski, S.J.; Taylor, Q.M.; Wilke, C.A.; Podsiad, A.B.; Crespo, J.; Bhan, U.; Moore, B.B. Influenza-induced immune suppression to methicillin-resistant Staphylococcus aureus is mediated by TLR9. PLoS Pathog. 2019, 15, e1007560. [Google Scholar] [CrossRef]
- Tian, X.; Xu, F.; Lung, W.Y.; Meyerson, C.; Ghaffari, A.A.; Cheng, G.; Deng, J.C. Poly I:C Enhances Susceptibility to Secondary Pulmonary Infections by Gram-Positive Bacteria. PLoS ONE 2012, 7, e41879. [Google Scholar] [CrossRef]
- Robinson, K.M.; Ramanan, K.; Clay, M.; McHugh, K.J.; Pilewski, M.J.; Nickolich, K.L.; Corey, C.; Shiva, S.; Wang, J.; Alcorn, J.F. The inflammasome potentiates influenza/Staphylococcus aureus superinfection in mice. JCI Insight 2018, 3, 97470. [Google Scholar] [CrossRef]
- Ghoneim, H.E.; Thomas, P.G.; McCullers, J.A. Depletion of Alveolar Macrophages during Influenza Infection Facilitates Bacterial Superinfections. J. Immunol. 2013, 191, 1250–1259. [Google Scholar] [CrossRef] [PubMed]
- Robinson, K.M.; McHugh, K.J.; Mandalapu, S.; Clay, M.E.; Lee, B.; Scheller, E.V.; Enelow, R.I.; Chan, Y.R.; Kolls, J.K.; Alcorn, J.F. Influenza A virus exacerbates Staphylococcus aureus pneumonia in mice by attenuating antimicrobial peptide production. J. Infect. Dis. 2014, 209, 865–875. [Google Scholar] [CrossRef] [PubMed]
- Gopal, R.; Lee, B.; McHugh, K.J.; Rich, H.; Ramanan, K.; Mandalapu, S.; Clay, M.; Seger, P.J.; Enelow, R.I.; Manni, M.L.; et al. STAT2 Signaling Regulates Macrophage Phenotype during Influenza and Bacterial Super-Infection. Front. Immunol. 2018, 9, 2151. [Google Scholar] [CrossRef]
- Sun, K.; Metzger, D. Inhibition of pulmonary antibacterial defense by interferon-γ during recovery from influenza infection. Nat. Med. 2008, 14, 558–564. [Google Scholar] [CrossRef]
- Raza, M.; Blackwell, C.; Elton, R.; Weir, D. Bactericidal activity of a monocytic cell line (THP-1) against common respiratory tract bacterial pathogens is depressed after infection with respiratory syncytial virus. J. Med. Microbiol. 2000, 49, 227–233. [Google Scholar] [CrossRef]
- Arrevillaga, G.; Gaona, J.; Sánchez, C.; Rosales, V.; Gómez, B. Respiratory Syncytial Virus Persistence in Macrophages Downregulates Intercellular Adhesion Molecule-1 Expression and Reduces Adhesion of Non-Typeable Haemophilus influenzae. Intervirology 2012, 55, 442–450. [Google Scholar] [CrossRef]
- Sun, K.; Metzger, D. Influenza Infection Suppresses NADPH Oxidase–Dependent Phagocytic Bacterial Clearance and Enhances Susceptibility to Secondary Methicillin-Resistant Staphylococcus aureus Infection. J. Immunol. 2014, 192, 3301–3307. [Google Scholar] [CrossRef] [PubMed]
- Damjanovic, D.; Lai, R.; Jeyanathan, M.; Hogaboam, C.M.; Xing, Z. Marked Improvement of Severe Lung Immunopathology by Influenza-Associated Pneumococcal Superinfection Requires the Control of Both Bacterial Replication and Host Immune Responses. Am. J. Pathol. 2013, 183, 868–880. [Google Scholar] [CrossRef]
- Smith, C.M.; Sandrini, S.; Datta, S.; Freestone, P.; Shafeeq, S.; Radhakrishnan, P.; Williams, G.; Glenn, S.M.; Kuipers, O.P.; Hirst, R.A.; et al. Respiratory Syncytial Virus Increases the Virulence of Streptococcus pneumoniae by Binding to Penicillin Binding Protein 1a. A New Paradigm in Respiratory Infection. Am. J. Respir. Crit. Care Med. 2014, 190, 196–207. [Google Scholar] [CrossRef]
- Engelich, G.; White, M.; Hartshorn, K. Neutrophil survival is markedly reduced by incubation with influenza virus and Streptococcus pneumoniae: Role of respiratory burst. J. Leukoc. Biol. 2001, 69, 50–56. [Google Scholar]
- McNamee, L.A.; Harmsen, A.G. Both Influenza-Induced Neutrophil Dysfunction and Neutrophil-Independent Mechanisms Contribute to Increased Susceptibility to a Secondary Streptococcus pneumoniae Infection. Infect. Immun. 2006, 74, 6707–6721. [Google Scholar] [CrossRef]
- Narasaraju, T.; Yang, E.; Samy, R.P.; Ng, H.H.; Poh, W.P.; Liew, A.-A.; Phoon, M.C.; van Rooijen, N.; Chow, V.T. Excessive Neutrophils and Neutrophil Extracellular Traps Contribute to Acute Lung Injury of Influenza Pneumonitis. Am. J. Pathol. 2011, 179, 199–210. [Google Scholar] [CrossRef]
- Moorthy, A.N.; Narasaraju, T.; Rai, P.; Perumalsamy, R.; Tan, K.B.; Wang, S.; Engelward, B.; Chow, V.T.K. In Vivo and in Vitro studies on the roles of neutrophil extracellular traps during secondary pneumococcal pneumonia after primary pulmonary influenza infection. Front. Immunol. 2013, 4, 56. [Google Scholar] [CrossRef]
- Ellis, G.T.; Davidson, S.; Crotta, S.; Branzk, N.; Papayannopoulos, V.; Wack, A. TRAIL + monocytes and monocyte-related cells cause lung damage and thereby increase susceptibility to influenza– S treptococcus pneumoniae coinfection. EMBO Rep. 2015, 16, 1203–1218. [Google Scholar] [CrossRef] [PubMed]
- Small, C.-L.; Shaler, C.R.; McCormick, S.; Jeyanathan, M.; Damjanovic, D.; Brown, E.G.; Arck, P.; Jordana, M.; Kaushic, C.; Ashkar, A.; et al. Influenza Infection Leads to Increased Susceptibility to Subsequent Bacterial Superinfection by Impairing NK Cell Responses in the Lung. J. Immunol. 2010, 184, 2048–2056. [Google Scholar] [CrossRef] [PubMed]
- McDermott, D.S.; Weiss, K.A.; Knudson, C.J.; Varga, S.M. Central role of dendritic cells in shaping the adaptive immune response during respiratory syncytial virus infection. Futur. Virol. 2011, 6, 963–973. [Google Scholar] [CrossRef] [PubMed]
- Smed-Sörensen, A.; Chalouni, C.; Chatterjee, B.; Cohn, L.; Blattmann, P.; Nakamura, N.; Delamarre, L.; Mellman, I. Influenza A Virus Infection of Human Primary Dendritic Cells Impairs Their Ability to Cross-Present Antigen to CD8 T Cells. PLoS Pathog. 2012, 8, e1002572. [Google Scholar] [CrossRef]
- Campana, P.; Parisi, V.; Leosco, D.; Bencivenga, D.; Della Ragione, F.; Borriello, A. Dendritic Cells and SARS-CoV-2 Infection: Still an Unclarified Connection. Cells 2020, 9, 2046. [Google Scholar] [CrossRef]
- Schultze, J.L.; Aschenbrenner, A.C. COVID-19 and the human innate immune system. Cell 2021, 184, 1671–1692. [Google Scholar] [CrossRef]
- Cavalcante-Silva, L.H.A.; Carvalho, D.C.M.; Lima, D.A.; Galvão, J.G.; Silva, J.S.D.F.D.; de Sales-Neto, J.M.; Rodrigues-Mascarenhas, S. Neutrophils and COVID-19: The road so far. Int. Immunopharmacol. 2021, 90, 107233. [Google Scholar] [CrossRef]
- Rynda-Apple, A.; Harmsen, A.; Erickson, A.S.; Larson, K.; Morton, R.V.; Richert, L.E.; Harmsen, A.G. Regulation of IFN-γ by IL-13 dictates susceptibility to secondary postinfluenza MRSA pneumonia. Eur. J. Immunol. 2014, 44, 3263–3272. [Google Scholar] [CrossRef]
- Van Der Sluijs, K.F.; Van Elden, L.; Nijhuis, M.; Schuurman, R.; Pater, J.M.; Florquin, S.; Goldman, M.; Jansen, H.M.; Lutter, R.; Van Der Poll, T. IL-10 Is an Important Mediator of the Enhanced Susceptibility to Pneumococcal Pneumonia after Influenza Infection. J. Immunol. 2004, 172, 7603–7609. [Google Scholar] [CrossRef] [PubMed]
- Van Der Sluijs, K.F.; Nijhuis, M.; Levels, J.H.M.; Florquin, S.; Mellor, A.L.; Jansen, H.M.; Van Der Poll, T.; Lutter, R. Influenza-Induced Expression of Indoleamine 2,3-Dioxygenase Enhances Interleukin-10 Production and Bacterial Outgrowth during Secondary Pneumococcal Pneumonia. J. Infect. Dis. 2006, 193, 214–222. [Google Scholar] [CrossRef] [PubMed]
- Machado, D.; Hoffmann, J.; Moroso, M.; Rosa-Calatrava, M.; Endtz, H.; Terrier, O.; Paranhos-Baccalà, G. RSV Infection in Human Macrophages Promotes CXCL10/IP-10 Expression during Bacterial Co-Infection. Int. J. Mol. Sci. 2017, 18, 2654. [Google Scholar] [CrossRef]
- Hoffmann, J.; Machado, D.; Terrier, O.; Pouzol, S.; Messaoudi, M.; Basualdo, W.; Espínola, E.E.; Guillen, R.M.; Rosa-Calatrava, M.; Picot, V.; et al. Viral and bacterial co-infection in severe pneumonia triggers innate immune responses and specifically enhances IP-10: A translational study. Sci. Rep. 2016, 6, 38532. [Google Scholar] [CrossRef] [PubMed]
- Kovarik, P.; Castiglia, V.; Ivin, M.; Ebner, F. Type I Interferons in Bacterial Infections: A Balancing Act. Front. Immunol. 2016, 7, 652. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.; Robinson, K.M.; McHugh, K.J.; Scheller, E.V.; Mandalapu, S.; Chen, C.; Di, Y.P.; Clay, M.; Enelow, R.I.; Dubin, P.J.; et al. Influenza-induced type I interferon enhances susceptibility to gram-negative and gram-positive bacterial pneumonia in mice. Am. J. Physiol. Cell. Mol. Physiol. 2015, 309, L158–L167. [Google Scholar] [CrossRef]
- Kudva, A.; Scheller, E.V.; Robinson, K.M.; Crowe, C.R.; Choi, S.M.; Slight, S.R.; Khader, S.A.; Dubin, P.J.; Enelow, R.I.; Kolls, J.K.; et al. Influenza A Inhibits Th17-Mediated Host Defense against Bacterial Pneumonia in Mice. J. Immunol. 2011, 186, 1666–1674. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Moltedo, B.; Moran, T.M. Type I Interferon Induction during Influenza Virus Infection Increases Susceptibility to Secondary Streptococcus pneumoniae Infection by Negative Regulation of T Cells. J. Virol. 2012, 86, 12304–12312. [Google Scholar] [CrossRef]
- Shahangian, A.; Chow, E.; Tian, X.; Kang, J.R.; Ghaffari, A.; Liu, S.Y.; Belperio, J.A.; Cheng, G.; Deng, J.C. Type I IFNs mediate development of postinfluenza bacterial pneumonia in mice. J. Clin. Investig. 2009, 119, 1910–1920. [Google Scholar] [CrossRef] [PubMed]
- Shepardson, K.M.; Larson, K.; Johns, L.L.; Stanek, K.; Cho, H.; Wellham, J.; Henderson, H.; Rynda-Apple, A. IFNAR2 Is Required for Anti-influenza Immunity and Alters Susceptibility to Post-influenza Bacterial Superinfections. Front. Immunol. 2018, 9, 2589. [Google Scholar] [CrossRef] [PubMed]
- Shepardson, K.M.; Larson, K.; Morton, R.V.; Prigge, J.R.; Schmidt, E.E.; Huber, V.C.; Rynda-Apple, A. Differential Type I Interferon Signaling Is a Master Regulator of Susceptibility to Postinfluenza Bacterial Superinfection. mBio 2016, 7, e00506-16. [Google Scholar] [CrossRef] [PubMed]
- Planet, P.J.; Parker, D.; Cohen, T.; Smith, H.; Leon, J.D.; Ryan, C.; Hammer, T.J.; Fierer, N.; Chen, E.I.; Prince, A.S. Lambda Interferon Restructures the Nasal Microbiome and Increases Susceptibility to Staphylococcus aureus Superinfection. mBio 2016, 7, e01939-15. [Google Scholar] [CrossRef]
- Parker, D. Impact of Type I and III Interferons on Respiratory Superinfections Due to Multidrug-Resistant Pathogens. J. Infect. Dis. 2017, 215, S58–S63. [Google Scholar] [CrossRef]
- Abood, R.N.; McHugh, K.J.; Rich, H.E.; Ortiz, M.A.; Tobin, J.M.; Ramanan, K.; Robinson, K.M.; Bomberger, J.M.; Kolls, J.K.; Manni, M.L.; et al. IL-22-binding protein exacerbates influenza, bacterial super-infection. Mucosal Immunol. 2019, 12, 1231–1243. [Google Scholar] [CrossRef]
- Robinson, K.M.; Lee, B.; Scheller, E.V.; Mandalapu, S.; Enelow, R.I.; Kolls, J.K.; Alcorn, J.F. The role of IL-27 in susceptibility to post-influenza Staphylococcus aureus pneumonia. Respir. Res. 2015, 16, 10. [Google Scholar] [CrossRef]
- Lee, B.; Gopal, R.; Manni, M.L.; McHugh, K.J.; Mandalapu, S.; Robinson, K.M.; Alcorn, J.F. STAT1 Is Required for Suppression of Type 17 Immunity during Influenza and Bacterial Superinfection. ImmunoHorizons 2017, 1, 81–91. [Google Scholar] [CrossRef] [PubMed]
- Blevins, L.K.; Wren, J.T.; Holbrook, B.C.; Hayward, S.L.; Swords, W.E.; Parks, G.D.; Alexander-Miller, M.A. Coinfection with Streptococcus pneumoniae Negatively Modulates the Size and Composition of the Ongoing Influenza-Specific CD8+T Cell Response. J. Immunol. 2015, 193, 5076–5087. [Google Scholar] [CrossRef]
- Wu, Y.; Tu, W.; Lam, K.-T.; Chow, K.-H.; Ho, P.-L.; Guan, Y.; Peiris, J.S.M.; Lau, Y.-L. Lethal Coinfection of Influenza Virus and Streptococcus pneumoniae Lowers Antibody Response to Influenza Virus in Lung and Reduces Numbers of Germinal Center B Cells, T Follicular Helper Cells, and Plasma Cells in Mediastinal Lymph Node. J. Virol. 2014, 89, 2013–2023. [Google Scholar] [CrossRef]
- Wolf, A.I.; Strauman, M.C.; Mozdzanowska, K.; Whittle, J.R.R.; Williams, K.L.; Sharpe, A.H.; Weiser, J.; Caton, A.J.; Hensley, S.E.; Erikson, J. Coinfection with Streptococcus pneumoniae Modulates the B Cell Response to Influenza Virus. J. Virol. 2014, 88, 11995–12005. [Google Scholar] [CrossRef] [PubMed]
- Cao, J.; Wang, D.; Xu, F.; Gong, Y.; Wang, H.; Song, Z.; Li, D.; Zhang, H.; Li, D.; Zhang, L.; et al. Activation of IL -27 signalling promotes development of postinfluenza pneumococcal pneumonia. EMBO Mol. Med. 2014, 6, 120–140. [Google Scholar] [CrossRef] [PubMed]
- Barthelemy, A.; Ivanov, S.; Fontaine, J.; Soulard, D.; Bouabe, H.; Paget, C.; Faveeuw, C.; Trottein, F. Influenza A virus-induced release of interleukin-10 inhibits the anti-microbial activities of invariant natural killer T cells during invasive pneumococcal superinfection. Mucosal Immunol. 2017, 10, 460–469. [Google Scholar] [CrossRef]
- McAuley, J.; Hornung, F.; Boyd, K.L.; Smith, A.; McKeon, R.; Bennink, J.; Yewdell, J.W.; McCullers, J.A. Expression of the 1918 Influenza A Virus PB1-F2 Enhances the Pathogenesis of Viral and Secondary Bacterial Pneumonia. Cell Host Microbe 2007, 2, 240–249. [Google Scholar] [CrossRef]
- McAuley, J.L.; Chipuk, J.E.; Boyd, K.L.; Van De Velde, N.; Green, D.R.; McCullers, J.A. PB1-F2 Proteins from H5N1 and 20th Century Pandemic Influenza Viruses Cause Immunopathology. PLoS Pathog. 2010, 6, e1001014. [Google Scholar] [CrossRef]
- Alymova, I.V.; Green, A.M.; Van De Velde, N.; McAuley, J.L.; Boyd, K.L.; Ghoneim, H.E.; McCullers, J.A. Immunopathogenic and Antibacterial Effects of H3N2 Influenza A Virus PB1-F2 Map to Amino Acid Residues 62, 75, 79, and 82. J. Virol. 2011, 85, 12324–12333. [Google Scholar] [CrossRef]
- Lyczak, J.B.; Cannon, C.L.; Pier, G.B. Lung Infections Associated with Cystic Fibrosis. Clin. Microbiol. Rev. 2002, 15, 194–222. [Google Scholar] [CrossRef]
- Hewitt, R.; Farne, H.; Ritchie, A.; Luke, E.; Johnston, S.; Mallia, P. The role of viral infections in exacerbations of chronic obstructive pulmonary disease and asthma. Ther. Adv. Respir. Dis. 2016, 10, 158–174. [Google Scholar] [CrossRef] [PubMed]
- Kotnala, S.; Dhasmana, A.; Kashyap, V.K.; Chauhan, S.C.; Yallapu, M.M.; Jaggi, M. A bird eye view on cystic fibrosis: An underestimated multifaceted chronic disorder. Life Sci. 2021, 268, 118959. [Google Scholar] [CrossRef] [PubMed]
- O’Sullivan, B.P.; Freedman, S.D. Cystic fibrosis. Lancet 2009, 373, 1891–1904. [Google Scholar] [CrossRef]
- Wat, D.; Gelder, C.; Hibbitts, S.; Cafferty, F.; Bowler, I.; Pierrepoint, M.; Evans, R.; Doull, I. The role of respiratory viruses in cystic fibrosis. J. Cyst. Fibros. 2008, 7, 320–328. [Google Scholar] [CrossRef]
- Hendricks, M.R.; Bomberger, J.M. Digging through the Obstruction: Insight into the Epithelial Cell Response to Respiratory Virus Infection in Patients with Cystic Fibrosis. J. Virol. 2016, 90, 4258–4261. [Google Scholar] [CrossRef] [PubMed]
- van Ewijk, B.E.; Wolfs, T.F.W.; Aerts, P.C.; Van Kessel, K.P.M.; Fleer, A.; Kimpen, J.L.L.; Van Der Ent, C.K. RSV Mediates Pseudomonas aeruginosa Binding to Cystic Fibrosis and Normal Epithelial Cells. Pediatr. Res. 2007, 61, 398–403. [Google Scholar] [CrossRef]
- Kiedrowski, M.R.; Bomberger, J.M. Viral-Bacterial Co-infections in the Cystic Fibrosis Respiratory Tract. Front. Immunol. 2018, 9, 3067. [Google Scholar] [CrossRef] [PubMed]
- De Serres, G.; Lampron, N.; La Forge, J.; Rouleau, I.; Bourbeau, J.; Weiss, K.; Barret, B.; Boivin, G. Importance of viral and bacterial infections in chronic obstructive pulmonary disease exacerbations. J. Clin. Virol. 2009, 46, 129–133. [Google Scholar] [CrossRef] [PubMed]
- Molyneaux, P.L.; Mallia, P.; Cox, M.; Footitt, J.; Willis-Owen, S.; Homola, D.; Trujillo-Torralbo, M.-B.; Elkin, S.; Kon, O.M.; Cookson, W.O.C.; et al. Outgrowth of the Bacterial Airway Microbiome after Rhinovirus Exacerbation of Chronic Obstructive Pulmonary Disease. Am. J. Respir. Crit. Care Med. 2013, 188, 1224–1231. [Google Scholar] [CrossRef]
- Sigurs, N.; Bjarnason, R.; Sigurbergsson, F.; Kjellman, B. Respiratory Syncytial Virus Bronchiolitis in Infancy Is an Important Risk Factor for Asthma and Allergy at Age 7. Am. J. Respir. Crit. Care Med. 2000, 161, 1501–1507. [Google Scholar] [CrossRef]
- Gern, J.E.; Rosenthal, L.A.; Sorkness, R.L.; Lemanske, R.F. Effects of viral respiratory infections on lung development and childhood asthma. J. Allergy Clin. Immunol. 2005, 115, 668–674. [Google Scholar] [CrossRef]
- Chang, D.; Cruz, C.S.D.; Sharma, L. Challenges in understanding lung microbiome: It is NOT like the gut microbiome. Respirology 2020, 25, 244–245. [Google Scholar] [CrossRef]
- Sommariva, M.; Le Noci, V.; Bianchi, F.; Camelliti, S.; Balsari, A.; Tagliabue, E.; Sfondrini, L. The lung microbiota: Role in maintaining pulmonary immune homeostasis and its implications in cancer development and therapy. Cell. Mol. Life Sci. 2020, 77, 2739–2749. [Google Scholar] [CrossRef] [PubMed]
- Piters, W.D.S.; Heinonen, S.; Hasrat, R.; Bunsow, E.; Smith, B.; Suarez-Arrabal, M.-C.; Chaussabel, D.; Cohen, D.M.; Sanders, E.A.M.; Ramilo, O.; et al. Nasopharyngeal Microbiota, Host Transcriptome, and Disease Severity in Children with Respiratory Syncytial Virus Infection. Am. J. Respir. Crit. Care Med. 2016, 194, 1104–1115. [Google Scholar] [CrossRef]
- Kaul, D.; Rathnasinghe, R.; Ferres, M.; Tan, G.S.; Barrera, A.; Pickett, B.E.; Methe, B.A.; Das, S.R.; Budnik, I.; Halpin, R.A.; et al. Microbiome disturbance and resilience dynamics of the upper respiratory tract during influenza A virus infection. Nat. Commun. 2020, 11, 2537. [Google Scholar] [CrossRef] [PubMed]
- Hanada, S.; Pirzadeh, M.; Carver, K.Y.; Deng, J.C. Respiratory Viral Infection-Induced Microbiome Alterations and Secondary Bacterial Pneumonia. Front. Immunol. 2018, 9, 2640. [Google Scholar] [CrossRef] [PubMed]
- Sonawane, A.R.; Tian, L.; Chu, C.-Y.; Qiu, X.; Wang, L.; Holden-Wiltse, J.; Grier, A.; Gill, S.R.; Caserta, M.T.; Falsey, A.R.; et al. Microbiome-Transcriptome Interactions Related to Severity of Respiratory Syncytial Virus Infection. Sci. Rep. 2019, 9, 13824. [Google Scholar] [CrossRef] [PubMed]
- Huffnagle, G.B.; Dickson, R.P.; Lukacs, N.W. The respiratory tract microbiome and lung inflammation: A two-way street. Mucosal Immunol. 2017, 10, 299–306. [Google Scholar] [CrossRef] [PubMed]
- Richard, A.L.; Siegel, S.J.; Erikson, J.; Weiser, J.N. TLR2 Signaling Decreases Transmission of Streptococcus pneumoniae by Limiting Bacterial Shedding in an Infant Mouse Influenza A Co-infection Model. PLoS Pathog. 2014, 10, e1004339. [Google Scholar] [CrossRef]
- Marks, L.; Davidson, B.A.; Knight, P.R.; Hakansson, A.P. Interkingdom Signaling Induces Streptococcus pneumoniae Biofilm Dispersion and Transition from Asymptomatic Colonization to Disease. mBio 2013, 4, e00438-13. [Google Scholar] [CrossRef]
- Reddinger, R.M.; Luke-Marshall, N.R.; Hakansson, A.P.; Campagnari, A.A. Host Physiologic Changes Induced by Influenza A Virus Lead to Staphylococcus aureus Biofilm Dispersion and Transition from Asymptomatic Colonization to Invasive Disease. mBio 2016, 7, e01235-16. [Google Scholar] [CrossRef]
- Pettigrew, M.M.; Marks, L.; Kong, Y.; Gent, J.F.; Roche-Hakansson, H.; Hakansson, A.P. Dynamic Changes in the Streptococcus pneumoniae Transcriptome during Transition from Biofilm Formation to Invasive Disease upon Influenza A Virus Infection. Infect. Immun. 2014, 82, 4607–4619. [Google Scholar] [CrossRef]
- Kanmani, P.; Clua, P.; Vizoso-Pinto, M.G.; Rodriguez, C.; Alvarez, S.; Melnikov, V.; Takahashi, H.; Kitazawa, H.; Villena, J. Respiratory Commensal Bacteria Corynebacterium pseudodiphtheriticum Improves Resistance of Infant Mice to Respiratory Syncytial Virus and Streptococcus pneumoniae Superinfection. Front. Microbiol. 2017, 8, 1613. [Google Scholar] [CrossRef] [PubMed]
- Bruchhagen, C.; Jarick, M.; Mewis, C.; Hertlein, T.; Niemann, S.; Ohlsen, K.; Peters, G.; Planz, O.; Ludwig, S.; Ehrhardt, C. Metabolic conversion of CI-1040 turns a cellular MEK-inhibitor into an antibacterial compound. Sci. Rep. 2018, 8, 9114. [Google Scholar] [CrossRef] [PubMed]
- Surmann, K.; Simon, M.; Hildebrandt, P.; Pförtner, H.; Michalik, S.; Stentzel, S.; Steil, L.; Dhople, V.M.; Bernhardt, J.; Schlüter, R.; et al. A proteomic perspective of the interplay of Staphylococcus aureus and human alveolar epithelial cells during infection. J. Proteom. 2015, 128, 203–217. [Google Scholar] [CrossRef] [PubMed]
- De Vrankrijker, A.M.; Wolfs, T.F.; Ciofu, O.; Høiby, N.; Van Der Ent, C.K.; Poulsen, S.S.; Johansen, H.K. Respiratory syncytial virus infection facilitates acute colonization of Pseudomonas aeruginosa in mice. J. Med. Virol. 2009, 81, 2096–2103. [Google Scholar] [CrossRef]
- Yan, T.; Tang, X.; Sun, L.; Tian, R.; Li, Z.; Liu, G. Co infection of respiratory syncytial viruses (RSV) and Streptococcus pneumonia modulates pathogenesis and dependent of serotype and phase variant. Microb. Pathog. 2020, 144, 104126. [Google Scholar] [CrossRef]
- LeVine, A.M.; Koeningsknecht, V.; Stark, J.M. Decreased pulmonary clearance of S. pneumoniae following influenza A infection in mice. J. Virol. Methods 2001, 94, 173–186. [Google Scholar] [CrossRef]
- Lee, M.; Arrecubieta, C.; Martin, F.J.; Prince, A.; Borczuk, A.C.; Lowy, F.D. A Postinfluenza Model of Staphylococcus aureus Pneumonia. J. Infect. Dis. 2010, 201, 508–515. [Google Scholar] [CrossRef]
- McCullers, J.A.; Webster, R.G. A mouse model of dual infection with influenza virus and Streptococcus pneumoniae. Int. Congr. Ser. 2001, 1219, 601–607. [Google Scholar] [CrossRef]
- Kobayashi, S.D.; Olsen, R.J.; LaCasse, R.A.; Safronetz, D.; Ashraf, M.; Porter, A.R.; Braughton, K.R.; Feldmann, F.; Clifton, D.R.; Kash, J.C.; et al. Seasonal H3N2 influenza A virus fails to enhance Staphylococcus aureus co-infection in a non-human primate respiratory tract infection model. Virulence 2013, 4, 707–715. [Google Scholar] [CrossRef]
- Miyake, T.; Soda, K.; Itoh, Y.; Sakoda, Y.; Ishigaki, H.; Nagata, T.; Ishida, H.; Nakayama, M.; Ozaki, H.; Tsuchiya, H.; et al. Amelioration of pneumonia with Streptococcus pneumoniae infection by inoculation with a vaccine against highly pathogenic avian influenza virus in a non-human primate mixed infection model. J. Med. Primatol. 2010, 39, 58–70. [Google Scholar] [CrossRef]
- Sanford, B.A.; Ramsay, M.A. In Vivo Localization of Staphylococcus aureus in Nasal Tissues of Healthy and Influenza A Virus-Infected Ferrets. Exp. Biol. Med. 1989, 191, 163–169. [Google Scholar] [CrossRef] [PubMed]
- Memoli, M.J.; Czajkowski, L.; Reed, S.; Athota, R.; Bristol, T.; Proudfoot, K.; Fargis, S.; Stein, M.; Dunfee, R.L.; Shaw, P.A.; et al. Validation of the Wild-type Influenza A Human Challenge Model H1N1pdMIST: An A(H1N1)pdm09 Dose-Finding Investigational New Drug Study. Clin. Infect. Dis. 2015, 60, 693–702. [Google Scholar] [CrossRef]
- Gkatzis, K.; Taghizadeh, S.; Huh, D.; Stainier, D.; Bellusci, S. Use of three-dimensional organoids and lung-on-a-chip methods to study lung development, regeneration and disease. Eur. Respir. J. 2018, 52, 1800876. [Google Scholar] [CrossRef]
- Colby, L.E.; Quenee, L.E.; Zitzow, L.A. Considerations for Infectious Disease Research Studies Using Animals. Comp. Med. 2017, 67, 222–231. [Google Scholar] [PubMed]
- Han, M.; Rajput, C.; Ishikawa, T.; Jarman, C.R.; Lee, J.; Hershenson, M.B. Small Animal Models of Respiratory Viral Infection Related to Asthma. Viruses 2018, 10, 682. [Google Scholar] [CrossRef]
- Bouvier, N.M.; Lowen, A.C. Animal Models for Influenza Virus Pathogenesis and Transmission. Viruses 2010, 2, 1530–1563. [Google Scholar] [CrossRef] [PubMed]
- Altamirano-Lagos, M.J.; Díaz, F.E.; Mansilla, M.A.; Rivera-Pérez, D.B.; Soto, D.A.; McGill, J.L.; Vasquez, A.E.; Kalergis, A.M. Current Animal Models for Understanding the Pathology Caused by the Respiratory Syncytial Virus. Front. Microbiol. 2019, 10, 873. [Google Scholar] [CrossRef]
- Muñoz-Fontela, C.; Dowling, W.E.; Funnell, S.G.P.; Gsell, P.-S.; Riveros-Balta, A.X.; Albrecht, R.A.; Andersen, H.; Baric, R.S.; Carroll, M.W.; Cavaleri, M.; et al. Animal models for COVID-19. Nature 2020, 586, 509–515. [Google Scholar] [CrossRef]
- Reyes, L.F.; Restrepo, M.I.; Hinojosa, C.A.; Soni, N.J.; Shenoy, A.; Gilley, R.P.; Gonzalez-Juarbe, N.; Noda, J.R.; Winter, V.T.; De La Garza, M.A.; et al. A Non-Human Primate Model of Severe Pneumococcal Pneumonia. PLoS ONE 2016, 11, e0166092. [Google Scholar] [CrossRef]
- Chen, L.; Welty-Wolf, K.E.; Kraft, B.D. Nonhuman primate species as models of human bacterial sepsis. Lab Anim. 2019, 48, 57–65. [Google Scholar] [CrossRef]
- Enkirch, T.; von Messling, V. Ferret models of viral pathogenesis. Virology 2015, 479–480, 259–270. [Google Scholar] [CrossRef] [PubMed]
- Peltola, V.T.; Boyd, K.L.; McAuley, J.L.; Rehg, J.E.; McCullers, J.A. Bacterial Sinusitis and Otitis Media following Influenza Virus Infection in Ferrets. Infect. Immun. 2006, 74, 2562–25627. [Google Scholar] [CrossRef] [PubMed]
- Diep, B.A.; Hilliard, J.J.; Le, V.T.M.; Tkaczyk, C.; Le, H.N.; Tran, V.G.; Rao, R.L.; Dip, E.C.; Pereira-Franchi, E.P.; Cha, P.; et al. Targeting Alpha Toxin to Mitigate Its Lethal Toxicity in Ferret and Rabbit Models of Staphylococcus aureus Necrotizing Pneumonia. Antimicrob. Agents Chemother. 2017, 61, e02456-16. [Google Scholar] [CrossRef]
- Peltola, V.T.; Rehg, J.E.; McCullers, J.E. A ferret model of synergism between influenza virus and Streptococcus pneumoniae. Int. Congr. Ser. 2004, 1263, 486–490. [Google Scholar] [CrossRef]
- McCullers, J.A.; McAuley, J.L.; Browall, S.; Iverson, A.R.; Boyd, K.L.; Normark, B.H. Influenza Enhances Susceptibility to Natural Acquisition of and Disease due to Streptococcus pneumoniae in Ferrets. J. Infect. Dis. 2010, 202, 1287–1295. [Google Scholar] [CrossRef]
- Nguyen, D.T.; Louwen, R.; Elberse, K.; Van Amerongen, G.; Yüksel, S.; Luijendijk, A.; Osterhaus, A.D.M.E.; Duprex, W.P.; De Swart, R.L. Streptococcus pneumoniae Enhances Human Respiratory Syncytial Virus Infection In Vitro and In Vivo. PLoS ONE 2015, 10, e0127098. [Google Scholar] [CrossRef]
- Shirey, K.A.; Perkins, D.J.; Lai, W.; Zhang, W.; Fernando, L.R.; Gusovsky, F.; Blanco, J.C.G.; Vogel, S.N. Influenza “Trains” the Host for Enhanced Susceptibility to Secondary Bacterial Infection. mBio 2019, 10. [Google Scholar] [CrossRef] [PubMed]
- Giebink, G.S.; Berzins, I.K.; Marker, S.C.; Schiffman, G. Experimental otitis media after nasal inoculation of Streptococcus pneumoniae and influenza A virus in chinchillas. Infect. Immun. 1980, 445, 450. [Google Scholar] [CrossRef]
- Brockson, M.E.; Novotny, L.A.; Jurcisek, J.A.; McGillivary, G.; Bowers, M.R.; Bakaletz, L.O. Respiratory Syncytial Virus Promotes Moraxella catarrhalis-Induced Ascending Experimental Otitis Media. PLoS ONE 2012, 7, e40088. [Google Scholar] [CrossRef][Green Version]
- Jochems, S.P.; Marcon, F.; Carniel, B.F.; Holloway, M.; Mitsi, E.; Smith, E.; Gritzfeld, J.F.; Solórzano, C.; Reiné, J.; Pojar, S.; et al. Inflammation induced by influenza virus impairs human innate immune control of pneumococcus. Nat. Immunol. 2018, 19, 1299–1308. [Google Scholar] [CrossRef]
| Experimental Models | Advantages | Drawbacks | Refs | |
|---|---|---|---|---|
| In vitro | Cell monolayers (Cell lines/primary cells) | Easy handling/practical Low cost | Limited biological relevance | [128,174,175] |
| Reconstituted HAE | Physiological relevance | High cost | [129] | |
| In vivo | Mouse | Low cost Availability Transgenic/Humanized/KO models | Limited biological relevance | [45,46,59,113,176,177,178,179,180] |
| NHP | Physiological relevance | High cost Ethical concern High complexity Availability of reagents | [43,44,181,182] | |
| Ferret | [183] | |||
| Human challenge | [184] | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oliva, J.; Terrier, O. Viral and Bacterial Co-Infections in the Lungs: Dangerous Liaisons. Viruses 2021, 13, 1725. https://doi.org/10.3390/v13091725
Oliva J, Terrier O. Viral and Bacterial Co-Infections in the Lungs: Dangerous Liaisons. Viruses. 2021; 13(9):1725. https://doi.org/10.3390/v13091725
Chicago/Turabian StyleOliva, Justine, and Olivier Terrier. 2021. "Viral and Bacterial Co-Infections in the Lungs: Dangerous Liaisons" Viruses 13, no. 9: 1725. https://doi.org/10.3390/v13091725
APA StyleOliva, J., & Terrier, O. (2021). Viral and Bacterial Co-Infections in the Lungs: Dangerous Liaisons. Viruses, 13(9), 1725. https://doi.org/10.3390/v13091725






