Acetylation, Methylation and Allysine Modification Profile of Viral and Host Proteins during Influenza A Virus Infection
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cells, Viruses, and Infection
2.2. In-Gel Digestion
2.3. Filter-Aided Sample Preparation (FASP)
2.4. Mass Spectrometry Analysis
2.5. Data Analysis
2.6. Bioinformatics Analysis
3. Results
3.1. Eight Viral Proteins Were Modified by Acetylation, Methylation and/or Allysine
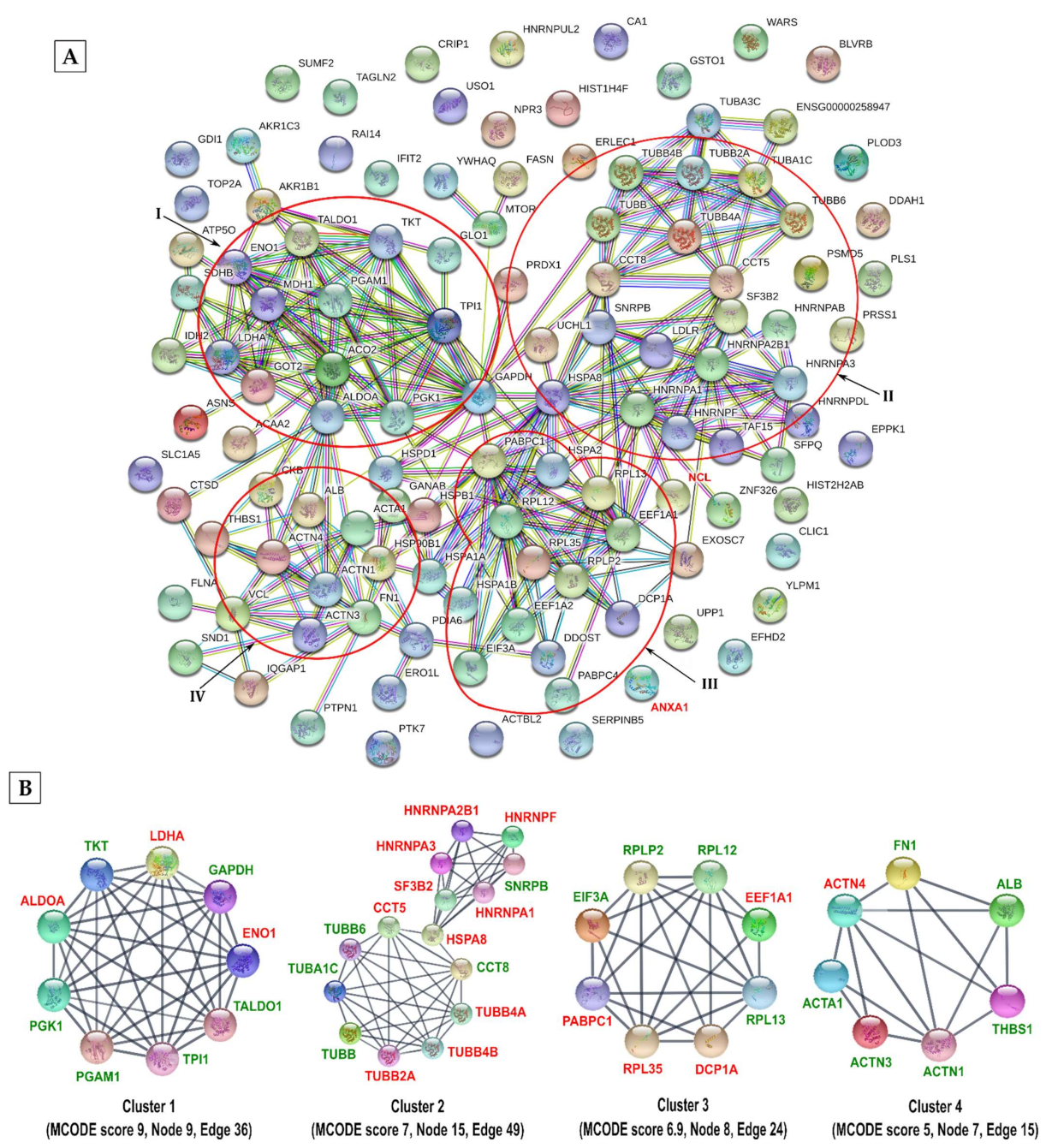
3.2. Two Hundred and Forty-Five Host Proteins Were Modified at 300 Sites by Methylation, Acetylation, and/or Allysine in Response to IAV Infection
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Husain, M. Host factors involved in influenza virus infection. Emerg. Top. Life Sci. 2020, 4, 389–398. [Google Scholar] [CrossRef] [PubMed]
- Drazic, A.; Myklebust, L.M.; Ree, R.; Arnesen, T. The world of protein acetylation. Biochim. Biophys. Acta 2016, 1864, 1372–1401. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Murn, J.; Shi, Y. The winding path of protein methylation research: Milestones and new frontiers. Nat. Rev. Mol. Cell Biol. 2017, 18, 517–527. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Zhang, L.; Liu, X. Role of Post-translational Modifications in Influenza A Virus Life Cycle and Host Innate Immune Response. Front. Microbiol. 2020, 11, 517461. [Google Scholar] [CrossRef] [PubMed]
- Dawson, A.R.; Wilson, G.M.; Coon, J.J.; Mehle, A. Post-Translation Regulation of Influenza Virus Replication. Annu Rev. Virol 2020, 7, 167–187. [Google Scholar] [CrossRef] [PubMed]
- Britton, L.M.; Newhart, A.; Bhanu, N.V.; Sridharan, R.; Gonzales-Cope, M.; Plath, K.; Janicki, S.M.; Garcia, B.A. Initial characterization of histone H3 serine 10 O-acetylation. Epigenetics 2013, 8, 1101–1113. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Beaver, J.E.; Waters, M.L. Molecular Recognition of Lys and Arg Methylation. ACS Chem. Biol. 2016, 11, 643–653. [Google Scholar] [CrossRef]
- Yang, X.-D.; Tajkhorshid, E.; Chen, L.-F. Functional Interplay between Acetylation and Methylation of the RelA Subunit of NF-κB. Mol. Cell. Biol. 2010, 30, 2170–2180. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ma, L.; Huang, C.; Wang, X.J.; Xin, D.E.; Wang, L.S.; Zou, Q.C.; Zhang, Y.S.; Tan, M.D.; Wang, Y.M.; Zhao, T.C.; et al. Lysyl Oxidase 3 Is a Dual-Specificity Enzyme Involved in STAT3 Deacetylation and Deacetylimination Modulation. Mol. Cell 2017, 65, 296–309. [Google Scholar] [CrossRef] [Green Version]
- Diedrich, D.L.; Schnaitman, C.A. Lysyl-Derived Aldehydes in Outer Membrane Proteins of Escherichia coli. Proc. Natl. Acad. Sci. USA 1978, 75, 3708–3712. [Google Scholar] [CrossRef] [Green Version]
- Pinnell, S.R.; Martin, G.R. The cross-linking of collagen and elastin: Enzymatic conversion of lysine in peptide linkage to alpha-aminoadipic-delta-semialdehyde (allysine) by an extract from bone. Proc. Natl. Acad. Sci. USA 1968, 61, 708–716. [Google Scholar] [CrossRef] [Green Version]
- Rowbottom, M.W.; Bain, G.; Calderon, I.; Lasof, T.; Lonergan, D.; Lai, A.; Huang, F.; Darlington, J.; Prodanovich, P.; Santini, A.M.; et al. Identification of 4-(Aminomethyl)-6-(trifluoromethyl)-2-(phenoxy)pyridine Derivatives as Potent, Selective, and Orally Efficacious Inhibitors of the Copper-Dependent Amine Oxidase, Lysyl Oxidase-Like 2 (LOXL2). J. Med. Chem. 2017, 60, 4403–4423. [Google Scholar] [CrossRef] [PubMed]
- Herranz, N.; Dave, N.; Millanes-Romero, A.; Pascual-Reguant, L.; Morey, L.; Diaz, V.M.; Lorenz-Fonfria, V.; Gutierrez-Gallego, R.; Jeronimo, C.; Iturbide, A.; et al. Lysyl oxidase-like 2 (LOXL2) oxidizes trimethylated lysine 4 in histone H3. FEBS J. 2016, 283, 4263–4273. [Google Scholar] [CrossRef]
- Nutsford, A.N.; Galvin, H.D.; Ahmed, F.; Husain, M. The Class IV human deacetylase, HDAC11, exhibits anti-influenza A virus properties via its involvement in host innate antiviral response. Cell. Microbiol. 2019, 21, e12989. [Google Scholar] [CrossRef]
- Galvin, H.D.; Husain, M. Influenza A virus-induced host caspase and viral PA-X antagonize the antiviral host factor, histone deacetylase 4. J. Biol. Chem. 2019, 294, 20207–20221. [Google Scholar] [CrossRef] [PubMed]
- Nagesh, P.T.; Hussain, M.; Galvin, H.D.; Husain, M. Histone Deacetylase 2 Is a Component of Influenza A Virus-Induced Host Antiviral Response. Front. Microbiol. 2017, 8, 1315. [Google Scholar] [CrossRef]
- Nagesh, P.T.; Husain, M. Influenza A Virus Dysregulates Host Histone Deacetylase 1 That Inhibits Viral Infection in Lung Epithelial Cells. J. Virol. 2016, 90, 4614–4625. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Husain, M.; Cheung, C.Y. Histone deacetylase 6 inhibits influenza A virus release by downregulating the trafficking of viral components to the plasma membrane via its substrate, acetylated microtubules. J. Virol. 2014, 88, 11229–11239. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koyuncu, E.; Budayeva, H.G.; Miteva, Y.V.; Ricci, D.P.; Silhavy, T.J.; Shenk, T.; Cristea, I.M. Sirtuins are evolutionarily conserved viral restriction factors. mBio 2014, 5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Giese, S.; Ciminski, K.; Bolte, H.; Moreira, É.A.; Lakdawala, S.; Hu, Z.; David, Q.; Kolesnikova, L.; Götz, V.; Zhao, Y.; et al. Role of influenza A virus NP acetylation on viral growth and replication. Nat. Commun. 2017, 8, 1259. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hatakeyama, D.; Shoji, M.; Yamayoshi, S.; Yoh, R.; Ohmi, N.; Takenaka, S.; Saitoh, A.; Arakaki, Y.; Masuda, A.; Komatsu, T.; et al. Influenza A virus nucleoprotein is acetylated by histone acetyltransferases PCAF and GCN5. J. Biol. Chem. 2018, 293, 7126–7138. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, H.; Qian, Y.; Chen, X.; Ruan, Z.; Ye, Y.; Chen, H.; Babiuk, L.A.; Jung, Y.S.; Dai, J. HDAC6 Restricts Influenza A Virus by Deacetylation of the RNA Polymerase PA Subunit. J. Virol. 2019, 93. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oishi, K.; Yamayoshi, S.; Kozuka-Hata, H.; Oyama, M.; Kawaoka, Y. N-Terminal Acetylation by NatB Is Required for the Shutoff Activity of Influenza A Virus PA-X. Cell Rep. 2018, 24, 851–860. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hutchinson, E.C.; Denham, E.M.; Thomas, B.; Trudgian, D.C.; Hester, S.S.; Ridlova, G.; York, A.; Turrell, L.; Fodor, E. Mapping the phosphoproteome of influenza A and B viruses by mass spectrometry. PLoS Pathog. 2012, 8, e1002993. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Husain, M.; Harrod, K.S. Enhanced acetylation of alpha-tubulin in influenza A virus infected epithelial cells. FEBS Lett. 2011, 585, 128–132. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; Peng, Q.; Zhao, W.; Sun, W.; Yang, J.; Liu, N. Proteomics in Influenza Research: The Emerging Role of Posttranslational Modifications. J. Proteome Res. 2021, 20, 110–121. [Google Scholar] [CrossRef]
- Liu, Z.; Wang, Y.; Gao, T.; Pan, Z.; Cheng, H.; Yang, Q.; Cheng, Z.; Guo, A.; Ren, J.; Xue, Y. CPLM: A database of protein lysine modifications. Nucleic Acids Res. 2013, 42, D531–D536. [Google Scholar] [CrossRef] [Green Version]
- Perillo, B.; Di Santi, A.; Cernera, G.; Galasso, G.; Pocsfalvi, G.; Castoria, G.; Migliaccio, A. Acetylation/methylation at lysine 9 in histone H3 as a mark of nucleosome asymmetry in human somatic breast cells. Cell Death Discov. 2020, 6, 39. [Google Scholar] [CrossRef]
- Yang, X.-J.; Seto, E. Lysine Acetylation: Codified Crosstalk with Other Posttranslational Modifications. Mol. Cell 2008, 31, 449–461. [Google Scholar] [CrossRef] [Green Version]
- Shevchenko, A.; Tomas, H.; Havli, J.; Olsen, J.V.; Mann, M. In-gel digestion for mass spectrometric characterization of proteins and proteomes. Nat. Protoc. 2006, 1, 2856–2860. [Google Scholar] [CrossRef]
- Wiśniewski, J.R.; Zougman, A.; Nagaraj, N.; Mann, M. Universal sample preparation method for proteome analysis. Nat. Methods 2009, 6, 359–362. [Google Scholar] [CrossRef] [PubMed]
- Käll, L.; Canterbury, J.D.; Weston, J.; Noble, W.S.; MacCoss, M.J. Semi-supervised learning for peptide identification from shotgun proteomics datasets. Nat. Methods 2007, 4, 923–925. [Google Scholar] [CrossRef]
- Li, Z.; Zhong, L.; He, J.; Huang, Y.; Zhao, Y. Development and application of reverse genetic technology for the influenza virus. Virus Genes 2021, 57, 151–163. [Google Scholar] [CrossRef] [PubMed]
- Arzt, S.; Baudin, F.; Barge, A.; Timmins, P.; Burmeister, W.P.; Ruigrok, R.W.H. Combined Results from Solution Studies on Intact Influenza Virus M1 Protein and from a New Crystal Form of Its N-Terminal Domain Show That M1 Is an Elongated Monomer. Virology 2001, 279, 439–446. [Google Scholar] [CrossRef] [Green Version]
- Peukes, J.; Xiong, X.; Erlendsson, S.; Qu, K.; Wan, W.; Calder, L.J.; Schraidt, O.; Kummer, S.; Freund, S.M.V.; Krausslich, H.G.; et al. The native structure of the assembled matrix protein 1 of influenza A virus. Nature 2020, 587, 495–498. [Google Scholar] [CrossRef] [PubMed]
- Burleigh, L.M.; Calder, L.J.; Skehel, J.J.; Steinhauer, D.A. Influenza a viruses with mutations in the m1 helix six domain display a wide variety of morphological phenotypes. J. Virol. 2005, 79, 1262–1270. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, L.; Du, Y.; Lu, M.; Li, T. ASEB: A web server for KAT-specific acetylation site prediction. Nucleic Acids Res. 2012, 40, W376–W379. [Google Scholar] [CrossRef] [Green Version]
- Chenavas, S.; Estrozi, L.F.; Slama-Schwok, A.; Delmas, B.; Di Primo, C.; Baudin, F.; Li, X.; Crépin, T.; Ruigrok, R.W.H. Monomeric Nucleoprotein of Influenza A Virus. PLOS Pathog. 2013, 9, e1003275. [Google Scholar] [CrossRef]
- Liu, C.-L.; Hung, H.-C.; Lo, S.-C.; Chiang, C.-H.; Chen, I.J.; Hsu, J.T.A.; Hou, M.-H. Using mutagenesis to explore conserved residues in the RNA-binding groove of influenza A virus nucleoprotein for antiviral drug development. Sci. Rep. 2016, 6, 21662. [Google Scholar] [CrossRef]
- Li, Z.; Watanabe, T.; Hatta, M.; Watanabe, S.; Nanbo, A.; Ozawa, M.; Kakugawa, S.; Shimojima, M.; Yamada, S.; Neumann, G.; et al. Mutational Analysis of Conserved Amino Acids in the Influenza A Virus Nucleoprotein. J. Virol. 2009, 83, 4153–4162. [Google Scholar] [CrossRef] [Green Version]
- Ye, Q.; Krug, R.M.; Tao, Y.J. The mechanism by which influenza A virus nucleoprotein forms oligomers and binds RNA. Nature 2006, 444, 1078–1082. [Google Scholar] [CrossRef] [PubMed]
- Chan, W.-H.; Ng, A.K.-L.; Robb, N.C.; Lam, M.K.-H.; Chan, P.K.-S.; Au, S.W.-N.; Wang, J.-H.; Fodor, E.; Shaw, P.-C. Functional Analysis of the Influenza Virus H5N1 Nucleoprotein Tail Loop Reveals Amino Acids That Are Crucial for Oligomerization and Ribonucleoprotein Activities. J. Virol. 2010, 84, 7337–7345. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chang, C.K.; Chen, C.J.; Wu, C.C.; Chen, S.W.; Shih, S.R.; Kuo, R.L. Cellular hnRNP A2/B1 interacts with the NP of influenza A virus and impacts viral replication. PLoS ONE 2017, 12, e0188214. [Google Scholar] [CrossRef] [Green Version]
- Moen, S.O.; Abendroth, J.; Fairman, J.W.; Baydo, R.O.; Bullen, J.; Kirkwood, J.L.; Barnes, S.R.; Raymond, A.C.; Begley, D.W.; Henkel, G.; et al. Structural analysis of H1N1 and H7N9 influenza A virus PA in the absence of PB1. Sci. Rep. 2014, 4, 5944. [Google Scholar] [CrossRef] [Green Version]
- Hara, K.; Schmidt, F.I.; Crow, M.; Brownlee, G.G. Amino Acid Residues in the N-Terminal Region of the PA Subunit of Influenza A Virus RNA Polymerase Play a Critical Role in Protein Stability, Endonuclease Activity, Cap Binding, and Virion RNA Promoter Binding. J. Virol. 2006, 80, 7789–7798. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, L.; Wu, A.; Wang, Y.E.; Quanquin, N.; Li, C.; Wang, J.; Chen, H.-W.; Liu, S.; Liu, P.; Zhang, H.; et al. Functional Genomics Reveals Linkers Critical for Influenza Virus Polymerase. J. Virol. 2016, 90, 2938–2947. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Obayashi, E.; Yoshida, H.; Kawai, F.; Shibayama, N.; Kawaguchi, A.; Nagata, K.; Tame, J.R.H.; Park, S.-Y. The structural basis for an essential subunit interaction in influenza virus RNA polymerase. Nature 2008, 454, 1127–1131. [Google Scholar] [CrossRef]
- Tarendeau, F.; Boudet, J.; Guilligay, D.; Mas, P.J.; Bougault, C.M.; Boulo, S.; Baudin, F.; Ruigrok, R.W.H.; Daigle, N.; Ellenberg, J.; et al. Structure and nuclear import function of the C-terminal domain of influenza virus polymerase PB2 subunit. Nat. Struct. Mol. Biol. 2007, 14, 229–233. [Google Scholar] [CrossRef]
- Han, C.W.; Jeong, M.S.; Jang, S.B. Structure and Function of the Influenza A Virus Non-Structural Protein 1. J. Microbiol Biotechnol. 2019, 29, 1184–1192. [Google Scholar] [CrossRef]
- Gamblin, S.J.; Haire, L.F.; Russell, R.J.; Stevens, D.J.; Xiao, B.; Ha, Y.; Vasisht, N.; Steinhauer, D.A.; Daniels, R.S.; Elliot, A.; et al. The structure and receptor binding properties of the 1918 influenza hemagglutinin. Science 2004, 303, 1838–1842. [Google Scholar] [CrossRef]
- Bahadoran, A.; Bezavada, L.; Smallwood, H.S. Fueling influenza and the immune response: Implications for metabolic reprogramming during influenza infection and immunometabolism. Immunol. Rev. 2020, 295, 140–166. [Google Scholar] [CrossRef]
- Smallwood, H.S.; Duan, S.; Morfouace, M.; Rezinciuc, S.; Shulkin, B.L.; Shelat, A.; Zink, E.E.; Milasta, S.; Bajracharya, R.; Oluwaseum, A.J.; et al. Targeting Metabolic Reprogramming by Influenza Infection for Therapeutic Intervention. Cell Rep. 2017, 19, 1640–1653. [Google Scholar] [CrossRef] [Green Version]
- Mishra, S.; Goyal, P.; Kumar, D.; Chaudhari, R.; Rajala, M.S. Experimental validation of influenza A virus matrix protein (M1) interaction with host cellular alpha enolase and pyruvate kinase. Virology 2020, 549, 59–67. [Google Scholar] [CrossRef]
- Bedi, S.; Ono, A. Friend or Foe: The Role of the Cytoskeleton in Influenza A Virus Assembly. Viruses 2019, 11, 46. [Google Scholar] [CrossRef] [Green Version]
- Thompson, M.G.; Munoz-Moreno, R.; Bhat, P.; Roytenberg, R.; Lindberg, J.; Gazzara, M.R.; Mallory, M.J.; Zhang, K.; Garcia-Sastre, A.; Fontoura, B.M.A.; et al. Co-regulatory activity of hnRNP K and NS1-BP in influenza and human mRNA splicing. Nat. Commun. 2018, 9, 2407. [Google Scholar] [CrossRef]
- Tsai, P.L.; Chiou, N.T.; Kuss, S.; García-Sastre, A.; Lynch, K.W.; Fontoura, B.M. Cellular RNA binding proteins NS1-BP and hnRNP K regulate influenza A virus RNA splicing. PLoS Pathog. 2013, 9, e1003460. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Lin, L.; Zhong, Y.; Feng, M.; Yu, T.; Yan, Y.; Zhou, J.; Liao, M. Cellular hnRNPAB binding to viral nucleoprotein inhibits flu virus replication by blocking nuclear export of viral mRNA. iScience 2021, 24, 102160. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Batra, J.; Stuchlik, O.; Reed, M.S.; Pohl, J.; Chow, V.T.K.; Sambhara, S.; Lal, S.K. Influenza A Virus Nucleoprotein Activates the JNK Stress-Signaling Pathway for Viral Replication by Sequestering Host Filamin A Protein. Front. Microbiol. 2020, 11, 581867. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Mayank, A.K.; Nailwal, H.; Tripathi, S.; Patel, J.R.; Bowzard, J.B.; Gaur, P.; Donis, R.O.; Katz, J.M.; Cox, N.J.; et al. Influenza A viral nucleoprotein interacts with cytoskeleton scaffolding protein alpha-actinin-4 for viral replication. FEBS J. 2014, 281, 2899–2914. [Google Scholar] [CrossRef] [PubMed]
- Ohno, M.; Sekiya, T.; Nomura, N.; Daito, T.J.; Shingai, M.; Kida, H. Influenza virus infection affects insulin signaling, fatty acid-metabolizing enzyme expressions, and the tricarboxylic acid cycle in mice. Sci. Rep. 2020, 10, 10879. [Google Scholar] [CrossRef]
- Schloer, S.; Hubel, N.; Masemann, D.; Pajonczyk, D.; Brunotte, L.; Ehrhardt, C.; Brandenburg, L.O.; Ludwig, S.; Gerke, V.; Rescher, U. The annexin A1/FPR2 signaling axis expands alveolar macrophages, limits viral replication, and attenuates pathogenesis in the murine influenza A virus infection model. FASEB J. 2019, 33, 12188–12199. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chan, C.-M.; Chu, H.; Zhang, A.J.; Leung, L.-H.; Sze, K.-H.; Kao, R.Y.-T.; Chik, K.K.-H.; To, K.K.-W.; Chan, J.F.-W.; Chen, H.; et al. Hemagglutinin of influenza A virus binds specifically to cell surface nucleolin and plays a role in virus internalization. Virology 2016, 494, 78–88. [Google Scholar] [CrossRef]
- Gao, Z.; Hu, J.; Liang, Y.; Yang, Q.; Yan, K.; Liu, D.; Wang, X.; Gu, M.; Liu, X.; Hu, S.; et al. Generation and Comprehensive Analysis of Host Cell Interactome of the PA Protein of the Highly Pathogenic H5N1 Avian Influenza Virus in Mammalian Cells. Front. Microbiol. 2017, 8. [Google Scholar] [CrossRef]
- Gao, Z.; Hu, J.; Wang, X.; Yang, Q.; Liang, Y.; Ma, C.; Liu, D.; Liu, K.; Hao, X.; Gu, M.; et al. The PA-interacting host protein nucleolin acts as an antiviral factor during highly pathogenic H5N1 avian influenza virus infection. Arch. Virol. 2018, 163, 2775–2786. [Google Scholar] [CrossRef] [PubMed]
- Terrier, O.; Carron, C.; De Chassey, B.; Dubois, J.; Traversier, A.; Julien, T.; Cartet, G.; Proust, A.; Hacot, S.; Ressnikoff, D.; et al. Nucleolin interacts with influenza A nucleoprotein and contributes to viral ribonucleoprotein complexes nuclear trafficking and efficient influenza viral replication. Sci Rep. 2016, 6, 29006. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.; Du, Y.; Wang, G.; Li, K. Non-structural protein 1 of H3N2 influenza A virus induces nucleolar stress via interaction with nucleolin. Sci Rep. 2017, 7, 17761. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dubois, J.; Terrier, O.; Rosa-Calatrava, M. Influenza Viruses and mRNA Splicing: Doing More with Less. mBio 2014, 5, e00070-14. [Google Scholar] [CrossRef] [Green Version]
- Li, X.; Fu, Z.; Liang, H.; Wang, Y.; Qi, X.; Ding, M.; Sun, X.; Zhou, Z.; Huang, Y.; Gu, H.; et al. H5N1 influenza virus-specific miRNA-like small RNA increases cytokine production and mouse mortality via targeting poly(rC)-binding protein 2. Cell Res. 2018, 28, 157–171. [Google Scholar] [CrossRef] [Green Version]
- Konig, R.; Stertz, S.; Zhou, Y.; Inoue, A.; Hoffmann, H.H.; Bhattacharyya, S.; Alamares, J.G.; Tscherne, D.M.; Ortigoza, M.B.; Liang, Y.; et al. Human host factors required for influenza virus replication. Nature 2010, 463, 813–817. [Google Scholar] [CrossRef]
- Tan, K.S.; Ng, W.C.; Seet, J.E.; Olfat, F.; Engelward, B.P.; Chow, V.T. Investigating the efficacy of pamidronate, a chemical inhibitor of farnesyl pyrophosphate synthase, in the inhibition of influenza virus infection in vitro and in vivo. Mol. Med. Rep. 2014, 9, 51–56. [Google Scholar] [CrossRef] [Green Version]
- Kuss-Duerkop, S.K.; Wang, J.; Mena, I.; White, K.; Metreveli, G.; Sakthivel, R.; Mata, M.A.; Muñoz-Moreno, R.; Chen, X.; Krammer, F.; et al. Influenza virus differentially activates mTORC1 and mTORC2 signaling to maximize late stage replication. PLOS Pathog. 2017, 13, e1006635. [Google Scholar] [CrossRef] [PubMed]
- Mayer, D.; Molawi, K.; Martinez-Sobrido, L.; Ghanem, A.; Thomas, S.; Baginsky, S.; Grossmann, J.; Garcia-Sastre, A.; Schwemmle, M. Identification of cellular interaction partners of the influenza virus ribonucleoprotein complex and polymerase complex using proteomic-based approaches. J. Proteome Res. 2007, 6, 672–682. [Google Scholar] [CrossRef] [Green Version]
- Mahesutihan, M.; Zheng, W.; Cui, L.; Li, Y.; Jiao, P.; Yang, W.; Liu, W.; Li, J.; Fan, W.; Yang, L.; et al. CypA Regulates AIP4-Mediated M1 Ubiquitination of Influenza A Virus. Virol. Sin. 2018, 33, 440–448. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Yu, M.; Zheng, W.; Liu, W. Nucleocytoplasmic shuttling of influenza A virus proteins. Viruses 2015, 7, 2668–2682. [Google Scholar] [CrossRef] [Green Version]
- Bedford, M.T.; Clarke, S.G. Protein arginine methylation in mammals: Who, what, and why. Mol. Cell 2009, 33, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Blackwell, E.; Ceman, S. Arginine methylation of RNA-binding proteins regulates cell function and differentiation. Mol. Reprod Dev. 2012, 79, 163–175. [Google Scholar] [CrossRef] [Green Version]
- Tarus, B.; Chevalier, C.; Richard, C.A.; Delmas, B.; Di Primo, C.; Slama-Schwok, A. Molecular dynamics studies of the nucleoprotein of influenza A virus: Role of the protein flexibility in RNA binding. PLoS ONE 2012, 7, e30038. [Google Scholar] [CrossRef] [Green Version]
- Shen, Y.F.; Chen, Y.H.; Chu, S.Y.; Lin, M.I.; Hsu, H.T.; Wu, P.Y.; Wu, C.J.; Liu, H.W.; Lin, F.Y.; Lin, G.; et al. E339...R416 salt bridge of nucleoprotein as a feasible target for influenza virus inhibitors. Proc. Natl. Acad. Sci. USA 2011, 108, 16515–16520. [Google Scholar] [CrossRef] [Green Version]
- Rehn, A.; Lawatscheck, J.; Jokisch, M.-L.; Mader, S.L.; Luo, Q.; Tippel, F.; Blank, B.; Richter, K.; Lang, K.; Kaila, V.R.I.; et al. A methylated lysine is a switch point for conformational communication in the chaperone Hsp90. Nat. Commun. 2020, 11, 1219. [Google Scholar] [CrossRef] [PubMed]
- Yu, M.; Liu, X.; Cao, S.; Zhao, Z.; Zhang, K.; Xie, Q.; Chen, C.; Gao, S.; Bi, Y.; Sun, L.; et al. Identification and Characterization of Three Novel Nuclear Export Signals in the Influenza A Virus Nucleoprotein. J. Virol. 2012, 86, 4970–4980. [Google Scholar] [CrossRef] [Green Version]
- Dai, J.; Bercury, K.K.; Jin, W.; Macklin, W.B. Olig1 Acetylation and Nuclear Export Mediate Oligodendrocyte Development. J. Neurosci. 2015, 35, 15875–15893. [Google Scholar] [CrossRef]
- Baltus, G.A.; Kowalski, M.P.; Zhai, H.; Tutter, A.V.; Quinn, D.; Wall, D.; Kadam, S. Acetylation of sox2 induces its nuclear export in embryonic stem cells. Stem Cells 2009, 27, 2175–2184. [Google Scholar] [CrossRef]
- Kumar, S.; Maiti, S. The Effect of N-acetylation and N-methylation of Lysine Residue of Tat Peptide on its Interaction with HIV-1 TAR RNA. PLoS ONE 2013, 8, e77595. [Google Scholar] [CrossRef]
- Pumroy, R.A.; Ke, S.; Hart, D.J.; Zachariae, U.; Cingolani, G. Molecular determinants for nuclear import of influenza A PB2 by importin α isoforms 3 and 7. Structure 2015, 23, 374–384. [Google Scholar] [CrossRef] [Green Version]
- Rachakonda, P.S.; Veit, M.; Korte, T.; Ludwig, K.; Böttcher, C.; Huang, Q.; Schmidt, M.F.G.; Herrmann, A. The relevance of salt bridges for the stability of the influenza virus hemagglutinin. FASEB J. 2007, 21, 995–1002. [Google Scholar] [CrossRef]
- Sanchez, E.L.; Lagunoff, M. Viral activation of cellular metabolism. Virology 2015, 479–480, 609–618. [Google Scholar] [CrossRef] [Green Version]
- Hsu, M.-C.; Tsai, Y.-L.; Lin, C.-H.; Pan, M.-R.; Shan, Y.-S.; Cheng, T.-Y.; Cheng, S.H.-C.; Chen, L.-T.; Hung, W.-C. Protein arginine methyltransferase 3-induced metabolic reprogramming is a vulnerable target of pancreatic cancer. J. Hematol. Oncol. 2019, 12. [Google Scholar] [CrossRef] [Green Version]
- Prakasam, G.; Iqbal, M.A.; Bamezai, R.N.K.; Mazurek, S. Posttranslational Modifications of Pyruvate Kinase M2: Tweaks that Benefit Cancer. Front. Oncol. 2018, 8, 22. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jin, Q.; Zhuang, L.; Lai, B.; Wang, C.; Li, W.; Dolan, B.; Lu, Y.; Wang, Z.; Zhao, K.; Peng, W.; et al. Gcn5 and PCAF negatively regulate interferon-β production through HAT-independent inhibition of TBK1. EMBO Rep. 2014, 15, 1192–1201. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ma, J.; Wu, R.; Xu, G.; Cheng, Y.; Wang, Z.; Wang, H.A.; Yan, Y.; Li, J.; Sun, J. Acetylation at K108 of the NS1 protein is important for the replication and virulence of influenza virus. Vet. Res. 2020, 51. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zee, B.M.; Levin, R.S.; DiMaggio, P.A.; Garcia, B.A. Global turnover of histone post-translational modifications and variants in human cells. Epigenetics Chromatin 2010, 3, 22. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kamieniarz, K.; Schneider, R. Tools to Tackle Protein Acetylation. Chem. Biol. 2009, 16, 1027–1029. [Google Scholar] [CrossRef] [PubMed] [Green Version]

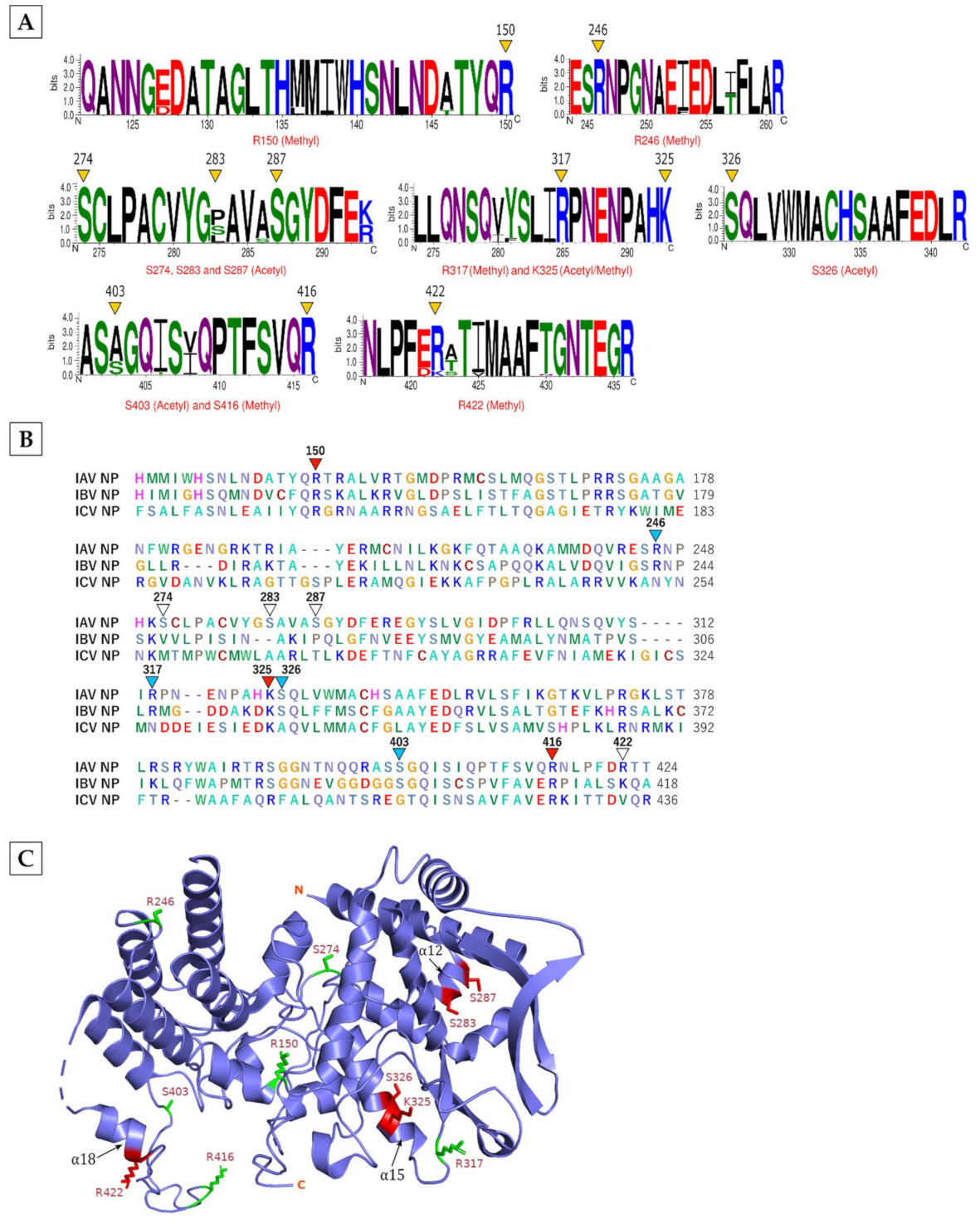





| Viral Protein | Modification | Modified Peptide |
|---|---|---|
| M1 | Methyl [K95] * | [R].FVQNALNGNGDPNNMDK.[A] |
| Methyl [K98] * | [R].FVQNALNGNGDPNNMDKAVK.[L] | |
| Methyl [R160] | [R].MGAVTTEVAFGLVCATCEQIADSQHR.[S] | |
| Methyl [K230] * | [R].TIGTHPSSSAGLKNDLLENLQAYQK.[R] | |
| Methyl [K242] | [K].NDLLENLQAYQK.[R] | |
| Acetyl [S195] | [K].AMEQMAGSSEQAAEAMEVASQAR.[Q] | |
| Acetyl [S196] | [K].AMEQMAGSSEQAAEAMEVASQAR.[Q] | |
| Acetyl [S207] | [K].AMEQMAGSSEQAAEAMEVASQAR.[Q] | |
| Acetyl [K95] * | [R].FVQNALNGNGDPNNMDK.[A] | |
| Allysine [K35] | [R].LEDVFAGKNTDLEVLMEWLK.[T] | |
| Allysine [K98] * | [R].FVQNALNGNGDPNNMDKAVK.[L] | |
| Allysine [K230] * | [R].TIGTHPSSSAGLKNDLLENLQAYQK.[R] | |
| NP | Methyl [R150] | [R].QANNGDDATAGLTHMMIWHSNLNDATYQR.[T] |
| Methyl [R246] | [R].ESRNPGNAEFEDLTFLAR.[S] | |
| Methyl [R317] | [R].LLQNSQVYSLIRPNENPAHK.[S] | |
| Methyl [K325] * | [R].LLQNSQVYSLIRPNENPAHK.[S] | |
| Methyl [R416] | [R].ASAGQISIQPTFSVQR.[N] | |
| Methyl [R422] | [R].NLPFDRTTVMAAFTGNTEGR.[T] | |
| Acetyl [S274] | [K].SCLPACVYGSAVASGYDFER.[E] | |
| Acetyl [S283] | [K].SCLPACVYGSAVASGYDFER.[E] | |
| Acetyl [S287] | [K].SCLPACVYGSAVASGYDFER.[E] | |
| Acetyl [S326] | [K].SQLVWMACHSAAFEDLR.[V] | |
| Acetyl [S403] | [R].ASSGQISIQPTFSVQR.[N] | |
| Acetyl [K325] * | [R].LLQNSQVYSLIRPNENPAHK.[S] | |
| PA | Methyl [K102] * | [R].TMAWTVVNSICNTTGAEKPK.[F] |
| Methyl [K104] * | [R].TMAWTVVNSICNTTGAEKPK.[F] | |
| Acetyl [K102] * | [R].TMAWTVVNSICNTTGAEKPK.[F] | |
| Acetyl [K104] * | [R].TMAWTVVNSICNTTGAEKPK.[F] | |
| Acetyl [S631] | [K].GVEESSIGK.[V] | |
| PB1 | N-term acetyl | [-].MDVNPTLLFLK.[V] |
| PB2 | Allysine [K718] | [R].YGPALSINELSNLAK.[G] |
| NS1 | Methyl [R193] | [K].NAVGVLIGGLEWNDNTVR.[V] |
| Allysine [K110] | [K].QKVAGPLCIR.[M] | |
| N-term acetyl | [-].MDPNTVSSFQVDCFLWHVR.[K] | |
| NS2 | N-term acetyl | [-].MDPNTVSSFQDILLR.[M] |
| HA | Methyl [R91] | [K].CNIAGWLLGNPECDLLLPVR.[S] |
| Methyl [R269] | [K].VRDQAGRMNYYWTLLKPGDTIIFEANGNLVAPR.[Y] | |
| Methyl [K252] | [R].MNYYWTLLKPGDTIIFEANGNLIAPR.[Y] | |
| Allysine [K62] | [R].LKGITPLQLGK.[C] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ahmed, F.; Kleffmann, T.; Husain, M. Acetylation, Methylation and Allysine Modification Profile of Viral and Host Proteins during Influenza A Virus Infection. Viruses 2021, 13, 1415. https://doi.org/10.3390/v13071415
Ahmed F, Kleffmann T, Husain M. Acetylation, Methylation and Allysine Modification Profile of Viral and Host Proteins during Influenza A Virus Infection. Viruses. 2021; 13(7):1415. https://doi.org/10.3390/v13071415
Chicago/Turabian StyleAhmed, Farjana, Torsten Kleffmann, and Matloob Husain. 2021. "Acetylation, Methylation and Allysine Modification Profile of Viral and Host Proteins during Influenza A Virus Infection" Viruses 13, no. 7: 1415. https://doi.org/10.3390/v13071415
APA StyleAhmed, F., Kleffmann, T., & Husain, M. (2021). Acetylation, Methylation and Allysine Modification Profile of Viral and Host Proteins during Influenza A Virus Infection. Viruses, 13(7), 1415. https://doi.org/10.3390/v13071415






