Oral Immunization with Lactobacillus casei Expressing the Porcine Circovirus Type 2 Cap and LTB Induces Mucosal and Systemic Antibody Responses in Mice
Abstract
1. Introduction
2. Materials and Methods
2.1. Bacterial Strain and Culture Conditions
2.2. Animals
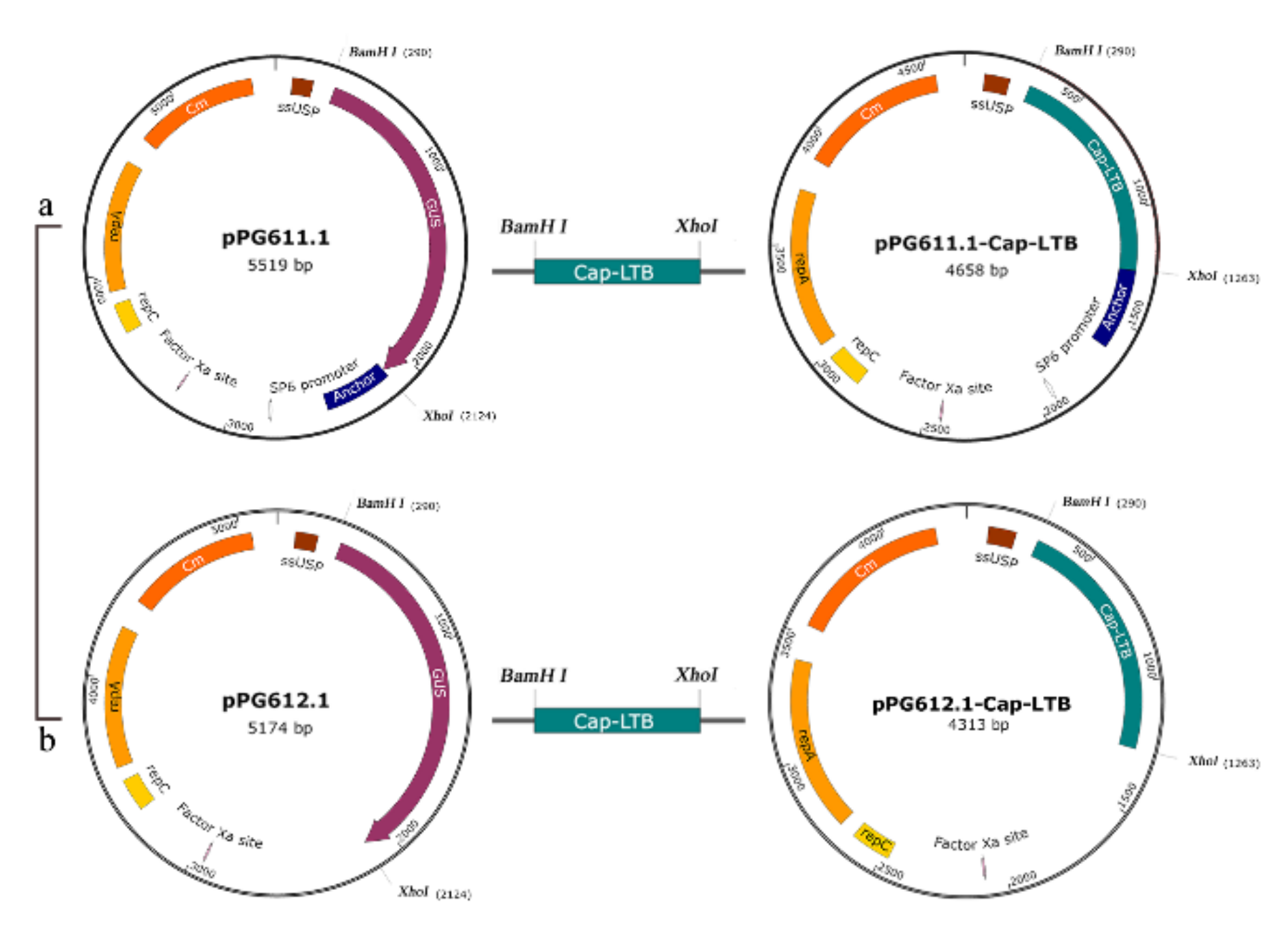
2.3. Plasmid Procedure and Electrotransformation
2.4. Protein Expression and Western Blotting Analysis
2.5. Surface Expression Analysis
2.6. Secretion Expression Analysis
2.7. Immunizations
2.8. Enzyme-Linked Immunosorbent Assay
2.9. Lymphocyte Proliferation
2.10. Statistical Analysis
3. Results
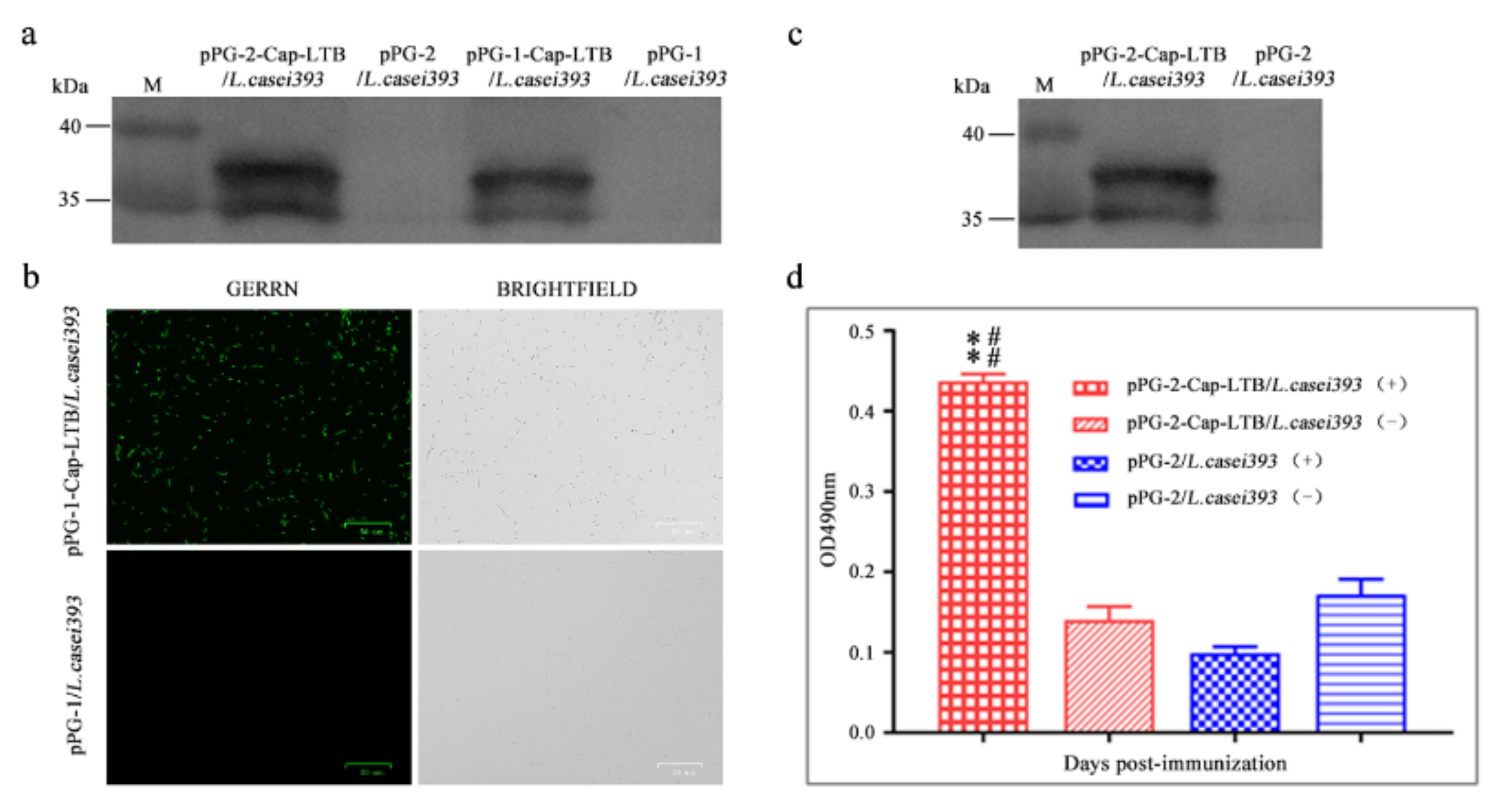
3.1. Expression of Cap-LTB in L. casei
3.2. Cap-LTB Protein Surface-Displayed Expression by L. casei
3.3. Cap-LTB Protein Secretory Expression by L. casei
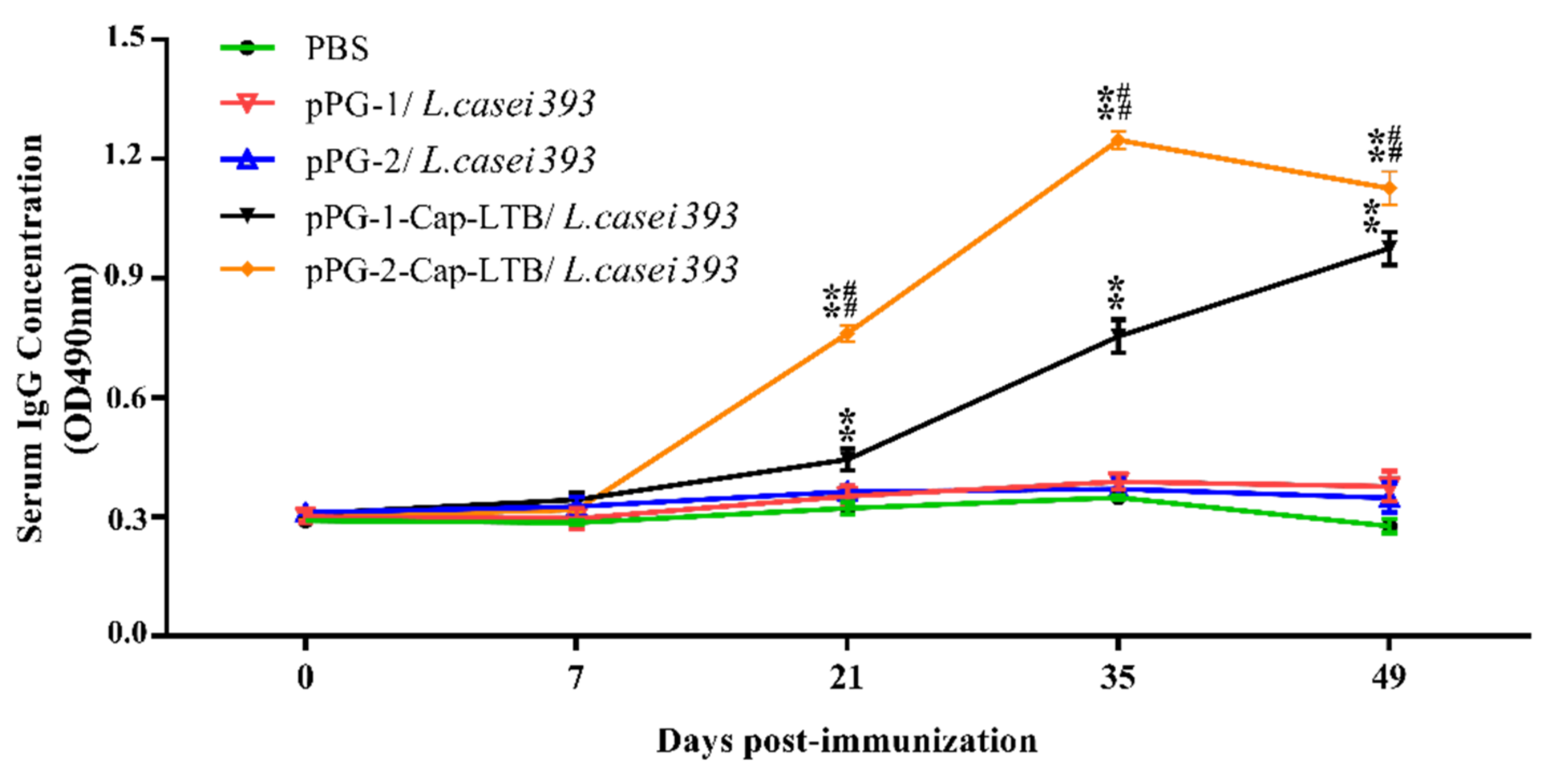
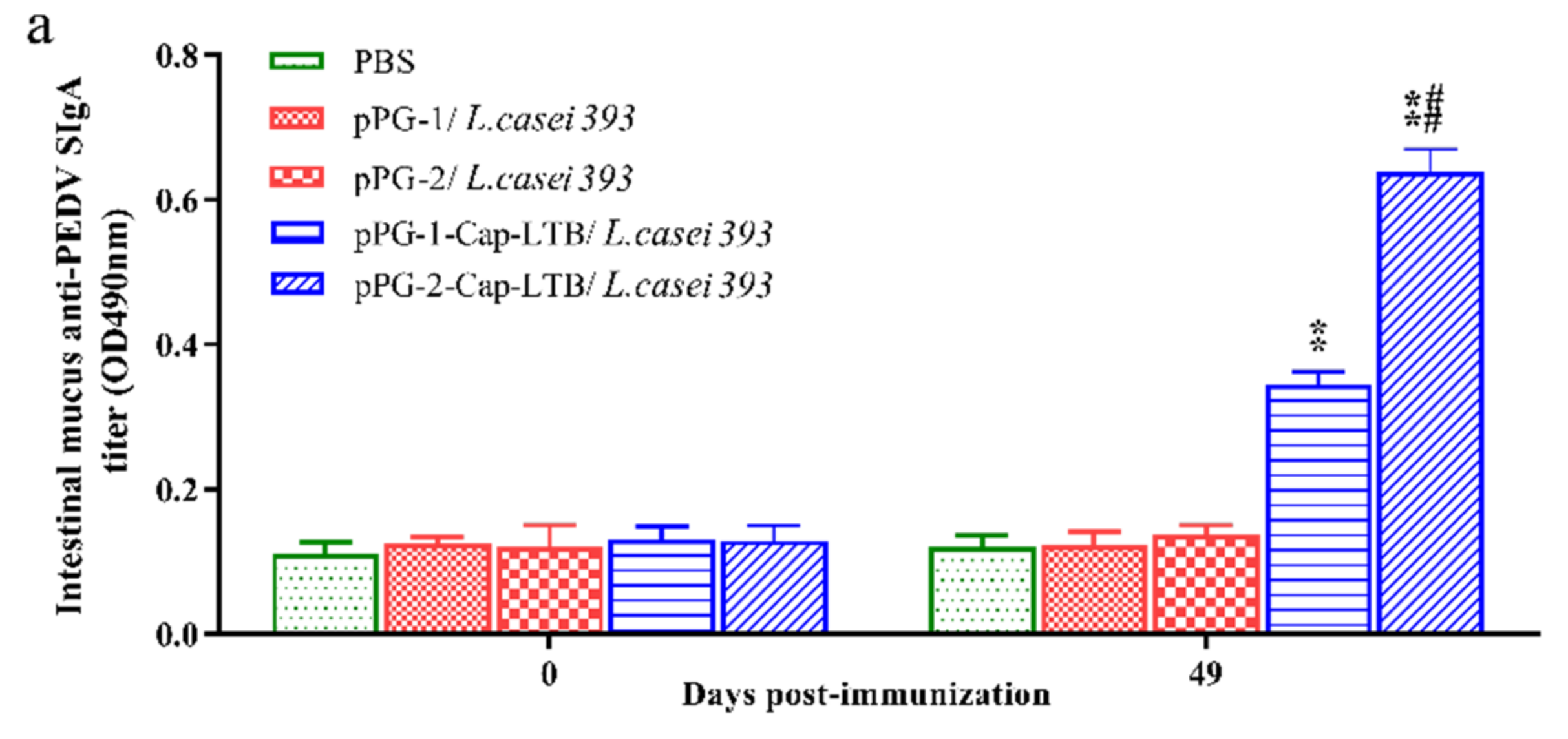
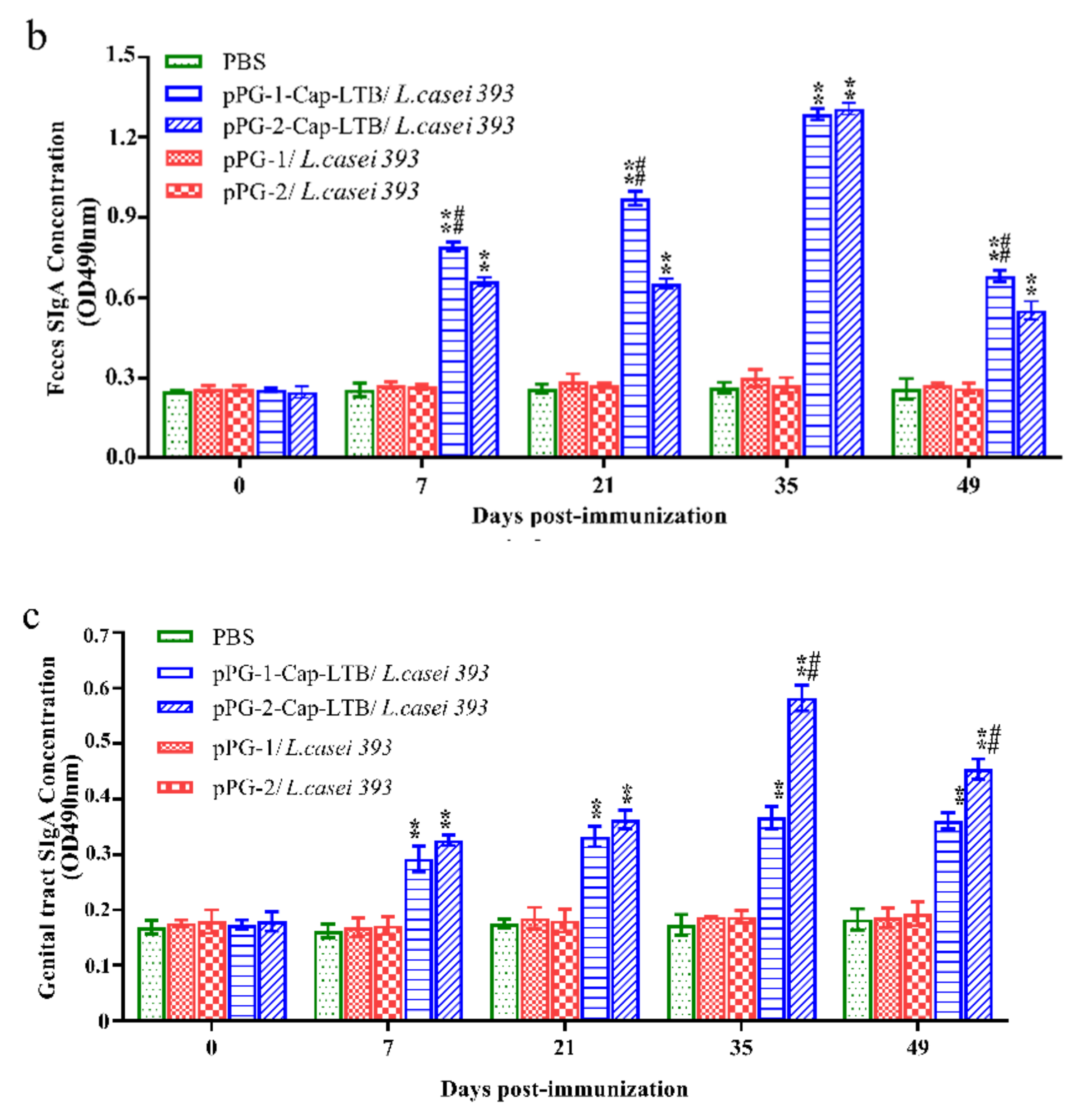
3.4. Antibody Responses Following Oral Immunizations
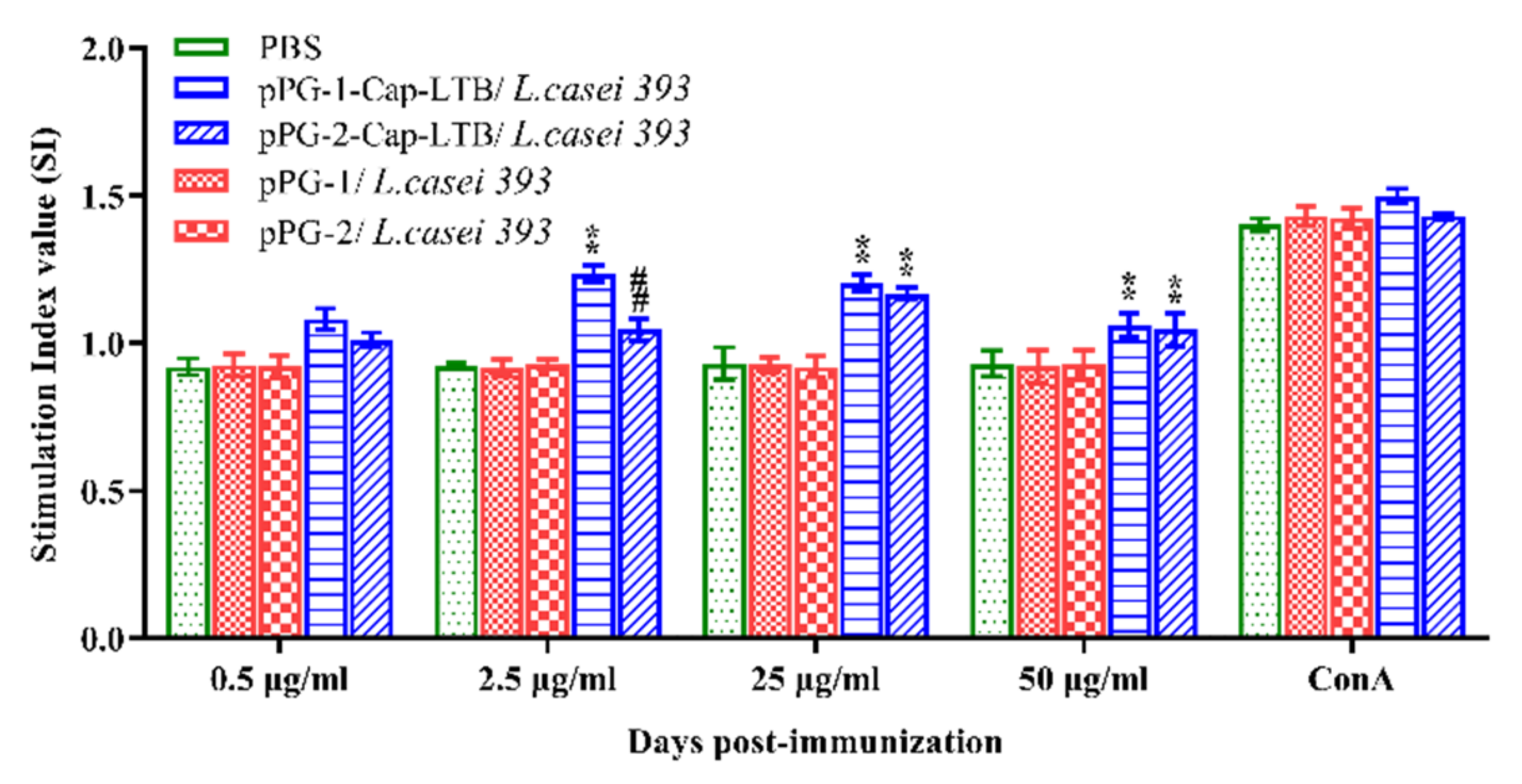
3.5. Lymphocyte Proliferation
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Segalés, J.; Allan, G.M.; Domingo, M. Porcine circovirus diseases. Anim Health Res. Rev. 2005, 6, 119–142. [Google Scholar] [CrossRef]
- Segalés, J.; Domingo, M.; Chianini, F.; Majó, N.; Domínguez, J.; Darwich, L.; Mateu, E. Immunosuppression in postweaning multisystemic wasting syndrome affected pigs. Vet. Microbiol. 2004, 98, 151–158. [Google Scholar] [CrossRef] [PubMed]
- Harding, J.C. The clinical expression and emergence of porcine circovirus 2. Vet. Microbiol. 2004, 98, 131–135. [Google Scholar] [CrossRef] [PubMed]
- Mankertz, A.; Domingo, M.; Folch, J.M.; LeCann, P.; Jestin, A.; Segalés, J.; Chmielewicz, B.; Plana-Durán, J.; Soike, D. Characterisation of PCV-2 isolates from Spain, Germany and France. Virus Res. 2000, 66, 65–77. [Google Scholar] [CrossRef]
- Nawagitgul, P.; Morozov, I.; Bolin, S.R.; Harms, P.A.; Sorden, S.D.; Paul, P.S. Open reading frame 2 of porcine circovirus type 2 encodes a major capsid protein. J. Gen. Virol. 2000, 81, 2281–2287. [Google Scholar] [CrossRef]
- Cheung, A.K.; Bolin, S.R. Kinetics of porcine circovirus type 2 replication. Arch. Virol. 2002, 147, 43–58. [Google Scholar] [CrossRef] [PubMed]
- McNeilly, F.; McNair, I.; Mackie, D.P.; Meehan, B.M.; Kennedy, S.; Moffett, D.; Ellis, J.; Krakowka, S.; Allan, G.M. Production, characterisation and applications of monoclonal antibodies to porcine circovirus 2. Arch. Virol. 2001, 146, 909–922. [Google Scholar] [CrossRef] [PubMed]
- Pogranichnyy, R.M.; Yoon, K.J.; Harms, P.A.; Swenson, S.L.; Zimmerman, J.J.; Sorden, S.D. Characterization of Immune Response of Young Pigs to Porcine Circovirus Type 2 Infection. Viral Immunol. 2000, 13, 143–153. [Google Scholar] [CrossRef] [PubMed]
- Nawagitgul, P.; Harms, P.A.; Morozov, I.; Thacker, B.J.; Sorden, S.D.; Lekcharoensuk, C.; Paul, P.S. Modified indirect porcine circovirus (PCV) type 2-based and recombinant capsid protein (ORF2)-based enzyme-linked immunosorbent assays for detection of antibodies to PCV. Clin. Vaccine Immunol. 2002, 9, 33–40. [Google Scholar] [CrossRef]
- Blanchard, P.; Mahé, D.; Cariolet, R.; Keranflec’h, A.; Baudouard, M.A.; Cordioli, P.; Albina, E.; Jestin, A. Protection of swine against post-weaning multisystemic wasting syndrome (PMWS) by porcine circovirus type 2 (PCV2) proteins. Vaccine 2003, 21, 4565–4575. [Google Scholar] [CrossRef]
- Uren, T.; Wijburg, O.L.; Simmons, C.; Johansen, F.E.; Brandtzaeg, P.; Strugnell, R.A. Vaccine-induced protection against gastrointestinal bacterial infections in the absence of secretory antibodies. Eur. J. Immunol. 2010, 35, 180–188. [Google Scholar] [CrossRef]
- Kodama, C.; Eguchi, M.; Sekiya, Y.; Yamamoto, T.; Kikuchi, Y.; Matsui, H. Evaluation of the Lon-deficient Salmonella strain as an oral vaccine candidate. Microbiol. Immunol. 2005, 49, 1035–1045. [Google Scholar] [CrossRef]
- Smith, B.P.; Reina-Guerra, M.; Hoiseth, S.K.; Stocker, B.A.; Habasha, F.; Johnson, E.; Merritt, F. Aromatic-dependent Salmonella typhimurium as modified live vaccines for calves. Am. J. Vet. Res. 1984, 45, 59–66. [Google Scholar] [PubMed]
- Segall, T.; Lindberg, A.A. Oral vaccination of calves with an aromatic-dependent Salmonella dublin (O9,12) hybrid expressing O4,12 protects against S. dublin (O9,12) but not against Salmonella typhimurium (O4,5,12). Infect. Immun. 1993, 61, 1222–1231. [Google Scholar] [CrossRef]
- Wray, C.; McLaren, I. Further Studies on the Use of Gal E Mutants of Salmonella typhimurium in Calves: Oral Vaccination and Toxicity Studies. J. Vet. Med. Ser. B 2010, 34, 22–29. [Google Scholar] [CrossRef]
- Smith, B.P.; Dilling, G.W.; Da Roden, L.; Stocker, B.A. Vaccination of calves with orally administered aromatic-dependent Salmonella dublin. Am. J. Vet. Res. 1993, 54, 1249. [Google Scholar] [PubMed]
- Pouwels, P.H.; Leer, R.J.; Boersma, W.J.J.o.B. The potential of Lactobacillus as a carrier for oral immunization: Development and preliminary characterization of vector systems for targeted delivery of antigens. J. Biotechnol. 1996, 44, 183–192. [Google Scholar] [CrossRef]
- Maassen, C.B.; Laman, J.D.; den Bak-Glashouwer, M.J.; Tielen, F.J.; van Holten-Neelen, J.C.; Hoogteijling, L.; Antonissen, C.; Leer, R.J.; Pouwels, P.H.; Boersma, W.J.; et al. Instruments for oral disease-intervention strategies: Recombinant Lactobacillus casei expressing tetanus toxin fragment C for vaccination or myelin proteins for oral tolerance induction in multiple sclerosis. Vaccine 1999, 17, 2117–2128. [Google Scholar] [CrossRef]
- Reveneau, N.; Geoffroy, M.C.; Locht, C.; Chagnaud, P.; Mercenier, A. Comparison of the immune responses induced by local immunizations with recombinant Lactobacillus plantarum producing tetanus toxin fragment C in different cellular locations. Vaccine 2002, 20, 1769–1777. [Google Scholar] [CrossRef]
- Oliveira, M.L.S.; Monedero, V.; Miyaji, E.N.; Leite, L.C.; Lee Ho, P.; Pérez-Martínez, G. Expression of Streptococcus pneumoniae antigens, PsaA and PspA by Lactobacillus casei. FEMS Microbiol. Lett. 2003, 227, 25–31. [Google Scholar] [CrossRef]
- Seegers, J.F. Lactobacilli as live vaccine delivery vectors: Progress and prospects. Trends Biotechnol. 2002, 20, 508–515. [Google Scholar] [CrossRef]
- Ho, P.S.; Kwang, J.; Lee, Y.K. Intragastric administration of Lactobacillus casei expressing transmissible gastroentritis coronavirus spike glycoprotein induced specific antibody production. Vaccine 2005, 23, 1335–1342. [Google Scholar] [CrossRef]
- Alander, M.; Satokari, R.; Korpela, R.; Saxelin, M.; Vilpponen-Salmela, T.; Mattila-Sandholm, T.; von Wright, A. Persistence of colonization of human colonic mucosa by a probiotic strain, Lactobacillus rhamnosus GG, after oral consumption. Appl. Environ. Microbiol. 1999, 65, 351–354. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.; Ho, P.S.; Low, C.S.; Arvilommi, H.; Salminen, S. Permanent colonization by Lactobacillus casei is hindered by the low rate of cell division in mouse gut. Appl. Environ. Microbiol. 2004, 70, 670–674. [Google Scholar] [CrossRef] [PubMed]
- Ogawa, T.; Asai, Y.; Yasuda, K.; Sakamoto, H. Oral immunoadjuvant activity of a new synbiotic Lactobacillus casei subsp casei in conjunction with dextran in BALB/c mice. Nutr. Res. 2005, 25, 295–304. [Google Scholar] [CrossRef]
- Verweij, W.R.; de Haan, L.; Holtrop, M.; Agsteribbe, E.; Brands, R.; van Scharrenburg, G.J.; Wilschut, J. Mucosal immunoadjuvant activity of recombinant Escherichia coli heat-labile enterotoxin and its B subunit: Induction of systemic IgG and secretory IgA responses in mice by intranasal immunization with influenza virus surface antigen. Vaccine 1998, 16, 2069–2076. [Google Scholar] [CrossRef]
- Yamamoto, M.; McGhee, J.R.; Hagiwara, Y.; Otake, S.; Kiyono, H. Genetically Manipulated Bacterial Toxin as a New Generation Mucosal Adjuvant. Scand. J. Immunol. 2001, 53, 211. [Google Scholar] [CrossRef]
- Tamura, S.I.; Hatori, E.; Tsuruhara, T.; Aizawa, C.; Kurata, T. Suppression of delayed-type hypersensitivity and IgE antibody responses to ovalbumin by intranasal administration of Escherichia coli heat-labile enterotoxin B subunit-conjugated ovalbumin. Vaccine 1997, 15, 225–229. [Google Scholar] [CrossRef]
- Saito, K.; Shoji, J.; Inada, N.; Iwasaki, Y.; Sawa, M. Immunosuppressive Effect of Cholera Toxin B on Allergic Conjunctivitis Model in Guinea Pig. Jpn. J. Ophthalmol. 2001, 45, 332–338. [Google Scholar] [CrossRef]
- De Haan, L.; Feil, I.K.; Verweij, W.R.; Holtrop, M.; Hol, W.G.; Agsteribbe, E.; Wilschut, J. Mutational analysis of the role of ADP-ribosylation activity and GM1-binding activity in the adjuvant properties of the Escherichia coli heat-labile enterotoxin towards intranasally administered keyhole limpet hemocyanin. Eur. J. Immunol. 1998, 28, 1243–1250. [Google Scholar] [CrossRef]
- Douce, G.; Fontana, M.; Pizza, M.; Rappuoli, R.; Dougan, G. Intranasal immunogenicity and adjuvanticity of site-directed mutant derivatives of cholera toxin. Infect. Immun. 1997, 65, 2821–2828. [Google Scholar] [CrossRef]
- Cortes-Perez, N.G.; Bermúdez-Humarán, L.G.; Le Loir, Y.; Rodriguez-Padilla, C.; Gruss, A.; Saucedo-Cárdenas, O.; Langella, P.; Montes-de-Oca-Luna, R. Mice immunization with live lactococci displaying a surface anchored HPV-16 E7 oncoprotein. FEMS Microbiol. Lett. 2003, 229, 37–42. [Google Scholar] [CrossRef]
- Wang, X.; Wang, L.; Huang, X.; Ma, S.; Yu, M.; Shi, W.; Qiao, X.; Tang, L.; Xu, Y.; Li, Y. Oral Delivery of Probiotics Expressing Dendritic Cell-Targeting Peptide Fused with Porcine Epidemic Diarrhea Virus COE Antigen: A Promising Vaccine Strategy against PEDV. Viruses 2017, 9, 312. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Zeng, X.; Yang, F.; Huang, Z.; Liu, H.; Ma, X.; Qiao, S. Dietary N-Carbamylglutamate Supplementation Boosts Intestinal Mucosal Immunity in Escherichia coli Challenged Piglets. PLoS ONE 2013, 8, e66280. [Google Scholar] [CrossRef]
- McCluskie, M.J.; Davis, H.L. CpG DNA is a potent enhancer of systemic and mucosal immune responses against hepatitis B surface antigen with intranasal administration to mice. J. Immunol. 1998, 161, 4463–4466. [Google Scholar]
- Robinson, K.; Chamberlain, L.M.; Schofield, K.M.; Wells, J.M.; Le Page, R.W. Oral vaccination of mice against tetanus with recombinant. Nat. Biotechnol. 1997, 15, 653–657. [Google Scholar] [CrossRef]
- Onate, A.A.; Cespedes, S.; Cabrera, A.; Rivers, R.; Gonzalez, A.; Munoz, C.; Folch, H.; Andrews, E. A DNA Vaccine Encoding Cu, Zn Superoxide Dismutase of Brucella abortus Induces Protective Immunity in BALB/c Mice. Infect. Immun. 2003, 71, 4857–4861. [Google Scholar] [CrossRef]
- Chae, C. Postweaning multisystemic wasting syndrome: A review of aetiology, diagnosis and pathology. Vet. J. 2004, 168, 41–49. [Google Scholar] [CrossRef]
- Chae, C. Porcine circovirus type 2 and its associated diseases in Korea. Virus Res. 2012, 164, 107–113. [Google Scholar] [CrossRef] [PubMed]
- Tischer, I.; Peters, D.; Rasch, R.; Pociuli, S. Replication of porcine circovirus: Induction by glucosamine and cell cycle dependence. Arch. Virol. 1987, 96, 39–57. [Google Scholar] [CrossRef]
- Kamstrup, S.; Barfoed, A.M.; Frimann, T.H.; Ladekjær-Mikkelsen, A.S.; Bøtner, A. Immunisation against PCV2 structural protein by DNA vaccination of mice. Vaccine 2004, 22, 1358–1361. [Google Scholar] [CrossRef]
- Ju, C.; Fan, H.; Tan, Y.; Liu, Z.; Xin, X.; Cao, S.; Wu, B.; Chen, H. Immunogenicity of a recombinant pseudorabies virus expressing ORF1–ORF2 fusion protein of porcine circovirus type 2. Vet. Microbiol. 2005, 109, 179–190. [Google Scholar] [CrossRef]
- Lekcharoensuk, P.; Morozov, I.; Paul, P.S.; Thangthumniyom, N.; Wajjawalku, W.; Meng, X.J. Epitope mapping of the major capsid protein of type 2 porcine circovirus (PCV2) by using chimeric PCV1 and PCV2. J. Virol. 2004, 78, 8135–8145. [Google Scholar] [CrossRef] [PubMed]
- Genmei, L.; Manlin, L.; Ruiai, C.; Hongliang, H.; Dangshuai, P. Construction and immunogenicity of recombinant adenovirus expressing ORF2 of PCV2 and porcine IFN gamma. Vaccine 2011, 29, 8677–8682. [Google Scholar] [CrossRef]
- Zepeda-Cervantes, J.; Cruz-Reséndiz, A.; Sampieri, A.; Carreón-Nápoles, R.; Sánchez-Betancourt, J.I.; Vaca, L. Incorporation of ORF2 from Porcine Circovirus Type 2(PCV2) into genetically encoded nanoparticles as a novel vaccine using a self-aggregating peptide. Vaccine 2019, 37, 1928–1937. [Google Scholar] [CrossRef] [PubMed]
- Hou, Z.; Wang, H.; Feng, Y.; Li, Q.; Li, J. A candidate DNA vaccine encoding a fusion protein of porcine complement C3d-P28 and ORF2 of porcine circovirus type 2 induces cross-protective immunity against PCV2b and PCV2d in pigs. Virol. J. 2019, 16, 1–8. [Google Scholar] [CrossRef]
- Mannam, P.; Jones, K.F.; Geller, B.L. Mucosal vaccine made from live, recombinant Lactococcus lactis protects mice against pharyngeal infection with Streptococcus pyogenes. Infect. Immun. 2004, 72, 3444–3450. [Google Scholar] [CrossRef][Green Version]
- Xin, K.Q.; Hoshino, Y.; Toda, Y.; Igimi, S.; Okuda, K. Immunogenicity and protective efficacy of orally administered recombinant Lactococcus lactis expressing surface-bound HIV Env. Blood 2003, 102, 223–228. [Google Scholar] [CrossRef]
- Fagarasan, S.; Honjo, T. Intestinal IgA synthesis: Regulation of front-line body defences. Nat. Rev. Immunol. 2003, 3, 63–72. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Hou, X.; Yu, L.; Wang, G.; Zhao, Z.; Gao, Y. Colonization and distribution of recombinant Lactobacillus casei with green fluorescent protein in mice intestine. Wei Sheng Wu Xue Bao Acta Microbiol. Sin. 2010, 50, 1232–1238. [Google Scholar]
- Xu, Y.; Cui, L.; Tian, C.; Zhang, G.; Huo, G.; Tang, L.; Li, Y. Immunogenicity of recombinant classic swine fever virus CD8(+) T lymphocyte epitope and porcine parvovirus VP2 antigen coexpressed by Lactobacillus casei in swine via oral vaccination. Clin. Vaccine Immunol. 2011, 18, 1979–1986. [Google Scholar] [CrossRef] [PubMed]
| Primer Names | Primer Sequences (5′-3′) |
|---|---|
| C0 | GGATCC a AGGCATCTTCAACACCCGC |
| C1 | ACTTTATTCATAACATACGGGCCCGGGCCGGGTTT b AAGTGGGGGGTC |
| L1 | GACCCCCCACTTAAACCCGGCCCGGGCCCG b TATGTTATGAATAAAGT |
| L2 | GGGCTCGAG a GTAGTTTTCCATACTGATTGCCGCA |
| A1 | CAACTGCTGTCCCAGCTGTAG |
| A2 | AGGAGGCGTTACCGCAGAAG |
| P1 | TCTTTAAGATTAAATTCTCT |
| P2 | ATGTAAACTACTCCTCCCGC |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, F.; Wang, X.; Ma, R.; Wu, W.; Teng, F.; Cheng, X.; Jiang, Y.; Zhou, H.; Wang, L.; Tang, L.; et al. Oral Immunization with Lactobacillus casei Expressing the Porcine Circovirus Type 2 Cap and LTB Induces Mucosal and Systemic Antibody Responses in Mice. Viruses 2021, 13, 1302. https://doi.org/10.3390/v13071302
Li F, Wang X, Ma R, Wu W, Teng F, Cheng X, Jiang Y, Zhou H, Wang L, Tang L, et al. Oral Immunization with Lactobacillus casei Expressing the Porcine Circovirus Type 2 Cap and LTB Induces Mucosal and Systemic Antibody Responses in Mice. Viruses. 2021; 13(7):1302. https://doi.org/10.3390/v13071302
Chicago/Turabian StyleLi, Fengsai, Xiaona Wang, Rumeng Ma, Wei Wu, Fei Teng, Xi Cheng, Yanping Jiang, Han Zhou, Li Wang, Lijie Tang, and et al. 2021. "Oral Immunization with Lactobacillus casei Expressing the Porcine Circovirus Type 2 Cap and LTB Induces Mucosal and Systemic Antibody Responses in Mice" Viruses 13, no. 7: 1302. https://doi.org/10.3390/v13071302
APA StyleLi, F., Wang, X., Ma, R., Wu, W., Teng, F., Cheng, X., Jiang, Y., Zhou, H., Wang, L., Tang, L., Qiao, X., & Li, Y. (2021). Oral Immunization with Lactobacillus casei Expressing the Porcine Circovirus Type 2 Cap and LTB Induces Mucosal and Systemic Antibody Responses in Mice. Viruses, 13(7), 1302. https://doi.org/10.3390/v13071302





