Specific Recognition of a Stem-Loop RNA Structure by the Alphavirus Capsid Protein
Abstract
1. Introduction
2. Materials and Methods
2.1. Cp Expression and Purification
2.2. Alexa488-RNAs
2.3. RNA Binding Assay
2.4. RNA Fraction Bound Calculations
3. Results
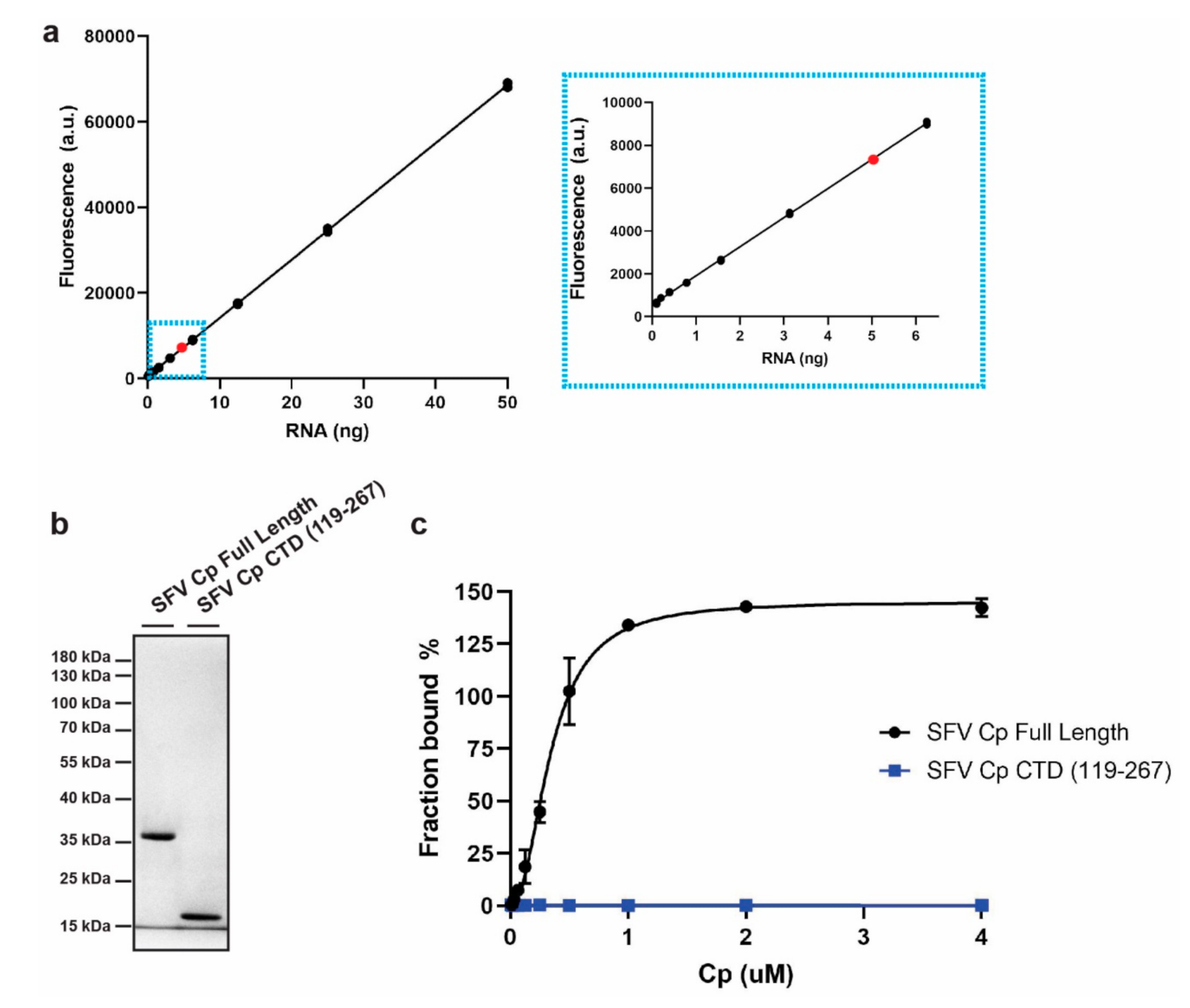
3.1. Development of an In Vitro Cp-RNA Binding Assay
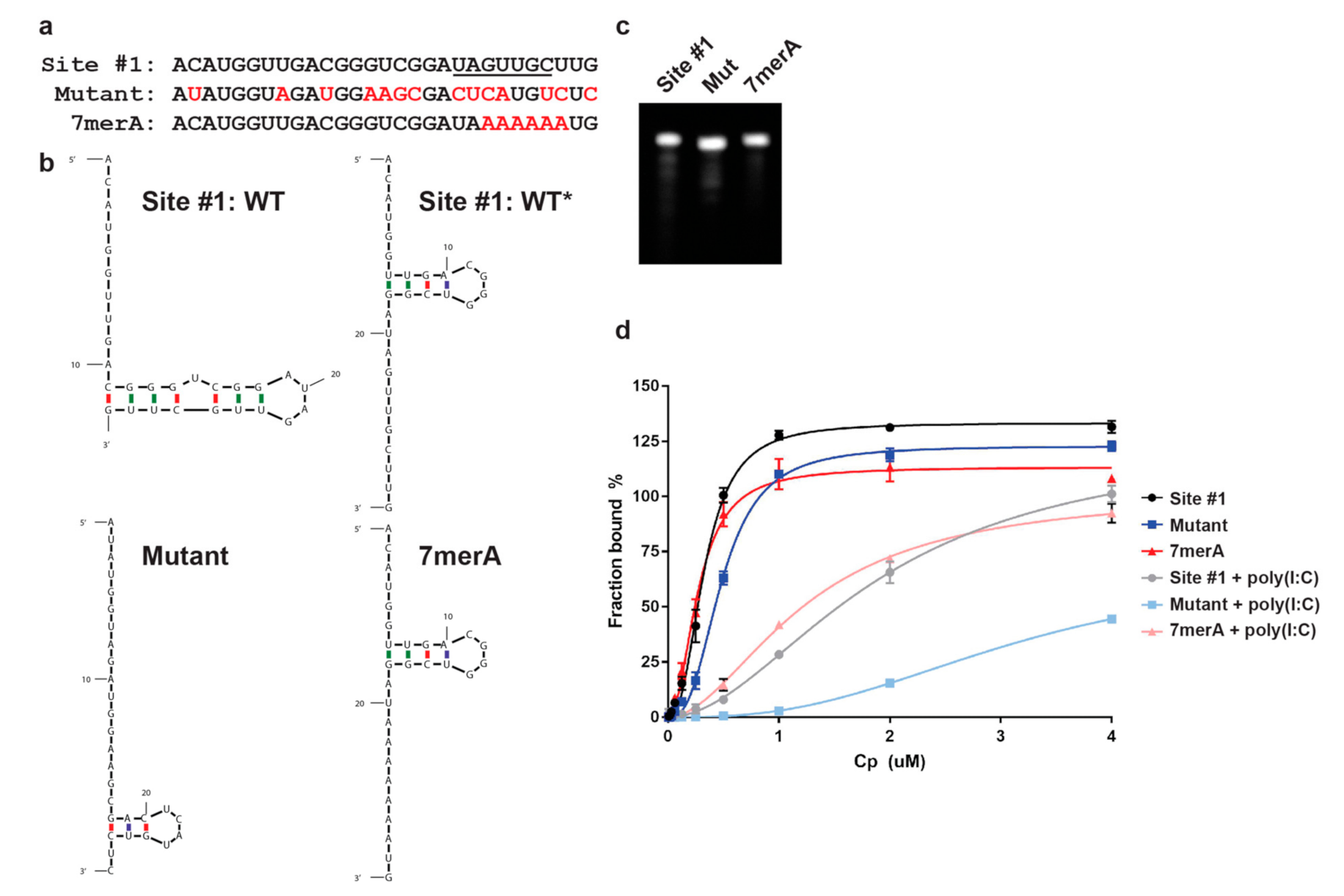
3.2. Cp Binding to Site #1 and Site #1 Mutant RNAs
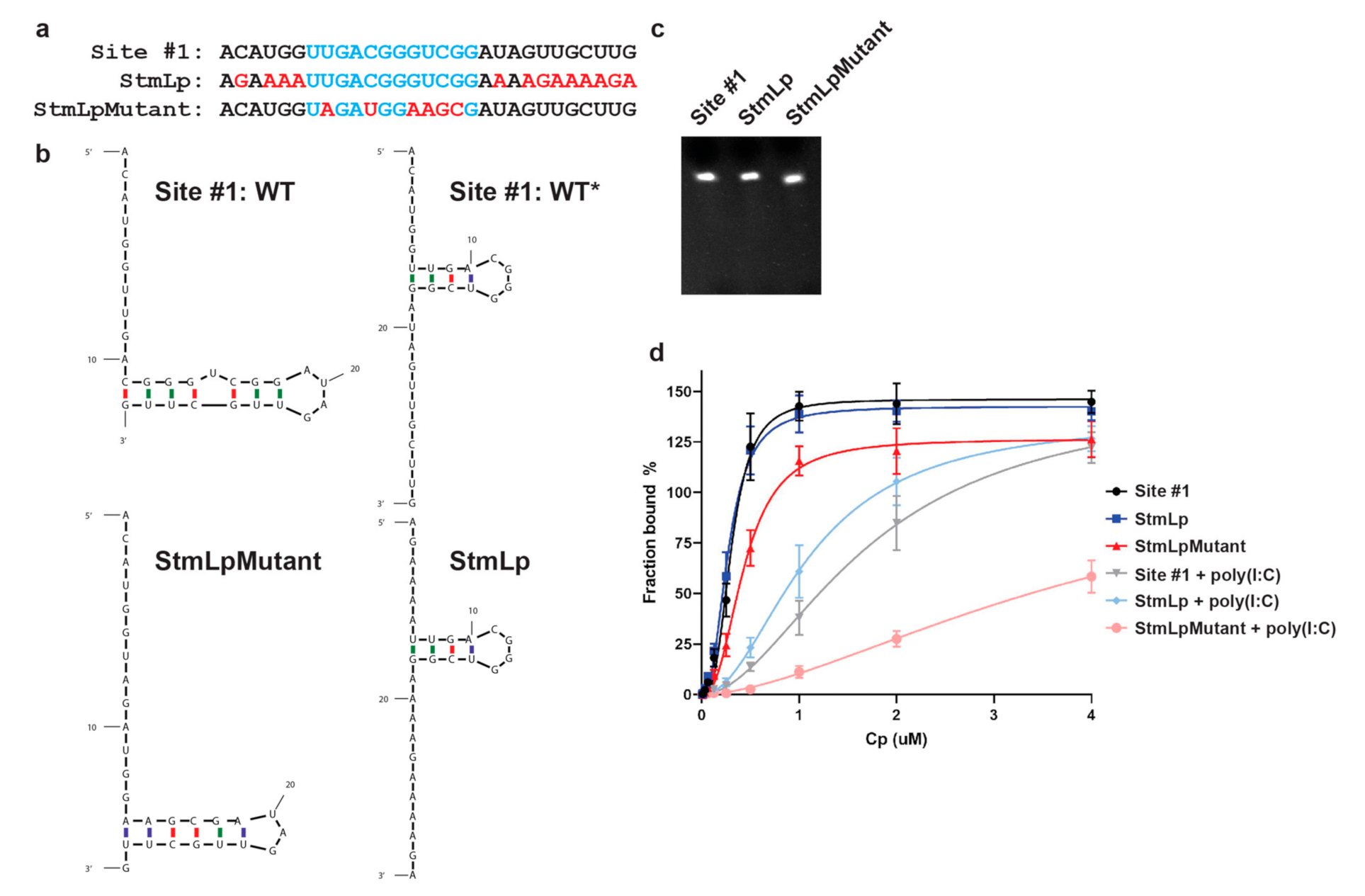
3.3. Role of the Predicted WT* Stem-Loop
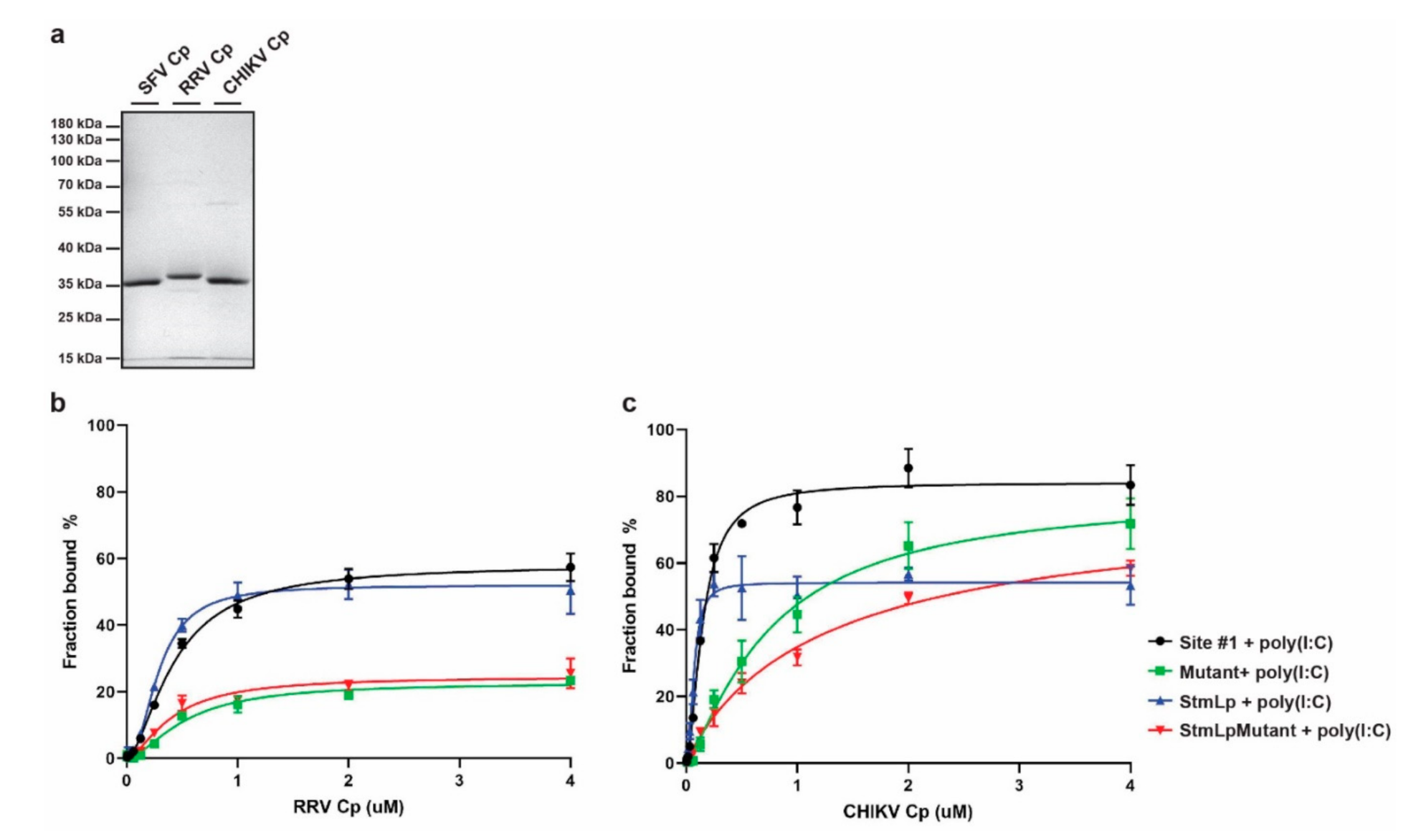
3.4. Binding of Other Alphavirus Cps to Site #1 RNA
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Kuhn, R.J. Togaviridae: The Viruses and Their Replication. In Fields Virology: Emerging Viruses, 7th ed.; Howley, P.M., Knipe, D.M., Eds.; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2021; Volume 1, pp. 170–193. [Google Scholar]
- Zaid, A.; Burt, F.J.; Liu, X.; Poo, Y.S.; Zandi, K.; Suhrbier, A.; Weaver, S.C.; Texeira, M.M.; Mahalingam, S. Arthritogenic alphaviruses: Epidemiological and clinical perspective on emerging arboviruses. Lancet. Infect. Dis. 2021, 21, e123–e133. [Google Scholar] [CrossRef]
- Baxter, V.K.; Heise, M.T. Immunopathogenesis of alphaviruses. Adv. Virus Res. 2020, 107, 315–382. [Google Scholar]
- Silva, L.A.; Dermody, T.S. Chikungunya virus: Epidemiology, replication, disease mechanisms, and prospective intervention strategies. J. Clin. Investig. 2017, 127, 737–749. [Google Scholar] [CrossRef]
- Weaver, S.C.; Winegar, R.; Manger, I.D.; Forrester, N.L. Alphaviruses: Population genetics and determinants of emergence. Antiviral Res. 2012, 94, 242–257. [Google Scholar] [CrossRef]
- Frolov, I.; Frolova, E.I. Molecular Virology of Chikungunya Virus. Curr. Top. Microbiol. Immunol. 2019. [Google Scholar] [CrossRef]
- Chen, G.L.; Coates, E.E.; Plummer, S.H.; Carter, C.A.; Berkowitz, N.; Conan-Cibotti, M.; Cox, J.H.; Beck, A.; O’Callahan, M.; Andrews, C.; et al. Effect of a Chikungunya Virus-Like Particle Vaccine on Safety and Tolerability Outcomes: A Randomized Clinical Trial. JAMA 2020, 323, 1369–1377. [Google Scholar] [CrossRef]
- Weaver, S.C.; Osorio, J.E.; Livengood, J.A.; Chen, R.; Stinchcomb, D.T. Chikungunya virus and prospects for a vaccine. Expert Rev. Vaccines 2012, 11, 1087–1101. [Google Scholar] [CrossRef] [PubMed]
- Brown, R.S.; Wan, J.J.; Kielian, M. The Alphavirus Exit Pathway: What We Know and What We Wish We Knew. Viruses 2018, 10, 89. [Google Scholar] [CrossRef]
- Mendes, A.; Kuhn, R.J. Alphavirus Nucleocapsid Packaging and Assembly. Viruses 2018, 10, 138. [Google Scholar] [CrossRef] [PubMed]
- Button, J.M.; Mukhopadhyay, S. Removing the Polyanionic Cargo Requirement for Assembly of Alphavirus Core-Like Particles to Make an Empty Alphavirus Core. Viruses 2020, 12, 846. [Google Scholar] [CrossRef] [PubMed]
- Wan, J.J.; Brown, R.S.; Kielian, M. Berberine Chloride is an Alphavirus Inhibitor That Targets Nucleocapsid Assembly. mBio 2020, 11, e01382-20. [Google Scholar] [CrossRef] [PubMed]
- Skoging-Nyberg, U.; Liljestrom, P. M-X-I motif of semliki forest virus capsid protein affects nucleocapsid assembly. J. Virol. 2001, 75, 4625–4632. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kim, B.; Arcos, S.; Rothamel, K.; Jian, J.; Rose, K.L.; McDonald, W.H.; Bian, Y.; Reasoner, S.; Barrows, N.J.; Bradrick, S.; et al. Discovery of Widespread Host Protein Interactions with the Pre-replicated Genome of CHIKV Using VIR-CLASP. Mol. Cell 2020, 78, 624–640. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.Y.; Firth, A.E.; Atasheva, S.; Frolova, E.I.; Frolov, I. Conservation of a packaging signal and the viral genome RNA packaging mechanism in alphavirus evolution. J. Virol. 2011, 85, 8022–8036. [Google Scholar] [CrossRef]
- Warrier, R.; Linger, B.R.; Golden, B.L.; Kuhn, R.J. Role of sindbis virus capsid protein region II in nucleocapsid core assembly and encapsidation of genomic RNA. J. Virol. 2008, 82, 4461–4470. [Google Scholar] [CrossRef]
- Frolova, E.; Frolov, I.; Schlesinger, S. Packaging signals in alphaviruses. J. Virol. 1997, 71, 248–258. [Google Scholar] [CrossRef]
- Owen, K.E.; Kuhn, R.J. Identification of a region in the sindbis virus nucleocapsid protein that is involved in specificity of RNA encapsidation. J. Virol. 1996, 70, 2757–2763. [Google Scholar] [CrossRef]
- Rumenapf, T.; Strauss, E.G.; Strauss, J.H. Subgenomic mRNA of Aura alphavirus is packaged into virions. J. Virol. 1994, 68, 56–62. [Google Scholar] [CrossRef]
- Brown, R.S.; Anastasakis, D.G.; Hafner, M.; Kielian, M. Multiple capsid protein binding sites mediate selective packaging of the alphavirus genomic RNA. Nat. Commun. 2020, 11, 4693. [Google Scholar] [CrossRef] [PubMed]
- Twarock, R.; Stockley, P.G. RNA-Mediated Virus Assembly: Mechanisms and Consequences for Viral Evolution and Therapy. Ann. Rev. Biophys. 2019, 48, 495–514. [Google Scholar] [CrossRef]
- Dykeman, E.C.; Stockley, P.G.; Twarock, R. Solving a Levinthal’s paradox for virus assembly identifies a unique antiviral strategy. Proc. Natl. Acad. Sci. USA 2014, 111, 5361–5366. [Google Scholar] [CrossRef]
- Jones-Burrage, S.E.; Tan, Z.; Li, L.; Zlotnick, A.; Mukhopadhyay, S. Identification of Chikungunya virus nucleocapsid core assembly modulators. bioRxiv 2019, 774943. [Google Scholar] [CrossRef]
- Kutchko, K.M.; Madden, E.A.; Morrison, C.; Plante, K.S.; Sanders, W.; Vincent, H.A.; Cruz Cisneros, M.C.; Long, K.M.; Moorman, N.J.; Heise, M.T.; et al. Structural divergence creates new functional features in alphavirus genomes. Nucl. Acids Res. 2018, 46, 3657–3670. [Google Scholar] [CrossRef] [PubMed]
- Madden, E.A.; Plante, K.S.; Morrison, C.R.; Kutchko, K.M.; Sanders, W.; Long, K.M.; Taft-Benz, S.; Cruz Cisneros, M.C.; White, A.M.; Sarkar, S.; et al. Using SHAPE-MaP To Model RNA Secondary Structure and Identify 3’UTR Variation in Chikungunya Virus. J. Virol. 2020, 94, e00701-20. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brown, R.S.; Kim, L.; Kielian, M. Specific Recognition of a Stem-Loop RNA Structure by the Alphavirus Capsid Protein. Viruses 2021, 13, 1517. https://doi.org/10.3390/v13081517
Brown RS, Kim L, Kielian M. Specific Recognition of a Stem-Loop RNA Structure by the Alphavirus Capsid Protein. Viruses. 2021; 13(8):1517. https://doi.org/10.3390/v13081517
Chicago/Turabian StyleBrown, Rebecca S., Lisa Kim, and Margaret Kielian. 2021. "Specific Recognition of a Stem-Loop RNA Structure by the Alphavirus Capsid Protein" Viruses 13, no. 8: 1517. https://doi.org/10.3390/v13081517
APA StyleBrown, R. S., Kim, L., & Kielian, M. (2021). Specific Recognition of a Stem-Loop RNA Structure by the Alphavirus Capsid Protein. Viruses, 13(8), 1517. https://doi.org/10.3390/v13081517





