Dual Effect of Organogermanium Compound THGP on RIG-I-Mediated Viral Sensing and Viral Replication during Influenza a Virus Infection
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Culture
2.2. Viruses, Antibodies, and Reagents
2.3. MAMPs Stimulation and Transfection
2.4. Viral Infection
2.5. ELISA
2.6. Plaque-Forming Assay
2.7. qRT-PCR
2.8. Immunofluorescence
2.9. FACS Analysis
2.10. Recombinant RIG-I Protein
2.11. In Vitro RNA Pull down Assay
2.12. Binding Assay Using THGP Immobilized Column
2.13. Luciferase Assay
2.14. RIP Assay
2.15. Mice Experiment
2.16. 1H-NMR
2.17. Quantification and Statistical Analysis
3. Results
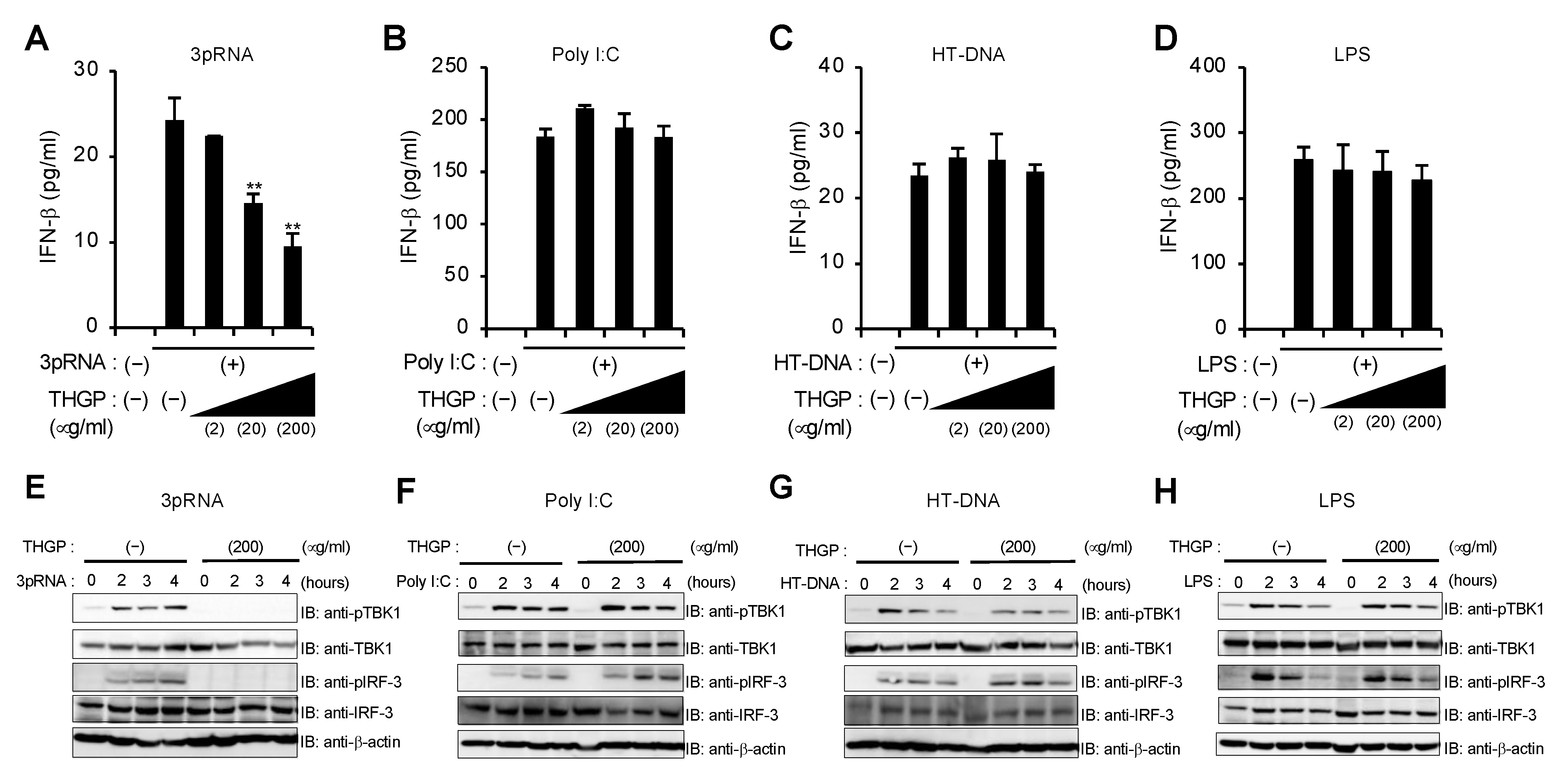
3.1. THGP Inhibits RIG-I-Mediated IFN-β mRNA Induction
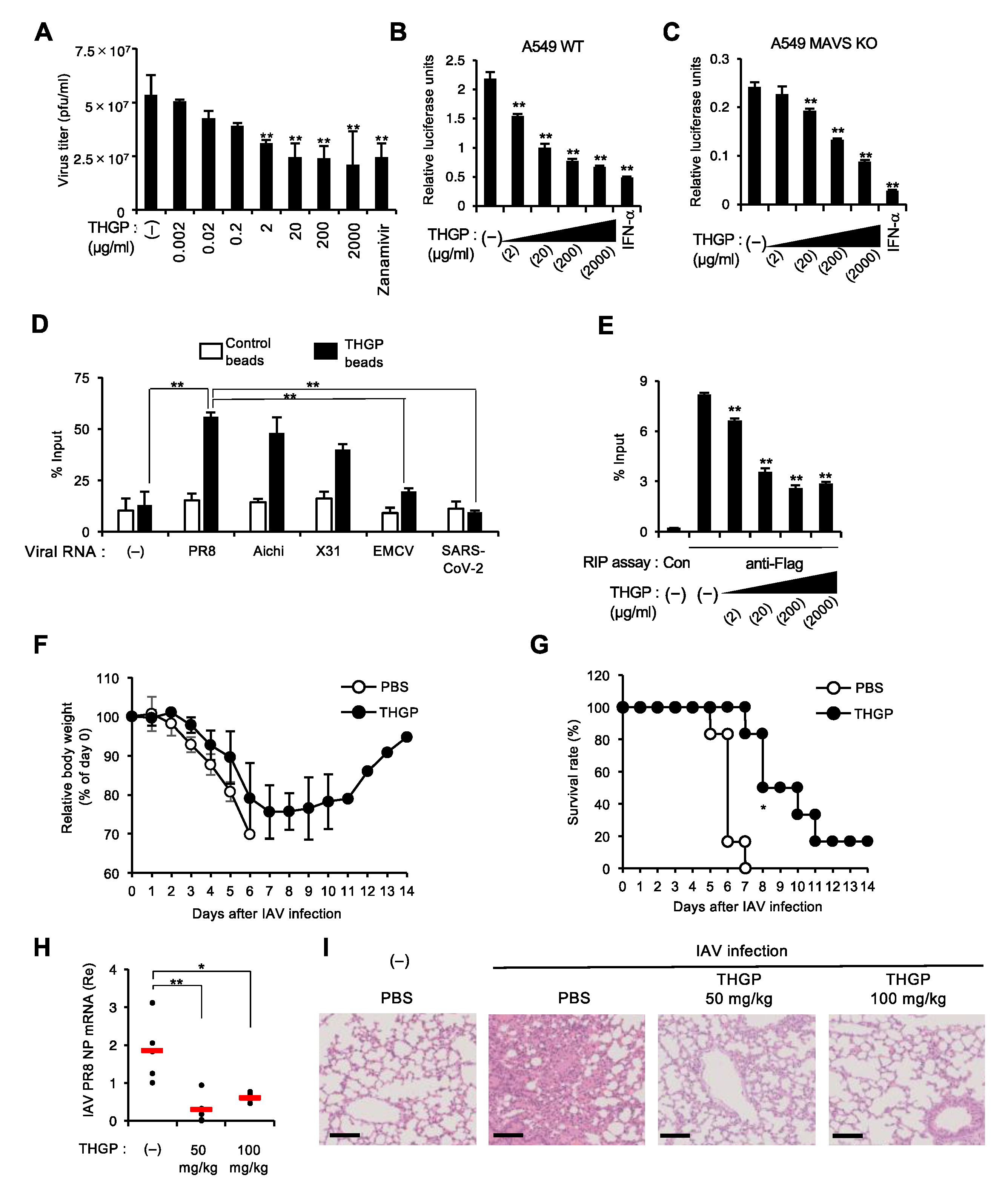
3.2. THGP Attenuates IFN-β mRNA Induction in Response to IAV and VSV Infections
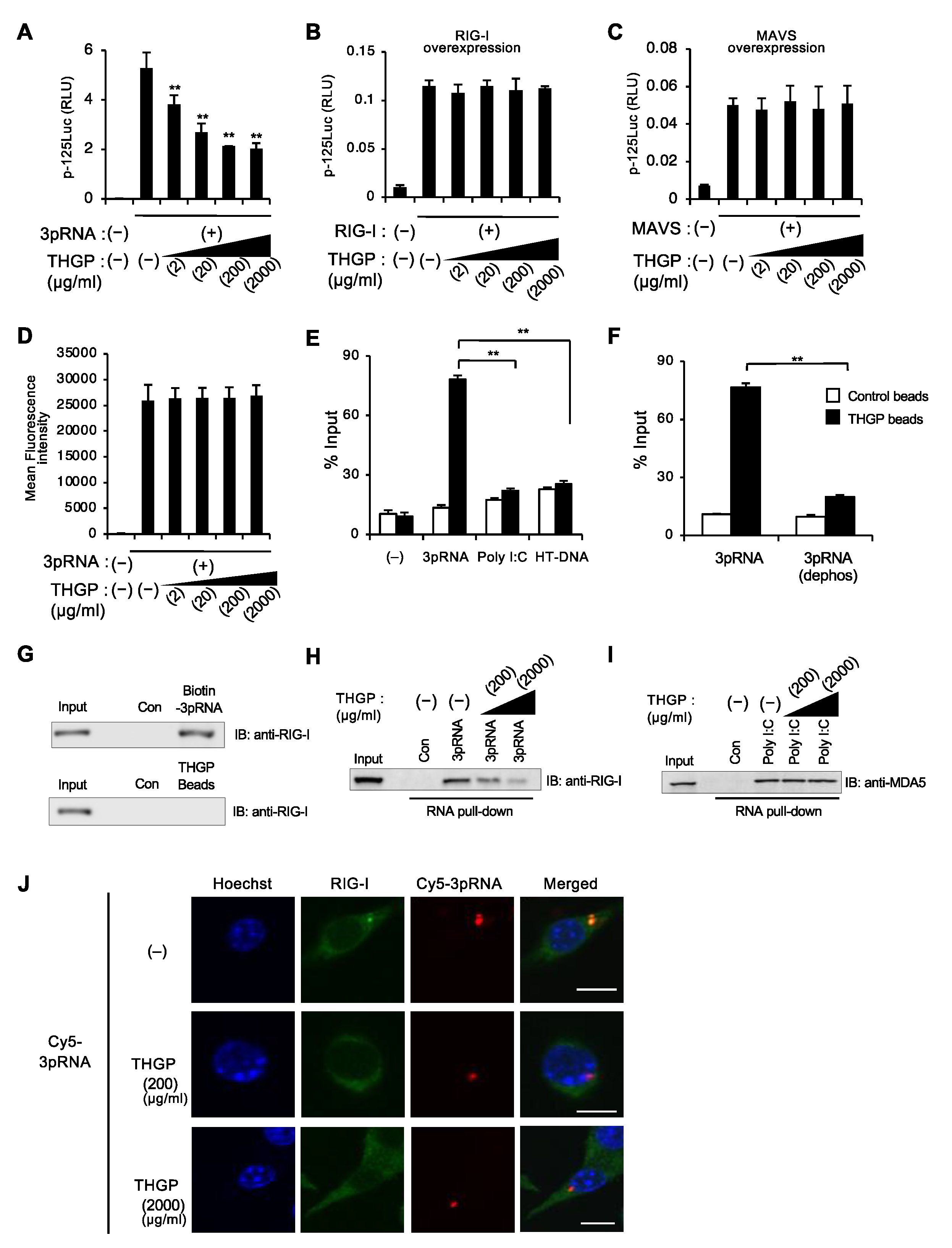
3.3. THGP Reduces the Interaction between RIG-I and Its Ligand
3.4. THGP Impedes the Interaction of IAV Polymerase with Viral RNA Genome
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Takeuchi, O.; Akira, S. Pattern recognition receptors and inflammation. Cell 2010, 140, 805–820. [Google Scholar] [CrossRef]
- McNab, F.; Mayer-Barber, K.; Sher, A.; Wack, A.; O’Garra, A. Type I interferons in infectious disease. Nat. Rev. Immunol. 2015, 15, 87–103. [Google Scholar] [CrossRef]
- Chen, K.; Liu, J.; Cao, X. Regulation of type I interferon signaling in immunity and inflammation: A comprehensive review. J. Autoimmun. 2017, 83, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Takaoka, A.; Yamada, T. Regulation of signaling mediated by nucleic acid sensors for innate interferon-mediated responses during viral infection. Int. Immunol. 2019, 31, 477–488. [Google Scholar] [CrossRef] [PubMed]
- Kato, H.; Takeuchi, O.; Sato, S.; Yoneyama, M.; Yamamoto, M.; Matsui, K.; Uematsu, S.; Jung, A.; Kawai, T.; Ishii, K.J.; et al. Differential roles of MDA5 and RIG-I helicases in the recognition of RNA viruses. Nature 2006, 441, 101–105. [Google Scholar] [CrossRef] [PubMed]
- Saito, T.; Owen, D.M.; Jiang, F.; Marcotrigiano, J.; Gale, M. Innate immunity induced by composition-dependent RIG-I recognition of hepatitis C virus RNA. Nature 2008, 454, 523–527. [Google Scholar] [CrossRef]
- Sato, S.; Li, K.; Kameyama, T.; Hayashi, T.; Ishida, Y.; Murakami, S.; Watanabe, T.; Iijima, S.; Sakurai, Y.; Watashi, K.; et al. The RNA sensor RIG-I dually functions as an innate sensor and direct antiviral factor for hepatitis B virus. Immunity 2015, 42, 123–132. [Google Scholar] [CrossRef]
- Plumet, S.; Herschke, F.; Bourhis, J.M.; Valentin, H.; Longhi, S.; Gerlier, D. Cytosolic 5′-triphosphate ended viral leader transcript of measles virus as activator of the RIG I-mediated interferon response. PLoS ONE 2007, 2, e279. [Google Scholar] [CrossRef]
- Kim, E.; Jeon, Y.; Kim, D.Y.; Lee, E.; Hyun, S.H. Antioxidative effect of carboxyethylgermanium sesquioxide (Ge-132) on IVM of porcine oocytes and subsequent embryonic development after parthenogenetic activation and IVF. Theriogenology 2015, 84, 226–236. [Google Scholar] [CrossRef]
- Wada, T.; Hanyu, T.; Nozaki, K.; Kataoka, K.; Kawatani, T.; Asahi, T.; Sawamura, N. Antioxidant Activity of Ge-132, a Synthetic Organic Germanium, on Cultured Mammalian Cells. Biol. Pharm. Bull. 2018, 41, 749–753. [Google Scholar] [CrossRef]
- Aso, H.; Shibuya, E.; Suzuki, F.; Nakamura, T.; Inoue, H.; Ebina, T.; Ishida, N. Antitumor effect in mice of an organic germanium compound (Ge-132) when different administration methods are used. Gan. Kagaku Ryoho 1985, 12, 2345–2351. [Google Scholar]
- Suzuki, F.; Brutkiewicz, R.R.; Pollard, R.B. Ability of sera from mice treated with Ge-132, an organic germanium compound, to inhibit experimental murine ascites tumours. Br. J. Cancer 1985, 52, 757–763. [Google Scholar] [CrossRef]
- Dozono, H.; Ikeda, K.; Onishi, T. Effectiveness of Ge-132 to relieve pain and smooth home care administration for the terminal cancer patient. Gan. Kagaku Ryoho 1996, 23, 291–295. [Google Scholar]
- Nakamura, T.; Saito, M.; Aso, H. Effects of a lactobacilli, oligosaccharide and organic germanium intake on the immune responses of mice. Biosci. Biotechnol. Biochem. 2012, 76, 375–377. [Google Scholar] [CrossRef]
- Nakamura, T.; Takeda, T.; Tokuji, Y. The Oral Intake of Organic Germanium, Ge-132, Elevates α-Tocopherol Levels in the Plas-ma and Modulates Hepatic Gene Expression Profiles to Promote Immune Activation in Mice. Int. J. Vitam. Nutr. Res. 2014, 84, 183–195. [Google Scholar] [CrossRef] [PubMed]
- Shimada, Y.; Sato, K.; Takeda, T.; Tokuji, Y. The Organogermanium Compound Ge-132 Interacts with Nucleic Acid Components and Inhibits the Catalysis of Adenosine Substrate by Adenosine Deaminase. Biol. Trace Elem. Res. 2018, 181, 164–172. [Google Scholar] [CrossRef] [PubMed]
- Azumi, J.; Takeda, T.; Shimada, Y.; Aso, H.; Nakamura, T. The Organogermanium Compound THGP Suppresses Melanin Synthesis via Complex Formation with L-DOPA on Mushroom Tyrosinase and in B16 4A5 Melanoma Cells. Int. J. Mol. Sci. 2019, 20, 4785. [Google Scholar] [CrossRef] [PubMed]
- Sugiya YSakamaki, S.; Sugita, T.; Abo, Y.; Sato, H. Subacute oral toxicity of carboxyethylgermanium sesquioxide (Ge-132) in rats. Ouyou Yakuri 1986, 31, 1181–1190. [Google Scholar]
- Nakamura, T.; Shimada, Y.; Takeda, T.; Sato, K.; Akiba, M.; Fukaya, H. Organogermanium compound, Ge-132, forms complexes with adrenaline, ATP and other physiological cis-diol compounds. Future Med. Chem. 2015, 7, 1233–1246. [Google Scholar] [CrossRef]
- Doi, Y.; Imai, N.; Suguro, M.; Numano, T.; Furukawa, F. No carcinogenicity of poly-trans-[(2-carboxyethyl) germasesquioxane] (Ge-132): 26-week feeding study using rasH2 mice. Fundam. Toxicol. Sci. 2017, 4, 137–150. [Google Scholar] [CrossRef]
- Iwadate, K.; Yamaguchi, Y.; Sasaki, M.; Nakatani, M.; Doi, Y.; Imai, N.; Tamano, S.; Nishihori, Y. Carcinogenicity study of poly-trans-[(2-carboxyethyl)germasesquioxane] (Ge-132) in F344 rats. Fundam. Toxicol. Sci. 2018, 5, 127–140. [Google Scholar] [CrossRef]
- Aso, H.; Suzuki, F.; Ebina, T.; Ishida, N. Antiviral activity of carboxyethylgermanium sesquioxide (Ge-132) in mice infected with influenza virus. J. Biol. Response. Mod. 1989, 8, 180–189. [Google Scholar] [PubMed]
- Cong, L.; Ran, F.A.; Cox, D.; Lin, S.; Barretto, R.; Habib, N.; Hsu, P.; Wu, X.; Jiang, W.; Marraffini, L.A.; et al. Multiplex genome engineering using CRISPR/Cas systems. Science 2013, 339, 819–823. [Google Scholar] [CrossRef] [PubMed]
- Yamada, T.; Horimoto, H.; Kameyama, T.; Hayakawa, S.; Yamato, H.; Dazai, M.; Takada, A.; Kida, H.; Bott, D.; Zhou, A.C.; et al. Constitutive aryl hydrocarbon receptor signaling constrains type I interferon-mediated antiviral innate defense. Nat. Immunol. 2016, 17, 687–694. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, H.; Kameyama, T.; Takaoka, A. BinCARD2 as a positive regulator of interferon response in innate immunity. Biochem. Biophys. Res. Commun. 2019, 511, 287–293. [Google Scholar] [CrossRef] [PubMed]
- Sato, S.; Li, K.; Sakurai, N.; Hashizume, M.; Baidya, S.; Nonaka, H.; Noguchi, K.; Ishikawa, K.; Obuse, C.; Takaoka, A. Regulation of an adaptor protein STING by Hsp90β to enhance innate immune responses against microbial infections. Cell Immunol. 2020, 356, 104188. [Google Scholar] [CrossRef]
- Yamada, T.; Sato, S.; Sotoyama, Y.; Orba, Y.; Sawa, H.; Yamauchi, H.; Sasaki, M.; Takaoka, A. RIG-I triggers a signaling-abortive anti-SARS-CoV-2 defense in human lung cells. Nat. Immunol. 2021, 22, 820–828. [Google Scholar] [CrossRef] [PubMed]
- Ozawa, M.; Fujii, K.; Muramoto, Y.; Yamada, S.; Yamayoshi, S.; Takada, A.; Goto, H.; Horimoto, T.; Kawaoka, Y. Contributions of two nuclear localization signals of influenza A virus nucleoprotein to viral replication. J. Virol. 2007, 81, 30–41. [Google Scholar] [CrossRef]
- Muramoto, Y.; Noda, T.; Kawakami, E.; Akkina, R.; Kawaoka, Y. Identification of novel influenza A virus proteins translated from PA mRNA. J. Virol. 2013, 87, 2455–2462. [Google Scholar] [CrossRef]
- Lee, M.T.M. Definition of the minimal viral components required for the initiation of unprimed RNA synthesis by influenza virus RNA polymerase. Nucleic Acids Res. 2002, 30, 429–438. [Google Scholar] [CrossRef][Green Version]
- Yoneyama, M.; Kikuchi, M.; Natsukawa, T.; Shinobu, N.; Imaizumi, T.; Miyagishi, M.; Taira, K.; Akira, S.; Fujita, T. The RNA helicase RIG-I has an essential function in double-stranded RNA-induced innate antiviral responses. Nat. Immunol. 2004, 5, 730–737. [Google Scholar] [CrossRef] [PubMed]
- Yoneyama, M.; Kikuchi, M.; Matsumoto, K.; Imaizumi, T.; Miyagishi, M.; Taira, K.; Foy, E.; Loo, Y.-M.; Gale, M., Jr.; Akira, S.; et al. Shared and unique functions of the DExD/H-box helicases RIG-I, MDA5, and LGP2 in antiviral innate immunity. J. Immunol. 2005, 175, 2851–2858. [Google Scholar] [CrossRef] [PubMed]
- Romano, M.; Ruggiero, A.; Squeglia, F.; Maga, G.; Berisio, R. A Structural View of SARS-CoV-2 RNA Replication Machinery: RNA Synthesis, Proofreading and Final Capping. Cells 2020, 9, 1267. [Google Scholar] [CrossRef] [PubMed]
- Weber, M.; Gawanbacht, A.; Habjan, M.; Rang, A.; Borner, C.; Schmidt, A.M.; Veitinger, S.; Jacob, R.; Devignot, S.; Kochs, G.; et al. Incoming RNA virus nucleocapsids containing a 5′-triphosphorylated genome activate RIG-I and antiviral signaling. Cell Host Microbe 2013, 13, 336–346. [Google Scholar] [CrossRef] [PubMed]
- Kolakofsky, D. Isolation and characterization of Sendai virus DI-RNAs. Cell 1976, 8, 547–555. [Google Scholar] [CrossRef]
- Dronova, M.; Ikeoka, H.; Itsumura, N.; Hirotsu, N.; Ansaripour, A.; Aballéa, S.; Onishi, Y.; Hill, M.; Igarashi, A. Cost-effectiveness of baloxavir marboxil compared with laninamivir for the treatment of influenza in patients at high risk for complications in Japan. Curr. Med. Res. Opin. 2021, 37, 1135–1148. [Google Scholar] [CrossRef]
- Eckard, S.C.; Rice, G.; Fabre, A.; Badens, C.; Gray, E.E.; Hartley, J.L.; Crow, Y.; Stetson, D.B. The SKIV2L RNA exosome limits activation of the RIG-I-like receptors. Nat. Immunol. 2014, 15, 839–845. [Google Scholar] [CrossRef]
- Tisoncik, J.R.; Korth, M.J.; Simmons, C.P.; Farrar, J.; Martin, T.R.; Katze, M.G. Into the eye of the cytokine storm. Microbiol. Mol. Biol. Rev. 2012, 76, 16–32. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baidya, S.; Nishimoto, Y.; Sato, S.; Shimada, Y.; Sakurai, N.; Nonaka, H.; Noguchi, K.; Kido, M.; Tadano, S.; Ishikawa, K.; et al. Dual Effect of Organogermanium Compound THGP on RIG-I-Mediated Viral Sensing and Viral Replication during Influenza a Virus Infection. Viruses 2021, 13, 1674. https://doi.org/10.3390/v13091674
Baidya S, Nishimoto Y, Sato S, Shimada Y, Sakurai N, Nonaka H, Noguchi K, Kido M, Tadano S, Ishikawa K, et al. Dual Effect of Organogermanium Compound THGP on RIG-I-Mediated Viral Sensing and Viral Replication during Influenza a Virus Infection. Viruses. 2021; 13(9):1674. https://doi.org/10.3390/v13091674
Chicago/Turabian StyleBaidya, Sunanda, Yoko Nishimoto, Seiichi Sato, Yasuhiro Shimada, Nozomi Sakurai, Hirotaka Nonaka, Koki Noguchi, Mizuki Kido, Satoshi Tadano, Kozo Ishikawa, and et al. 2021. "Dual Effect of Organogermanium Compound THGP on RIG-I-Mediated Viral Sensing and Viral Replication during Influenza a Virus Infection" Viruses 13, no. 9: 1674. https://doi.org/10.3390/v13091674
APA StyleBaidya, S., Nishimoto, Y., Sato, S., Shimada, Y., Sakurai, N., Nonaka, H., Noguchi, K., Kido, M., Tadano, S., Ishikawa, K., Li, K., Okubo, A., Yamada, T., Orba, Y., Sasaki, M., Sawa, H., Miyamoto, H., Takada, A., Nakamura, T., & Takaoka, A. (2021). Dual Effect of Organogermanium Compound THGP on RIG-I-Mediated Viral Sensing and Viral Replication during Influenza a Virus Infection. Viruses, 13(9), 1674. https://doi.org/10.3390/v13091674








