Cytomegalovirus Infection Impairs the Mobilization of Tissue-Resident Innate Lymphoid Cells into the Peripheral Blood Compartment in Response to Acute Exercise
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Experimental Design
2.3. Experimental Procedures
2.3.1. Height, Weight, PARQ, Medical and Health History Form (V1)
2.3.2. VO2Peak Test (V1)
2.3.3. Sub-Maximal Exercise Protocol (Visit 2)
2.4. Outcome Assessments
2.4.1. Cell Isolation and Antibody Preparation
2.4.2. Flow Cytometry Analysis
2.4.3. Determination of CMV Serostatus
2.5. Statistical Analysis
3. Results
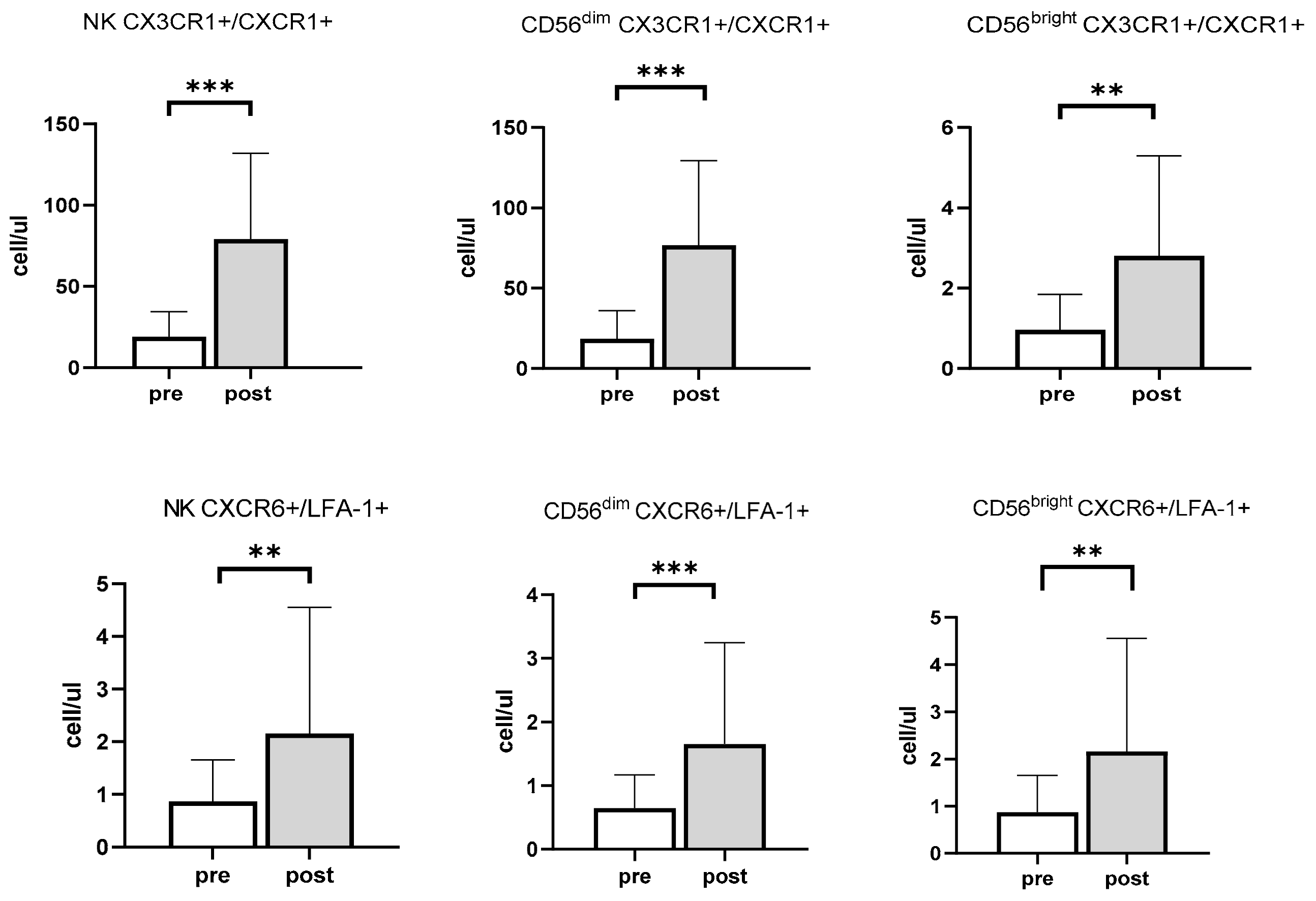
3.1. Acute Exercise Preferentially Mobilizes NK Cell-Expressing Inflammatory Receptors and Cytotoxic Effector Functions
3.2. Effects of Exercise and Sex on Chemokine Receptors in NK Cells
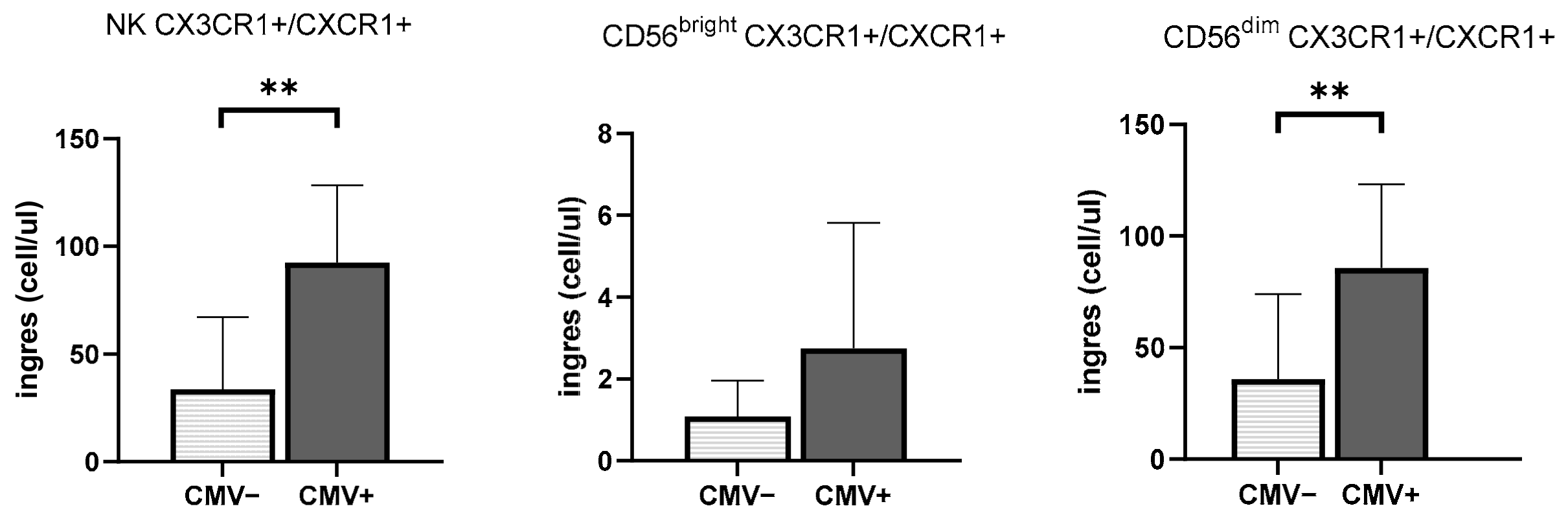
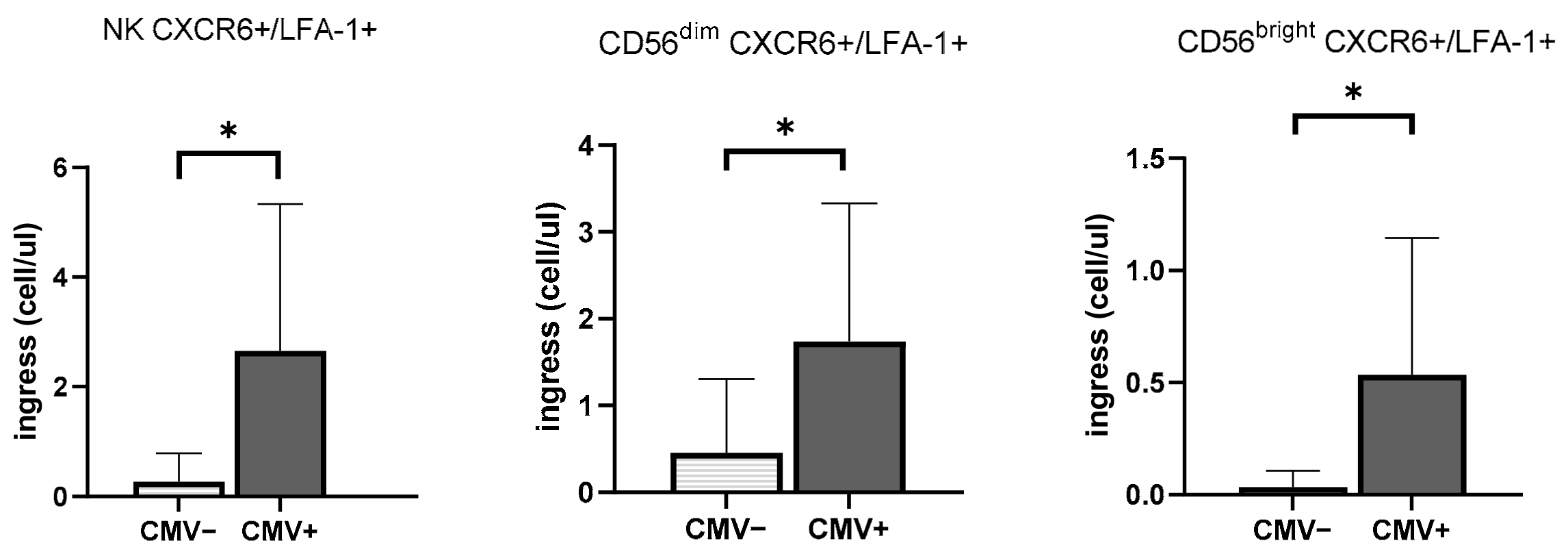
3.3. CMV Infection History Increases the Mobilization of NK Cells Expressing Inflammasome and Cytotoxic Receptors
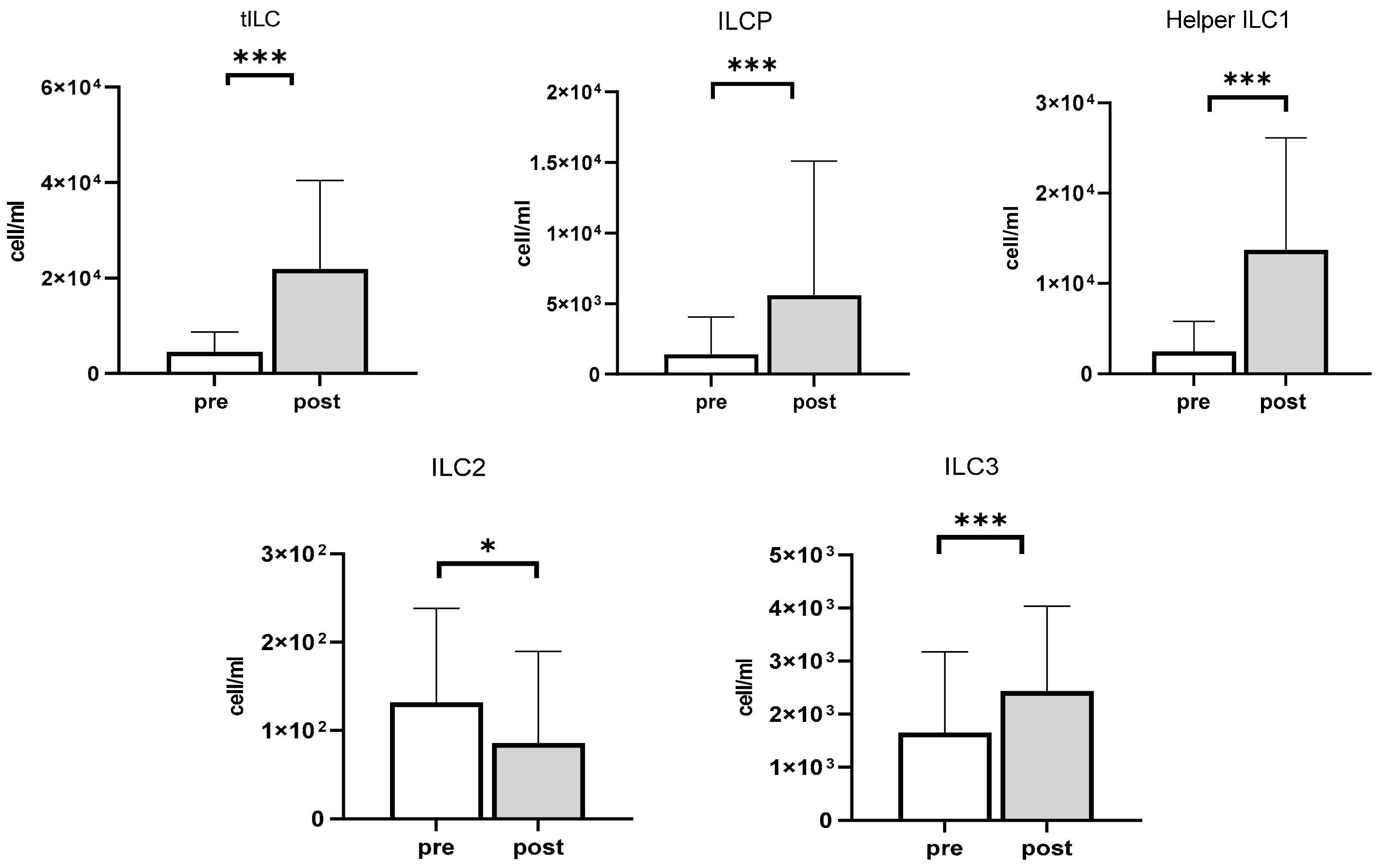
3.4. The Impact of Exercise on ILCP and ILCs
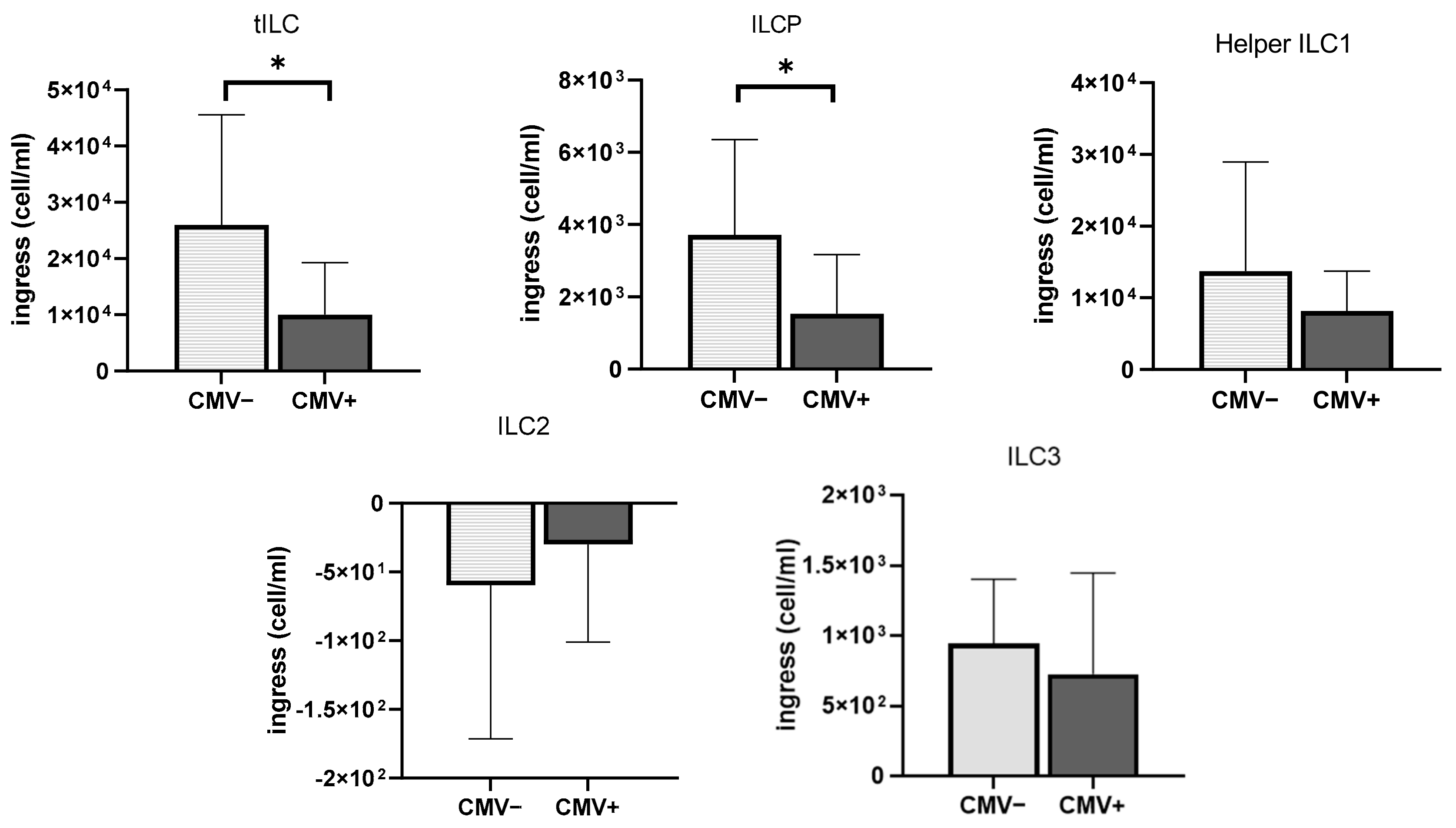
3.5. The Impact of CMV Serostatus and Response to Exercise on ILCs and Chemokine Receptors
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Walsh, N.P.; Gleeson, M.; Shephard, R.J.; Gleeson, M.; Woods, J.A.; Bishop, N.C.; Fleshner, M.; Green, C.; Pedersen, B.K.; Hoffman-Goetz, L.; et al. Position statement. Part one: Immune function and exercise. Exerc. Immunol. Rev. 2011, 17, 6–63. [Google Scholar]
- Shephard, R.J. Adhesion molecules, catecholamines and leucocyte redistribution during and following exercise. Sports Med. 2003, 33, 261–284. [Google Scholar] [CrossRef] [PubMed]
- Simpson, R.J.; Kunz, H.; Agha, N.; Graff, R. Exercise and the regulation of immune functions. Prog. Mol. Biol. Transl. Sci. 2015, 135, 355–380. [Google Scholar] [PubMed]
- Dimitrov, S.; Lange, T.; Born, J. Selective mobilization of cytotoxic leukocytes by epinephrine. J. Immunol. 2010, 184, 503–511. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Campbell, J.P.; Riddell, N.E.; Burns, V.E.; Turner, M.; van Zanten, J.J.V.; Drayson, M.T.; Bosch, J.A. Acute exercise mobilises CD8+ T lymphocytes exhibiting an effector-memory phenotype. Brain Behav. Immun. 2009, 23, 767–775. [Google Scholar] [CrossRef]
- Turner, J.E.; Spielmann, G.; Wadley, A.J.; Aldred, S.; Simpson, R.J.; Campbell, J.P. Exercise-induced B cell mobilisation: Preliminary evidence for an influx of immature cells into the bloodstream. Physiol. Behav. 2016, 164, 376–382. [Google Scholar] [CrossRef] [Green Version]
- Hanson, E.D.; Danson, E.; Evans, W.S.; Wood, W.A.; Battaglini, C.L.; Sakkal, S. Exercise Increases Mucosal-associated Invariant T Cell Cytokine Expression but Not Activation or Homing Markers. Med. Sci. Sports Exerc. 2019, 51, 379–388. [Google Scholar] [CrossRef]
- Hanson, E.D.; Sakkal, S.; Que, S.; Cho, E.; Spielmann, G.; Kadife, E.; Violet, J.A.; Battaglini, C.L.; Stoner, L.; Bartlett, D.B. Natural killer cell mobilization and egress following acute exercise in men with prostate cancer. Exp. Physiol. 2020, 105, 1524–1539. [Google Scholar] [CrossRef] [PubMed]
- Spits, H.; Artis, D.; Colonna, M.; Diefenbach, A.; Di Santo, J.P.; Eberl, G.; Koyasu, S.; Locksley, R.M.; McKenzie, A.N.; Mebius, R.E. Innate lymphoid cells—A proposal for uniform nomenclature. Nat. Rev. Immunol. 2013, 13, 145–149. [Google Scholar] [CrossRef]
- Lim, A.I.; Li, Y.; Lopez-Lastra, S.; Stadhouders, R.; Paul, F.; Casrouge, A.; Serafini, N.; Puel, A.; Bustamante, J.; Surace, L. Systemic human ILC precursors provide a substrate for tissue ILC differentiation. Cell 2017, 168, 1086–1100.e10. [Google Scholar] [CrossRef] [Green Version]
- Willinger, T. Metabolic control of innate lymphoid cell migration. Front. Immunol. 2019, 10, 2010. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vivier, E.; Artis, D.; Colonna, M.; Diefenbach, A.; Di Santo, J.P.; Eberl, G.; Koyasu, S.; Locksley, R.M.; McKenzie, A.N.; Mebius, R.E. Innate lymphoid cells: 10 years on. Cell 2018, 174, 1054–1066. [Google Scholar] [CrossRef] [Green Version]
- Mjösberg, J.M.; Trifari, S.; Crellin, N.K.; Peters, C.P.; Van Drunen, C.M.; Piet, B.; Fokkens, W.J.; Cupedo, T.; Spits, H. Human IL-25-and IL-33-responsive type 2 innate lymphoid cells are defined by expression of CRTH2 and CD161. Nat. Immunol. 2011, 12, 1055–1062. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.H.; Hashimoto-Hill, S.; Kim, M. Migration and Tissue Tropism of Innate Lymphoid Cells. Trends Immunol. 2016, 37, 68–79. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bigley, A.B.; Spielmann, G.; Agha, N.; Simpson, R.J. The effects of age and latent cytomegalovirus infection on NK-cell phenotype and exercise responsiveness in man. Oxidative Med. Cell. Longev. 2015, 2015. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bigley, A.B.; Lowder, T.W.; Spielmann, G.; Rector, J.L.; Pircher, H.; Woods, J.A.; Simpson, R.J. NK-cells have an impaired response to acute exercise and a lower expression of the inhibitory receptors KLRG1 and CD158a in humans with latent cytomegalovirus infection. Brain Behav. Immun. 2012, 26, 177–186. [Google Scholar] [CrossRef]
- Dogra, P.; Rancan, C.; Ma, W.; Toth, M.; Senda, T.; Carpenter, D.J.; Kubota, M.; Matsumoto, R.; Thapa, P.; Szabo, P.A. Tissue determinants of human NK cell development, function, and residence. Cell 2020, 180, 749–763.e713. [Google Scholar] [CrossRef] [Green Version]
- Vély, F.; Barlogis, V.; Vallentin, B.; Neven, B.; Piperoglou, C.; Ebbo, M.; Perchet, T.; Petit, M.; Yessaad, N.; Touzot, F.; et al. Evidence of innate lymphoid cell redundancy in humans. Nat. Immunol. 2016, 17, 1291–1299. [Google Scholar] [CrossRef] [Green Version]
- Borg, G. Borg’s Perceived Exertion and Pain Scales; Human Kinetics: Champaign, IL, USA, 1998. [Google Scholar]
- Spielmann, G.; McFarlin, B.K.; O’Connor, D.P.; Smith, P.J.; Pircher, H.; Simpson, R.J. Aerobic fitness is associated with lower proportions of senescent blood T-cells in man. Brain Behav. Immun. 2011, 25, 1521–1529. [Google Scholar] [CrossRef]
- Mayol, K.; Biajoux, V.; Marvel, J.; Balabanian, K.; Walzer, T. Sequential desensitization of CXCR4 and S1P5 controls natural killer cell trafficking. Blood 2011, 118, 4863–4871. [Google Scholar] [CrossRef]
- Campbell, J.J.; Qin, S.; Unutmaz, D.; Soler, D.; Murphy, K.E.; Hodge, M.R.; Wu, L.; Butcher, E.C. Unique Subpopulations of CD56+ NK and NK-T Peripheral Blood Lymphocytes Identified by Chemokine Receptor Expression Repertoire. J. Immunol. 2001, 166, 6477–6482. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Imai, T.; Hieshima, K.; Haskell, C.; Baba, M.; Nagira, M.; Nishimura, M.; Kakizaki, M.; Takagi, S.; Nomiyama, H.; Schall, T.J. Identification and molecular characterization of fractalkine receptor CX3CR1, which mediates both leukocyte migration and adhesion. Cell 1997, 91, 521–530. [Google Scholar] [CrossRef] [Green Version]
- Casilli, F.; Bianchini, A.; Gloaguen, I.; Biordi, L.; Alesse, E.; Festuccia, C.; Cavalieri, B.; Strippoli, R.; Cervellera, M.N.; Di Bitondo, R.; et al. Inhibition of interleukin-8 (CXCL8/IL-8) responses by repertaxin, a new inhibitor of the chemokine receptors CXCR1 and CXCR2. Biochem. Pharmacol. 2005, 69, 385–394. [Google Scholar] [CrossRef] [PubMed]
- Bernardini, G.; Gismondi, A.; Santoni, A. Chemokines and NK cells: Regulators of development, trafficking and functions. Immunol. Lett. 2012, 145, 39–46. [Google Scholar] [CrossRef]
- Stegmann, K.A.; Robertson, F.; Hansi, N.; Gill, U.; Pallant, C.; Christophides, T.; Pallett, L.J.; Peppa, D.; Dunn, C.; Fusai, G.; et al. CXCR6 marks a novel subset of T-betloEomeshi natural killer cells residing in human liver. Sci. Rep. 2016, 6, 26157. [Google Scholar] [CrossRef] [PubMed]
- Cook, D.N.; Prosser, D.M.; Forster, R.; Zhang, J.; Kuklin, N.A.; Abbondanzo, S.J.; Niu, X.-D.; Chen, S.-C.; Manfra, D.J.; Wiekowski, M.T. CCR6 mediates dendritic cell localization, lymphocyte homeostasis, and immune responses in mucosal tissue. Immunity 2000, 12, 495–503. [Google Scholar] [CrossRef] [Green Version]
- Al-Banna, N.A.; Vaci, M.; Slauenwhite, D.; Johnston, B.; Issekutz, T.B. CCR4 and CXCR3 play different roles in the migration of T cells to inflammation in skin, arthritic joints, and lymph nodes. Eur. J. Immunol. 2014, 44, 1633–1643. [Google Scholar] [CrossRef]
- Theall, B.; Wang, H.; Kuremsky, C.A.; Cho, E.; Hardin, K.; Robelot, L.; Marucci, J.; Mullenix, S.; Lemoine, N., Jr.; Johannsen, N.M. Allostatic stress load and CMV serostatus impact immune response to maximal exercise in collegiate swimmers. J. Appl. Physiol. 2020, 128, 178–188. [Google Scholar] [CrossRef]
- Turner, J.E.; Aldred, S.; Witard, O.C.; Drayson, M.T.; Moss, P.M.; Bosch, J.A. Latent Cytomegalovirus infection amplifies CD8 T-lymphocyte mobilisation and egress in response to exercise. Brain Behav. Immun. 2010, 24, 1362–1370. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Spielmann, G.; Bollard, C.M.; Bigley, A.B.; Hanley, P.J.; Blaney, J.W.; LaVoy, E.C.; Pircher, H.; Simpson, R.J. The effects of age and latent cytomegalovirus infection on the redeployment of CD8+ T cell subsets in response to acute exercise in humans. Brain Behav. Immun. 2014, 39, 142–151. [Google Scholar] [CrossRef]
- Emmons, R.; Niemiro, G.M.; De Lisio, M. Exercise as an Adjuvant Therapy for Hematopoietic Stem Cell Mobilization. Stem Cells Int. 2016, 2016, 7131359. [Google Scholar] [CrossRef] [Green Version]
- Gasteiger, G.; Fan, X.; Dikiy, S.; Lee, S.Y.; Rudensky, A.Y. Tissue residency of innate lymphoid cells in lymphoid and nonlymphoid organs. Science 2015, 350, 981–985. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cobb, L.M.; Verneris, M.R. Therapeutic manipulation of innate lymphoid cells. JCI Insight 2021, 6, e146006. [Google Scholar] [CrossRef] [PubMed]
- Munneke, J.M.; Björklund, A.T.; Mjösberg, J.M.; Garming-Legert, K.; Bernink, J.H.; Blom, B.; Huisman, C.; van Oers, M.H.; Spits, H.; Malmberg, K.J.; et al. Activated innate lymphoid cells are associated with a reduced susceptibility to graft-versus-host disease. Blood 2014, 124, 812–821. [Google Scholar] [CrossRef] [PubMed]
- Moriyama, S.; Brestoff, J.R.; Flamar, A.-L.; Moeller, J.B.; Klose, C.S.; Rankin, L.C.; Yudanin, N.A.; Monticelli, L.A.; Putzel, G.G.; Rodewald, H.-R. β2-adrenergic receptor–mediated negative regulation of group 2 innate lymphoid cell responses. Science 2018, 359, 1056–1061. [Google Scholar] [CrossRef] [Green Version]
- Freeman, A.T.; Staples, K.J.; Wilkinson, T.M. Defining a role for exercise training in the management of asthma. Eur. Respir. Rev. 2020, 29, 190106. [Google Scholar] [CrossRef] [PubMed]
- Fong, A.M.; Robinson, L.A.; Steeber, D.A.; Tedder, T.F.; Yoshie, O.; Imai, T.; Patel, D.D. Fractalkine and CX3CR1 mediate a novel mechanism of leukocyte capture, firm adhesion, and activation under physiologic flow. J. Exp. Med. 1998, 188, 1413–1419. [Google Scholar] [CrossRef]
- Campbell, D.J.; Kim, C.H.; Butcher, E.C. Chemokines in the systemic organization of immunity. Immunol. Rev. 2003, 195, 58–71. [Google Scholar] [CrossRef]
- Bolovan-Fritts, C.A.; Spector, S.A. Endothelial damage from cytomegalovirus-specific host immune response can be prevented by targeted disruption of fractalkine-CX3CR1 interaction. Blood J. Am. Soc. Hematol. 2008, 111, 175–182. [Google Scholar] [CrossRef]
- Parolini, S.; Santoro, A.; Marcenaro, E.; Luini, W.; Massardi, L.; Facchetti, F.; Communi, D.; Parmentier, M.; Majorana, A.; Sironi, M.; et al. The role of chemerin in the colocalization of NK and dendritic cell subsets into inflamed tissues. Blood 2007, 109, 3625–3632. [Google Scholar] [CrossRef] [PubMed]
- Yamin, R.; Lecker, L.S.; Weisblum, Y.; Vitenshtein, A.; Le-Trilling, V.T.K.; Wolf, D.G.; Mandelboim, O. HCMV vCXCL1 binds several chemokine receptors and preferentially attracts neutrophils over NK cells by interacting with CXCR2. Cell Rep. 2016, 15, 1542–1553. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lüttichau, H.R. The cytomegalovirus UL146 gene product vCXCL1 targets both CXCR1 and CXCR2 as an agonist. J. Biol. Chem. 2010, 285, 9137–9146. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bigley, A.B.; Rezvani, K.; Chew, C.; Sekine, T.; Pistillo, M.; Crucian, B.; Bollard, C.M.; Simpson, R.J. Acute exercise preferentially redeploys NK-cells with a highly-differentiated phenotype and augments cytotoxicity against lymphoma and multiple myeloma target cells. Brain Behav. Immun. 2014, 39, 160–171. [Google Scholar] [CrossRef] [PubMed]
- Melsen, J.E.; Lugthart, G.; Lankester, A.C.; Schilham, M.W. Human Circulating and Tissue-Resident CD56bright Natural Killer Cell Populations. Front. Immunol. 2016, 7, 262. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Geary, C.D.; Sun, J.C. Memory responses of natural killer cells. Semin. Immunol. 2017, 31, 11–19. [Google Scholar] [CrossRef] [PubMed]
- Simpson, R.J.; Campbell, J.P.; Gleeson, M.; Krüger, K.; Nieman, D.C.; Pyne, D.B.; Turner, J.E.; Walsh, N.P. Can exercise affect immune function to increase susceptibility to infection? Exerc. Immunol. Rev. 2020, 26, 8–22. [Google Scholar] [PubMed]
- Walzer, T.; Chiossone, L.; Chaix, J.; Calver, A.; Carozzo, C.; Garrigue-Antar, L.; Jacques, Y.; Baratin, M.; Tomasello, E.; Vivier, E. Natural killer cell trafficking in vivo requires a dedicated sphingosine 1-phosphate receptor. Nat. Immunol. 2007, 8, 1337–1344. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, B.K.; Steensberg, A.; Fischer, C.; Keller, C.; Ostrowski, K.; Schjerling, P. Exercise and cytokines with particular focus on muscle derived IL-6. Exerc. Immunol. Rev. 2001, 7, 18–31. [Google Scholar] [PubMed]
- Hanson, E.D.; Bates, L.C.; Bartlett, D.B.; Campbell, J.P. Does exercise attenuate age- and disease-associated dysfunction in unconventional T cells? Shining a light on overlooked cells in exercise immunology. Eur. J. Appl. Physiol. 2021, 121, 1815–1834. [Google Scholar] [CrossRef]
| Cell Type | Phenotype | References |
|---|---|---|
| Total ILC | Lin-CD56-CD127+ | [10] |
| ILCP | Lin-CD56-CD117+CD127+ | [10] |
| Helper ILC1 | Lin-CD56-CD127+ CD117- | [9] |
| ILC2 | Lin-CD56-CRTH2+ ST2+ | [9,13] |
| ILC3 | Lin-CD56-CXCR5-S1P5+ | [11,14] |
| Chemokine Receptors | Roles | References |
| CCR7+/CXCR4+ | Homing receptor into lymph organs/ Retention of cells in bone marrow | [21,22] |
| CXCR4+/CD62L+ | Retention of cells in bone marrow/ Regulation of homing into lymph nodes | [21,22] |
| CXCR3+/CXCR1+ | Homing to inflamed tissues/ Homing to inflamed lymph node and airways | [23,24,25] |
| CXCR6+/LFA-1+ | Homing into liver/ Regulation of activation and cytotoxicity | [2,26] |
| CCR6+/CCR4+ | Homing to gut, mucosal tissues/ Homing to skin and lung | [25,27] |
| CXCR3+/CCR4+ | Recruitment, activation of immune cell within inflamed tissue/ Homing to skin and lung | [25,28] |
| CXCR5+/S1P5+ | Required for homing into lymphoid follicle/ Required to exit from bone marrow into inflamed tissue | [21,25] |
| All subjects (n = 22) | CMV- (n = 12) | CMV+ (n = 10) | |
|---|---|---|---|
| % female | 41% (n = 9) | 25% (n = 3) | 60% (n = 6) |
| Age (yr) | 27.18 ± 5.34 | 28.08 ± 4.42 | 26.10 ± 6.35 |
| Height (cm) | 173.00 ± 9.87 | 174.45 ± 11.54 | 171.27 ± 7.64 |
| Body weight (kg) | 77.61 ± 16.03 | 83.24 ± 16.41 | 70.85 ± 13.33 |
| BMI | 25.79 ± 3.90 | 27.22 ± 3.66 | 24.08 ± 3.63 |
| VO2Peak (mL/kg/min) | 36.51 ± 6.20 | 38.16 ± 5.82 | 34.53 ± 6.34 |
| Max power (Watts) | 216.25 ± 37.94 | 240.00 ± 29.44 | 192.50 ± 30.30 ** |
| Max HR (beats/min) | 186.05 ± 13.37 | 192.09 ± 9.30 | 179.40 ± 14.40 * |
| Sub-max exercise measures | |||
| Mean VO2 (mL/kg/min) | 24.95 ± 4.56 | 25.43 ± 4.83 | 24.41 ± 4.44 |
| Mean VO2 (% max) | 69.19 ± 14.1 | 65.74 ± 5.23 | 72.98 ± 19.51 |
| Mean HR (beats/min) | 156.76 ± 16.26 | 156.50 ± 12.04 | 157.05 ± 20.64 |
| Mean HR (% max) | 84.30 ± 6.74 | 81.46 ± 4.67 | 87.42 ± 7.49 |
| Pre | Post | Ingress Cells | |
|---|---|---|---|
| Lymphocytes | 1963.41 ± 389.71 | 3194.09 ± 907.64 *** | +1230.68 |
| NK cell (cells/μL) | 195.91 ± 84.40 | 596.60 ± 207.24 *** | +400.69 |
| CD56dim (cells/μL) | 181.33 ± 84.36 | 566.58 ± 206.43 *** | +385.25 |
| CD56bright (cells/μL) | 14.45 ± 6.20 | 30.04 ± 17.36 *** | +15.59 |
| Total ILC (cell/mL) | 4377.90 ± 4096.87 | 23,200.18 ± 18,271.89 *** | +18,822.28 |
| ILCP (cell/mL) | 871.06 ± 853.31 | 3604.12 ± 2739.67 *** | +2733.06 |
| Helper ILC1 (cell/mL) | 2492.44 ± 3307.85 | 13,718.13 ± 12,413.57 *** | +11,225.69 |
| ILC2 (cell/mL) | 131.73 ± 106.70 | 85.71 ± 103.94 * | ‒37.72 |
| ILC3 (cell/mL) | 1648.66 ± 1523.97 | 2435.39 ± 1597.79 *** | +786.72 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cho, E.; Theall, B.; Stampley, J.; Granger, J.; Johannsen, N.M.; Irving, B.A.; Spielmann, G. Cytomegalovirus Infection Impairs the Mobilization of Tissue-Resident Innate Lymphoid Cells into the Peripheral Blood Compartment in Response to Acute Exercise. Viruses 2021, 13, 1535. https://doi.org/10.3390/v13081535
Cho E, Theall B, Stampley J, Granger J, Johannsen NM, Irving BA, Spielmann G. Cytomegalovirus Infection Impairs the Mobilization of Tissue-Resident Innate Lymphoid Cells into the Peripheral Blood Compartment in Response to Acute Exercise. Viruses. 2021; 13(8):1535. https://doi.org/10.3390/v13081535
Chicago/Turabian StyleCho, Eunhan, Bailey Theall, James Stampley, Joshua Granger, Neil M. Johannsen, Brian A. Irving, and Guillaume Spielmann. 2021. "Cytomegalovirus Infection Impairs the Mobilization of Tissue-Resident Innate Lymphoid Cells into the Peripheral Blood Compartment in Response to Acute Exercise" Viruses 13, no. 8: 1535. https://doi.org/10.3390/v13081535
APA StyleCho, E., Theall, B., Stampley, J., Granger, J., Johannsen, N. M., Irving, B. A., & Spielmann, G. (2021). Cytomegalovirus Infection Impairs the Mobilization of Tissue-Resident Innate Lymphoid Cells into the Peripheral Blood Compartment in Response to Acute Exercise. Viruses, 13(8), 1535. https://doi.org/10.3390/v13081535






