Response to Vaccination against Mumps in Medical Students: Two Doses Are Needed
Abstract
1. Introduction
2. Materials and Methods
2.1. Population
2.2. Antibody Measurement
2.3. Statistics
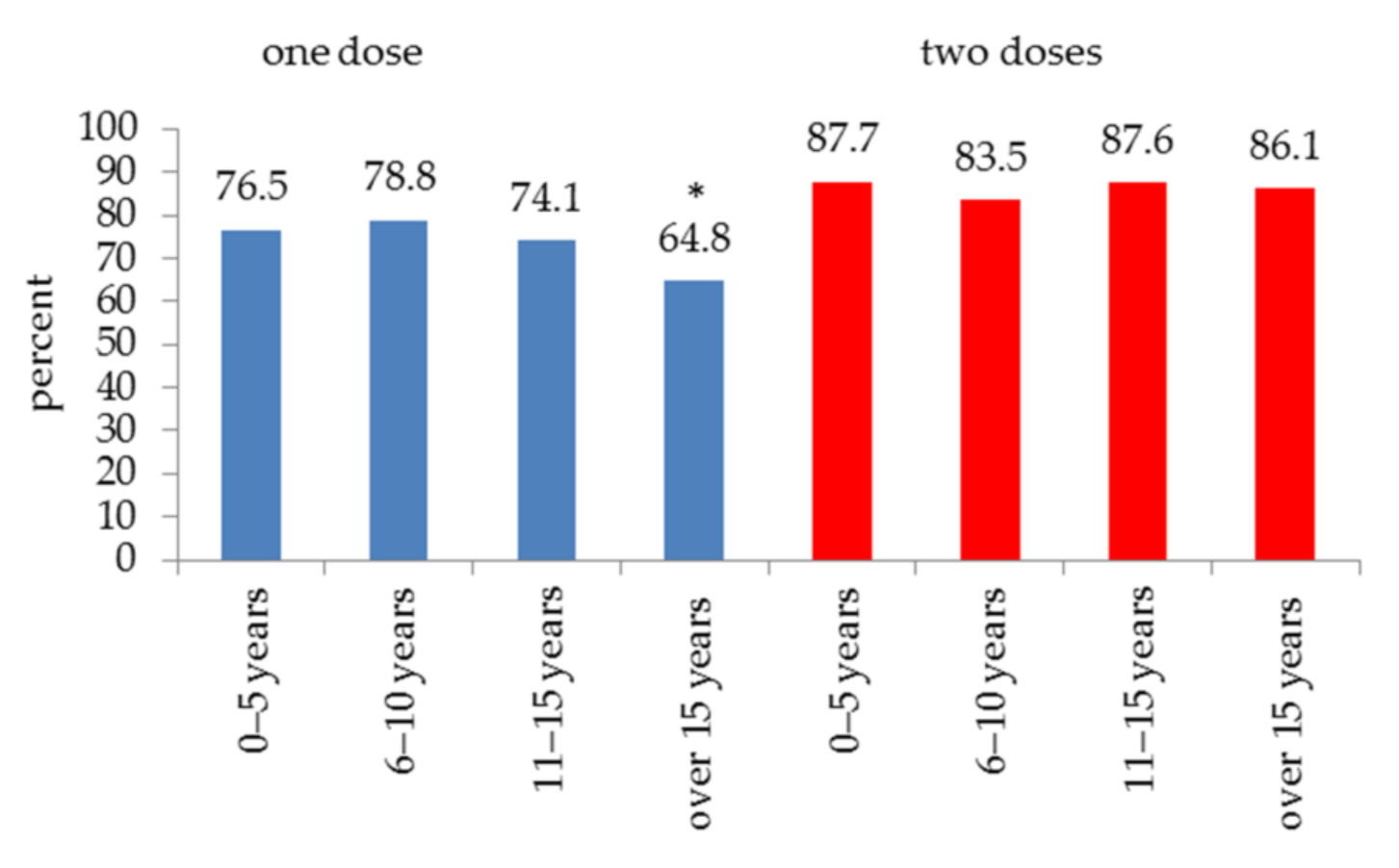
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wellington, K.; Goa, K.L. Measles, mumps, rubella vaccine (Priorix; GSK-MMR): A review of its use in the prevention of measles, mumps and rubella. Drugs 2003, 63, 2107–2126. [Google Scholar] [CrossRef] [PubMed]
- Rubin, S.; Kennedy, R.; Poland, G. Emerging mumps infection. Pediatr. Infect. Dis. J. 2016, 35, 799–801. [Google Scholar] [CrossRef] [PubMed]
- Anderson, R.M.; May, R.M. Immunisation and herd immunity. Lancet 1990, 335, 641–645. [Google Scholar] [CrossRef]
- Ministero della Salute. Circolare n° 12. Controllo ed Eliminazione di Morbillo, Parotite e Rosolia per Mezzo Della Vaccinazione (Circular 12. Control and Elimination of Measles, Mumps and Rubella by Means of Vaccination); Ministry of Health: Rome, Italy, 1999.
- European Center for Disease Prevention and Control. Mumps—Annual Epidemiological Report for 2018. Available online: www.ecdc.europa.eu/en/surveillance-and-disease-data/surveillance-reports (accessed on 16 February 2021).
- Van Loon, F.P.; Holmes, S.J.; Sirotkin, B.I.; Williams, W.W.; Cochi, S.L.; Hadler, S.C.; Lindegren, M.L. Mumps surveillance-United States, 1988–1993. MMWR CDC Surveill. Summ. 1995, 44, 1–14. [Google Scholar]
- Peltola, H.; Heinonen, O.P.; Valle, M.; Paunio, M.; Virtanen, M.; Karanko, V.; Cantell, K. The elimination of indigenous measles, mumps, and rubella from Finland by a 12-year, two-dose vaccination program. N. Engl. J. Med. 1994, 331, 1397–1402. [Google Scholar] [CrossRef] [PubMed]
- Peltola, H.; Davidkin, I.; Paunio, M.; Valle, M.; Leinikki, P.; Heinonen, O.P. Mumps and rubella eliminated from Finland. JAMA 2000, 284, 2643–2647. [Google Scholar] [CrossRef] [PubMed]
- Gabutti, G.; Rota, M.C.; Salmaso, S.; Bruzzone, B.M.; Bella, A.; Crovari, P.; Serological Study Group. Epidemiology of measles, mumps and rubella in Italy. Epidemiol. Infect. 2002, 129, 543–550. [Google Scholar] [CrossRef] [PubMed]
- Gabutti, G.; Guido, M.; Rota, M.C.; De Donno, A.; Ciofi Degli Atti, M.L.; Crovari, P.; Seroepidemiology Group. The epidemiology of mumps in Italy. Vaccine 2008, 26, 2906–2911. [Google Scholar] [CrossRef] [PubMed]
- Nardone, A.; Pebody, R.G.; van den Hof, S.; Levy-Bruhl, D.; Plesner, A.M.; Rota, M.C.; Tischer, A.; Andrews, N.; Berbers, G.; Crovari, P.; et al. Sero-epidemiology of mumps in western Europe. Epidemiol. Infect. 2003, 131, 691–701. [Google Scholar] [CrossRef]
- Eriksen, J.; Davidkin, I.; Kafatos, G.; Andrews, N.; Barbara, C.; Cohen, D.; Duks, A.; Griskevicius, A.; Johansen, K.; Bartha, K.; et al. Seroepidemiology of mumps in Europe (1996–2008): Why do outbreaks occur in highly vaccinated populations? Epidemiol. Infect. 2013, 141, 651–666. [Google Scholar] [CrossRef]
- Decreto legge n. 73. Disposizioni urgenti in tema di prevenzione vaccinale (Law decree N. 73. Urgent provisions on vaccine prevention). Rome, Italy, 7 June 2017. Gazzetta Ufficiale N. 130, 7 June 2017. Available online: https://www.gazzettaufficiale.it/eli/id/2017/06/7/17G00095/sg (accessed on 6 July 2021).
- Piano Nazionale Prevenzione Vaccinale 2017–2019 (National Vaccination Prevention Plan 2017–2019). Rome, Italy, 17 January 2017. Available online: https://www.salute.gov.it (accessed on 6 July 2021).
- Position paper. La Carta di Pisa delle vaccinazioni negli operatori sanitari (The Pisas’ Paper of Vaccinations in Healthcare Professionals). GlmPIOS 2017, 7, 155–157.
- Marlow, M.A.; Marin, M.; Moore, K.; Patel, M. CDC guidance for use of a third dose of MMR vaccine during outbreaks. J. Public Health Manag. Pract. 2020, 26, 109–115. [Google Scholar] [CrossRef] [PubMed]
- Fiebelkorn, A.P.; Coleman, L.A.; Belongia, E.A.; Freeman, S.K.; York, D.; Bi, D.; Zhang, C.; Ngo, L.; Rubin, S. Mumps antibody response in young adults after a third dose of measles-mumps-rubella vaccine. Open Forum Infect. Dis. 2014, 1, ofu094. [Google Scholar] [CrossRef] [PubMed]
- Trevisan, A.; Bertoncello, C.; Artuso, E.; Frasson, C.; Lago, L.; De Nuzzo, D.; Nicolli, A.; Maso, S. Will we have a cohort of healthcare workers full vaccinated against measles, mumps, and rubella? Vaccines 2020, 8, 104. [Google Scholar] [CrossRef] [PubMed]
- Decreto Legislativo n. 81. Tutela della salute e della sicurezza nei luoghi di lavoro (protection of health and safety in the workplace). Rome, 9 April 2008. Gazzetta Ufficiale N. 101, 30 April 2008. Available online: https://www.gazzettaufficiale.it/eli/id/2008/04/30/008G0104/sg (accessed on 6 July 2021).
- Centers for Disease Control and Prevention. Prevention of varicella. Update recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR 1999, 48, 1–5. [Google Scholar]
- Ravault, S.; Friel, D.; Di Paolo, E.; Caplanusi, A.; Gillard, P.; Povey, M.; Carryn, S. Assessment of Mumps Virus-Specific Antibodies: Comparison of Plaque Reduction Neutralization Test and Enzyme-Linked Immunosorbent Assay Estimates. J. Infect. Dis. 2019, 220, 1462–1468. [Google Scholar] [CrossRef] [PubMed]
- Latner, D.R.; McGrew, M.; Williams, N.; Lowe, L.; Werman, R.; Warnock, E.; Gallagher, K.; Doyle, P.; Smole, S.; Lett, S.; et al. Enzyme-linked immunospot assay detection of mumps-specific antibody-secreting B cells as an alternative method of laboratory diagnosis. Clin. Vaccine Immunol. 2011, 18, 35–42. [Google Scholar] [CrossRef] [PubMed]
- Mercader, S.; McGrew, M.; Sowers, S.B.; Williams, N.J.; Bellini, W.J.; Hickman, C.J. Development and Use of an Endpoint Titration Assay To Characterize Mumps IgG Avidity following Measles, Mumps, and Rubella Vaccination and Wild-Type Mumps Infection. mSphere 2018, 3, e00320-18. [Google Scholar] [CrossRef]
- Trevisan, A.; Morandin, M.; Frasson, C.; Paruzzolo, P.; Davanzo, E.; Di Marco, L.; Fabrello, A.; Borella-Venturini, M. Prevalence of childhood exanthematic disease antibodies in paramedical students: Need of vaccination. Vaccine 2006, 24, 171–176. [Google Scholar] [CrossRef]
- Trevisan, A.; Frasson, C.; Morandin, M.; Beggio, M.; Bruno, A.; Davanzo, E.; Di Marco, L.; Simioni, L.; Amato, G. Immunity against infectious diseases: Predictive value of self-reported history of vaccination and disease. Infect. Control. Hosp. Epidemiol. 2007, 28, 564–569. [Google Scholar] [CrossRef]
- Ciofi Degli Atti, M.L.; Salmaso, S.; Bella, A.; Arigliani, R.; Gangemi, M.; Chiamenti, G.; Brusoni, G.; Tozzi, A.E.; Pediatric Sentinel Surveillance Study Group. Pediatric sentinel surveillance of vaccine-preventable diseases in Italy. Pediatr. Infect. Dis. J. 2002, 21, 763–768. [Google Scholar] [CrossRef] [PubMed]
- Ong, G.; Goh, K.T.; Ma, S.; Chew, S.K. Comparative efficacy of Rubini, Jeryl-Lynn and Urabe mumps vaccine in an Asian population. J. Infect. 2005, 51, 294–298. [Google Scholar] [CrossRef]
- Yang, L.; Grenfell, B.T.; Mina, M.J. Waning immunity and re-emergence of measles and mumps in the vaccine era. Curr. Opin. Virol. 2020, 40, 48–54. [Google Scholar] [CrossRef] [PubMed]
- Markowitz, L.E.; Preblud, S.R.; Fine, P.E.; Orenstein, W.A. Duration of live measles vaccine-induced immunity. Pediatr. Infect. Dis. J. 1990, 9, 101–110. [Google Scholar] [CrossRef] [PubMed]
- Dayan, G.H.; Quinlisk, M.P.; Parker, A.A.; Barskey, A.E.; Harris, M.L.; Schwartz, J.M.; Hunt, K.; Finley, C.G.; Leschinsky, D.P.; O’Keefe, A.L.; et al. Recent resurgence of mumps in the United States. N. Engl. J. Med. 2008, 358, 1580–1589. [Google Scholar] [CrossRef]
- Anis, E.; Grotto, I.; Moerman, L.; Warshavsky, B.; Slater, P.E.; Lev, B. Mumps outbreak in Israel’s highly vaccinated society: Are two doses enough? Epidemiol. Infect. 2012, 140, 439–446. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Yang, L.; Grenfell, B.T.; Mina, M.J. Measles vaccine immune escape: Should we be concerned? Eur. J. Epidemiol. 2019, 34, 893–896. [Google Scholar] [CrossRef] [PubMed]
- Bankamp, B.; Hickman, C.; Icenogle, J.P.; Rota, P.A. Successes and challenges for preventing measles, mumps and rubella by vaccination. Curr. Opin. Virol. 2019, 34, 110–116. [Google Scholar] [CrossRef] [PubMed]
- Pang, H.; Zhou, Y.; Zhao, W.; Jiang, Q. Epidemiological changes in mumps infections between 1990 and 2017 in urban area of Shanghai, China. Hum. Vaccin. Immunother. 2021, 17, 1358–1365. [Google Scholar] [CrossRef]
- Marin, M.; Marlow, M.; Moore, K.L.; Patel, M. Recommendation of the Advisory Committee on Immunization Practices for Use of a Third Dose of Mumps Virus-Containing Vaccine in Persons at Increased Risk for Mumps During an Outbreak. MMWR Morb. Mortal. Wkly Rep. 2018, 67, 33–38. [Google Scholar] [CrossRef] [PubMed]
- Su, S.B.; Chang, H.L.; Chen, A.K. Current Status of Mumps Virus Infection: Epidemiology, Pathogenesis, and Vaccine. Int. J. Environ. Res. Public Health 2020, 17, 1686. [Google Scholar] [CrossRef]
- Ministero della Salute. “Vaccinazioni Dell’adolescente. Anno 2019 (Coorte 2001–18 Anni) Coperture Vaccinali (per 100 abitanti) per Vaccinazioni Dell’adolescente, Calcolate sui Riepiloghi Inviati Dalle Regioni e PP.AA. (per Singolo Antigene)”. Aggiornamento 18 Giugno 2020. Available online: https://www.salute.gov.it/imgs/C_17_tavole_20_8_7_file.pdf (accessed on 1 July 2021).
- Position paper. Mumps virus vaccines: WHO position paper. Wkly Epidemiol. Rec. 2007, 82, 49–60.
- Pérez-Alba, E.; García-Ortiz, A.; Salazar-Montalvo, R.G.; Hernández-Guedea, M.A.; Camacho-Ortiz, A. Mumps outbreak with high complication rates among residents in a university teaching hospital. Am. J. Infect. Control 2019, 47, 337–339. [Google Scholar] [CrossRef] [PubMed]
- Karadeniz, A.; Akduman Alaşehir, E. Seroepidemiology of hepatitis viruses, measles, mumps, rubella and varicella among healthcare workers and students: Should we screen before vaccination? J. Infect. Public Health 2020, 13, 480–484. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Hu, Y.; Deng, X.; Wang, Z.; Lu, P.; Ma, F.; Zhou, M.; Liu, P.; Min, J. Seroepidemiology of mumps in the general population of Jiangsu province, China after introduction of a one-dose measles-mumps-rubella vaccine. Sci. Rep. 2015, 5, 14660. [Google Scholar] [CrossRef] [PubMed]
- Klein, S.L.; Jedlicka, A.; Pekosz, A. The Xs and Y of immune responses to viral vaccines. Lancet Infect. Dis. 2010, 10, 338–349. [Google Scholar] [CrossRef]
- Cook, I.F. Sexual dimorphism of humoral immunity with human vaccines. Vaccine 2008, 26, 3551–3555. [Google Scholar] [CrossRef]
- Kennedy, R.B.; Ovsyannikova, I.G.; Pankratz, V.S.; Vierkant, R.A.; Jacobson, R.M.; Ryan, M.A.; Poland, G.A. Gender effects on humoral immune responses to smallpox vaccine. Vaccine 2009, 27, 3319–3323. [Google Scholar] [CrossRef]
- Trevisan, A.; Frasson, C.; De Nuzzo, D.; Nicolli, A.; Scapellato, M.L. Significance of anti-HB levels below 10 IU/L after vaccination against hepatitis B in infancy or adolescence: An update in relation to sex. Hum. Vaccin. Immunother. 2020, 16, 460–464. [Google Scholar] [CrossRef] [PubMed]
- Hoes, J.; Knol, M.J.; Mollema, L.; Buisman, A.; de Melker, H.E.; van der Klis, F.R.M. Comparison of antibody response between boys and girls after infant and childhood vaccinations in the Netherlands. Vaccine 2019, 37, 4504–4510. [Google Scholar] [CrossRef]
- Trevisan, A.; Nicolli, A.; De Nuzzo, D.; Lago, L.; Artuso, E.; Maso, S. Varicella seroepidemiology and immunization in a cohort of future healthcare workers in the pre-vaccination era. Int. J. Infect. Dis. 2020, 96, 228–232. [Google Scholar] [CrossRef] [PubMed]
| Year of Birth | N. | No Vaccine (N.) | % | One Dose (N.) | % | Two Doses (N.) | % |
|---|---|---|---|---|---|---|---|
| before 1980 | 506 | 492 | 97.2 | 12 | 2.4 | 2 | 0.4 |
| 1980–1985 | 1562 | 1146 | 73.4 | 376 | 24.1 | 40 | 2.6 |
| 1986–1990 | 2718 | 742 | 27.3 | 1216 | 44.7 | 760 | 28.0 |
| 1991–1995 | 3927 | 219 | 5.6 | 547 | 14.6 | 3161 | 80.5 |
| after 1995 | 2614 | 73 | 2.8 | 190 | 7.3 | 2351 | 89.9 |
| all | 11,327 | 2672 | 23.6 | 2341 | 20.7 | 6314 | 55.7 |
| Vaccination | N. | Positives | % | χ2 | p | χ2 | p |
|---|---|---|---|---|---|---|---|
| differences between doses | differences between sexes | ||||||
| all students | 11,327 | 8999 | 79.4 | ||||
| no vaccine | 2672 | 1937 | 72.5 | ||||
| one dose | 2341 | 1667 | 71.2 | ||||
| two doses | 6314 | 5395 | 85.4 | 229.523 | <0.0001 | ||
| all males | 3939 | 3000 | 76.2 | ||||
| no vaccine | 930 | 651 | 70.0 | ||||
| one dose | 801 | 518 | 64.7 | ||||
| two doses | 2208 | 1831 | 82.9 | 113.346 | <0.0001 | ||
| all females | 7388 | 5999 | 81.2 | 39.625 | <0.0001 | ||
| no vaccine | 1742 | 1286 | 73.8 | 4.255 | =0.0391 | ||
| one dose | 1540 | 1149 | 74.6 | 24.918 | <0.0001 | ||
| two doses | 4106 | 3564 | 86.8 | 119.751 | <0.0001 | 17.018 | <0.0001 |
| Year of Birth | All Students | Males | Females | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| no vaccine | N. | positives | % | N. | positives | % | N. | positives | % | χ2 | p |
| before 1980 | 492 | 382 | 77.6 | 219 | 160 | 73.1 | 273 | 222 | 81.3 | 4.311 | =0.0379 |
| 1980–1985 | 1146 | 909 | 79.3 | 351 | 272 | 77.5 | 795 | 637 | 80.1 | 0.874 | n.s. |
| 1986–1990 | 742 | 536 | 72.2 | 236 | 171 | 72.5 | 506 | 365 | 72.1 | 0.000 | n.s. |
| 1991–1995 | 219 | 96 | 43.8 | 87 | 38 | 43.7 | 132 | 58 | 43.9 | 0.000 | n.s. |
| after 1995 | 73 | 14 | 19.2 | 37 | 10 | 27.0 | 36 | 4 | 11.1 | 2.044 | n.s. |
| one dose | N. | positives | % | N. | positives | % | N. | positives | % | χ2 | p |
| before 1980 | 12 | 11 | 91.7 | 8 | 7 | 87.5 | 4 | 4 | 100.0 | 0.000 | n.s. |
| 1980–1985 | 376 | 291 | 77.4 | 122 | 86 | 70.5 | 254 | 205 | 80.7 | 4.350 | =0.0370 |
| 1986–1990 | 1216 | 897 | 73.8 | 378 | 256 | 67.7 | 838 | 641 | 76.5 | 9.898 | =0.0017 |
| 1991–1995 | 547 | 334 | 61.1 | 209 | 108 | 51.7 | 338 | 226 | 66.9 | 11.901 | =0.0006 |
| after 1995 | 190 | 134 | 70.5 | 84 | 61 | 72.6 | 106 | 73 | 68.9 | 0.162 | n.s. |
| two doses | N. | positives | % | N. | positives | % | N. | positives | % | χ2 | p |
| before 1980 | 2 | 2 | 100.0 | 1 | 1 | 100.0 | 1 | 1 | 100.0 | 0.000 | n.s. |
| 1980–1985 | 40 | 39 | 97.5 | 22 | 21 | 95.5 | 18 | 18 | 100.0 | 0.000 | n.s. |
| 1986–1990 | 760 | 637 | 83.8 | 262 | 199 | 76.0 | 498 | 438 | 88.0 | 17.344 | <0.0001 |
| 1991–1995 | 3161 | 2630 | 83.2 | 1129 | 931 | 82.5 | 2032 | 1699 | 83.6 | 0.687 | n.s. |
| after 1995 | 2351 | 2087 | 88.8 | 794 | 679 | 85.5 | 1557 | 1408 | 90.4 | 12.249 | =0.0005 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Trevisan, A.; Moretto, A.; Bertoncello, C.; Nicolli, A.; Maso, S.; Scapellato, M.L.; Mason, P. Response to Vaccination against Mumps in Medical Students: Two Doses Are Needed. Viruses 2021, 13, 1311. https://doi.org/10.3390/v13071311
Trevisan A, Moretto A, Bertoncello C, Nicolli A, Maso S, Scapellato ML, Mason P. Response to Vaccination against Mumps in Medical Students: Two Doses Are Needed. Viruses. 2021; 13(7):1311. https://doi.org/10.3390/v13071311
Chicago/Turabian StyleTrevisan, Andrea, Angelo Moretto, Chiara Bertoncello, Annamaria Nicolli, Stefano Maso, Maria Luisa Scapellato, and Paola Mason. 2021. "Response to Vaccination against Mumps in Medical Students: Two Doses Are Needed" Viruses 13, no. 7: 1311. https://doi.org/10.3390/v13071311
APA StyleTrevisan, A., Moretto, A., Bertoncello, C., Nicolli, A., Maso, S., Scapellato, M. L., & Mason, P. (2021). Response to Vaccination against Mumps in Medical Students: Two Doses Are Needed. Viruses, 13(7), 1311. https://doi.org/10.3390/v13071311








