Functional Cure of Hepatitis B Virus Infection in Individuals With HIV-Coinfection: A Literature Review
Abstract
1. Introduction
2. Functional Cure in HIV-HBV Co-Infected Individuals Undergoing Potent Anti-HBV Therapy
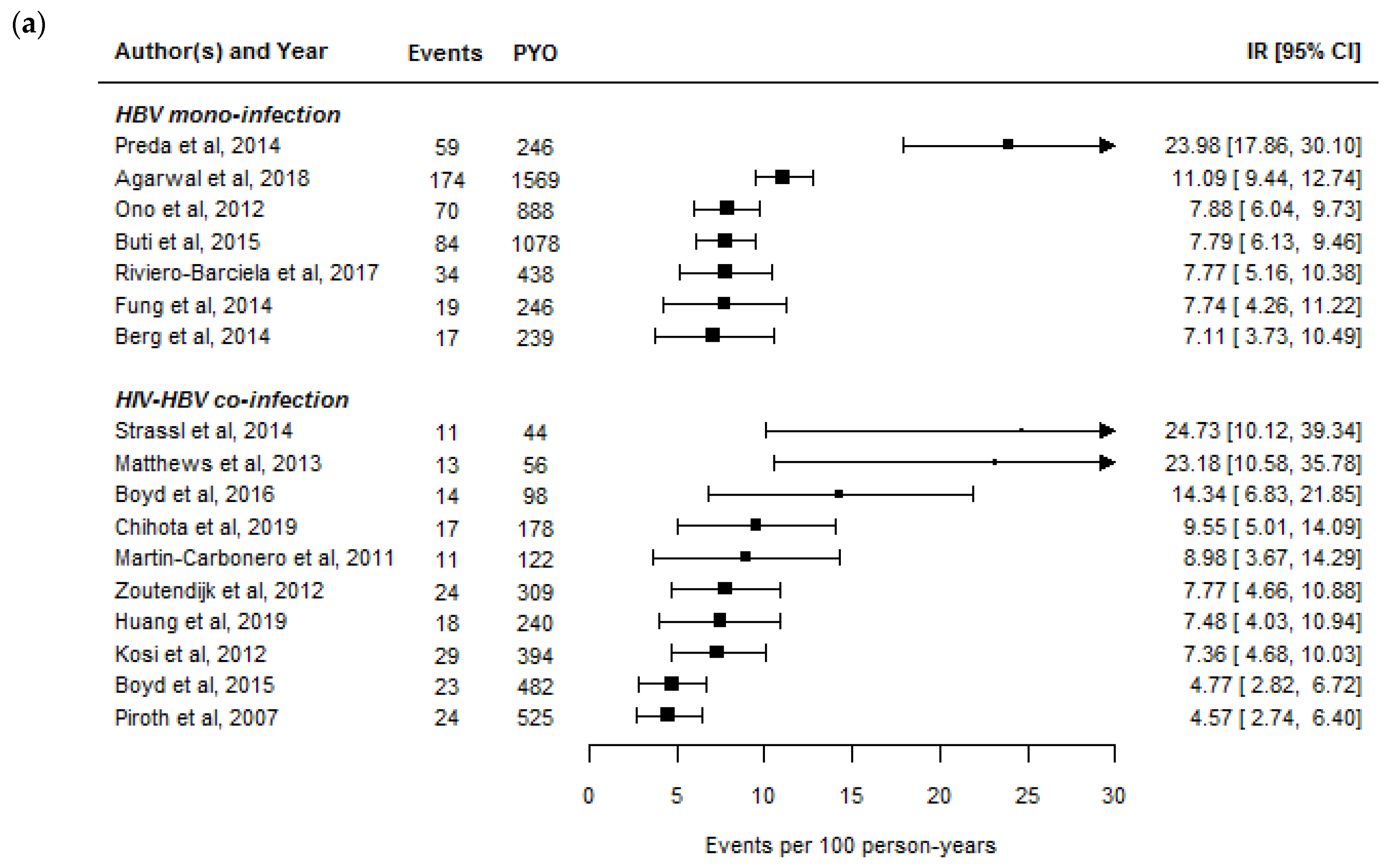
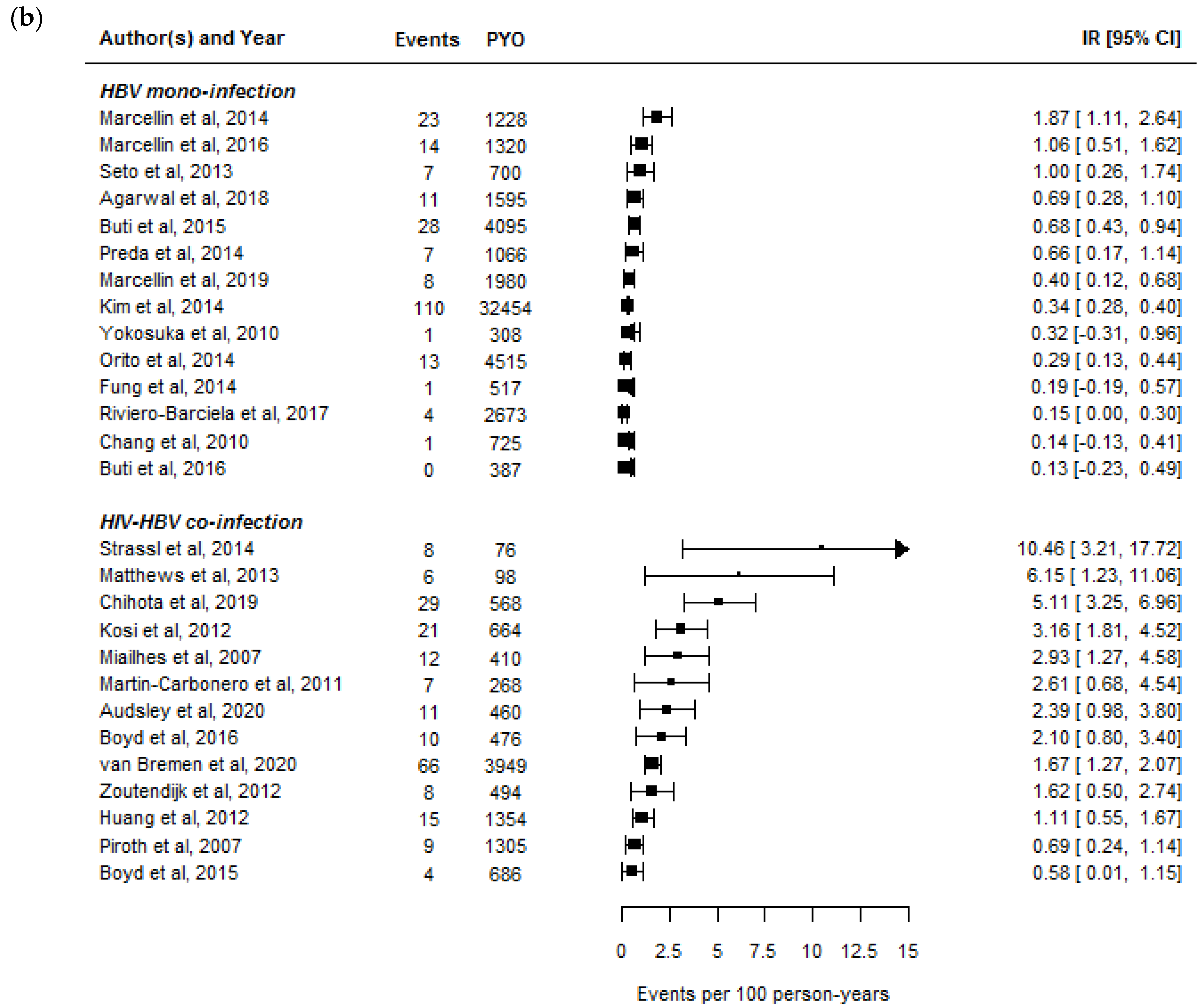
2.1. Rates of HBeAg- and HBsAg-Seroclearance in HIV-HBV Co-Infected Individuals
2.2. Rates of HBeAg- and HBsAg-Seroclearance between HBV Mono-Infected and HIV-HBV Co-Infected Individuals
3. Determinants of Functional Cure during Treated HIV-HBV Co-Infection
3.1. Phase of HBV Infection
3.2. HBV (Sub-)Genotypes and HBeAg-Negative Variants
3.3. Genetic Variability of the Host
3.4. Immunosuppression
4. Surrogate Markers of HBV Functional Cure in HIV-HBV Co-Infection
4.1. Direct Markers of HBV Activity
4.1.1. Quantification of Hepatitis B Surface Antigen
4.1.2. Quantification of Hepatitis B Surface Antigen Protein Composition
4.1.3. Quantification of Hepatitis B Core-Related Antigen
4.1.4. Quantification of HBV RNA
4.2. Indirect Markers of HBV Activity
4.2.1. Quantification of Interferon-Gamma-Inducible Protein 10
4.2.2. Quantification of Anti-Hepatitis B Core Antibodies
5. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Leumi, S.; Bigna, J.J.; Amougou, M.A.; Ngouo, A.; Nyaga, U.F.; Noubiap, J.J. Global burden of hepatitis b infection in people living with human immunodeficiency virus: A systematic review and meta-analysis. Clin. Infect. Dis. 2020, 71, 2799–2806. [Google Scholar] [CrossRef]
- Falade-Nwulia, O.; Seaberg, E.C.; Rinaldo, C.R.; Badri, S.; Witt, M.; Thio, C.L. Comparative risk of liver-related mortality from chronic hepatitis B versus chronic hepatitis C virus infection. Clin. Infect. Dis. 2012, 55, 507–513. [Google Scholar] [CrossRef]
- Nina Kim, H.; Newcomb, C.W.; Carbonari, D.M.; Roy, J.A.; Torgersen, J.; Althoff, K.N.; Kitahata, M.M.; Rajender Reddy, K.; Lim, J.K.; Silverberg, M.J.; et al. Risk of Hepatocellular carcinoma with hepatitis B viremia among HIV/hepatitis B virus-coinfected persons in North America. Hepatology 2021. [Google Scholar] [CrossRef]
- Matthews, G.V.; Bartholomeusz, A.; Locarnini, S.; Ayres, A.; Sasaduesz, J.; Seaberg, E.; Cooper, D.A.; Lewin, S.; Dore, G.J.; Thio, C.L. Characteristics of drug resistant HBV in an international collaborative study of HIV-HBV-infected individuals on extended lamivudine therapy. AIDS 2006, 20, 863–870. [Google Scholar] [CrossRef] [PubMed]
- Boyd, A.; Moh, R.; Gabillard, D.; le Carrou, J.; Danel, C.; Anglaret, X.; Eholie, S.P.; Maylin, S.; Delaugerre, C.; Zoulim, F.; et al. Low risk of lamivudine-resistant HBV and hepatic flares in treated HIV-HBV-coinfected patients from Cote d’Ivoire. Antivir. Ther. 2015, 20, 643–654. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Xie, J.; Han, Y.; Wang, H.; Zhu, T.; Wang, N.; Lv, W.; Guo, F.; Qiu, Z.; Li, Y.; et al. Lamivudine monotherapy-based cART is efficacious for HBV treatment in HIV/HBV coinfection when baseline HBV DNA <20,000 IU/mL. J. Acquir. Immune Defic. Syndr. 2016, 72, 39–45. [Google Scholar] [CrossRef] [PubMed]
- Thio, C.L.; Smeaton, L.; Hollabaugh, K.; Saulynas, M.; Hwang, H.; Saravanan, S.; Kulkarni, S.; Hakim, J.; Nyirenda, M.; Iqbal, H.S.; et al. Comparison of HBV-active HAART regimens in an HIV-HBV multinational cohort: Outcomes through 144 weeks. AIDS 2015, 29, 1173–1182. [Google Scholar] [CrossRef]
- Boyd, A.; Gozlan, J.; Maylin, S.; Delaugerre, C.; Peytavin, G.; Girard, P.M.; Zoulim, F.; Lacombe, K. Persistent viremia in human immunodeficiency virus/hepatitis B coinfected patients undergoing long-term tenofovir: Virological and clinical implications. Hepatology 2014, 60, 497–507. [Google Scholar] [CrossRef] [PubMed]
- Gallant, J.; Brunetta, J.; Crofoot, G.; Benson, P.; Mills, A.; Brinson, C.; Oka, S.; Cheng, A.; Garner, W.; Fordyce, M.; et al. Brief report: Efficacy and safety of switching to a single-tablet regimen of elvitegravir/cobicistat/emtricitabine/tenofovir alafenamide in HIV-1/hepatitis B-coinfected adults. J. Acquir. Immune Defic. Syndr. 2016, 73, 294–298. [Google Scholar] [CrossRef] [PubMed]
- Boyd, A.; Bottero, J.; Miailhes, P.; Lascoux-Combe, C.; Rougier, H.; Girard, P.M.; Serfaty, L.; Lacombe, K. Liver fibrosis regression and progression during controlled hepatitis B virus infection among HIV-HBV patients treated with tenofovir disoproxil fumarate in France: A prospective cohort study. J. Int. AIDS Soc. 2017, 20, 21426. [Google Scholar] [CrossRef]
- Marcellin, P.; Gane, E.; Buti, M.; Afdhal, N.; Sievert, W.; Jacobson, I.M.; Washington, M.K.; Germanidis, G.; Flaherty, J.F.; Aguilar Schall, R.; et al. Regression of cirrhosis during treatment with tenofovir disoproxil fumarate for chronic hepatitis B: A 5-year open-label follow-up study. Lancet 2013, 381, 468–475. [Google Scholar] [CrossRef]
- Sterling, R.K.; King, W.C.; Khalili, M.; Chung, R.T.; Sulkowski, M.; Jain, M.K.; Lisker-Melman, M.; Ghany, M.G.; Wong, D.K.; Hinerman, A.S.; et al. A prospective study evaluating changes in histology, clinical and virologic outcomes in HBV-HIV co-infected adults in North America. Hepatology 2021. [Google Scholar] [CrossRef]
- European Association for the Study of the Liver. EASL 2017 clinical practice guidelines on the management of hepatitis B virus infection. J. Hepatol. 2017, 67, 370–398. [Google Scholar] [CrossRef]
- Servant-Delmas, A.; Mercier-Darty, M.; Ly, T.D.; Wind, F.; Alloui, C.; Sureau, C.; Laperche, S. Variable capacity of 13 hepatitis B virus surface antigen assays for the detection of HBsAg mutants in blood samples. J. Clin. Virol. 2012, 53, 338–345. [Google Scholar] [CrossRef] [PubMed]
- Cornberg, M.; Lok, A.S.; Terrault, N.A.; Zoulim, F.; EASL-AASLD HBV Treatment Endpoints Conference Faculty. Guidance for design and endpoints of clinical trials in chronic hepatitis B—Report from the 2019 EASL-AASLD HBV Treatment Endpoints Conference. J. Hepatol. 2020, 72, 539–557. [Google Scholar] [CrossRef] [PubMed]
- Dore, G.J.; Soriano, V.; Rockstroh, J.; Kupfer, B.; Tedaldi, E.; Peters, L.; Neuhaus, J.; Puoti, M.; Klein, M.B.; Mocroft, A.; et al. Frequent hepatitis B virus rebound among HIV-hepatitis B virus-coinfected patients following antiretroviral therapy interruption. AIDS 2010, 24, 857–865. [Google Scholar] [CrossRef] [PubMed]
- Boyd, A.; Maylin, S.; Gozlan, J.; Delaugerre, C.; Simon, F.; Girard, P.M.; Lacombe, K. Use of hepatitis B surface and “e” antigen quantification during extensive treatment with tenofovir in patients co-infected with HIV-HBV. Liver Int. 2015, 35, 795–804. [Google Scholar] [CrossRef]
- Boyd, A.; Maylin, S.; Moh, R.; Mahjoub, N.; Gabillard, D.; Eholie, S.P.; Danel, C.; Anglaret, X.; Zoulim, F.; Girard, P.M.; et al. Hepatitis B surface antigen quantification as a predictor of seroclearance during treatment in HIV-hepatitis B virus coinfected patients from Sub-Saharan Africa. J. Gastroenterol. Hepatol. 2016, 31, 634–644. [Google Scholar] [CrossRef] [PubMed]
- Chihota, B.V.; Wandeler, G.; Chilengi, R.; Mulenga, L.; Chung, R.T.; Bhattacharya, D.; Egger, M.; Vinikoor, M.J. High rates of hepatitis B virus (HBV) functional cure among human immunodeficiency virus-HBV coinfected patients on antiretroviral therapy in Zambia. J. Infect. Dis. 2020, 221, 218–222. [Google Scholar] [CrossRef]
- Huang, Y.S.; Sun, H.Y.; Chang, S.Y.; Chuang, Y.C.; Cheng, A.; Huang, S.H.; Huang, Y.C.; Chen, G.J.; Lin, K.Y.; Su, Y.C.; et al. Long-term virological and serologic responses of chronic hepatitis B virus infection to tenofovir disoproxil fumarate-containing regimens in patients with HIV and hepatitis B coinfection. Hepatol. Int. 2019, 13, 431–439. [Google Scholar] [CrossRef]
- Kosi, L.; Reiberger, T.; Payer, B.A.; Grabmeier-Pfistershammer, K.; Strassl, R.; Rieger, A.; Peck-Radosavljevic, M. Five-year on-treatment efficacy of lamivudine-, tenofovir- and tenofovir + emtricitabine-based HAART in HBV-HIV-coinfected patients. J. Viral Hepat. 2012, 19, 801–810. [Google Scholar] [CrossRef]
- Martin-Carbonero, L.; Teixeira, T.; Poveda, E.; Plaza, Z.; Vispo, E.; Gonzalez-Lahoz, J.; Soriano, V. Clinical and virological outcomes in HIV-infected patients with chronic hepatitis B on long-term nucleos(t)ide analogues. AIDS 2011, 25, 73–79. [Google Scholar] [CrossRef]
- Matthews, G.V.; Ali, R.J.; Avihingsanon, A.; Amin, J.; Hammond, R.; Bowden, S.; Lewin, S.R.; Sasadeusz, J.; Littlejohn, M.; Locarnini, S.L.; et al. Quantitative HBsAg and HBeAg predict hepatitis B seroconversion after initiation of HAART in HIV-HBV coinfected individuals. PLoS ONE 2013, 8, e61297. [Google Scholar] [CrossRef]
- Piroth, L.; Sene, D.; Pol, S.; Goderel, I.; Lacombe, K.; Martha, B.; Rey, D.; Loustau-Ratti, V.; Bergmann, J.F.; Pialoux, G.; et al. Epidemiology, diagnosis and treatment of chronic hepatitis B in HIV-infected patients (EPIB 2005 STUDY). AIDS 2007, 21, 1323–1331. [Google Scholar] [CrossRef] [PubMed]
- Strassl, R.; Reiberger, T.; Honsig, C.; Payer, B.A.; Mandorfer, M.; Grabmeier-Pfistershammer, K.; Rieger, A.; Kundi, M.; Grundtner, P.; Peck-Radosavljevic, M.; et al. Viral determinants predicting hepatitis B surface antigen (HBsAg) seroclearance in HIV-/HBV-coinfected patients. J. Viral Hepat. 2014, 21, 508–516. [Google Scholar] [CrossRef] [PubMed]
- Dezanet, L.N.C.; Miailhes, P.; Lascoux-Combe, C.; Chas, J.; Maylin, S.; Gabassi, A.; Rougier, H.; Delaugerre, C.; Lacombe, K.; Boyd, A. Persistent HBV replication and serological response during up to fifteen years of tenofovir-based antiretroviral therapy in HIV-hepatitis B coinfected patients: A multicenter prospective cohort study. MedRxiv 2021. [Google Scholar] [CrossRef]
- Zoutendijk, R.; Zaaijer, H.L.; de Vries-Sluijs, T.E.; Reijnders, J.G.; Mulder, J.W.; Kroon, F.P.; Richter, C.; van der Eijk, A.A.; Sonneveld, M.J.; Hansen, B.E.; et al. Hepatitis B surface antigen declines and clearance during long-term tenofovir therapy in patients coinfected with HBV and HIV. J. Infect. Dis. 2012, 206, 974–980. [Google Scholar] [CrossRef]
- Miailhes, P.; Trabaud, M.A.; Pradat, P.; Lebouche, B.; Chevallier, M.; Chevallier, P.; Zoulim, F.; Trepo, C. Impact of highly active antiretroviral therapy (HAART) on the natural history of hepatitis B virus (HBV) and HIV coinfection: Relationship between prolonged efficacy of HAART and HBV surface and early antigen seroconversion. Clin. Infect. Dis. 2007, 45, 624–632. [Google Scholar] [CrossRef][Green Version]
- Van Bremen, K.; Hoffmann, C.; Mauss, S.; Lutz, T.; Ingiliz, P.; Spinner, C.D.; Scholten, S.; Schwarze-Zander, C.; Berger, F.; Breitschwerdt, S.; et al. Obstacles to HBV functional cure: Late presentation in HIV and its impact on HBV seroconversion in HIV/HBV coinfection. Liver Int. 2020, 40, 2978–2981. [Google Scholar] [CrossRef]
- Audsley, J.; Avihingsanon, A.; Littlejohn, M.; Bowden, S.; Matthews, G.V.; Fairley, C.K.; Lewin, S.R.; Sasadeusz, J. Long-term TDF-inclusive ART and Progressive rates of HBsAg loss in HIV-HBV coinfection-lessons for functional HBV cure? J. Acquir. Immune Defic. Syndr. 2020, 84, 527–533. [Google Scholar] [CrossRef] [PubMed]
- Boyd, A.; Canini, L.; Gozlan, J.; Lascoux-Combe, C.; Miailhes, P.; Fonquernie, L.; Girard, P.M.; Lacombe, K. Development of anti-hepatitis B surface (HBs) antibodies after HBs antigen loss in HIV-hepatitis B virus co-infected patients. J. Clin. Virol. 2017, 95, 55–60. [Google Scholar] [CrossRef]
- Agarwal, K.; Brunetto, M.; Seto, W.K.; Lim, Y.S.; Fung, S.; Marcellin, P.; Ahn, S.H.; Izumi, N.; Chuang, W.L.; Bae, H.; et al. 96weeks treatment of tenofovir alafenamide vs. tenofovir disoproxil fumarate for hepatitis B virus infection. J. Hepatol. 2018, 68, 672–681. [Google Scholar] [CrossRef]
- Buti, M.; Tsai, N.; Petersen, J.; Flisiak, R.; Gurel, S.; Krastev, Z.; Aguilar Schall, R.; Flaherty, J.F.; Martins, E.B.; Charuworn, P.; et al. Seven-year efficacy and safety of treatment with tenofovir disoproxil fumarate for chronic hepatitis B virus infection. Dig. Dis. Sci. 2015, 60, 1457–1464. [Google Scholar] [CrossRef]
- Fung, S.; Kwan, P.; Fabri, M.; Horban, A.; Pelemis, M.; Hann, H.W.; Gurel, S.; Caruntu, F.A.; Flaherty, J.F.; Massetto, B.; et al. Randomized comparison of tenofovir disoproxil fumarate vs. emtricitabine and tenofovir disoproxil fumarate in patients with lamivudine-resistant chronic hepatitis B. Gastroenterology 2014, 146, 980–988. [Google Scholar] [CrossRef]
- Ono, A.; Suzuki, F.; Kawamura, Y.; Sezaki, H.; Hosaka, T.; Akuta, N.; Kobayashi, M.; Suzuki, Y.; Saitou, S.; Arase, Y.; et al. Long-term continuous entecavir therapy in nucleos(t)ide-naive chronic hepatitis B patients. J. Hepatol. 2012, 57, 508–514. [Google Scholar] [CrossRef]
- Preda, C.M.; Baicus, C.; Negreanu, L.; Tugui, L.; Olariu, S.V.; Andrei, A.; Zambatu, I.; Diculescu, M.M. Effectiveness of entecavir treatment and predictive factors for virologic response. Rev. Esp. Enferm. Dig. 2014, 106, 305–311. [Google Scholar]
- Riveiro-Barciela, M.; Tabernero, D.; Calleja, J.L.; Lens, S.; Manzano, M.L.; Rodriguez, F.G.; Crespo, J.; Piqueras, B.; Pascasio, J.M.; Comas, C.; et al. Effectiveness and safety of entecavir or tenofovir in a Spanish cohort of chronic hepatitis B patients: Validation of the page-B score to predict hepatocellular carcinoma. Dig. Dis. Sci. 2017, 62, 784–793. [Google Scholar] [CrossRef]
- Berg, T.; Zoulim, F.; Moeller, B.; Trinh, H.; Marcellin, P.; Chan, S.; Kitrinos, K.M.; Dinh, P.; Flaherty, J.F., Jr.; McHutchison, J.G.; et al. Long-term efficacy and safety of emtricitabine plus tenofovir DF vs. tenofovir DF monotherapy in adefovir-experienced chronic hepatitis B patients. J. Hepatol. 2014, 60, 715–722. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Sinn, D.H.; Kang, W.; Gwak, G.Y.; Paik, Y.H.; Choi, M.S.; Lee, J.H.; Koh, K.C.; Paik, S.W. Low-level viremia and the increased risk of hepatocellular carcinoma in patients receiving entecavir treatment. Hepatology 2017, 66, 335–343. [Google Scholar] [CrossRef] [PubMed]
- Chang, T.T.; Lai, C.L.; Kew Yoon, S.; Lee, S.S.; Coelho, H.S.; Carrilho, F.J.; Poordad, F.; Halota, W.; Horsmans, Y.; Tsai, N.; et al. Entecavir treatment for up to 5 years in patients with hepatitis B e antigen-positive chronic hepatitis B. Hepatology 2010, 51, 422–430. [Google Scholar] [CrossRef] [PubMed]
- Yokosuka, O.; Takaguchi, K.; Fujioka, S.; Shindo, M.; Chayama, K.; Kobashi, H.; Hayashi, N.; Sato, C.; Kiyosawa, K.; Tanikawa, K.; et al. Long-term use of entecavir in nucleoside-naive Japanese patients with chronic hepatitis B infection. J. Hepatol. 2010, 52, 791–799. [Google Scholar] [CrossRef]
- Orito, E.; Hasebe, C.; Kurosaki, M.; Osaki, Y.; Joko, K.; Watanabe, H.; Kimura, H.; Nishijima, N.; Kusakabe, A.; Izumi, N.; et al. Risk of hepatocellular carcinoma in cirrhotic hepatitis B virus patients during nucleoside/nucleotide analog therapy. Hepatol. Res. 2015, 45, 872–879. [Google Scholar] [CrossRef]
- Buti, M.; Gane, E.; Seto, W.K.; Chan, H.L.; Chuang, W.L.; Stepanova, T.; Hui, A.J.; Lim, Y.S.; Mehta, R.; Janssen, H.L.; et al. Tenofovir alafenamide versus tenofovir disoproxil fumarate for the treatment of patients with HBeAg-negative chronic hepatitis B virus infection: A randomised, double-blind, phase 3, non-inferiority trial. Lancet Gastroenterol. Hepatol. 2016, 1, 196–206. [Google Scholar] [CrossRef]
- Kim, G.A.; Lim, Y.S.; An, J.; Lee, D.; Shim, J.H.; Kim, K.M.; Lee, H.C.; Chung, Y.H.; Lee, Y.S.; Suh, D.J. HBsAg seroclearance after nucleoside analogue therapy in patients with chronic hepatitis B: Clinical outcomes and durability. Gut 2014, 63, 1325–1332. [Google Scholar] [CrossRef]
- Marcellin, P.; Wong, D.K.; Sievert, W.; Buggisch, P.; Petersen, J.; Flisiak, R.; Manns, M.; Kaita, K.; Krastev, Z.; Lee, S.S.; et al. Ten-year efficacy and safety of tenofovir disoproxil fumarate treatment for chronic hepatitis B virus infection. Liver Int. 2019, 39, 1868–1875. [Google Scholar] [CrossRef] [PubMed]
- Marcellin, P.; Zoulim, F.; Hezode, C.; Causse, X.; Roche, B.; Truchi, R.; Pauwels, A.; Ouzan, D.; Dumortier, J.; Pageaux, G.P.; et al. Effectiveness and safety of tenofovir disoproxil fumarate in chronic hepatitis B: A 3-year, prospective, real-world study in France. Dig. Dis. Sci. 2016, 61, 3072–3083. [Google Scholar] [CrossRef] [PubMed]
- Seto, W.K.; Wong, D.K.; Fung, J.; Huang, F.Y.; Lai, C.L.; Yuen, M.F. Reduction of hepatitis B surface antigen levels and hepatitis B surface antigen seroclearance in chronic hepatitis B patients receiving 10 years of nucleoside analogue therapy. Hepatology 2013, 58, 923–931. [Google Scholar] [CrossRef] [PubMed]
- Taniguchi, H.; Iwasaki, Y.; Aimi, M.; Shimazaki, G.; Moriya, A. Clinical features of chronic hepatitis B patients with low hepatitis B surface antigen levels and determinants of hepatitis B surface antigen seroclearance. JGH Open 2020, 4, 698–706. [Google Scholar] [CrossRef] [PubMed]
- Maylin, S.; Boyd, A.; Lavocat, F.; Gozlan, J.; Lascoux-Combe, C.; Miailhes, P.; Lassel, L.; Delaugerre, C.; Girard, P.M.; Zoulim, F.; et al. Kinetics of hepatitis B surface and e antigen and prediction of treatment response to tenofovir in antiretroviral-experienced HIV-hepatitis B virus-infected patients. AIDS 2012, 26, 939–949. [Google Scholar] [CrossRef]
- Lacombe, K.; Rockstroh, J. HIV and viral hepatitis coinfections: Advances and challenges. Gut 2012, 61 (Suppl. S1), i47–i58. [Google Scholar] [CrossRef]
- Gantner, P.; Cotte, L.; Allavena, C.; Bani-Sadr, F.; Huleux, T.; Duvivier, C.; Valantin, M.A.; Jacomet, C.; Joly, V.; Cheret, A.; et al. Higher rates of HBsAg clearance with tenofovir-containing therapy in HBV/HIV co-infection. PLoS ONE 2019, 14, e0215464. [Google Scholar] [CrossRef]
- Yang, R.; Gui, X.; Ke, H.; Xiong, Y.; Gao, S. Long-term observation on hepatitis B surface antigen seroclearance in therapy experienced HIV/HBV co-infected Chinese. J. Viral Hepat. 2020, 27, 127–134. [Google Scholar] [CrossRef]
- Lin, C.L.; Kao, J.H. Hepatitis B virus genotypes and variants. Cold Spring Harb. Perspect. Med. 2015, 5, a021436. [Google Scholar] [CrossRef] [PubMed]
- Bayliss, J.; Yuen, L.; Rosenberg, G.; Wong, D.; Littlejohn, M.; Jackson, K.; Gaggar, A.; Kitrinos, K.M.; Subramanian, G.M.; Marcellin, P.; et al. Deep sequencing shows that HBV basal core promoter and precore variants reduce the likelihood of HBsAg loss following tenofovir disoproxil fumarate therapy in HBeAg-positive chronic hepatitis B. Gut 2017, 66, 2013–2023. [Google Scholar] [CrossRef] [PubMed]
- Boyd, A.; Moh, R.; Maylin, S.; Abdou Chekaraou, M.; Mahjoub, N.; Gabillard, D.; Anglaret, X.; Eholie, S.P.; Delaugerre, C.; Danel, C.; et al. Precore G1896A mutation is associated with reduced rates of HBsAg seroclearance in treated HIV hepatitis B virus co-infected patients from western Africa. J. Viral Hepat. 2018, 25, 1121–1131. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; Guindon, S.; Rodrigo, A.; Wee, L.Y.; Inoue, M.; Thompson, A.J.; Locarnini, S.; Lim, S.G. Cumulative viral evolutionary changes in chronic hepatitis B virus infection precedes hepatitis B e antigen seroconversion. Gut 2013, 62, 1347–1355. [Google Scholar] [CrossRef]
- Kramvis, A.; Arakawa, K.; Yu, M.C.; Nogueira, R.; Stram, D.O.; Kew, M.C. Relationship of serological subtype, basic core promoter and precore mutations to genotypes/subgenotypes of hepatitis B virus. J. Med. Virol. 2008, 80, 27–46. [Google Scholar] [CrossRef]
- Tong, S.; Revill, P. Overview of hepatitis B viral replication and genetic variability. J. Hepatol. 2016, 64, S4–S16. [Google Scholar] [CrossRef]
- Lacombe, K.; Boyd, A.; Gozlan, J.; Lavocat, F.; Girard, P.M.; Zoulim, F. Drug-resistant and immune-escape HBV mutants in HIV-infected hosts. Antivir. Ther. 2010, 15, 493–497. [Google Scholar] [CrossRef][Green Version]
- Eschlimann, M.; Malve, B.; Velay, A.; Fenaux, H.; Berger, S.; Frippiat, J.P.; Zoulim, F.; Bensenane, M.; Bronowicki, J.P.; Goehringer, F.; et al. The variability of hepatitis B envelope is associated with HBs antigen persistence in either chronic or acute HBV genotype A infection. J. Clin. Virol. 2017, 94, 115–122. [Google Scholar] [CrossRef]
- Velay, A.; Jeulin, H.; Eschlimann, M.; Malve, B.; Goehringer, F.; Bensenane, M.; Frippiat, J.P.; Abraham, P.; Ismail, A.M.; Murray, J.M.; et al. Characterization of hepatitis B virus surface antigen variability and impact on HBs antigen clearance under nucleos(t)ide analogue therapy. J. Viral Hepat. 2016, 23, 387–398. [Google Scholar] [CrossRef]
- Li, S.; Zhao, K.; Liu, S.; Wu, C.; Yao, Y.; Cao, L.; Hu, X.; Zhou, Y.; Wang, Y.; Pei, R.; et al. HBsAg sT123N mutation induces stronger antibody responses to HBsAg and HBcAg and accelerates in vivo HBsAg clearance. Virus Res. 2015, 210, 119–125. [Google Scholar] [CrossRef]
- Boyd, A.; Moh, R.; Maylin, S.; Abdou Chekaraou, M.; Mahjoub, N.; Gabillard, D.; Anglaret, X.; Eholie, S.P.; Danel, C.; Delaugerre, C.; et al. Effect of hepatitis B virus (HBV) surface-gene variability on markers of replication during treated human immunodeficiency virus-HBV infection in Western Africa. Liver Int. 2019, 39, 280–289. [Google Scholar] [CrossRef] [PubMed]
- Koukoulioti, E.; Fischer, J.; Schott, E.; Fulop, B.; Heyne, R.; Berg, T.; van Bommel, F. Association of HLA-DPA1 and HLA-DPB1 polymorphisms with spontaneous HBsAg seroclearance in Caucasians. Liver Int. 2019, 39, 646–654. [Google Scholar] [CrossRef] [PubMed]
- Hosaka, T.; Suzuki, F.; Kobayashi, M.; Fukushima, T.; Kawamura, Y.; Sezaki, H.; Akuta, N.; Suzuki, Y.; Saitoh, S.; Arase, Y.; et al. HLA-DP genes polymorphisms associate with hepatitis B surface antigen kinetics and seroclearance during nucleot(s)ide analogue therapy. Liver Int. 2015, 35, 1290–1302. [Google Scholar] [CrossRef]
- Xu, J.; Zhan, Q.; Fan, Y.; Yu, Y.; Zeng, Z. Human genetic susceptibility to hepatitis B virus infection. Infect. Genet. Evol. 2021, 87, 104663. [Google Scholar] [CrossRef] [PubMed]
- Fischer, J.; Koukoulioti, E.; Schott, E.; Fulop, B.; Heyne, R.; Berg, T.; van Bommel, F. Polymorphisms in the Toll-like receptor 3 (TLR3) gene are associated with the natural course of hepatitis B virus infection in Caucasian population. Sci. Rep. 2018, 8, 12737. [Google Scholar] [CrossRef] [PubMed]
- Rybicka, M.; Woziwodzka, A.; Sznarkowska, A.; Romanowski, T.; Stalke, P.; Dreczewski, M.; Verrier, E.R.; Baumert, T.F.; Bielawski, K.P. Genetic variation in IL-10 influences the progression of hepatitis B infection. Int. J. Infect. Dis. 2020, 96, 260–265. [Google Scholar] [CrossRef] [PubMed]
- Liao, J.; Zhang, Q.; Liao, Y.; Cai, B.; Chen, J.; Li, L.; Wang, L. Association of T-cell immunoglobulin and mucin domain-containing molecule 3 (Tim-3) polymorphisms with susceptibility and disease progression of HBV infection. PLoS ONE 2014, 9, e98280. [Google Scholar] [CrossRef] [PubMed]
- Martin, M.P.; Qi, Y.; Goedert, J.J.; Hussain, S.K.; Kirk, G.D.; Hoots, W.K.; Buchbinder, S.; Carrington, M.; Thio, C.L. IL28B polymorphism does not determine outcomes of hepatitis B virus or HIV infection. J. Infect. Dis. 2010, 202, 1749–1753. [Google Scholar] [CrossRef]
- Michel Wolf, J.; Zingalli Bueno Pereira, V.R.; Zanetti Ballardin Roncato, P.A.; Castagna Wortmann, A.; Stumm, G.Z.; Oliveira da Silva, F.; Lunge, V.R.; Simon, D. The HLA-G 14-bp insertion/deletion polymorphism is associated with chronic hepatitis B in Southern Brazil: A case-control study. Hum. Immunol. 2020, 81, 79–84. [Google Scholar] [CrossRef]
- Yoshikawa, S.; Yoshio, S.; Yoshida, Y.; Tsutsui, Y.; Kawai, H.; Yamazoe, T.; Mori, T.; Osawa, Y.; Sugiyama, M.; Iwamoto, M.; et al. Impact of immune reconstitution-induced hepatic flare on HBsAg loss in HBV/HIV-1-coinfected patients. J. Infect. Dis. 2020. [Google Scholar] [CrossRef]
- Tout, I.; Loureiro, D.; Mansouri, A.; Soumelis, V.; Boyer, N.; Asselah, T. Hepatitis B surface antigen seroclearance: Immune mechanisms, clinical impact, importance for drug development. J. Hepatol. 2020, 73, 409–422. [Google Scholar] [CrossRef] [PubMed]
- Iser, D.M.; Avihingsanon, A.; Wisedopas, N.; Thompson, A.J.; Boyd, A.; Matthews, G.V.; Locarnini, S.A.; Slavin, J.; Desmond, P.V.; Lewin, S.R. Increased intrahepatic apoptosis but reduced immune activation in HIV-HBV co-infected patients with advanced immunosuppression. AIDS 2011, 25, 197–205. [Google Scholar] [CrossRef]
- Coffin, C.S.; Zhou, K.; Terrault, N.A. New and old biomarkers for diagnosis and management of chronic hepatitis B virus infection. Gastroenterology 2019, 156, 355–368. [Google Scholar] [CrossRef] [PubMed]
- Charre, C.; Levrero, M.; Zoulim, F.; Scholtes, C. Non-invasive biomarkers for chronic hepatitis B virus infection management. Antivir. Res. 2019, 169, 104553. [Google Scholar] [CrossRef]
- Thompson, A.J.; Nguyen, T.; Iser, D.; Ayres, A.; Jackson, K.; Littlejohn, M.; Slavin, J.; Bowden, S.; Gane, E.J.; Abbott, W.; et al. Serum hepatitis B surface antigen and hepatitis B e antigen titers: Disease phase influences correlation with viral load and intrahepatic hepatitis B virus markers. Hepatology 2010, 51, 1933–1944. [Google Scholar] [CrossRef] [PubMed]
- Janssen, H.L.; Sonneveld, M.J.; Brunetto, M.R. Quantification of serum hepatitis B surface antigen: Is it useful for the management of chronic hepatitis B? Gut 2012, 61, 641–645. [Google Scholar] [CrossRef] [PubMed]
- Boyd, A.; Lacombe, K.; Lavocat, F.; Maylin, S.; Miailhes, P.; Lascoux-Combe, C.; Delaugerre, C.; Girard, P.M.; Zoulim, F. Decay of ccc-DNA marks persistence of intrahepatic viral DNA synthesis under tenofovir in HIV-HBV co-infected patients. J. Hepatol. 2016, 65, 683–691. [Google Scholar] [CrossRef] [PubMed]
- Martinot-Peignoux, M.; Lapalus, M.; Asselah, T.; Marcellin, P. The role of HBsAg quantification for monitoring natural history and treatment outcome. Liver Int. 2013, 33 (Suppl. S1), 125–132. [Google Scholar] [CrossRef] [PubMed]
- Heermann, K.H.; Goldmann, U.; Schwartz, W.; Seyffarth, T.; Baumgarten, H.; Gerlich, W.H. Large surface proteins of hepatitis B virus containing the pre-s sequence. J. Virol. 1984, 52, 396–402. [Google Scholar] [CrossRef]
- Pfefferkorn, M.; Bohm, S.; Schott, T.; Deichsel, D.; Bremer, C.M.; Schroder, K.; Gerlich, W.H.; Glebe, D.; Berg, T.; van Bommel, F. Quantification of large and middle proteins of hepatitis B virus surface antigen (HBsAg) as a novel tool for the identification of inactive HBV carriers. Gut 2018, 67, 2045–2053. [Google Scholar] [CrossRef]
- Pfefferkorn, M.; Schott, T.; Bohm, S.; Deichsel, D.; Felkel, C.; Gerlich, W.H.; Glebe, D.; Wat, C.; Pavlovic, V.; Heyne, R.; et al. Composition of HBsAg is predictive of HBsAg loss during treatment in patients with HBeAg-positive chronic hepatitis B. J. Hepatol. 2021, 74, 283–292. [Google Scholar] [CrossRef]
- Rokuhara, A.; Tanaka, E.; Matsumoto, A.; Kimura, T.; Yamaura, T.; Orii, K.; Sun, X.; Yagi, S.; Maki, N.; Kiyosawa, K. Clinical evaluation of a new enzyme immunoassay for hepatitis B virus core-related antigen; a marker distinct from viral DNA for monitoring lamivudine treatment. J. Viral Hepat. 2003, 10, 324–330. [Google Scholar] [CrossRef] [PubMed]
- Testoni, B.; Lebosse, F.; Scholtes, C.; Berby, F.; Miaglia, C.; Subic, M.; Loglio, A.; Facchetti, F.; Lampertico, P.; Levrero, M.; et al. Serum hepatitis B core-related antigen (HBcrAg) correlates with covalently closed circular DNA transcriptional activity in chronic hepatitis B patients. J. Hepatol. 2019, 70, 615–625. [Google Scholar] [CrossRef] [PubMed]
- Dezanet, L.N.C.; Maylin, S.; Gabassi, A.; Rougier, H.; Miailhes, P.; Lascoux-Combe, C.; Chas, J.; Girard, P.M.; Delaugerre, C.; Zoulim, F.; et al. Correlation of serum hepatitis B core-related antigen with hepatitis B virus total intrahepatic DNA and covalently closed circular-DNA viral load in HIV-hepatitis B coinfection. AIDS 2020, 34, 1943–1949. [Google Scholar] [CrossRef] [PubMed]
- Chuaypen, N.; Posuwan, N.; Payungporn, S.; Tanaka, Y.; Shinkai, N.; Poovorawan, Y.; Tangkijvanich, P. Serum hepatitis B core-related antigen as a treatment predictor of pegylated interferon in patients with HBeAg-positive chronic hepatitis B. Liver Int. 2016, 36, 827–836. [Google Scholar] [CrossRef] [PubMed]
- Sonneveld, M.J.; van Oord, G.W.; van Campenhout, M.J.; De Man, R.A.; Janssen, H.L.A.; de Knegt, R.J.; Boonstra, A.; van der Eijk, A.A. Relationship between hepatitis B core-related antigen levels and sustained HBeAg seroconversion in patients treated with nucleo(s)tide analogues. J. Viral Hepat. 2019, 26, 828–834. [Google Scholar] [CrossRef] [PubMed]
- Dezanet, L.N.C.; Maylin, S.; Gabassi, A.; Rougier, H.; Miailhes, P.; Lascoux-Combe, C.; Chas, J.; Girard, P.M.; Delaugerre, C.; Lacombe, K.; et al. Kinetics of Hepatitis B Core-Related Antigen and Anti-Hepatitis B Core Antibody and Their Association With Serological Response in Human Immunodeficiency Virus-Hepatitis B Coinfection. J. Infect. Dis. 2020, 221, 1826–1837. [Google Scholar] [CrossRef]
- Volz, T.; Lutgehetmann, M.; Wachtler, P.; Jacob, A.; Quaas, A.; Murray, J.M.; Dandri, M.; Petersen, J. Impaired intrahepatic hepatitis B virus productivity contributes to low viremia in most HBeAg-negative patients. Gastroenterology 2007, 133, 843–852. [Google Scholar] [CrossRef] [PubMed]
- Giersch, K.; Allweiss, L.; Volz, T.; Dandri, M.; Lutgehetmann, M. Serum HBV pgRNA as a clinical marker for cccDNA activity. J. Hepatol. 2017, 66, 460–462. [Google Scholar] [CrossRef]
- van Bommel, F.; Bartens, A.; Mysickova, A.; Hofmann, J.; Kruger, D.H.; Berg, T.; Edelmann, A. Serum hepatitis B virus RNA levels as an early predictor of hepatitis B e antigen seroconversion during treatment with polymerase inhibitors. Hepatology 2015, 61, 66–76. [Google Scholar] [CrossRef]
- Van Bommel, F.; van Bommel, A.; Krauel, A.; Wat, C.; Pavlovic, V.; Yang, L.; Deichsel, D.; Berg, T.; Bohm, S. Serum HBV RNA as a predictor of peginterferon Alfa-2a response in patients with HBeAg-positive chronic hepatitis B. J. Infect. Dis. 2018, 218, 1066–1074. [Google Scholar] [CrossRef]
- Wang, J.; Zhao, J.H.; Wang, P.P.; Xiang, G.J. Expression of CXC chemokine IP-10 in patients with chronic hepatitis B. Hepatobiliary Pancreat. Dis. Int. 2008, 7, 45–50. [Google Scholar]
- Bayard, F.; Godon, O.; Nalpas, B.; Costentin, C.; Zhu, R.; Soussan, P.; Vallet-Pichard, A.; Fontaine, H.; Mallet, V.; Pol, S.; et al. T-cell responses to hepatitis B splice-generated protein of hepatitis B virus and inflammatory cytokines/chemokines in chronic hepatitis B patients. ANRS study: HB EP 02 HBSP-FIBRO. J. Viral Hepat. 2012, 19, 872–880. [Google Scholar] [CrossRef]
- Sonneveld, M.J.; Arends, P.; Boonstra, A.; Hansen, B.E.; Janssen, H.L. Serum levels of interferon-gamma-inducible protein 10 and response to peginterferon therapy in HBeAg-positive chronic hepatitis B. J. Hepatol. 2013, 58, 898–903. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhao, C.; Zhang, L.; Yu, W.; Shen, C.; Wang, W.; Zhen, Z.; Zhou, J. Predictive value of interferon-gamma inducible protein 10 kD for hepatitis B e antigen clearance and hepatitis B surface antigen decline during pegylated interferon alpha therapy in chronic hepatitis B patients. Antivir. Res. 2014, 103, 51–59. [Google Scholar] [CrossRef] [PubMed]
- Papatheodoridis, G.; Goulis, J.; Manolakopoulos, S.; Margariti, A.; Exarchos, X.; Kokkonis, G.; Hadziyiannis, E.; Papaioannou, C.; Manesis, E.; Pectasides, D.; et al. Changes of HBsAg and interferon-inducible protein 10 serum levels in naive HBeAg-negative chronic hepatitis B patients under 4-year entecavir therapy. J. Hepatol. 2014, 60, 62–68. [Google Scholar] [CrossRef] [PubMed]
- Hattab, S.; Guiguet, M.; Carcelain, G.; Fourati, S.; Guihot, A.; Autran, B.; Caby, F.; Marcelin, A.G.; Costagliola, D.; Katlama, C. Soluble biomarkers of immune activation and inflammation in HIV infection: Impact of 2 years of effective first-line combination antiretroviral therapy. HIV Med. 2015, 16, 553–562. [Google Scholar] [CrossRef]
- Noel, N.; Lerolle, N.; Lecuroux, C.; Goujard, C.; Venet, A.; Saez-Cirion, A.; Avettand-Fenoel, V.; Meyer, L.; Boufassa, F.; Lambotte, O.; et al. Immunologic and virologic progression in HIV controllers: The role of viral “Blips” and immune activation in the ANRS CO21 CODEX study. PLoS ONE 2015, 10, e0131922. [Google Scholar] [CrossRef]
- Simmons, R.P.; Scully, E.P.; Groden, E.E.; Arnold, K.B.; Chang, J.J.; Lane, K.; Lifson, J.; Rosenberg, E.; Lauffenburger, D.A.; Altfeld, M. HIV-1 infection induces strong production of IP-10 through TLR7/9-dependent pathways. AIDS 2013, 27, 2505–2517. [Google Scholar] [CrossRef]
- Giarda, P.; Avihingsanon, A.; Sasadeusz, J.; Audsley, J.; Marks, P.; Matthews, G.; Ruxrungtham, K.; Lewin, S.R.; Crane, M. CXCL-10, interleukin-12 and interleukin-21 are not immunological predictors of HBeAg seroconversion in HIV-1-HBV coinfection following HBV-active antiretroviral therapy. Antivir. Ther. 2014, 19, 429–433. [Google Scholar] [CrossRef]
- Yuan, Q.; Song, L.W.; Liu, C.J.; Li, Z.; Liu, P.G.; Huang, C.H.; Yan, Y.; Ge, S.X.; Wang, Y.B.; Peng, C.Y.; et al. Quantitative hepatitis B core antibody level may help predict treatment response in chronic hepatitis B patients. Gut 2013, 62, 182–184. [Google Scholar] [CrossRef] [PubMed]
- Fan, R.; Sun, J.; Yuan, Q.; Xie, Q.; Bai, X.; Ning, Q.; Cheng, J.; Yu, Y.; Niu, J.; Shi, G.; et al. Baseline quantitative hepatitis B core antibody titre alone strongly predicts HBeAg seroconversion across chronic hepatitis B patients treated with peginterferon or nucleos(t)ide analogues. Gut 2016, 65, 313–320. [Google Scholar] [CrossRef] [PubMed]
- Kardava, L.; Moir, S.; Wang, W.; Ho, J.; Buckner, C.M.; Posada, J.G.; O’Shea, M.A.; Roby, G.; Chen, J.; Sohn, H.W.; et al. Attenuation of HIV-associated human B cell exhaustion by siRNA downregulation of inhibitory receptors. J. Clin. Investig. 2011, 121, 2614–2624. [Google Scholar] [CrossRef] [PubMed]
- Cruchet, R.; Dezanet, L.N.C.; Maylin, S.; Gabassi, A.; Rougier, H.; Miailhes, P.; Lascoux-Combe, C.; Chas, J.; Girard, P.M.; Delaugerre, C.; et al. Association of hepatitis B core-related antigen and antihepatitis B core antibody with liver fibrosis evolution in human immunodeficiency virus-hepatitis B virus coinfected patients during treatment with tenofovir. Open Forum Infect. Dis. 2020, 7, ofaa215. [Google Scholar] [CrossRef] [PubMed]
- Brouwer, W.P.; Xie, Q.; Sonneveld, M.J.; Zhang, N.; Zhang, Q.; Tabak, F.; Streinu-Cercel, A.; Wang, J.Y.; Idilman, R.; Reesink, H.W.; et al. Adding pegylated interferon to entecavir for hepatitis B e antigen-positive chronic hepatitis B: A multicenter randomized trial (ARES study). Hepatology 2015, 61, 1512–1522. [Google Scholar] [CrossRef] [PubMed]
- Bourliere, M.; Rabiega, P.; Ganne-Carrie, N.; Serfaty, L.; Marcellin, P.; Barthe, Y.; Thabut, D.; Guyader, D.; Hezode, C.; Picon, M.; et al. Effect on HBs antigen clearance of addition of pegylated interferon alfa-2a to nucleos(t)ide analogue therapy versus nucleos(t)ide analogue therapy alone in patients with HBe antigen-negative chronic hepatitis B and sustained undetectable plasma hepatitis B virus DNA: A randomised, controlled, open-label trial. Lancet Gastroenterol. Hepatol. 2017, 2, 177–188. [Google Scholar] [CrossRef] [PubMed]
- Liaw, Y.F. Finite nucleos(t)ide analog therapy in HBeAg-negative chronic hepatitis B: An emerging paradigm shift. Hepatol. Int. 2019, 13, 665–673. [Google Scholar] [CrossRef] [PubMed]
- Boyd, A.; Piroth, L.; Maylin, S.; Maynard-Muet, M.; Lebosse, F.; Bouix, C.; Lascoux-Combe, C.; Mahjoub, N.; Girard, P.M.; Delaugerre, C.; et al. Intensification with pegylated interferon during treatment with tenofovir in HIV-hepatitis B virus co-infected patients. J. Viral Hepat. 2016, 23, 1017–1026. [Google Scholar] [CrossRef]
- Boyd, A.; Houghtaling, L.; Moh, R.; Chekaraou, M.A.; Gabillard, D.; Eholie, S.P.; Anglaret, X.; Zoulim, F.; Danel, C.; Lacombe, K.; et al. Clinical outcomes during treatment interruptions in human immunodeficiency virus-hepatitis B virus co-infected patients from Sub-Saharan Africa. Am. J. Trop. Med. Hyg. 2017, 97, 1936–1942. [Google Scholar] [CrossRef] [PubMed]
- Raimondo, G.; Locarnini, S.; Pollicino, T.; Levrero, M.; Zoulim, F.; Lok, A.S.; Taormina Workshop on Occult HBV Infection Faculty Members. Update of the statements on biology and clinical impact of occult hepatitis B virus infection. J. Hepatol. 2019, 71, 397–408. [Google Scholar] [CrossRef]
- Martinez, M.G.; Villeret, F.; Testoni, B.; Zoulim, F. Can we cure hepatitis B virus with novel direct-acting antivirals? Liver Int. 2020, 40 (Suppl. S1), 27–34. [Google Scholar] [CrossRef] [PubMed]
- Dusheiko, G. Will we need novel combinations to cure HBV infection? Liver Int. 2020, 40 (Suppl. S1), 35–42. [Google Scholar] [CrossRef] [PubMed]
- Iser, D.M.; Lewin, S.R. The pathogenesis of liver disease in the setting of HIV-hepatitis B virus coinfection. Antivir. Ther. 2009, 14, 155–164. [Google Scholar] [PubMed]
| Review Sections | Search and Selection Strategy |
|---|---|
| Rates of functional cure | We conducted a literature search using PubMed on 31 March 2021 to retrieve relevant articles in English. We searched using the terms “HBeAg loss”, “HBeAg seroclearance”, “HBeAg seroconversion”, “HBsAg loss”, “HBsAg seroclearance”, “HBsAg seroconversion”, “functional cure”, “tenofovir”, “entecavir”, “nucleoside analogue”, “nucleotide analogue”, and “hepatitis B virus” alone and in combinations to retrieve an initial list of publications. We restricted studies to those including individuals with HBV mono-infection or HIV-HBV co-infection for whom the majority were undergoing treatment with potent NA. We included studies from this search that had results on the number of events, number of individuals included in analysis, and median/mean time or person-years of follow-up. |
| Determinants of functional cure | We included studies from the search on rates of functional cure that included an analysis describing characteristics of individuals with higher rates of HBsAg-seroclearance. Since no study from this search included data on genetic polymorphisms, we removed the treatment search terms and included the terms “polymorphism”, “allele”, and “genetic mutation” alone and in combinations. |
| Markers predicting functional cure | We conducted a literature search using PubMed on 31 March 2021 to retrieve relevant articles in English. We used each marker of HBV replication as a search term (i.e., “HBsAg quantification”, “HBsAg composition”, “hepatitis B core-related antigen”, “pre-genomic HBV RNA”, “HBV RNA”, “IP-10”, and “anti-hepatitis B core antibody”). We restricted studies to those including treated individuals with HBV mono-infection and HIV-HBV co-infection. |
| Serological Endpoint | HBV Mono-Infected | HIV-HBV Co-Infected |
|---|---|---|
| HBeAg-seroclearance 1 | 7.8 (7.1–24.0) | 8.4 (4.6–24.7) |
| HBeAg-seroconversion 1 | 5.7 (2.2–14.3) | 4.1 (1.0–23.2) |
| HBsAg-seroclearance 2 | 0.37 (0–1.06) | 2.39 (0.6–10.46) |
| HBsAg-seroconversion 2 | 0.44 (0–0.92) | 0.92 (0.15–7.85) |
| Biomarker | HBV Mono-Infected | HIV-HBV Co-Infected |
|---|---|---|
| Direct Markers of Activity | ||
| HBsAg levels | HBeAg-seroclearance, HBsAg-seroclearance | HBeAg-seroclearance, HBsAg-seroclarance (preliminary) |
| HBsAg protein composition (L, M, S) | HBsAg-seroclearance (preliminary) | None |
| HBcrAg levels | HBeAg-seroclearance 1 HBsAg-seroclearance (preliminary) | HBeAg-seroclearance, None on HBsAg-seroclarance |
| pgHBV RNA/HBV RNA levels | HBeAg-seroclearance 2 HBsAg-seroclearance | None |
| Indirect markers of activity | ||
| IP-10 levels | HBeAg-seroclearance, HBsAg-seroclearance | None |
| anti-HBc antibody levels | HBeAg-seroconversion, HBsAg-seroconversion | HBeAg-seroclearance, None on HBsAg-seroclearance |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Boyd, A.; Dezanet, L.N.C.; Lacombe, K. Functional Cure of Hepatitis B Virus Infection in Individuals With HIV-Coinfection: A Literature Review. Viruses 2021, 13, 1341. https://doi.org/10.3390/v13071341
Boyd A, Dezanet LNC, Lacombe K. Functional Cure of Hepatitis B Virus Infection in Individuals With HIV-Coinfection: A Literature Review. Viruses. 2021; 13(7):1341. https://doi.org/10.3390/v13071341
Chicago/Turabian StyleBoyd, Anders, Lorenza N. C. Dezanet, and Karine Lacombe. 2021. "Functional Cure of Hepatitis B Virus Infection in Individuals With HIV-Coinfection: A Literature Review" Viruses 13, no. 7: 1341. https://doi.org/10.3390/v13071341
APA StyleBoyd, A., Dezanet, L. N. C., & Lacombe, K. (2021). Functional Cure of Hepatitis B Virus Infection in Individuals With HIV-Coinfection: A Literature Review. Viruses, 13(7), 1341. https://doi.org/10.3390/v13071341






