Antivirals against the Chikungunya Virus
Abstract
:1. Introduction
2. CHIKV Replication Cycle
3. Strategies for Identification of Antiviral Compounds
4. Virus Targeting Inhibitors
4.1. Viral Entry and Membrane Fusion
4.2. Capsid Protease
4.3. 6K Protein
4.4. Non-Structural Proteins
4.4.1. Non-Structural Protein 1 (nsP1)
4.4.2. Non-Structural Protein 2 (nsP2)
4.4.3. Non-Structural Protein 3 (nsP3)
4.4.4. Non-Structural Protein 4 (nsP4)
5. Targeting Host Factors
5.1. Viral Entry and Membrane Fusion
5.2. Lipid Pathway Inhibitors
5.3. Pyrimidine and Purine Synthesis Inhibitors
5.4. Protein Synthesis Inhibitors
5.5. Cellular Protein Inhibitors
5.5.1. Cellular Enzyme Inhibitors
Hydrolases
Kinases
Lyases/transferases
5.6. Cellular Receptor Inhibitors
5.6.1. Inhibitors of Channel-Linked Receptors
5.6.2. Inhibitors of Enzyme-linked Receptors
5.7. Immunomodulatory Agents
6. Undefined Targets
7. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Robinson, M.C. An epidemic of virus disease in Southern Province, Tanganyika Territory, in 1952-53. I. Clinical features. Trans. R. Soc. Trop. Med. Hyg. 1955, 49, 28–32. [Google Scholar] [CrossRef]
- Chretien, J.P.; Anyamba, A.; Bedno, S.A.; Breiman, R.F.; Sang, R.; Sergon, K.; Powers, A.M.; Onyango, C.O.; Small, J.; Tucker, C.J.; et al. Drought-associated chikungunya emergence along coastal East Africa. Am. J. Trop. Med. Hyg. 2007, 76, 405–407. [Google Scholar] [CrossRef] [Green Version]
- Vazeille, M.; Moutailler, S.; Coudrier, D.; Rousseaux, C.; Khun, H.; Huerre, M.; Thiria, J.; Bastien Dehecq, J.-S.; Fontenille, D.; Schuffenecker, I.; et al. Two Chikungunya Isolates from Two Chikungunya Isolates from the Outbreak of La Reunion (Indian Ocean) Exhibit Different Patterns of Infection in the Mosquito, Aedes albopictus. PLoS ONE 2007, 2, e1168. [Google Scholar] [CrossRef]
- Cassadou, S.; Boucau, S.; Petit-Sinturel, M.; Huc, P.; Leparc-Goffart, I.; Ledrans, M. Emergence of chikungunya fever on the French side of Saint Martin island, October to December 2013. Eurosurveillance 2014, 19, 20752. [Google Scholar] [CrossRef] [Green Version]
- Zeller, H.; Van Bortel, W.; Sudre, B. Chikungunya: Its History in Africa and Asia and Its Spread to New Regions in 2013–2014. J. Infect. Dis. 2015, 214, 436–440. [Google Scholar] [CrossRef] [PubMed]
- Rezza, G.; Nicoletti, L.; Angelini, R.; Romi, R.; Finarelli, A.C.; Panning, M.; Cordioli, P.; Fortuna, C.; Boros, S.; Magurano, F.; et al. CHIKV study group. Infection with chikungunya virus in Italy: An outbreak in a temperate region. Lancet 2007, 370, 1840–1846. [Google Scholar] [CrossRef]
- Venturi, G.; Di Luca, M.; Fortuna, C.; Remoli, M.E.; Riccardo, F.; Severini, F.; Toma, L.; Del Manso, M.; Benedetti, E.; Caporali, M.G.; et al. Detection of a chikungunya outbreak in central Italy, August to September 2017. Eurosurveillance 2017, 22, 11–14. [Google Scholar] [CrossRef] [Green Version]
- Delisle, E.; Rousseau, C.; Broche, B.; Leparc-Goffart, I.; L’ambert, G.; Cochet, A.; Prat, C.; Foulongne, V.; Ferré, J.B.; Catelinois, O.; et al. Chikungunya outbreak in Montpellier, France, September to October 2014. Eurosurveillance 2015, 20. [Google Scholar] [CrossRef] [Green Version]
- Calba, C.; Guerbois-Galla, M.; Franke, F.; Jeannin, C.; Auzet-Caillaud, M.; Grard, G.; Pigaglio, L.; Decoppet, A.; Weicherding, J.; Savaill, M.C.; et al. Preliminary report of an autochthonous chikungunya outbreak in France, July to September 2017. Eurosurveillance 2017, 22, 5–10. [Google Scholar] [CrossRef] [Green Version]
- Campbell, L.P.; Luther, C.; Moo-Llanes, D.; Ramsey, J.M.; Danis-Lozano, R.; Peterson, A.T. Climate change influences on global distributions of dengue and chikungunya virus vectors. Philos. Trans. R. Soc. B Biol. Sci. 2015, 370, 1–9. [Google Scholar] [CrossRef]
- WHO Media Centre. Chikungunya—Key Facts. Available online: https://www.who.int/news-room/fact-sheets/detail/chikungunya (accessed on 31 May 2021).
- Thiberville, S.-D.; Moyen, N.; Dupuis-Maguiraga, L.; Nougairede, A.; Gould, E.A.; Roques, P.; De Lamballerie, X. Chikungunya fever: Epidemiology, clinical syndrome, pathogenesis and therapy. Antivir. Res. 2013, 99, 345–370. [Google Scholar] [CrossRef]
- Borgherini, G.; Poubeau, P.; Jossaume, A.; Gouix, A.; Cotte, L.; Michault, A.; Arvin-Berod, C.; Paganin, F. Persistent arthralgia associated with chikungunya virus: A study of 88 adult patients on reunion island. Clin. Infect. Dis. 2008, 47, 469–475. [Google Scholar] [CrossRef] [Green Version]
- Lebrun, G.; Chadda, K.; Reboux, A.H.; Martinet, O.; Gaüzère, B.A. Guillain-Barré Syndrome after Chikungunya Infection. Emerg. Infect. Dis. 2009, 15, 495–496. [Google Scholar] [CrossRef]
- Kennedy Amaral Pereira, J.; Schoen, R.T. Management of chikungunya arthritis. Clin. Rheumatol. 2017, 36, 2179–2186. [Google Scholar] [CrossRef] [PubMed]
- Kielian, M.; Chanel-Vos, C.; Liao, M. Alphavirus Entry and Membrane Fusion. Viruses 2010, 2, 796–825. [Google Scholar] [CrossRef] [Green Version]
- Voss, J.E.; Vaney, M.-C.; Duquerroy, S.; Vonrhein, C.; Girard-Blanc, C.; Crublet, E.; Thompson, A.; Bricogne, G.; Rey, F.A. Glycoprotein organization of Chikungunya virus particles revealed by X-ray crystallography. Nature 2010, 468, 709–712. [Google Scholar] [CrossRef] [PubMed]
- Solignat, M.; Gay, B.; Higgs, S.; Briant, L.; Devaux, C. Replication cycle of chikungunya: A re-emerging arbovirus. Virology 2009, 393, 183–197. [Google Scholar] [CrossRef] [Green Version]
- Van Der Heijden, M.W.; Bol, J.F. Composition of alphavirus-like replication complexes: Involvement of virus and host encoded proteins. Arch Virol 2002, 147, 875–898. [Google Scholar] [CrossRef] [PubMed]
- Ashbrook, A.W.; Lentscher, A.J.; Zamora, P.F.; Silva, L.A.; May, N.A.; Bauer, J.A.; Morrison, T.E.; Dermody, T.S. Antagonism of the Sodium-Potassium ATPase Impairs Chikungunya Virus Infection. MBio 2016, 7, e00693-16. [Google Scholar] [CrossRef] [Green Version]
- Abdelnabi, R.; Neyts, J.; Delang, L. Towards antivirals against chikungunya virus. Antivir. Res. 2015, 121, 59–68. [Google Scholar] [CrossRef]
- Jose, J.; Snyder, J.E.; Kuhn, R.J.; Snyder, E.J.; Kuhn, R.J. A structural and functional perspective of alphavirus replication and assembly. Futur. Med. 2009, 4, 837–856. [Google Scholar] [CrossRef] [Green Version]
- Strauss, J.H.; Strauss, E.G. The alphaviruses: Gene expression, replication, and evolution. Microbiol. Rev 1994, 58, 491–562. [Google Scholar] [CrossRef] [PubMed]
- Delogu, I.; Pastorino, B.; Baronti, C.; Nougairède, A.; Bonnet, E.; de Lamballerie, X. In vitro antiviral activity of arbidol against Chikungunya virus and characteristics of a selected resistant mutant. Antivir. Res. 2011, 90, 99–107. [Google Scholar] [CrossRef] [PubMed]
- Ho, Y.-J.; Wang, Y.-M.; Lu, J.; Wu, T.-Y.; Lin, L.-I.; Kuo, S.-C.; Lin, C.-C. Suramin Inhibits Chikungunya Virus Entry and Transmission. PLoS ONE 2015, 10, e0133511. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kuo, S.-C.; Wang, Y.-M.; Ho, Y.-J.; Chang, T.-Y.; Lai, Z.-Z.; Tsui, P.-Y.; Wu, T.-Y.; Lin, C.-C. Suramin treatment reduces chikungunya pathogenesis in mice. Antivir. Res. 2016, 134, 89–96. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Albulescu, I.C.; Van Hoolwerff, M.; Wolters, L.A.; Bottaro, E.; Nastruzzi, C.; Yang, S.C.; Tsay, S.-C.; Hwu, J.R.; Snijder, E.J.; Van Hemert, M.J. Suramin inhibits chikungunya virus replication through multiple mechanisms. Antivir. Res. 2015, 121, 39–46. [Google Scholar] [CrossRef] [PubMed]
- Sharma, R.; Fatma, B.; Saha, A.; Bajpai, S.; Sistla, S.; Dash, P.K.; Parida, M.; Kumar, P.; Tomar, S. Inhibition of chikungunya virus by picolinate that targets viral capsid protein. Virology 2016, 498, 265–276. [Google Scholar] [CrossRef] [PubMed]
- Dey, D.; Siddiqui, S.I.; Mamidi, P.; Ghosh, S.; Kumar, C.S.; Chattopadhyay, S.; Ghosh, S.; Banerjee, M. The effect of amantadine on an ion channel protein from Chikungunya virus. PLoS Negl. Trop. Dis. 2019, 13, e0007548. [Google Scholar] [CrossRef] [Green Version]
- Gigante, A.; Canela, M.-D.D.; Delang, L.; Priego, E.-M.M.; Camarasa, M.-J.J.; Querat, G.; Neyts, J.; Leyssen, P.; Pérez-Pérez, M.-J.J. Identification of [1,2,3]triazolo[4,5-d]pyrimidin-7(6H)-ones as novel inhibitors of Chikungunya virus replication. J. Med. Chem. 2014, 57, 4000–4008. [Google Scholar] [CrossRef]
- Delang, L.; Li, C.; Tas, A.; Quérat, G.; Albulescu, I.C.; De Burghgraeve, T.; Guerrero, N.A.S.; Gigante, A.; Piorkowski, G.; Decroly, E.; et al. The viral capping enzyme nsP1: A novel target for the inhibition of chikungunya virus infection. Sci. Rep. 2016, 6, 31819. [Google Scholar] [CrossRef]
- Gigante, A.; Gómez-SanJuan, A.; Delang, L.; Li, C.; Bueno, O.; Gamo, A.-M.; Priego, E.-M. Antiviral activity of [1,2,3]triazolo[4,5-d]pyrimidin-7(6H)-ones against chikungunya virus targeting the viral capping nsP1. Antivir. Res. 2017, 144, 216–222. [Google Scholar] [CrossRef] [PubMed]
- Gómez-SanJuan, A.; Gamo, A.-M.; Delang, L.; Pérez-Sánchez, A.; Amrun, S.N.; Abdelnabi, R.; Jacobs, S.; Priego, E.-M.; Camarasa, M.-J.; Jochmans, D.; et al. Inhibition of the Replication of Different Strains of Chikungunya Virus by 3-Aryl-[1,2,3]triazolo[4,5- d]pyrimidin-7(6 H)-ones. ACS Infect. Dis. 2018, 4, 605–619. [Google Scholar] [CrossRef]
- Moesslacher, J.; Battisti, V.; Delang, L.; Neyts, J.; Abdelnabi, R.; Pürstinger, G.; Urban, E.; Langer, T. Identification of 2-(4-(Phenylsulfonyl)piperazine-1-yl)pyrimidine Analogues as Novel Inhibitors of Chikungunya Virus. ACS Med. Chem. Lett. 2020, 11, 906–912. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abdelnabi, R.; Kovacikova, K.; Moesslacher, J.; Donckers, K.; Battisti, V.; Leyssen, P.; Langer, T.; Puerstinger, G.; Quérat, G.; Li, C.; et al. Novel Class of Chikungunya Virus Small Molecule Inhibitors That Targets the Viral Capping Machinery. Antimicrob. Agents Chemother. 2020, 64, e00649-20. [Google Scholar] [CrossRef]
- Feibelman, K.M.; Fuller, B.P.; Li, L.; LaBarbera, D.V.; Geiss, B.J. Identification of small molecule inhibitors of the Chikungunya virus nsP1 RNA capping enzyme. Antivir. Res. 2018, 154, 124–131. [Google Scholar] [CrossRef] [PubMed]
- Shin, Y.S.; Jarhad, D.B.; Jang, M.H.; Kovacikova, K.; Kim, G.; Yoon, J.; Kim, H.R.; Hyun, Y.E.; Tipnis, A.S.; Chang, T.S.; et al. Identification of 6′-β-fluoro-homoaristeromycin as a potent inhibitor of chikungunya virus replication. Eur. J. Med. Chem. 2020, 187, 111956. [Google Scholar] [CrossRef] [PubMed]
- Kovacikova, K.; Morren, B.M.; Tas, A.; Albulescu, I.C.; Van Rijswijk, R.; Jarhad, D.B.; Shin, Y.S.; Jang, M.H.; Kim, G.; Lee, H.W.; et al. 6′-β-Fluoro-Homoaristeromycin and 6′-Fluoro-Homoneplanocin A Are Potent Inhibitors of Chikungunya Virus Replication through Their Direct Effect on Viral Nonstructural Protein 1. Antimicrob. Agents Chemother. 2020, 64, e02532-19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mudgal, R.; Mahajan, S.; Tomar, S. Inhibition of Chikungunya virus by an adenosine analog targeting the SAM-dependent nsP1 methyltransferase. FEBS Lett. 2020, 594, 678–694. [Google Scholar] [CrossRef] [PubMed]
- Bassetto, M.; De Burghgraeve, T.; Delang, L.; Massarotti, A.; Coluccia, A.; Zonta, N.; Gatti, V.; Colombano, G.; Sorba, G.; Silvestri, R.; et al. Computer-aided identification, design and synthesis of a novel series of compounds with selective antiviral activity against chikungunya virus. Antivir. Res. 2013, 98, 12–18. [Google Scholar] [CrossRef] [PubMed]
- Tardugno, R.; Giancotti, G.; De Burghgraeve, T.; Delang, L.; Neyts, J.; Leyssen, P.; Brancale, A.; Bassetto, M. Design, synthesis and evaluation against Chikungunya virus of novel small-molecule antiviral agents. Bioorg. Med. Chem. 2018, 26, 869–874. [Google Scholar] [CrossRef]
- Giancotti, G.; Cancellieri, M.; Balboni, A.; Giustiniano, M.; Novellino, E.; Delang, L.; Neyts, J.; Leyssen, P.; Brancale, A.; Bassetto, M. Rational modifications on a benzylidene-acrylohydrazide antiviral scaffold, synthesis and evaluation of bioactivity against Chikungunya virus. Eur. J. Med. Chem. 2018, 149, 56–68. [Google Scholar] [CrossRef]
- Das, P.K.; Puusepp, L.; Varghese, F.S.; Utt, A.; Ahola, T.; Kananovich, D.G.; Lopp, M.; Merits, A.; Karelson, M. Design and Validation of Novel Chikungunya Virus Protease Inhibitors. Antimicrob. Agents Chemother. 2016, 60, 7382–7395. [Google Scholar] [CrossRef] [Green Version]
- Lucas-Hourani, M.; Lupan, A.; Desprès, P.; Thoret, S.; Pamlard, O.; Dubois, J.; Guillou, C.; Tangy, F.; Vidalain, P.-O.; Munier-Lehmann, H. A phenotypic assay to identify Chikungunya virus inhibitors targeting the nonstructural protein nsP2. J. Biomol. Screen. 2013, 18, 172–179. [Google Scholar] [CrossRef] [Green Version]
- Tripathi, P.K.; Soni, A.; Singh Yadav, S.P.; Kumar, A.; Gaurav, N.; Raghavendhar, S.; Sharma, P.; Sunil, S.; Ashish; Jayaram, B.; et al. Evaluation of novobiocin and telmisartan for anti-CHIKV activity. Virology 2020, 548, 250–260. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Garzan, A.; Haese Id, N.; Bostwick, R.; Martinez-Gzegozewska, Y.; Rasmussen, L.; Streblow, D.N.; Haise, M.T.; Pathak, A.K.; Augelli-Szafran, C.E.; et al. Pyrimidone inhibitors targeting Chikungunya Virus nsP3 macrodomain by fragment-based drug design. PLoS ONE 2021, 16, e0245013. [Google Scholar]
- Delang, L.; Segura Guerrero, N.; Tas, A.; Quérat, G.; Pastorino, B.; Froeyen, M.; Dallmeier, K.; Jochmans, D.; Herdewijn, P.; Bello, F.; et al. Mutations in the chikungunya virus non-structural proteins cause resistance to favipiravir (T-705), a broad-spectrum antiviral. J. Antimicrob. Chemother. 2014, 69, 2770–2784. [Google Scholar] [CrossRef]
- Abdelnabi, R.; Jochmans, D.; Verbeken, E.; Neyts, J.; Delang, L. Antiviral treatment efficiently inhibits chikungunya virus infection in the joints of mice during the acute but not during the chronic phase of the infection. Antivir. Res. 2017, 149, 113–117. [Google Scholar] [CrossRef] [PubMed]
- Ehteshami, M.; Tao, S.; Zandi, K.; Hsiao, H.-M.; Jiang, Y.; Hammond, E.; Amblard, F.; Russell, O.O.; Merits, A.; Schinazi, R.F. Characterization of β-d-N4-Hydroxycytidine as a Novel Inhibitor of Chikungunya Virus. Antimicrob. Agents Chemother. 2017, 61, e02395-16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Urakova, N.; Kuznetsova, V.; Crossman, D.K.; Sokratian, A.; Guthrie, D.B.; Kolykhalov, A.A.; Lockwood, M.A.; Natchus, M.G.; Crowley, M.R.; Painter, G.R.; et al. β-D-N4- Hydroxycytidine is a potent anti-alphavirus compound that induces a high level of mutations in the viral genome. J. Virol. 2017, 92, e01965-17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ferreira, A.C.; Reis, P.A.; de Freitas, C.S.; Sacramento, C.Q.; Hoelz, L.V.B.; Bastos, M.M.; Mattos, M.; Rocha, N.; de Azevedo Quintanilha, I.G.; da Silva Gouveia Pedrosa, C.; et al. Beyond members of the Flaviviridae family, sofosbuvir also inhibits chikungunya virus replication. Antimicrob. Agents Chemother. 2019, 63, e01389-18. [Google Scholar] [CrossRef] [Green Version]
- Wada, Y.; Orba, Y.; Sasaki, M.; Kobayashi, S.; Carr, M.J.; Nobori, H.; Sato, A.; Hall, W.W.; Sawa, H. Discovery of a novel antiviral agent targeting the nonstructural protein 4 (nsP4) of chikungunya virus. Virology. Virology 2017, 505, 102–112. [Google Scholar] [CrossRef]
- Scuotto, M.; Abdelnabi, R.; Collarile, S.; Schiraldi, C.; Delang, L.; Massa, A.; Ferla, S.; Brancale, A.; Leyssen, P.; Neyts, J.; et al. Discovery of novel multi-target indole-based derivatives as potent and selective inhibitors of chikungunya virus replication. Bioorg. Med. Chem. 2017, 25, 327–337. [Google Scholar] [CrossRef] [PubMed]
- Hwu, J.R.; Gupta, N.K.; Tsay, S.C.; Huang, W.C.; Albulescu, I.C.; Kovacikova, K.; van Hemert, M.J. Bis(benzofuran-thiazolidinone)s and bis(benzofuran-thiazinanone)s as inhibiting agents for chikungunya virus. Antivir. Res. 2017, 146, 96–101. [Google Scholar] [CrossRef] [Green Version]
- Passos, G.F.S.; Gomes, M.G.M.; de Aquino, T.M.; de Araújo-Júnior, J.X.; de Souza, S.J.M.; Cavalcante, J.P.M.; Dos Santos, E.C.; Bassi, Ê.J.; da Silva-Júnior, E.F. Computer-Aided Design, Synthesis, and Antiviral Evaluation of Novel Acrylamides as Potential Inhibitors of E3-E2-E1 Glycoproteins Complex from Chikungunya Virus. Pharmaceuticals 2020, 13, 141. [Google Scholar] [CrossRef] [PubMed]
- Ho, Y.J.; Liu, F.C.; Yeh, C.T.; Yang, C.M.; Lin, C.C.; Lin, T.Y.; Hsieh, P.S.; Hu, M.K.; Gong, Z.; Lu, J.W. Micafungin is a novel anti-viral agent of chikungunya virus through multiple mechanisms. Antivir. Res. 2018, 159, 134–142. [Google Scholar] [CrossRef]
- Rothan, H.A.; Bahrani, H.; Mohamed, Z.; Teoh, T.C.; Shankar, E.M.; Rahman, N.A.; Yusof, R. A Combination of Doxycycline and Ribavirin Alleviated Chikungunya Infection. PLoS ONE 2015, 10, e0126360. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fatma, B.; Kumar, R.; Singh, V.A.; Nehul, S.; Sharma, R.; Kesari, P.; Kuhn, R.J.; Tomar, S. Alphavirus capsid protease inhibitors as potential antiviral agents for Chikungunya infection. Antivir. Res. 2020, 179, 104808. [Google Scholar] [CrossRef]
- Mishra, P.; Kumar, A.; Mamidi, P.; Kumar, S.; Basantray, I.; Saswat, T.; Das, I.; Nayak, T.K.; Chattopadhyay, S.; Subudhi, B.B.; et al. Inhibition of Chikungunya Virus Replication by 1-[(2-Methylbenzimidazol-1-yl) Methyl]-2-Oxo-Indolin-3-ylidene] Amino] Thiourea (MBZM-N-IBT). Sci. Rep. 2016, 6, 20122. [Google Scholar] [CrossRef]
- Singh Jadav, S.; Sinha, B.N.; Hilgenfeld, R.; Pastorino, B.; De Lamballerie, X.; Jayaprakash, V. Thiazolidone derivatives as inhibitors of chikungunya virus. Eur. J. Med. Chem. 2015, 89, 172–178. [Google Scholar] [CrossRef]
- El-labbad, E.M.; Ismail, M.A.H.; Abou Ei Ella, D.A.; Ahmed, M.; Wang, F.; Barakat, K.H.; Abouzid, K.A.M. Discovery of Novel Peptidomimetics as Irreversible CHIKV NsP2 Protease Inhibitors Using Quantum Mechanical-Based Ligand Descriptors. Chem. Biol. Drug Des. 2015, 86, 1518–1527. [Google Scholar] [CrossRef]
- Singh, H.; Mudgal, R.; Narwal, M.; Kaur, R.; Singh, V.A.; Malik, A.; Chaudhary, M.; Tomar, S. Chikungunya virus inhibition by peptidomimetic inhibitors targeting virus-specific cysteine protease. Biochimie 2018, 149, 51–61. [Google Scholar] [CrossRef] [PubMed]
- Bhakat, S.; Delang, L.; Kaptein, S.; Neyts, J.; Leyssen, P.; Jayaprakash, V. Reaching beyond HIV/HCV: Nelfinavir as a potential starting point for broad-spectrum protease inhibitors against dengue and chikungunya virus. RSC Adv. 2015, 5, 85938–85949. [Google Scholar] [CrossRef]
- Di Mola, A.; Peduto, A.; La Gatta, A.; Delang, L.; Pastorino, B.; Neyts, J.; Leyssen, P.; de Rosa, M.; Filosa, R. Structure–activity relationship study of arbidol derivatives as inhibitors of chikungunya virus replication. Bioorg. Med. Chem. 2014, 22, 6014–6025. [Google Scholar] [CrossRef] [PubMed]
- Henß, L.; Beck, S.; Weidner, T.; Biedenkopf, N.; Sliva, K.; Weber, C.; Becker, S.; Schnierle, B.S. Suramin is a potent inhibitor of Chikungunya and Ebola virus cell entry. Virol. J. 2016, 13, 149. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Albulescu, I.C.; White-Scholten, L.; Tas, A.; Hoornweg, T.E.; Ferla, S.; Kovacikova, K.; Smit, J.M.; Brancale, A.; Snijder, E.J.; van Hemert, M.J. Suramin Inhibits Chikungunya Virus Replication by Interacting with Virions and Blocking the Early Steps of Infection. Viruses 2020, 12, 314. [Google Scholar] [CrossRef] [Green Version]
- Lu, J.W.; Hsieh, P.S.; Lin, C.C.; Hu, M.K.; Huang, S.M.; Wang, Y.M.; Liang, C.Y.; Gong, Z.; Ho, Y.J. Synergistic effects of combination treatment using EGCG and suramin against the chikungunya virus. Biochem. Biophys. Res. Commun. 2017, 491, 595–602. [Google Scholar] [CrossRef]
- Weber, C.; Sliva, K.; von Rhein, C.; Kümmerer, B.M.; Schnierle, B.S. The green tea catechin, epigallocatechin gallate inhibits chikungunya virus infection. Antivir. Res. 2015, 113, 1–3. [Google Scholar] [CrossRef]
- Loke, R.H.T.; Anderson, M.G.; Coleman, J.C.; Murray-Lyon, I.M.; Tsiquaye, K.N.; Zuckerman, A.J. Suramin treatment for chronic active hepatitis B—toxic and ineffective. J. Med. Virol. 1987, 21, 97–99. [Google Scholar] [CrossRef]
- Kaplan, L.D.; Wolfe, P.R.; Volberding, P.A.; Feorino, P.; Abrams, D.I.; Levy, J.A.; Wong, R.; Kaufman, L.; Gottlieb, M.S. Lack of response to suramin in patients with AIDS and AIDS-related complex. Am. J. Med. 1987, 82, 615–620. [Google Scholar] [CrossRef]
- Agarwal, G.; Gupta, S.; Gabrani, R.; Gupta, A.; Chaudhary, V.K.; Gupta, V. Virtual screening of inhibitors against Envelope glycoprotein of Chikungunya Virus: A drug repositioning approach. Bioinformation 2019, 15, 439–447. [Google Scholar] [CrossRef]
- Fernandez-Pol, J.A.; Fernandez-Pol, S. Method to Control Dengue Viruses in Humans by Picolinic Acid and Derivates Thereof 2010. U.S. Patent 12/175,277, 21 January 2010. [Google Scholar]
- Kim, H.Y.; Kuhn, R.J.; Patkar, C.; Warrier, R.; Cushman, M. Synthesis of dioxane-based antiviral agents and evaluation of their biological activities as inhibitors of Sindbis virus replication. Bioorganic Med. Chem. 2007, 15, 2667–2679. [Google Scholar] [CrossRef] [Green Version]
- Aggarwal, M.; Tapas, S.; Preeti; Siwach, A.; Kumar, P.; Kuhn, R.J.; Tomar, S. Crystal Structure of Aura Virus Capsid Protease and Its Complex with Dioxane: New Insights into Capsid-Glycoprotein Molecular Contacts. PLoS ONE 2012, 7, e51288. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aggarwal, M.; Kaur, R.; Saha, A.; Mudgal, R.; Yadav, R.; Dash, P.K.; Parida, M.; Kumar, P.; Tomar, S. Evaluation of antiviral activity of piperazine against Chikungunya virus targeting hydrophobic pocket of alphavirus capsid protein. Antivir. Res. 2017, 146, 102–111. [Google Scholar] [CrossRef]
- Sharma, R.; Kesari, P.; Kumar, P.; Tomar, S. Structure-function insights into chikungunya virus capsid protein: Small molecules targeting capsid hydrophobic pocket. Virology 2018, 515, 223–234. [Google Scholar] [CrossRef]
- Jing, X.; Ma, C.; Ohigashi, Y.; Oliveira, A.F.; Jardetzky, S.T.; Pinto, H.L.; Lamb, A.R. Functional studies indicate amantadine binds to the pore of the influenza A virus M2 proton-selective ion channel. Proc. Natl. Acad. Sci. USA 2008, 105, 10967–10972. [Google Scholar] [CrossRef] [Green Version]
- Cheung, J.; Frankling, M.; Mancia, F.; Rudolph, M.; Cassidy, M.; Gary, E.; Burshteyn, F.; Love, J. RCSB PDB—3TRK: Structure of the Chikungunya Virus nsP2 Protease; 2011. Available online: https://www.rcsb.org/structure/3TRK (accessed on 2 June 2021).
- Meena, M.K.; Kumar, D.; Kumari, K.; Kaushik, N.K.; Kumar, R.V.; Bahadur, I.; Vodwal, L.; Singh, P. Promising inhibitors of nsp2 of CHIKV using molecular docking and temperature-dependent molecular dynamics simulations. J. Biomol. Struct. Dyn. 2021, 21, 1–9. [Google Scholar] [CrossRef]
- Montes-Grajales, D.; Puerta-Guardo, H.; Espinosa, D.A.; Harris, E.; Caicedo-Torres, W.; Olivero-Verbel, J.; Martínez-Romero, E. In silico drug repurposing for the identification of potential candidate molecules against arboviruses infection. Antivir. Res. 2020, 173, 104668. [Google Scholar] [CrossRef] [PubMed]
- Khan, N.; Bhat, R.; Patel, A.K.; Ray, P. Discovery of small molecule inhibitors of chikungunya virus proteins (nsP2 and E1) using in silico approaches. J. Biomol. Struct. Dyn. 2021, 39, 1373–1385. [Google Scholar] [CrossRef]
- Kumar, P.; Kumar, D.; Giri, R. Targeting the nsp2 cysteine protease of Chikungunya virus using FDA approved library and selected Cysteine protease inhibitors. Pathogens 2019, 8, 128. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jain, J.; Kumari, A.; Somvanshi, P.; Grover, A.; Pai, S.; Sunil, S. In silico analysis of natural compounds targeting structural and nonstructural proteins of chikungunya virus. F1000Research 2017, 6, 1601. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, T.; Asthana, S.; Bissoyi, A. Molecular modeling and docking study to elucidate novel chikungunya virus nsP2 protease inhibitors. Indian J. Pharm. Sci. 2015, 77, 453–460. [Google Scholar]
- Nguyen, P.T.V.; Yu, H.; Keller, P.A. Identification of chikungunya virus nsP2 protease inhibitors using structure-base approaches. J. Mol. Graph. Model. 2015, 57, 1–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bora, L. Homology Modeling and Docking to Potential Novel Inhibitor for Chikungunya (37997) Protein nsP2 Protease. J Proteomics Bioinform 2012, 5, 54–59. [Google Scholar] [CrossRef] [Green Version]
- Singh, K.D.; Kirubakaran, P.; Nagarajan, S.; Sakkiah, S.; Muthusamy, K.; Velmurgan, D.; Jeyakanthan, J. Homology modeling, molecular dynamics, e-pharmacophore mapping and docking study of Chikungunya virus nsP2 protease. J. Mol. Model. 2012, 18, 39–51. [Google Scholar] [CrossRef] [PubMed]
- Dhindwal, S.; Kesari, P.; Singh, H.; Kumar, P.; Tomar, S.; Sarma, R.H. Conformer and pharmacophore based identification of peptidomimetic inhibitors of chikungunya virus nsP2 protease. J. Biomol. Struct. Dyn. 2017, 35, 3522–3539. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, P.T.V.; Yu, H.; Keller, P.A. Discovery of in silico hits targeting the nsP3 macro domain of chikungunya virus. J. Mol. Model. 2014, 20, 2216. [Google Scholar] [CrossRef]
- Abu Bakar, F.; Ng, L. Nonstructural Proteins of Alphavirus—Potential Targets for Drug Development. Viruses 2018, 10, 71. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abdelnabi, R.; de Morais, A.T.S.; Leyssen, P.; Imbert, I.; Beaucourt, S.; Blanc, H.; Froeyen, M.; Vignuzzi, M.; Canard, B.; Neyts, J.; et al. Understanding the Mechanism of the Broad-Spectrum Antiviral Activity of Favipiravir (T-705): Key Role of the F1 Motif of the Viral Polymerase. J. Virol. 2017, 91, e00487-17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Franco, E.J.; Rodriquez, J.L.; Pomeroy, J.J.; Hanrahan, K.C.; Brown, A.N. The effectiveness of antiviral agents with broad-spectrum activity against chikungunya virus varies between host cell lines. Antivir. Chem. Chemother. 2018, 26, 204020661880758. [Google Scholar] [CrossRef]
- Julander, J.G.; Dagley, A.; Gebre, M.; Komeno, T.; Nakajima, N.; Smee, D.F.; Furuta, Y. Strain-dependent disease and response to favipiravir treatment in mice infected with Chikungunya virus. Antivir. Res. 2020, 182, 104904. [Google Scholar] [CrossRef]
- Kumar, S.P.; Kapopara, R.G.; Patni, M.I.; Pandya, H.A.; Jasrai, Y.T.; Patel, S.K. Exploring the polymerase activity of chikungunya viral non structural protein 4 (nsP4) using molecular modeling, e-pharmacophore and docking studies. Int. J. Pharm. Life Sci. 2012, 3, 1752–1765. [Google Scholar]
- Khan, M.; Santhosh, S.R.; Tiwari, M.; Lakshmana Roa, P.V.; Parida, M. Assessment of in vitro prophylactic and therapeutic efficacy of chloroquine against chikungunya virus in Vero cells. J. Med. Virol. 2010, 88, 817–824. [Google Scholar] [CrossRef] [PubMed]
- Varghese, F.S.; Rausalu, K.; Hakanen, M.; Saul, S.; Kümmerer, B.M.; Susi, P.; Merits, A.; Ahola, T. Obatoclax Inhibits Alphavirus Membrane Fusion by Neutralizing the Acidic Environment of Endocytic Compartments. Antimicrob. Agents Chemother. 2017, 61, e02227-16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Y.-M.; Lu, J.-W.; Lin, C.-C.; Chin, Y.-F.; Wu, T.-Y.; Lin, L.-I.; Lai, Z.-Z.; Kuo, S.-C.; Ho, Y.-J. Antiviral activities of niclosamide and nitazoxanide against chikungunya virus entry and transmission. Antivir. Res. 2016, 135, 81–90. [Google Scholar] [CrossRef]
- Hua, C.; Lee, R.; Hussain, K.M.; Jang, J.; Chuid, H. Macropinocytosis dependent entry of Chikungunya virus into human muscle cells. PLoS Negl. Trop. Dis. 2019, 13, e0007610. [Google Scholar]
- Karlas, A.; Berre, S.; Couderc, T.; Varjak, M.; Braun, P.; Meyer, M.; Gangneux, N.; Karo-Astover, L.; Weege, F.; Raftery, M.; et al. A human genome-wide loss-of-function screen identifies effective chikungunya antiviral drugs. Nat. Commun. 2016, 7, 11320. [Google Scholar] [CrossRef]
- Bakhache, W.; Neyret, A.; McKellar, J.; Clop, C.; Bernard, E.; Weger-Lucarelli, J.; Briant, L. Fatty acid synthase and stearoyl-CoA desaturase-1 are conserved druggable cofactors of Old World Alphavirus genome replication. Antivir. Res. 2019, 172, 104642. [Google Scholar] [CrossRef]
- Hitakarun, A.; Khongwichit, S.; Wikan, N.; Roytrakul, S.; Yoksan, S.; Rajakam, S.; Davidson, A.D.; Smith, D.R. Evaluation of the antiviral activity of orlistat (tetrahydrolipstatin) against dengue virus, Japanese encephalitis virus, Zika virus and chikungunya virus. Sci. Rep. 2020, 10, 1499. [Google Scholar] [CrossRef] [Green Version]
- Wichit, S.; Hamel, R.; Bernard, E.; Talignani, L.; Diop, F.; Ferraris, P.; Liegeois, F.; Ekchariyawat, P.; Luplertlop, N.; Surasombatpattana, P.; et al. Imipramine Inhibits Chikungunya Virus Replication in Human Skin Fibroblasts through Interference with Intracellular Cholesterol Trafficking. Sci. Rep. 2017, 7, 3145. [Google Scholar] [CrossRef] [Green Version]
- Hwang, J.; Wang, Y.; Fikrig, E. Inhibition of chikungunya virus replication in primary human fibroblasts by liver X receptor agonist. Antimicrob. Agents Chemother. 2019, 63, e01220-19. [Google Scholar] [CrossRef] [Green Version]
- Briolant, S.; Garin, D.; Scaramozzino, N.; Jouan, A.; Crance, J.M. In vitro inhibition of Chikungunya and Semliki Forest viruses replication by antiviral compounds: Synergistic effect of interferon-alpha and ribavirin combination. Antivir. Res. 2004, 61, 111–117. [Google Scholar] [CrossRef]
- Tong, X.; Smith, J.; Bukreyeva, N.; Koma, T.; Manning, J.T.; Kalkeri, R.; Kwong, A.D.; Paessler, S. Merimepodib, an IMPDH inhibitor, suppresses replication of Zika virus and other emerging viral pathogens. Antivir. Res. 2018, 149, 34–40. [Google Scholar] [CrossRef] [PubMed]
- Lucas-Hourani, M.; Dauzonne, D.; Jorda, P.; Lle Cousin, G.; Lupan, A.; Helynck, O.; Gory Caignard, G.; Ve Janvier, G.; Naë Lle André -Leroux, G.; Khiar, S.; et al. Inhibition of Pyrimidine Biosynthesis Pathway Suppresses Viral Growth through Innate Immunity. PLoS Pathog. 2013, 9, e1003678. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lucas-Hourani, M.; Dauzonne, D.; Munier-Lehmann, H.; Khiar, S.; Nisole, S.; Dairou, J.; Helynck, O.; Afonso, P.V.; Tangy, F.; Vidalain, P.O. Original chemical series of pyrimidine biosynthesis inhibitors that boost the antiviral interferon response. Antimicrob. Agents Chemother. 2017, 61, e00383-17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, Y.; Cao, L.; Gao, H.; Wu, Y.; Wang, Y.; Fang, F.; Lan, T.; Lou, Z.; Rao, Y. Discovery, Optimization, and Target Identification of Novel Potent Broad-Spectrum Antiviral Inhibitors. J. Med. Chem. 2019, 62, 4056–4073. [Google Scholar] [CrossRef] [PubMed]
- Cifuentes Kottkamp, A.; De Jesus, E.; Grande, R.; Brown, J.A.; Jacobs, A.R.; Lim, J.K.; Stapleford, K.A. Atovaquone Inhibits Arbovirus Replication through the Depletion of Intracellular Nucleotides. J. Virol. 2019, 93, e00389-19. [Google Scholar] [CrossRef] [Green Version]
- Hwang, J.; Jiang, A.; Fikrig, E. A potent prolyl tRNA synthetase inhibitor antagonizes Chikungunya and Dengue viruses. Antivir. Res. 2019, 161, 163–168. [Google Scholar] [CrossRef]
- Kaur, P.; Thiruchelvan, M.; Lee, R.C.H.; Chen, H.; Chen, K.C.; Ng, M.L.; Chu, J.J.H. Inhibition of chikungunya virus replication by harringtonine, a novel antiviral that suppresses viral protein expression. Antimicrob. Agents Chemother. 2013, 57, 155–167. [Google Scholar] [CrossRef] [Green Version]
- Lundberg, L.; Brahms, A.; Hooper, I.; Carey, B.; Lin, S.C.; Dahal, B.; Narayanan, A.; Kehn-Hall, K. Repurposed FDA-Approved drug sorafenib reduces replication of Venezuelan equine encephalitis virus and other alphaviruses. Antivir. Res. 2018, 157, 57–67. [Google Scholar] [CrossRef]
- Kaur, P.; Lello, L.S.; Utt, A.; Dutta, S.K.; Merits, A.; Chu, J.J.H. Bortezomib inhibits chikungunya virus replication by interfering with viral protein synthesis. PLoS Negl. Trop. Dis. 2020, 14, e0008336. [Google Scholar] [CrossRef]
- Hwang, J.; Jiang, A.; Fikrig, E. Rev-erb Agonist Inhibits Chikungunya and O’nyong’nyong Virus Replication. Open Forum Infect. Dis. 2018, 5, ofy315. [Google Scholar] [CrossRef]
- Hackett, B.A.; Dittmar, M.; Segrist, E.; Pittenger, N.; To, J.; Griesman, T.; Gordesky-Gold, B.; Schultz, D.C.; Cherry, S. Sirtuin inhibitors are broadly antiviral against arboviruses. MBio 2019, 10, e01446-19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rathore, A.P.S.; Haystead, T.; Das, P.K.; Merits, A.; Ng, M.L.; Vasudevan, S.G. Chikungunya virus nsP3 & nsP4 interacts with HSP-90 to promote virus replication: HSP-90 inhibitors reduce CHIKV infection and inflammation in vivo. Antivir. Res. 2014, 103, 7–16. [Google Scholar]
- Barrera, M.D.; Callahan, V.; Akhrymuk, I.; Bhalla, N.; Zhou, W.; Campbell, C.; Narayanan, A.; Kehn-hall, K. Proteomic discovery of veev e2-host partner interactions identifies grp78 inhibitor ha15 as a potential therapeutic for alphavirus infections. Pathogens 2021, 10, 283. [Google Scholar] [CrossRef]
- Langsjoen, R.M.; Auguste, A.J.; Rossi, S.L.; Roundy, C.M.; Penate, H.N.; Kastis, M.; Schnizlein, M.K.; Le, K.C.; Haller, S.L.; Chen, R.; et al. Host oxidative folding pathways offer novel anti-chikungunya virus drug targets with broad spectrum potential. Antivir. Res. 2017, 143, 246–251. [Google Scholar] [CrossRef] [PubMed]
- Zilbermintz, L.; Leonardi, W.; Jeong, S.Y.; Sjodt, M.; McComb, R.; Ho, C.L.C.; Retterer, C.; Gharaibeh, D.; Zamani, R.; Soloveva, V.; et al. Identification of agents effective against multiple toxins and viruses by host-oriented cell targeting. Sci. Rep. 2015, 5, 13476. [Google Scholar] [CrossRef] [Green Version]
- Yoon, J.S.; Kim, G.; Jarhad, D.B.; Kim, H.R.; Shin, Y.S.; Qu, S.; Sahu, P.K.; Kim, H.O.; Lee, H.W.; Wang, S.B.; et al. Design, Synthesis, and Anti-RNA Virus Activity of 6′-Fluorinated-Aristeromycin Analogues. J. Med. Chem. 2019, 62, 6346–6362. [Google Scholar] [CrossRef] [PubMed]
- Broeckel, R.; Sarkar, S.; May, N.A.; Totonchy, J.; Kreklywich, C.N.; Smith, P.; Graves, L.; DeFilippis, V.R.; Heise, M.T.; Morrison, T.E.; et al. Src family kinase inhibitors block translation of alphavirus subgenomic mRNAs. Antimicrob. Agents Chemother. 2019, 63, e02325-18. [Google Scholar] [CrossRef] [Green Version]
- Cruz, D.J.M.; Bonotto, R.M.; Gomes, R.G.B.; da Silva, C.T.; Taniguchi, J.B.; No, J.H.; Lombardot, B.; Schwartz, O.; Hansen, M.A.E.; Freitas-Junior, L.H. Identification of Novel Compounds Inhibiting Chikungunya Virus-Induced Cell Death by High Throughput Screening of a Kinase Inhibitor Library. PLoS Negl. Trop. Dis. 2013, 7, e2471. [Google Scholar] [CrossRef] [Green Version]
- Varghese, F.S.; Kaukinen, P.; Gläsker, S.; Bespalov, M.; Hanski, L.; Wennerberg, K.; Kümmerer, B.M.; Ahola, T. Discovery of berberine, abamectin and ivermectin as antivirals against chikungunya and other alphaviruses. Antivir. Res. 2016, 126, 117–124. [Google Scholar] [CrossRef]
- Varghese, F.S.; Thaa, B.; Amrun, S.N.; Simarmata, D.; Rausalu, K.; Nyman, T.A.; Merits, A.; McInerney, G.M.; Ng, L.F.P.; Ahola, T. The Antiviral Alkaloid Berberine Reduces Chikungunya Virus-Induced Mitogen-Activated Protein Kinase Signaling. J. Virol. 2016, 90, 9743–9757. [Google Scholar] [CrossRef] [Green Version]
- Sharma, A.; Bhomia, M.; Yeh, T.-J.J.; Singh, J.; Maheshwari, R.K. Miltefosine inhibits Chikungunya virus replication in human primary dermal fibroblasts. F1000Research 2018, 7, 9. [Google Scholar] [CrossRef]
- Abdelnabi, R.; Amrun, S.N.; Ng, L.F.P.; Leyssen, P.; Neyts, J.; Delang, L. Protein kinases C as potential host targets for the inhibition of chikungunya virus replication. Antivir. Res. 2017, 139, 79–87. [Google Scholar] [CrossRef] [PubMed]
- Staveness, D.; Abdelnabi, R.; Schrier, A.J.; Loy, B.A.; Verma, V.A.; DeChristopher, B.A.; Near, K.E.; Neyts, J.; Delang, L.; Leyssen, P.; et al. Simplified Bryostatin Analogues Protect Cells from Chikungunya Virus-Induced Cell Death. J. Nat. Prod. 2016, 79, 675–679. [Google Scholar] [CrossRef] [PubMed]
- Staveness, D.; Abdelnabi, R.; Near, K.E.; Nakagawa, Y.; Neyts, J.; Delang, L.; Leyssen, P.; Wender, P.A. Inhibition of Chikungunya Virus-Induced Cell Death by Salicylate-Derived Bryostatin Analogues Provides Additional Evidence for a PKC-Independent Pathway. J. Nat. Prod. 2016, 79, 680–684. [Google Scholar] [CrossRef] [Green Version]
- Pu, S.-Y.; Wouters, R.; Schor, S.; Rozenski, J.; Barouch-Bentov, R.; Prugar, L.I.; O’brien, C.M.; Brannan, J.M.; Dye, J.M.; Herdewijn, P.; et al. Optimization of Isothiazolo[4,3-b]pyridine-Based Inhibitors of Cyclin G Associated Kinase (GAK) with Broad-Spectrum Antiviral Activity. J. Med. Chem. 2018, 61, 6178–6192. [Google Scholar] [CrossRef] [PubMed]
- Mounce, B.C.; Cesaro, T.; Moratorio, G.; Hooikaas, P.J.; Yakovleva, A.; Werneke, S.W.; Smith, E.C.; Poirier, E.Z.; Simon-Loriere, E.; Prot, M.; et al. Inhibition of Polyamine Biosynthesis Is a Broad-Spectrum Strategy against RNA Viruses. J. Virol. 2016, 90, 9683–9692. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheung, Y.Y.; Chen, K.C.; Chen, H.; Seng, E.K.; Chu, J.J.H. Antiviral activity of lanatoside C against dengue virus infection. Antivir. Res. 2014, 111, 93–99. [Google Scholar] [CrossRef] [PubMed]
- Müller, M.; Slivinski, N.; Todd, E.J.A.A.; Khalid, H.; Li, R.; Karwatka, M.; Merits, A.; Mankouri, J.; Tuplin, A. Chikungunya virus requires cellular chloride channels for efficient genome replication. PLoS Negl. Trop. Dis. 2019, 13, e0007703. [Google Scholar] [CrossRef] [Green Version]
- Bouma, E.M.; van de Pol, D.P.I.; Sanders, I.D.; Rodenhuis-Zybert, I.A.; Smit, J.M. Serotonergic Drugs Inhibit Chikungunya Virus Infection at Different Stages of the Cell Entry Pathway. J. Virol. 2020, 94, e00274-20. [Google Scholar] [CrossRef]
- Mainou, B.A.; Ashbrook, A.W.; Smith, E.C.; Dorset, D.C.; Denison, M.R.; Dermody, T.S. Serotonin Receptor Agonist 5-Nonyloxytryptamine Alters the Kinetics of Reovirus Cell Entry. J. Virol. 2015, 89, 8701–8712. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ekins, S.; Madrid, P.B. Tilorone, a broad-spectrum antiviral for emerging viruses. Antimicrob. Agents Chemother. 2020, 64, e00440-20. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gall, B.; Pryke, K.; Abraham, J.; Mizuno, N.; Botto, S.; Sali, T.M.; Broeckel, R.; Haese, N.; Nilsen, A.; Placzek, A.; et al. Emerging Alphaviruses Are Sensitive to Cellular States Induced by a Novel Small-Molecule Agonist of the STING Pathway. J. Virol. 2017, 92, e01913-17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sali, T.M.; Pryke, K.M.; Abraham, J.; Liu, A.; Archer, I.; Broeckel, R.; Staverosky, J.A.; Smith, J.L.; Al-Shammari, A.; Amsler, L.; et al. Characterization of a Novel Human-Specific STING Agonist that Elicits Antiviral Activity Against Emerging Alphaviruses. PLoS Pathog. 2015, 11, e1005324. [Google Scholar] [CrossRef]
- Pryke, K.M.; Abraham, J.; Sali, T.M.; Gall, B.J.; Archer, I.; Liu, A.; Bambina, S.; Baird, J.; Gough, M.; Chakhtoura, M.; et al. A novel agonist of the trif pathway induces a cellular state refractory to replication of Zika, Chikungunya, and dengue viruses. MBio 2017, 8, e00452-17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Herrero, L.J.; Foo, S.-S.; Sheng, K.-C.; Chen, W.; Forwood, M.R.; Bucala, R.; Mahalingam, S. Pentosan Polysulfate: A Novel Glycosaminoglycan-Like Molecule for Effective Treatment of Alphavirus-Induced Cartilage Destruction and Inflammatory Disease. J. Virol. 2015, 89, 8063–8076. [Google Scholar] [CrossRef] [Green Version]
- Supramaniam, A.; Liu, X.; Ferro, V.; Herrero, L.J. Prophylactic antiheparanase activity by PG545 is antiviral in vitro and protects against Ross River virus disease in mice. Antimicrob. Agents Chemother. 2018, 62, e01959-17. [Google Scholar] [CrossRef] [Green Version]
- Pou, S.; Winter, R.W.; Nilsen, A.; Kelly, J.X.; Li, Y.; Doggett, J.S.; Riscoe, E.W.; Wegmann, K.W.; Hinrichs, D.J.; Riscoe, M.K. Sontochin as a guide to the development of drugs against chloroquine-resistant malaria. Antimicrob. Agents Chemother. 2012, 56, 3475–3480. [Google Scholar] [CrossRef] [Green Version]
- Inglot, A.D. Comparison of the Antiviral Activity in vitro of some Non-steroidal Anti-inflammatory Drugs. J. Gen. Virol 1969, 4, 203–214. [Google Scholar] [CrossRef]
- Shimizu, Y.; Yamamoto, S.; Homma, M.; Ishida, N. Effect of chloroquine on the growth of animal viruses. Arch. Gesamte Virusforsch. 1972, 36, 93–104. [Google Scholar] [CrossRef]
- Helenius, A.; Marsht, M.; Whiter, J. Inhibition of Semliki Forest Virus Penetration by Lysosomotropic Weak Bases. J. Gen. Virol 1982, 58, 47–61. [Google Scholar] [CrossRef] [PubMed]
- Cassell, S.; Edwards, J.; Brown, D.T. Effects of lysosomotropic weak bases on infection of BHK-21 cells by Sindbis virus. J. Virol. 1984, 52, 857–864. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Freedman, A.; Steinberg, V.L. Chloroquine in rheumatoid arthritis; a double blindfold trial of treatment for one year. Ann. Rheum. Dis 1960, 19, 243–250. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brighton, S.W. Chloroquine phosphate treatment of chronic Chikungunya arthritis An open pilot study. S. Afr. Med. J. 1984, 66, 217–218. [Google Scholar]
- De Lamballerie, X.; Boisson, V.; Reynier, J.-C.; Enault, S.; Charrel, R.N.; Flahault, A.; Roques, P.; Le Grand, R. On Chikungunya Acute Infection and Chloroquine Treatment. Vector-Borne Zoonotic Dis. 2008, 8, 837–839. [Google Scholar] [CrossRef] [PubMed]
- Thiberville, S.D.; Boisson, V.; Gaudart, J.; Simon, F.; Flahault, A.; de Lamballerie, X. Chikungunya Fever: A Clinical and Virological Investigation of Outpatients on Reunion Island, South-West Indian Ocean. PLoS Negl. Trop. Dis. 2013, 7, e2004. [Google Scholar] [CrossRef]
- Chopra, A.; Saluja, M.; Venugopalan, A. Effectiveness of chloroquine and inflammatory cytokine response in patients with early persistent musculoskeletal pain and arthritis following chikungunya virus infection. Arthritis Rheumatol. 2014, 66, 319–326. [Google Scholar] [CrossRef] [Green Version]
- Rodrigo, C.; Fernando, S.D.; Rajapakse, S.; Paul, M. Systematic review Clinical evidence for repurposing chloroquine and hydroxychloroquine as antiviral agents: A systematic review. Clin. Microbiol. Infect. 2020, 26, 979–987. [Google Scholar] [CrossRef]
- Roques, P.; Thiberville, S.D.; Dupuis-Maguiraga, L.; Lum, F.M.; Labadie, K.; Martinon, F.; Gras, G.; Lebon, P.; Ng, L.F.P.; de Lamballerie, X.; et al. Paradoxical effect of chloroquine treatment in enhancing chikungunya virus infection. Viruses 2018, 10, 268. [Google Scholar] [CrossRef] [Green Version]
- Ozden, S.; Lucas-Hourani, M.; Ceccaldi, P.-E.; Basak, A.; Valentine, M.; Benjannet, S.; Hamelin, J.; Jacob, Y.; Mamchaoui, K.; Mouly, V.; et al. Inhibition of Chikungunya virus infection in cultured human muscle cells by furin inhibitors: Impairment of the maturation of the E2 surface glycoprotein. J. Biol. Chem. 2008, 283, 21899–21908. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Faraone, I.; Labanca, F.; Ponticelli, M.; De Tommasi, N.; Milella, L. Recent Clinical and Preclinical Studies of Hydroxychloroquine on RNA Viruses and Chronic Diseases: A Systematic Review. Molecules 2020, 25, 5318. [Google Scholar] [CrossRef] [PubMed]
- Padmakumar, B.; Jayan, J.B.; Menon, R.M.R.; Krishnankutty, B.; Payippallil, R.; Nisha, R.S. Comparative evaluation of four therapeutic regimes in Chikungunya arthritis: A prospective randomized parallel-group study. Indian J. Rheumatol. 2009, 4, 94–101. [Google Scholar] [CrossRef]
- Ravindran, V.; Alias, G. Efficacy of combination DMARD therapy vs. hydroxychloroquine monotherapy in chronic persistent chikungunya arthritis: A 24-week randomized controlled open label study. Clin. Rheumatol. 2017, 36, 1335–1340. [Google Scholar] [CrossRef] [PubMed]
- Pandya, S. Methotrexate and hydroxychloroquine combination therapy in chronic chikungunya arthritis: A 16 week study. Indian J. Rheumatol. 2008, 3, 93–97. [Google Scholar] [CrossRef]
- Bouquillard, E.; Fianu, A.; Bangil, M.; Charlette, N.; Ribéra, A.; Michault, A.; Favier, F.; Simon, F.; Flipo, R.M. Rheumatic manifestations associated with Chikungunya virus infection: A study of 307 patients with 32-month follow-up (RHUMATOCHIK study). Jt. Bone Spine 2018, 85, 207–210. [Google Scholar] [CrossRef]
- Sourisseau, M.; Schilte, C.; Casartelli, N.; Trouillet, C.; Guivel-Benhassine, F.; Rudnicka, D.; Sol-Foulon, N.; Le Roux, K.; Prevost, M.-C.; Fsihi, H.; et al. Characterization of Reemerging Chikungunya Virus. PLoS Pathog. 2007, 3, e89. [Google Scholar] [CrossRef]
- Irurzun, A.; Nieva, J.L.; Carrasco, L. Entry of Semliki forest virus into cells: Effects of concanamycin A and nigericin on viral membrane fusion and infection. Virology 1997, 227, 488–492. [Google Scholar] [CrossRef] [Green Version]
- Jurgeit, A.; Mcdowell, R.; Moese, S.; Meldrum, E.; Schwendener, R. Niclosamide Is a Proton Carrier and Targets Acidic Endosomes with Broad Antiviral Effects. PLoS Pathog. 2012, 8, e1002976. [Google Scholar] [CrossRef] [Green Version]
- Koivusalo, M.; Welch, C.; Hayashi, H.; Scott, C.C.; Kim, M.; Alexander, T.; Touret, N.; Hahn, K.M.; Grinstein, S. Amiloride inhibits macropinocytosis by lowering submembranous pH and preventing Rac1 and Cdc42 signaling. J. Cell Biol. 2010, 188, 547–563. [Google Scholar] [CrossRef] [Green Version]
- Shu, Q.; Nair, V. Inosine monophosphate dehydrogenase (IMPDH) as a target in drug discovery. Med. Res. Rev. 2008, 28, 219–232. [Google Scholar] [CrossRef] [PubMed]
- Turner, T.L.; Kopp, B.T.; Paul, G.; Landgrave, L.C.; Hayes, D.; Thompson, R. Respiratory syncytial virus: Current and emerging treatment options. Clin. Outcomes Res. 2014, 6, 217–225. [Google Scholar] [CrossRef] [Green Version]
- Paeshuyse, J.; Dallmeier, K.; Neyts, J. Ribavirin for the treatment of chronic hepatitis C virus infection: A review of the proposed mechanisms of action. Curr. Opin. Virol. 2011, 1, 590–598. [Google Scholar] [CrossRef] [PubMed]
- Gallegos, K.M.; Drusano, G.L.; D′Argenio, D.Z.; Brown, A.N. Chikungunya Virus: In Vitro Response to Combination Therapy With Ribavirin and Interferon Alfa 2a. J. Infect. Dis. 2016, 214, 1192–1197. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rothan, H.A.; Bahrani, H.; Abdulrahman, A.Y.; Mohamed, Z.; Teoh, T.C.; Othman, S.; Rashid, N.N.; Rahman, N.A.; Yusof, R. Mefenamic acid in combination with ribavirin shows significant effects in reducing chikungunya virus infection in vitro and in vivo. Antivir. Res. 2016, 127, 50–56. [Google Scholar] [CrossRef] [PubMed]
- Ravichandran, R.; Manian, M. Ribavirin therapy for Chikungunya arthritis. J. Infect. Dev. Ctries. 2008, 2, 140–142. [Google Scholar] [CrossRef] [PubMed]
- Rashad, A.A.; Neyts, J.; Leyssen, P.; Keller, P.A. A reassessment of mycophenolic acid as a lead compound for the development of inhibitors of chikungunya virus replication. Tetrahedron 2018, 74, 1294–1306. [Google Scholar] [CrossRef] [Green Version]
- Khan, M.; Dhanwani, R.; Patro, I.K.; Rao, P.V.L.; Parida, M.M. Cellular IMPDH enzyme activity is a potential target for the inhibition of Chikungunya virus replication and virus induced apoptosis in cultured mammalian cells. Antivir. Res. 2010, 89, 1–8. [Google Scholar] [CrossRef]
- Rada, B.; Dragúň, M. Antiviral Action and Selectivity of 6-Azauridine. Ann. N. Y. Acad. Sci. 1977, 284, 410–417. [Google Scholar] [CrossRef]
- Deneau, D.G.; Farber, E.M. The treatment of psoriasis with azaribine. Dermatologica 1975, 151, 158–163. [Google Scholar] [CrossRef]
- Cruthcher, W.A.; Moschella, S.L. Double-blind controlled crossover high-dose study of Azaribine in psoriasis. Br. J. Dermatol. 1975, 92, 199–205. [Google Scholar] [CrossRef]
- Raveh, A.; Delekta, P.C.; Dobry, C.J.; Peng, W.; Schultz, P.J.; Blakely, P.K.; Tai, A.W.; Matainaho, T.; Irani, D.N.; Sherman, D.H.; et al. Discovery of Potent Broad Spectrum Antivirals Derived from Marine Actinobacteria. PLoS ONE 2013, 8, e82318. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ashton, T.M.; Fokas, E.; Kunz-Schughart, L.A.; Folkes, L.K.; Anbalagan, S.; Huether, M.; Kelly, C.J.; Pirovano, G.; Buffa, F.M.; Hammond, E.M.; et al. The anti-malarial atovaquone increases radiosensitivity by alleviating tumour hypoxia. Nat. Commun. 2016, 7, 12308. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Keller, T.L.; Zocco, D.; Sundrud, M.S.; Hendrick, M.; Edenius, M.; Yum, J.; Kim, Y.-J.; Lee, H.-K.; Cortese, J.F.; Wirth, D.F.; et al. Halofuginone and other febrifugine derivatives inhibit prolyl-tRNA synthetase. Nat. Chem. Biol. 2012, 8, 311–317. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fresno, M.; Jiménez, A.; Vázquez, D. Inhibition of Translation in Eukaryotic Systems by Harringtonine. Eur. J. Biochem. 1977, 72, 323–330. [Google Scholar] [CrossRef] [PubMed]
- McKendrick, L.; Morley, S.J.; Pain, V.M.; Jagus, R.; Joshi, B. Phosphorylation of eukaryotic initiation factor 4E (eIF4E) at Ser209 is not required for protein synthesis in vitro and in vivo. Eur. J. Biochem. 2001, 268, 5375–5385. [Google Scholar] [CrossRef]
- Henss, L.; Scholz, T.; Grünweller, A.; Schnierle, B.; Henss, L.; Scholz, T.; Grünweller, A.; Schnierle, B.S. Silvestrol Inhibits Chikungunya Virus Replication. Viruses 2018, 10, 592. [Google Scholar] [CrossRef] [Green Version]
- Blum, L.; Geisslinger, G.; Parnham, M.J.; Grünweller, A.; Schiffmann, S. Natural antiviral compound silvestrol modulates human monocyte-derived macrophages and dendritic cells. J. Cell. Mol. Med. 2020, 24, 6988–6999. [Google Scholar] [CrossRef] [PubMed]
- Luo, H. Interplay between the virus and the ubiquitin-proteasome system: Molecular mechanism of viral pathogenesis. Curr. Opin. Virol. 2016, 17, 1–10. [Google Scholar] [CrossRef]
- Geller, R.; Taguwa, S.; Frydman, J. Broad action of Hsp90 as a host chaperone required for viral replication. Biochim. Biophys. Acta Mol. Cell Res. 2012, 1823, 698–706. [Google Scholar] [CrossRef] [Green Version]
- Das, I.; Basantray, I.; Mamidi, P.; Nayak, T.K.; Pratheek, B.M.; Chattopadhyay, S.; Chattopadhyay, S. Heat Shock Protein 90 Positively Regulates Chikungunya Virus Replication by Stabilizing Viral Non-Structural Protein nsP2 during Infection. PLoS ONE 2014, 9, e100531. [Google Scholar] [CrossRef] [Green Version]
- Izumida, M.; Hayashi, H.; Tanaka, A.; Kubo, Y. Cathepsin B Protease Facilitates Chikungunya Virus Envelope Protein-Mediated Infection Via Endocytosis or Macropinocytosis. Viruses 2020, 12, 722. [Google Scholar] [CrossRef]
- Klimstra, W.B.; Heidner, H.W.; Johnston, R.E.; Klimstra, B.; Ryman, K.D.; Johnston, R.E.; Virol, J. The Furin Protease Cleavage Recognition Sequence of Sindbis Virus PE2 Can Mediate Virion Attachment to Cell Surface Heparan Sulfate. J. Virol. 1999, 73, 6299–6306. [Google Scholar] [CrossRef] [Green Version]
- Heidner, H.W.; Knott, T.A.; Johnston, R.E.; Heidner, H.W.; McKnight, K.L.; Davis, N.L.; Johnston, R.E.; Virol, J. Differential Processing of Sindbis Virus Glycoprotein PE2 in Cultured Vertebrate and Arthropod Cells. J. Virol. 1996, 70, 2069–2073. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.; Fugère, M.; Day, R.; Kielian, M. Furin Processing and Proteolytic Activation of Semliki Forest Virus. J. Virol. 2003, 77, 2981–2989. [Google Scholar] [CrossRef] [Green Version]
- Hardes, K.; Ivanova, T.; Thaa, B.; McInerney, G.M.; Klokk, T.I.; Sandvig, K.; Künzel, S.; Lindberg, I.; Steinmetzer, T. Elongated and Shortened Peptidomimetic Inhibitors of the Proprotein Convertase Furin. ChemMedChem 2017, 12, 613–620. [Google Scholar] [CrossRef]
- Wolfe, M.S.; Borchardt, R.T. S-Adenosyl-L-homocysteine Hydrolase as a Target for Antiviral Chemotherapy. J. Med. Chem. 1991, 34, 1521–1530. [Google Scholar] [CrossRef]
- Keating, J.A.; Striker, R. Phosphorylation events during viral infections provide potential therapeutic targets. Rev. Med. Virol. 2012, 22, 166–181. [Google Scholar] [CrossRef]
- Wan, J.J.; Brown, R.S.; Kielian, M. Berberine chloride is an alphavirus inhibitor that targets nucleocapsid assembly. MBio 2020, 11, e01382-20. [Google Scholar] [CrossRef]
- Bourjot, M.; Delang, L.; Nguyen, V.H.; Neyts, J.; Oise Gueitte, F.; Leyssen, P.; Litaudon, M. Prostratin and 12-O-Tetradecanoylphorbol 13-Acetate Are Potent and Selective Inhibitors of Chikungunya Virus Replication. J. Nat. Prod. 2012, 75, 2183–2187. [Google Scholar] [CrossRef]
- Abdelnabi, R.; Staveness, D.; Near, K.E.; Wender, P.A.; Delang, L.; Neyts, J.; Leyssen, P. Comparative analysis of the anti-chikungunya virus activity of novel bryostatin analogs confirms the existence of a PKC-independent mechanism. Biochem. Pharmacol. 2016, 120, 15–21. [Google Scholar] [CrossRef] [Green Version]
- Mounce, B.C.; Poirier, E.Z.; Passoni, G.; Simon-Loriere, E.; Cesaro, T.; Prot, M.; Stapleford, K.A.; Moratorio, G.; Sakuntabhai, A.; Levraud, J.-P.; et al. Interferon-Induced Spermidine-Spermine Acetyltransferase and Polyamine Depletion Restrict Zika and Chikungunya Viruses. Cell Host Microbe 2016, 20, 167–177. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mounce, B.C.; Cesaro, T.; Vlajnić, L.; Vidiņa, A.; Vallet, T.; Weger-Lucarelli, J.; Passoni, G.; Stapleford, K.A.; Levraud, J.-P.; Vignuzzi, M. Chikungunya Virus Overcomes Polyamine Depletion by Mutation of nsP1 and the Opal Stop Codon To Confer Enhanced Replication and Fitness. J. Virol. 2017, 91, e00344-17. [Google Scholar] [CrossRef] [Green Version]
- Hover, S.; Foster, B.; Barr, J.N.; Mankouri, J. Viral dependence on cellular ion channels—An emerging antiviral target? J. Gen. Virol. 2017, 98, 345–351. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cook, L.E.; Locke, M.C.; Young, A.R.; Monte, K.; Hedberg, M.L.; Shimak, R.M.; Sheehan, K.C.F.; Veis, D.J.; Diamond, M.S.; Lenschow, D.J. Distinct Roles of Interferon Alpha and Beta in Controlling Chikungunya Virus Replication and Modulating Neutrophil-Mediated Inflammation. J. Virol. 2019, 94, e00841-19. [Google Scholar] [CrossRef] [PubMed]
- Krueger, R.F.; Mayer, G.D. Tilorone hydrochloride: An orally active antiviral agent. Science 1970, 169, 1213–1214. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.G.; Siripanyaphinyo, U.; Tumkosit, U.; Noranate, N.; A-Nuegoonpipat, A.; Pan, Y.; Kameoka, M.; Kurosu, T.; Ikuta, K.; Takeda, N.; et al. Poly (I:C), an agonist of toll-like receptor-3, inhibits replication of the Chikungunya virus in BEAS-2B cells. Virol. J. 2012, 9, 114. [Google Scholar] [CrossRef] [Green Version]
- Priya, R.; Patro, I.K.; Parida, M.M. TLR3 mediated innate immune response in mice brain following infection with Chikungunya virus. Virus Res. 2014, 189, 194–205. [Google Scholar] [CrossRef]
- Ichinohe, T.; Watanabe, I.; Ito, S.; Fujii, H.; Moriyama, M.; Tamura, S.; Takahashi, H.; Sawa, H.; Chiba, J.; Kurata, T.; et al. Synthetic Double-Stranded RNA Poly(I:C) Combined with Mucosal Vaccine Protects against Influenza Virus Infection. J. Virol. 2005, 79, 2910–2919. [Google Scholar] [CrossRef] [Green Version]
- Beljanski, V.; Chiang, C.; Kirchenbaum, G.A.; Olagnier, D.; Bloom, C.E.; Wong, T.; Haddad, E.K.; Trautmann, L.; Ross, T.M.; Hiscott, J. Enhanced Influenza Virus-Like Particle Vaccination with a Structurally Optimized RIG-I Agonist as Adjuvant. J. Virol. 2015, 89, 10612–10624. [Google Scholar] [CrossRef] [Green Version]
- Goulet, M.-L.; Olagnier, D.; Xu, Z.; Paz, S.; Belgnaoui, S.M.; Lafferty, E.I.; Janelle, V.; Arguello, M.; Paquet, M.; Ghneim, K.; et al. Systems Analysis of a RIG-I Agonist Inducing Broad Spectrum Inhibition of Virus Infectivity. PLoS Pathog. 2013, 9, e1003298. [Google Scholar] [CrossRef]
- Olagnier, D.; Scholte, F.E.M.; Chiang, C.; Albulescu, I.C.; Nichols, C.; He, Z.; Lin, R.; Snijder, E.J.; van Hemert, M.J.; Hiscott, J. Inhibition of Dengue and Chikungunya Virus Infections by RIG-I-Mediated Type I Interferon-Independent Stimulation of the Innate Antiviral Response. J. Virol. 2014, 88, 4180–4194. [Google Scholar] [CrossRef] [Green Version]
- Chiang, C.; Beljanski, V.; Yin, K.; Olagnier, D.; Ben Yebdri, F.; Steel, C.; Goulet, M.-L.; DeFilippis, V.R.; Streblow, D.N.; Haddad, E.K.; et al. Sequence-Specific Modifications Enhance the Broad-Spectrum Antiviral Response Activated by RIG-I Agonists. J. Virol. 2015, 89, 8011–8025. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Teng, T.S.; Foo, S.S.; Simamarta, D.; Lum, F.M.; Teo, T.H.; Lulla, A.; Yeo, N.K.W.; Koh, E.G.L.; Chow, A.; Leo, Y.S.; et al. Viperin restricts chikungunya virus replication and pathology. J. Clin. Investig. 2012, 122, 4447–4460. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carissimo, G.; Teo, T.H.; Chan, Y.H.; Lee, C.Y.P.; Lee, B.; Torres-Ruesta, A.; Tan, J.J.L.; Chua, T.K.; Fong, S.W.; Lum, F.M.; et al. Viperin controls chikungunya virus-specific pathogenic T cell IFNγ Th1 stimulation in mice. Life Sci. Alliance 2019, 2, e201900298. [Google Scholar] [CrossRef] [Green Version]
- Hammond, E.; Haynes, N.M.; Cullinane, C.; Brennan, T.V.; Bampton, D.; Handley, P.; Karoli, T.; Lanksheer, F.; Lin, L.; Yang, Y.; et al. Immunomodulatory activities of pixatimod: Emerging nonclinical and clinical data, and its potential utility in combination with PD-1 inhibitors. J. Immunother. Cancer 2018, 6, 54. [Google Scholar] [CrossRef] [Green Version]
- Dredge, K.; Brennan, T.V.; Hammond, E.; Lickliter, J.D.; Lin, L.; Bampton, D.; Handley, P.; Lankesheer, F.; Morrish, G.; Yang, Y.; et al. A Phase i study of the novel immunomodulatory agent PG545 (pixatimod) in subjects with advanced solid tumours. Br. J. Cancer 2018, 118, 1035–1041. [Google Scholar] [CrossRef] [Green Version]
- Krishnan, R.; Duiker, M.; Rudd, P.A.; Skerrett, D.; D Pollard, J.G.; Siddel, C.; Rifat, R.; K Ng, J.H.; Georgius, P.; Hererro, L.J.; et al. Pentosan polysulfate sodium for Ross River virus-induced arthralgia: A phase 2a, randomized, double-blind, placebo-controlled study. BMC Musculoskelet. Disord. 2021, 1, 271. [Google Scholar]
- Marra, R.K.F.; Kümmerle, A.E.; Guedes, G.P.; de Barros, S.C.; Gomes, R.S.P.; Cirne-Santos, C.C.; Paixão, I.C.N.P.; Neves, A.P. Quinolone-N-acylhydrazone hybrids as potent Zika and Chikungunya virus inhibitors. Bioorganic Med. Chem. Lett. 2020, 30, 126881. [Google Scholar] [CrossRef]
- Ching, K.-C.; Kam, Y.-W.; Merits, A.; Ng, L.F.P.; Chai, C.L.L. Trisubstituted Thieno[3,2-b]pyrrole 5-Carboxamides as Potent Inhibitors of Alphaviruses. J. Med. Chem. 2015, 58, 9196–9213. [Google Scholar] [CrossRef] [PubMed]
- Ching, K.-C.; Ngoc Quy Tran, T.; Naqiah Amrun, S.; Kam, Y.-W.; P Ng, L.F.; L Chai, C.L. Structural Optimizations of Thieno[3,2-b]pyrrole Derivatives for the Development of Metabolically Stable Inhibitors of Chikungunya Virus. J. Med. Chem. 2017, 60, 3165–3186. [Google Scholar] [CrossRef]
- Fares, M.; McCosker, P.M.; Alsherbiny, M.A.; Willis, A.C.; Clark, T.; Neyts, J.; Jochmans, D.; Keller, P.A. Regioselective convergent synthesis of 2-arylidene thiazolo[3,2- a] pyrimidines as potential anti-chikungunya agents. RSC Adv. 2020, 10, 5191–5195. [Google Scholar] [CrossRef] [Green Version]
- Hwu, J.R.; Kapoor, M.; Tsay, S.-C.; Lin, C.-C.; Hwang, K.C.; Horng, J.-C.; Chen, I.-C.; Shieh, F.-K.; Leyssen, P.; Neyts, J. Benzouracil–coumarin–arene conjugates as inhibiting agents for chikungunya virus. Antivir. Res. 2015, 118, 103–109. [Google Scholar] [CrossRef] [PubMed]
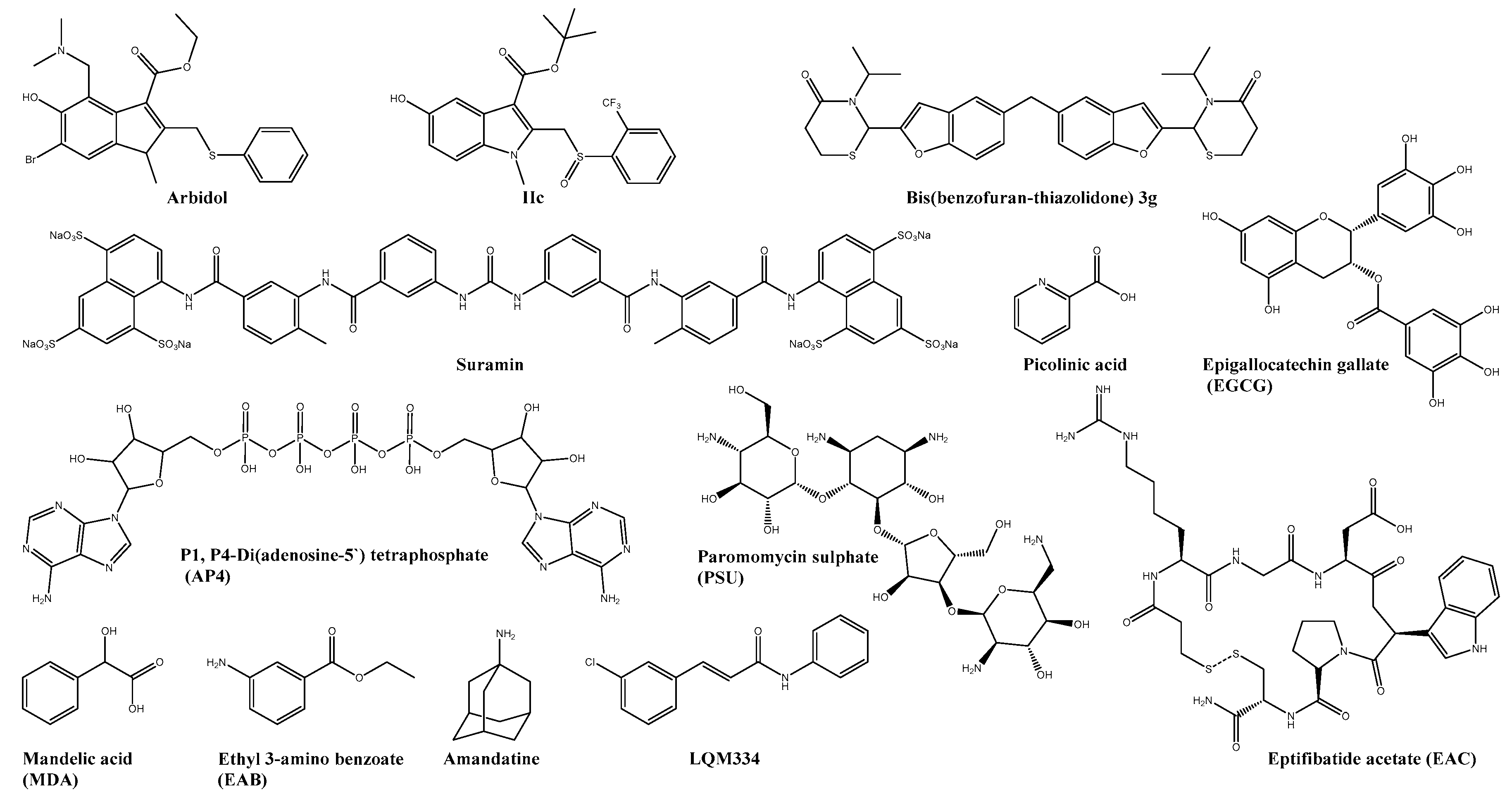
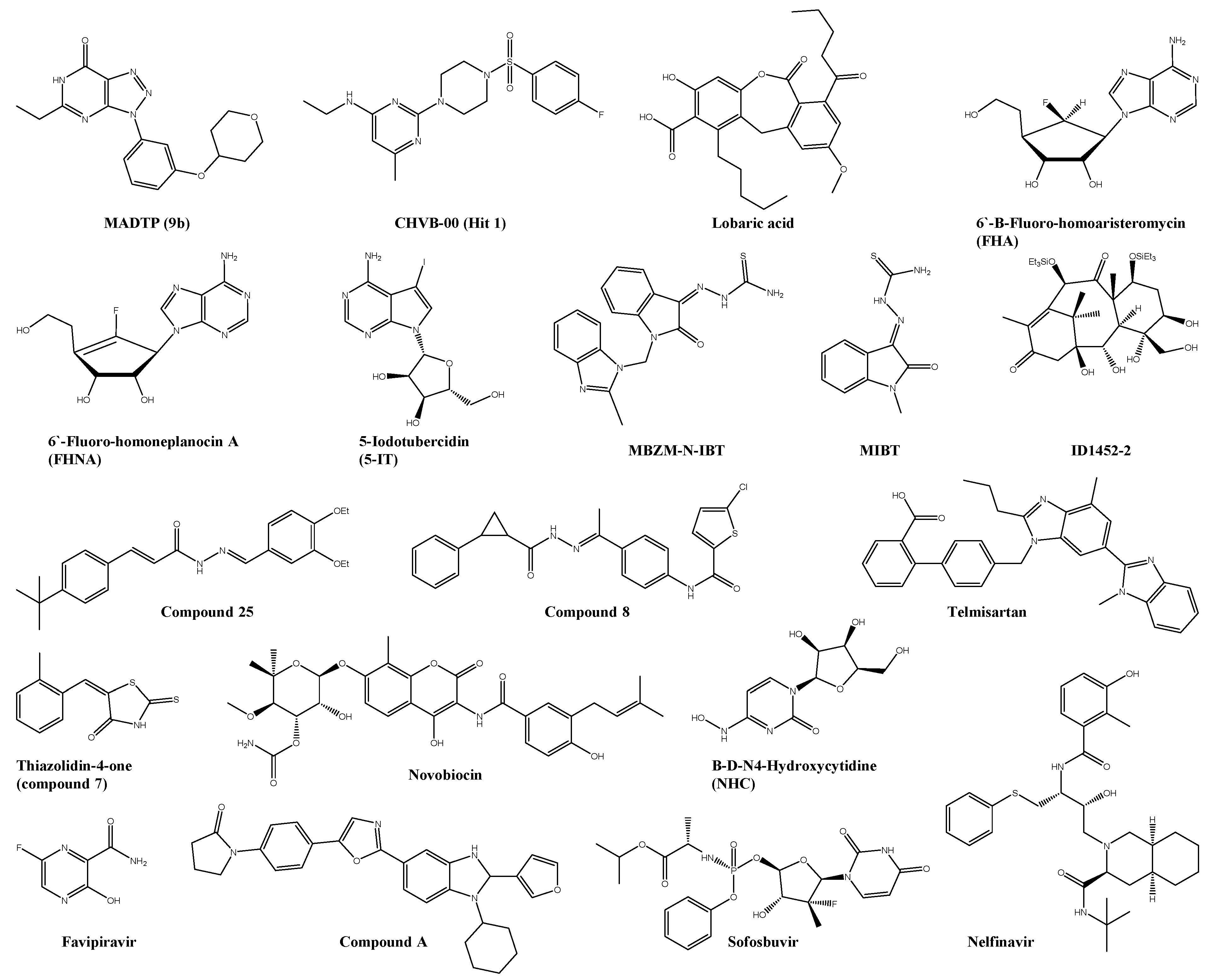
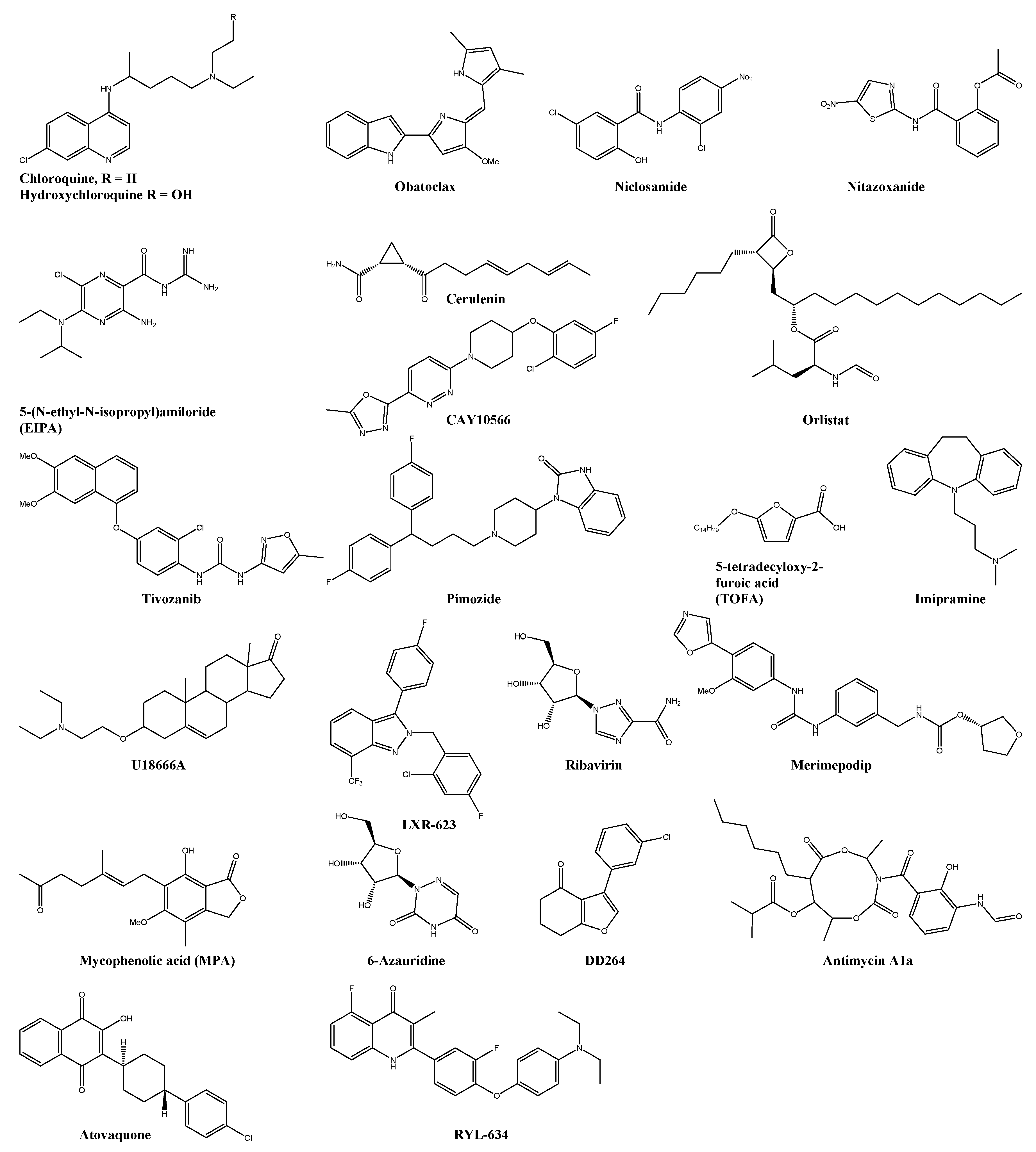
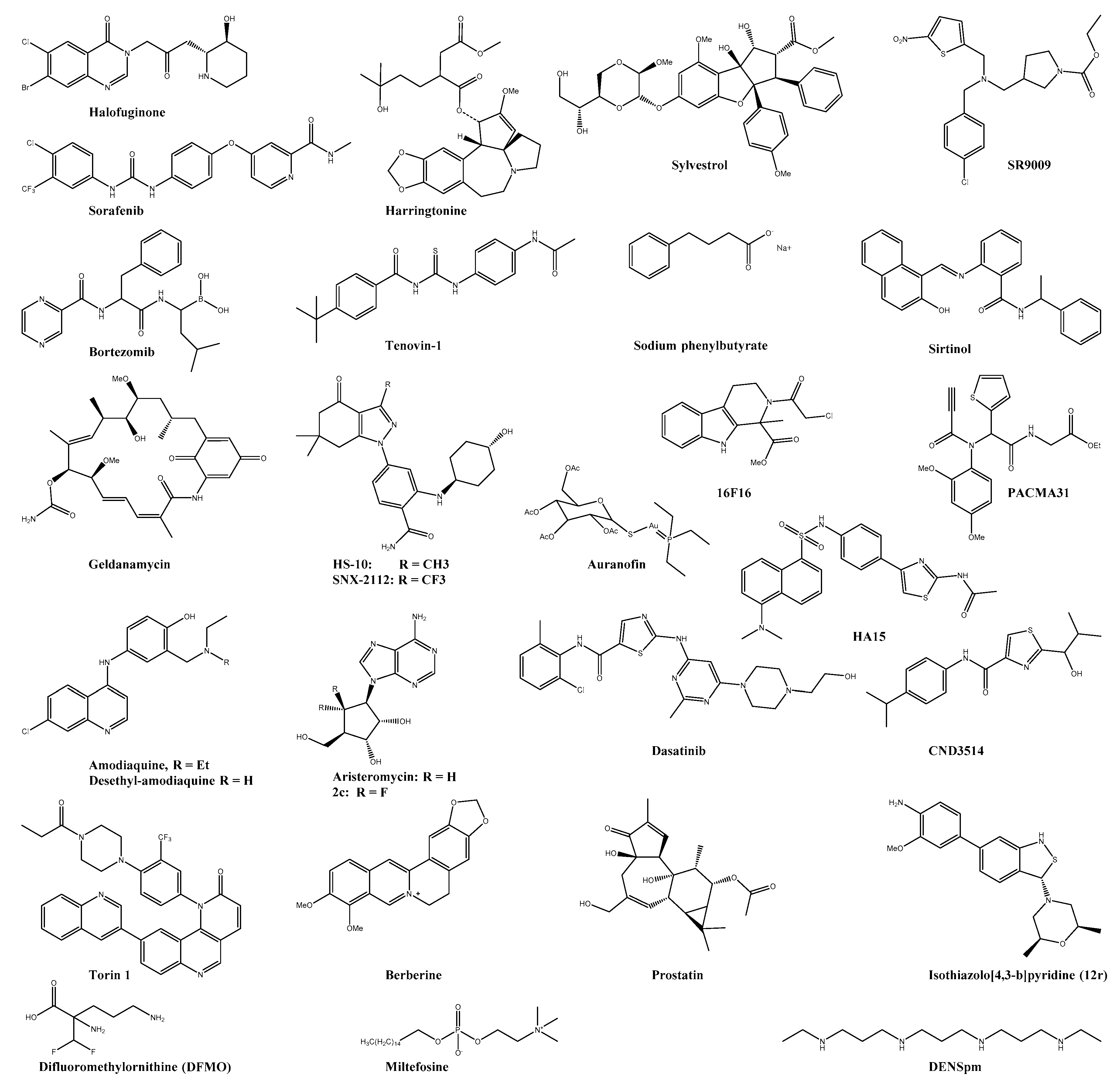
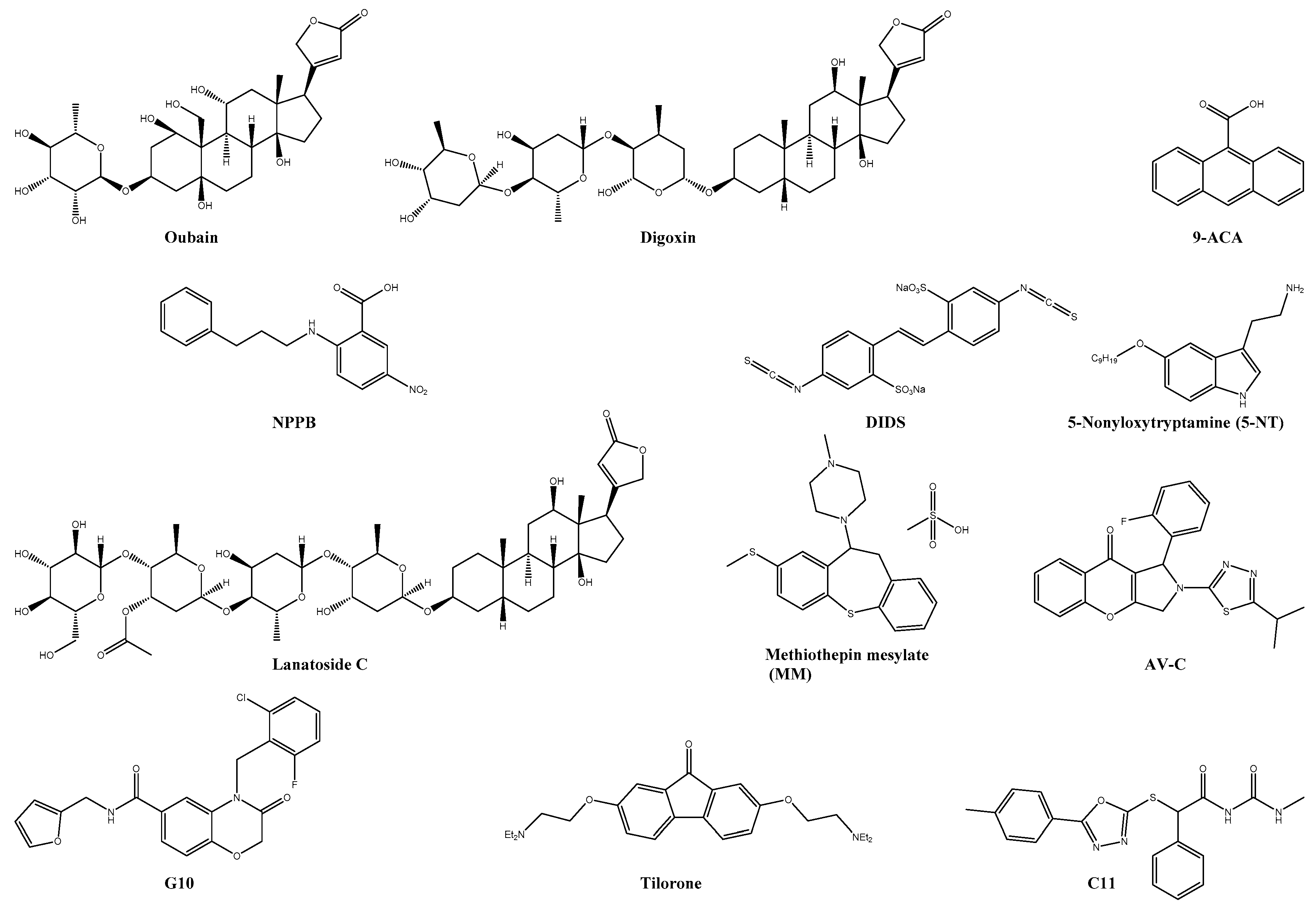
| In Vitro | In Vivo | |||||||
|---|---|---|---|---|---|---|---|---|
| Compound b | Viral Target c | EC50 (µM) d | CC50 (µM) | SI | Cell Line | Efficacy | Mouse Model | Ref. |
| Arbidol * | E2 | 12.2 ± 2.2 | 376 | 36 | MRC5 | ― | ― | [24] |
| Suramin * | E2 | 8.8 ± 0.5 | >700 | >39.1 | BHK-21 | Reduced viral burden and decreased foot swelling | C57BL/6 | [25,26,27] |
| Picolinic acid | C | 60.63% inhibition with 2 mM | n.s. | n.s. | Vero | ― | ― | [28] |
| Amantadine * | 6K | 29.51 | n.s. | n.s. | Vero | ― | ― | [29] |
| MADTP (9b) | nsP1 | 1.2 ± 0.009 | 84 ± 19 | 70 | Vero | ― | ― | [30,31,32,33] |
| CHVB-032 | nsP1 | 2.7 | >75 | n.s. | Vero | ― | ― | [34,35] |
| Lobaric acid | nsP1 | 5.3 ± 0.4 | 50 ± 1.3 | 7 | Huh-7 | ― | ― | [36] |
| FHA | nsP1 | 0.12 ± 0.04 | >250 | >1000 | Vero | ― | ― | [37,38] |
| FHNA | nsP1 | 0.18 ± 0.11 | >250 | >1000 | Vero | ― | ― | [37,38] |
| 5-IT | nsP1 | 0.409 | >50 | n.s. | Vero | ― | ― | [39] |
| Compound 25 | nsP2 | 3.2 ± 1.8 | 101 ± 50 | 32 | Vero | ― | ― | [40,41,42] |
| Compound 8 | nsP2 | 1.5 | >200 | >133.3 | BHK-21 | ― | ― | [43] |
| ID1452-2 | nsP2 | 31 | >31 | n.s. | HEK293T | ― | ― | [44] |
| Novobiocin * | nsP2 | 20 | n.s. | n.s. | Vero | ― | ― | [45] |
| Telmisartan * | nsP2 | 45 | n.s. | n.s. | Vero | ― | ― | [45] |
| SRI-43750 | nsP3 | 23 | >40 | n.s. | NHDF | ― | ― | [46] |
| Favipiravir * | nsP4 | 25 ± 3 | >636 | n.s. | Vero | decreased mortality by >50% and improved disease outcome | AG129 | [47] |
| Reduced viral replication in joints | C57BL/6J | [48] | ||||||
| NHC | nsP4 | 0.2 ± 0.1 | 7.7 | n.s. | Vero | ― | ― | [49,50] |
| Sofosbuvir * | nsP4 | 2.7 ± 0.5 | 402 ± 3 2 | 149 | Huh-7 | Reduced viremia and joint pain | SwissWebster | [51] |
| Compound-A | nsP4 | 0.54 ± 0.08 | 3.70 ± 0.32 | n.s. | Vero | ― | ― | [52] |
| In Vitro | In Vivo | |||||||
|---|---|---|---|---|---|---|---|---|
| Compound b | Suggested Viral Target | EC50 (µM) c | CC50 (µM) | SI | Cell Line | Efficacy | Mouse Model | Ref. |
| IIc | E2 | 6.5 ± 1 | 156 | 22 | Vero | ― | ― | [53] |
| Bis(benzofuran-thiazolidone)s (3g) | 1.5 | >200 | <133 | Vero | ― | ― | [54] | |
| LQM334 | E2 | 81.1 ± 6.4% viral inhibition | n.s. | n.s. | Vero | ― | ― | [55] |
| Micafungin * | E1/E2 | 17.2 ± 1.08 | >100 | >5.81 | U2OS | ― | ― | [56] |
| Doxycycline * | E2/nsP2 | 15.51 ± 1.62 | n.s. | n.s. | Vero | + ribavirin: Reduction of pathological signs and virus titre | Adult ICR | [57] |
| AP4 | C | 10.66 ± 2.25 | 2172 ± 104 | n.s. | Vero | ― | ― | [58] |
| EAC | C | 4.01 ± 1.96 | 1657 ± 1109 | n.s. | Vero | ― | ― | [58] |
| PSU | C | 22.91 ± 3.83 | 2505 ± 0683 | n.s. | Vero | ― | ― | [58] |
| MBZM-N-IBT | nsP2 | 38.68 | >800 | >21 | Vero | ― | ― | [59] |
| 1,3-thiazolidin-4-one (compound 7) | nsP2 | 0.42 | >100 | n.s. | Vero | ― | ― | [60] |
| peptidomimetic 3a | nsP2 | 8.76 | n.s. | n.s. | Vero | ― | ― | [61] |
| PEP-I | nsP2 | 34 | Maximum nontoxic dose is 50 µM | n.s. | BHK-21 | ― | ― | [62] |
| Nelfinavir * | nsP2 | 14 ± 1 | 22 ± 6 | 1.6 | Vero | ― | ― | [63] |
| In Vitro | In Vivo | |||||||
|---|---|---|---|---|---|---|---|---|
| Compound b | Host Target c | EC50 (µM) d | CC50 (µM) | SI | Cell Line | Efficacy | Mouse Model | Ref. |
| Viral Entry and Membrane Fusion | ||||||||
| Chloroquine * | pH | 7.0 ± 1.15 | >260 | 37.14 | Vero | ― | ― | [95] |
| Obatoclax * | Bcl-2/E1 | 0.03 ± 0.01 | 20.1 ± 4.8 | 670 | BHK-21 | ― | ― | [96] |
| Niclosamide * | pH | 0.36 ± 0.08 | >20 | >55.55 | U2OS | ― | ― | [97] |
| Nitazoxanide * | pH | 2.96 ± 0.18 | 25 | 8.45 | BHK-21 | ― | ― | [97] |
| EIPA | pH ** | Detectable inhibition observed at 0.03 µM | n.s. | n.s. | HSMM | ― | ― | [98] |
| Lipid Pathway Inhibitors | ||||||||
| Orlistat * | FASN | 0.82 | 8.67 | 10.57 | HEK293T | ― | ― | [99,100,101] |
| Cerulenin * | FASN | 3 | 7.57 | 2.53 | HEK293T | ― | ― | [99,100] |
| CAY10566 | SCD1 | HEK293T | ― | ― | [100] | |||
| TOFA | FASN | 0.15 | >60 | n.s. | HEK293T | Reduction of the viral replication and joint swelling | C57BL/6 | [99] |
| Tivozanib * | FLT4 | 0.8 | 8.34 | n.s. | HEK293T | ― | ― | [99] |
| Pimozide * | calmodulin | 0.28 | 19.18 | 69.75 | HEK293T | Reduction of the viral replication and joint swelling | C57BL/6 | [99] |
| Imipramine * | NPC ** | Detectable inhibition observed at 10 µM | n.s. | n.s. | HFF1 | ― | ― | [102] |
| U18666A | NPC ** | Detectable inhibition observed at 0.63 µM | n.s. | n.s. | HFF1 | ― | ― | [102] |
| LXR-623 | LXRβ | 2.50 | 63.30 | 25.3 | HFF | ― | ― | [103] |
| Pyrimidine and Purine Synthesis Inhibitors | ||||||||
| Ribavirin * | IMPDH/ (viral) RdRp ** | 341.1 | >30,000 | 24 | Vero | + doxycycline: Reduction of pathological signs and virus titre | Adult ICR | [57,104] |
| Merimepodib * | IMPDH | 1.8 ± 1.0 | 27 ± 3 | n.s. | Vero | ― | ― | [105] |
| 6-Azauridine | OMP | 0.816 | 208 | 204 | Vero | ― | ― | [104] |
| DD363 | DHODH | 3.6 ± 0.6 | 87 ± 7 | n.s. | HEK293T | ― | ― | [106,107] |
| RYL-634 | DHODH | 0.26 | >2.5 | >10 | Vero | ― | ― | [108] |
| Atovaquone * | DHODH ** | <0.75 | >11.25 | n.s. | Vero | ― | ― | [109] |
| Protein Synthesis Inhibitors | ||||||||
| Halofuginone | EPRS | 3 log10 viral titer reduction at 100 nM | n.s. | n.s. | HFF | ― | ― | [110] |
| Harringtonine | 0.24 | n.s | n.s. | BHK21 | ― | ― | [111] | |
| Sorafenib * | FLT4 | 0.16 | n.s. | n.s. | Vero | ― | ― | [99,112] |
| Bortezomib * | UPS ** | 0.023 | 0.47 | 20.6 | HeLA | ― | ― | [113] |
| SR9009 * | Rev-erb α/β ** | 100-fold reduction in viral titer at 10 µM | n.s. | n.s. | Huh7 | ― | ― | [114] |
| Cellular Protein Inhibitors | ||||||||
| Sirtinol | SIRT | >2 log10 viral titer reduction at 200 µM | n.s. | n.s. | U2OS | ― | ― | [115] |
| Geldanamycin * | HSP90 | 2.5 log10 viral titer reduction at 1.4 µM | >100 | n.s. | HEK293T | ― | ― | [116] |
| HS-10 | HSP90 | >2 log10 viral reduction in titre with 6.25 µM | >100 | n.s. | HEK293T | Reduced viral titer, inflammation, and swelling | SVA129 | [116] |
| SNX-2112 | HSP90 | >2 log10 viral reduction in titre with 6.25 µM | >100 | n.s. | HEK293T | Reduced viral titer, inflammation, and swelling | SVA129 | [116] |
| HA15 | GRP78 | reduction in titre with 25 µM | n.s. | n.s. | Vero | ― | ― | [117] |
| 16F16 | PDI | 6.6 ± 0.45 | 8.9 ± 9.2 | 1.35 | HEK293T | ― | ― | [118] |
| PACMA31 | PDI | 12.1 ± 0.3 | 12.2 ± 9.7 | 1.00 | HEK293T | Less reduction in footbed swelling and viremia than in auranofin group | C57BL/6 | [118] |
| Auranofin * | TRX | 1.0 ± 0.13 | 1.6 ± 8.6 | 1.6 | HEK293T | reduced footbed swelling and viremia | C57BL/6 | [118] |
| Cellular Enzyme Inhibitors-Hydrolases | ||||||||
| Amodiaquine * | Cathepsin B | 18.3 | >50 | >2 | HFF | ― | ― | [119] |
| DEAQ | Cathepsin B | 17.3 | >50 | >2.9 | HFF | ― | ― | [119] |
| Aristeromycin | SAH | 0.8 | 6.3 | 7.9 | Vero | ― | ― | [120] |
| 6,6′-Difluoroaristeromycin (2c) | SAH | 0.13 | 1.25 | >9.6 | Vero | ― | ― | [120] |
| Cellular Enzyme Inhibitors-Kinases | ||||||||
| Dasatinib * | SFK | >10-fold reduction in viral titer at 20 µM | >50 | n.s. | NHDF | ― | ― | [121] |
| Torin 1 | mTORC1/2 | >10-fold reduction in viral titer at 1 µM | >1 | n.s. | NHDF | ― | ― | [121] |
| CND3514 | PKR ** | 2.2 | >50 | >22.7 | HuH-7 | ― | ― | [122] |
| Berberine | MAPK | 1.8 ± 0.5 | >100 | >55.6 | BHK21 | Reduced viremia and disease symptoms | C57BL6/J | [123,124] |
| Miltefosine * | Pi3-Akt | antiviral activity was observed at 20–40 µM | n.s. | n.s. | hPDF | ― | ― | [125] |
| Prostatin | PCK | 0.2 ± 0.05 | 50 | n.s. | CRL-2522 | ― | ― | [126] |
| Bryostatin analogue (4) | PCK | 0.8 ± 0.1 | >50 | n.s. | BGM | [127,128] | ||
| Isothiazolo[4,3-b]pyridine (12r) | GAK | antiviral activity was observed <10 µM | n.s. | n.s. | Vero | ― | ― | [129] |
| Cellular Enzyme Inhibitors–Lyases/Transferases | ||||||||
| DFMO * | ODC1 | 200-fold reduction in viral titer at 500 µM | n.s. | n.s. | BHK-21 | Low reduction in viral titer | C57BL6/J | [130] |
| Cellular Receptor Inhibitors–Channel-linked Receptors | ||||||||
| Digoxin * | Na+/K+ ATPase | 0.049 | >10 | n.s. | U2OS | ― | ― | [20] |
| Lanatoside C * | Na+/K+ ATPase | 38.99% reduction of viral titer with 1 µM | >1 | n.s. | BHK-21 | ― | ― | [131] |
| DIDS | CLIC1/4 | 8-fold reduction in viral titer | n.s. | n.s. | HuH-7 | ― | ― | [132] |
| 9-ACA | CLIC1/4 | 8-fold reduction in viral titer | n.s. | n.s. | HuH-7 | ― | ― | [132] |
| NPPB | CLIC1/4 | 18-fold reduction in viral titer | n.s. | n.s. | HuH-7 | ― | ― | [132] |
| Cellular Receptor Inhibitors–Enzyme-linked Receptors | ||||||||
| 5-NT | 5-HT | 2.8 | >5 | n.s. | U2OS | ― | ― | [133,134] |
| MM | 5-HT | 97 ± 1.0% viral reduction 10 µM | >10 | n.s. | U2OS | ― | ― | [133] |
| Immunomodulatory Agents | ||||||||
| Tilorone * | IFN-inducer | 4.2 | 32 | 7.6 | Vero76 | ― | ― | [135] |
| C11 | STING | EC90: 16.44 µM | >50 | n.s. | THF | ― | ― | [136] |
| G10 | STING | IC90: 8.01 μM | n.s. | n.s. | THF | ― | ― | [137] |
| AV-10 | TRIF | IC90: 3.54 μM | n.s. | n.s. | THF | ― | ― | [138] |
| Pentosan polysulfate * | IL-10 inducer; decreased proinflammatory cytokines levels | ― | ― | ― | ― | Reduced disease symptoms | C57BL/6 | [139] |
| Pixatimod * | HPSE ** | 0.51 ± 0.50 | n.s. | n.s. | Vero | Reduced disease symptoms | C57BL/6 | [140] |
| In Vitro | In Vivo | ||||||
|---|---|---|---|---|---|---|---|
| Compound b | EC50 (µM) c | CC50 (µM) | SI | Cell Line | Efficacy | Mouse Model | Ref. |
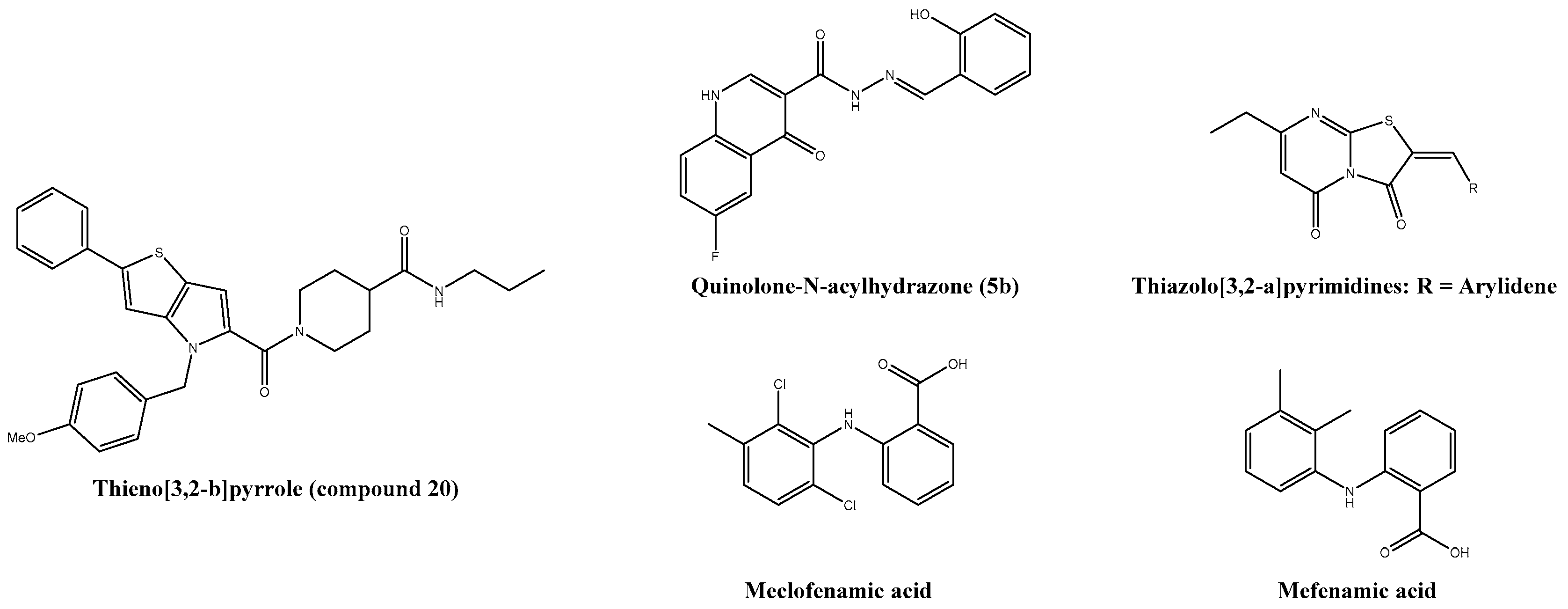
| Quinolone-N-acylhydrazone (5b) | 1.06 ± 0.08 | 669 ± 4.33 | 631.7 | Vero | ― | ― | [211] |
| Thieno[3,2-b]pyrrole (compound 20) | 3-4 | >100 | n.s. | HEK293T | Good in vivo pharmacokinetics | C57BL/6 | [212,213] |
| Compound 15 | 42 | n.s. | n.s. | MCF-7 | ― | ― | [214] |
| Mefenamic acid * | 13 | >100 | n.s. | Vero | + ribavirin: Reduction of viral titre and hypertrophic effects in liver and spleen | Adult ICR | [167] |
| Meclofenamic acid | 18 | >100 | n.s. | Vero | ― | ― | [167] |
| Ivermectin * | 0.6 ± 0.1 | 37.9 ± 7.6 | 62.4 | BHK-21 | ― | ― | [123] |
| Abamectin * | 1.5 ± 0.6 | 28.2 ± 1.1 | 19.2 | BHK-21 | ― | ― | [123] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Battisti, V.; Urban, E.; Langer, T. Antivirals against the Chikungunya Virus. Viruses 2021, 13, 1307. https://doi.org/10.3390/v13071307
Battisti V, Urban E, Langer T. Antivirals against the Chikungunya Virus. Viruses. 2021; 13(7):1307. https://doi.org/10.3390/v13071307
Chicago/Turabian StyleBattisti, Verena, Ernst Urban, and Thierry Langer. 2021. "Antivirals against the Chikungunya Virus" Viruses 13, no. 7: 1307. https://doi.org/10.3390/v13071307
APA StyleBattisti, V., Urban, E., & Langer, T. (2021). Antivirals against the Chikungunya Virus. Viruses, 13(7), 1307. https://doi.org/10.3390/v13071307






