Abstract
Many viruses, especially RNA viruses, utilize programmed ribosomal frameshifting and/or stop codon readthrough in their expression, and in the decoding of a few a UGA is dynamically redefined to specify selenocysteine. This recoding can effectively increase viral coding capacity and generate a set ratio of products with the same N-terminal domain(s) but different C-terminal domains. Recoding can also be regulatory or generate a product with the non-universal 21st directly encoded amino acid. Selection for translation speed in the expression of many viruses at the expense of fidelity creates host immune defensive opportunities. In contrast to host opportunism, certain viruses, including some persistent viruses, utilize recoding or adventitious frameshifting as part of their strategy to evade an immune response or specific drugs. Several instances of recoding in small intensively studied viruses escaped detection for many years and their identification resolved dilemmas. The fundamental importance of ribosome ratcheting is consistent with the initial strong view of invariant triplet decoding which however did not foresee the possibility of transitory anticodon:codon dissociation. Deep level dynamics and structural understanding of recoding is underway, and a high level structure relevant to the frameshifting required for expression of the SARS CoV-2 genome has just been determined.
1. Introduction
Both the meaning of individual codons and the framing of the readout process can be altered by specific information in individual mRNAs or their encoded product. Such translational recoding is dynamic—it is in competition with standard decoding—and it enriches gene expression. It is especially utilized in the decoding of positive sense single-stranded RNA genomes, though also employed by various DNA viruses [1,2,3,4]. One reason for this preferential usage is to expand the coding repertoire of generally (relatively) small genomes—it is also widely used by the diminutive bacterial IS elements—but it also performs various regulatory functions.
Here to honor the memory of Steve Oroszlan, we review usage of programmed stop codon readthrough, ribosomal frameshifting and other forms of translational recoding in the expression of selected animal viruses. Stop codon readthrough results in a proportion of the product having a C-terminal extension. Switching to an alternative frame, frameshifting, can, depending on the location of the stop codon in the new frame, yield a product shorter than the product of standard decoding (e.g., alphavirus TF protein). Bioinformatic identification of alphavirus-type occurrences is more difficult when a stop codon occurs immediately in the new frame resulting in few, or no, amino acids encoded by the new frame, the consequence is only synthesis of a proportion of product lacking a C-terminal domain. Many cases of viral frameshifting involve the reading frame switch occurring near the end of the coding sequence and yielding a product longer than the product of standard decoding. With orthoretroviruses the existence of the GagPol polyprotein was well known in advance of the discovery of recoding in their expression. However, for some well-studied viruses such as influenza A virus and especially alphavirus, the existence of the coding sequence for the additional protein whose expression involves a recoding event was not appreciated in advance—for alphaviruses, this extended even after the initial discovery. As even existence of the extra ORFs was ‘hidden’ during a long time of intense investigation, use of the term ‘genetic steganography’ seems appropriate [5,6] even though in several cases, the extra ORFs were apparent at an early stage. One example of the latter is in the RNA phage Qβ where the occurrence of in-frame stop codon readthrough was first discovered [7]. Many overlapping genes in eukaryotes are accessed by ribosomal frameshifting. However, many others are accessed by leaky scanning, and the first overlapping sequences discovered in any DNA [8] or RNA [9] viruses also did not involve programmed ribosomal frameshifting in their expression.
Since ribosomes translate 5′ to 3′ and replication of single stranded RNA viruses progresses 3′ to 5′, switching from translation to replication is a key step as ribosomes can block replicases [10]. One of the functions of frameshifting, as proposed a long time ago for certain plant RNA viruses is to facilitate the switch from translation to replication [11], and another is generation of different levels of structural and enzymatic products [6]. Others include appending an extension to a proportion of the viral capsid proteins [12]. A different type of recoding, StopGo (2A), yields the equivalent of two proteolytic products but via a translational phenomenon without the involvement of a stop codon. Relevant to the timing of the earliest examples of recoding considered below is the start, in 1984, of the crucial general availability of synthetic oligonucleotides of predetermined sequence. Together with other aspects of gene readout, recoding studies have since put to rest the old adage: from tapes to shapes—mRNA can be just as shapely as protein and importantly so.
2. Pox Virus, Alphavirus and Retrovirus Dynamic Codon Redefinition
When contextual features in particular mRNAs dynamically redefine UGA to specify the non-universal amino acid selenocysteine, the key feature is the identity of the amino acid specified. Selenocysteine is a structural and functional analog of cysteine in which a selenium atom replaces sulfur to confer an enhanced catalytic activity due to a lower side chain pKa and stronger nucleophilicity. Several selenocysteine-containing cellular proteins, selenoproteins, have important cellular functions in antioxidant defense, cell signaling and redox homeostasis, and one, selenoprotein P serves a key selenium transport and storage function. Low levels of reactive oxygen species (ROS)—side products derived from molecular oxygen—play important roles in the maintenance of the immune system, but excess ROS leads to oxidative stress. Such stress can be caused by infection by a number of viruses including human immunodeficiency virus, influenza viruses, hepatitis C virus, herpes simplex virus type 1 and Epstein–Barr virus [13]. Though the mechanisms are diverse, in some cases the host antioxidant defense enzymes, and especially members of the selenoproteome, are targeted [14,15]. Among the selenoproteome are Glutathione peroxidases—they reduce hydrogen peroxides, and glutathione peroxidase 4 reduces lipid peroxides [16,17]. Deficiency of selenium and associated oxidative stress impacts the host’s innate immune response [18,19]. It can also result in viral selection whereby a normally benign or mildly pathogenic virus becomes highly virulent not only in the deficient host but also in hosts with ‘normal’ selenium levels [20]. In contrast, at least two viruses with a long term survival strategy have incorporated a selenoprotein gene, perhaps to modulate for their benefit, the oxidative stress and inflammation they cause [21,22,23]. The pox viruses molluscum contagiosum and fowlpox have acquired cellular glutathione peroxidase 1 and 4 genes, respectively (the former successfully manipulates the host environment as evidenced by the papules it causes that often need at least 6 months, and sometimes years, to resolve). For most selenoprotein mRNAs including those for glutathione peroxidases, the efficiency of redefining UGA to specify selenocysteine is rather low [24,25] (though selective forces can lead to high efficiency as evidenced by coding sequences with more than 45 UGAs effectively specifying selenocysteine [26,27]). As shown by Gladyshev and colleagues, in the relevant fowlpox mRNA, the SECIS structural RNA element 3′ of the selenocysteine-specifying UGA required for codon redefinition, is in the coding sequence, 3′ of the selenocysteine specifying UGA [22], but not in the 3′ UTR where all cellular counterparts are located [22,28]. It can even function 5′ of that UGA [22]. The exceptional location of the fowlpox SECIS may have been influenced by pox viruses, unlike many other DNA viruses, replicating in the cytoplasm [22]. In eukaryotes 3′ SECIS elements and their associated proteins ‘inform’ ribosomes that a UGA(s) far 5′ should specify selenocysteine by at least mainly acting close to, or at the (first) UGA. How is unknown. Since in vitro transcribed mRNA can function to specify selenocysteine, it is clear that splice junction complex proteins located close to the (first) UGA are not essential. However, especially for mRNAs where selenocysteine specification is efficient, it remains possible that they perform some guiding role, or did so at an evolutionary earlier time [29]. A substantial number of proteins, including eIF4A3 [30] are involved in selenocysteine specification, with several having nuclear and/or cytoplasmic roles [31]. To what extent, exclusively cytoplasmic fowlpox glutathione peroxidase 4 mRNA selenocysteine specification merits investigation. Recently a mass spectrometric based approach revealed facultative incorporation of selenocysteine (or selenomethionine) at regulatory sites of key metabolic proteins whose coding sequence lacks the features thought until now to be required for its presence. The mechanism involved is unknown as is its relevance to viral infection and whether any viral encoded proteins contain selenocysteine by the process involved [32].
Dynamically redefining UGA to specify selenocysteine contrasts with what is commonly known as stop codon readthrough where the identity of the universal amino acid specified by UAG or UGA (rarely UAA) is often of secondary or no importance. Instead, the key feature is continued translation by a proportion of ribosomes. Decoding the genomes of Sindbis, Venezuelan equine encephalitis and related alphaviruses, involves readthrough of a UGA stop codon to synthesize the polymerase as a fusion with other nonstructural proteins. A phylogenetically conserved stem loop within the ∼ 150 nt 3’-adjacent to the UGA increases readthrough by up to 10-fold [33]. Mammalian termination involves four mRNA nts being pulled into the A site by eRF1 [34,35]. Possible 3′ mRNA stem loop structure inhibition of this process with reduced termination efficiency may explain its readthrough enhancement.
After studies of adenovirus led to the discovery of splicing in 1977, it was widely assumed, and even stated by a leader of the field in a voluminous monograph, that retroviral GagPol synthesis involved inefficient splicing to generate a subpopulation of RNA with fused gag and pol coding sequences, permitting synthesis of GagPol by standard translation. An in vitro protein synthesis experiment by Philipson, Gesteland and their colleagues with Moloney murine leukemia virus (MuLV) RNA in the presence of yeast amber suppressor tRNA yielded an enhanced ratio of GagPol fusion protein to Gag [36]. Before sequence information was available, this led to the proposal that a single UAG separated gag from pol which was in-frame with respect to it. However, it failed to dispel the widely held assumption that a splicing event was involved in its natural synthesis. Several of the N-terminal amino acids of the viral protease that cleaves the viral polyprotein, are encoded by the 3′ end of gag, and the main, more C-terminal part of it is encoded by the 5′end of pol (this viral protease is itself proteolytically released from its GagPol junction position). The key result which definitively showed that MuLV GagPol naturally results from readthrough of the gag terminator rather than splicing, was the sequencing of MuLV protease by Steve Oroszlan and his NIH colleagues [37] (Figure 1). They demonstrated that in GagPol the amino acid, glutamine, was present at the position corresponding to UAG in the mRNA. Glutamine tRNA is near-cognate for UAG, Since UAG causes almost 95% of the ribosomes translating gag to terminate, the remaining 5%+ of product, GagPol derives from readthrough of the gag terminator. J. Levin, A. Rein and their colleagues then showed that viral infection does not alter the tRNA(s) that decodes the UAG [38], so the readthrough is mediated by the standard cellular glutamine tRNA. Later an mRNA pseudoknot 3′ of the UAG was shown to be needed to yield significant levels of readthrough [39,40]. More detailed mutagenic analysis, and also structural probing of the pseudoknot and the 8 nt separating it from the UAG followed [40,41,42,43]. Later this pseudoknot became a prototype for studies addressing whether a protonation-dependent switch occurs to induce an active conformation [44]. In addition to the readthrough stimulatory role of the 3′ pseudoknot, Goff, Song and their colleagues discovered that after some GagPol is synthesized, an intriguing secondary stimulator that is also relevant to inhibition of nonsense mediated decay (NMD) comes into play. They identified an interaction of the RNase H domain of the reverse transcriptase component of Pol with the C-terminal domain of peptidyl release factor eRF1. This precludes eRF1 from binding release factor 3, eRF3 [45,46]. This promotes readthrough since delivery of eRF1 via an eRF1/eRF3/GTP ternary complex, is a requisite early step in the termination pathway. The interaction of the RNase H domain with eRF1 is weaker than that of eRF3, but if it is predominately an in cis effect that would likely be relevant [46]. This consideration is also pertinent to whether a temporal difference of readthrough efficiency would be expected. Further ribosome profiling experiments [47] could be helpful in this regard. In vivo imaging of one case of recoding, HIV gag-pol frameshifting, suggests that only a small subset of the translating pool undergoes recoding, but that subset does so at a high efficiency [48]. More studies are needed to assess this radical proposal. Whether it is relevant to murine leukemia virus stop codon readthrough is unknown.
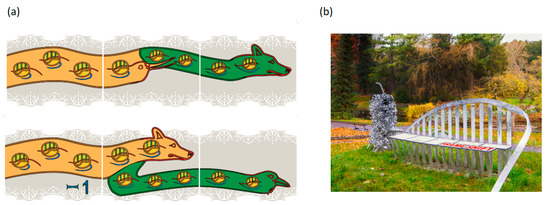
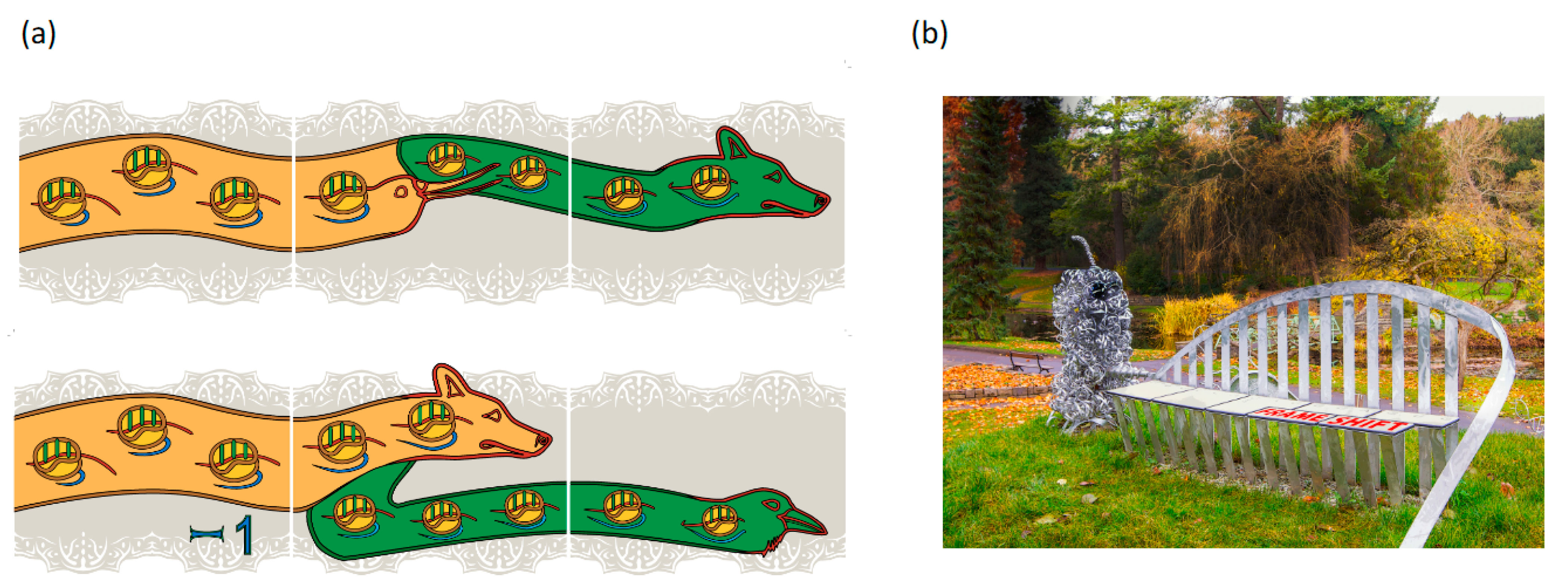
Figure 1.
Outreach representations of frameshifting and readthrough. Panel (a) depicts retroviral recoding where protease sequencing was important for mechanistic understanding: top, Murine Leukemia Virus gag stop codon Readthrough; bottom, Mouse Mammary Tumor Virus gag pol Frameshifting. The head motifs used to depict directionality were inspired by the 1200-year old ‘Book of Kells’ in Trinity College Dublin library. The embedded ribosomes have their A, P, and E sites in green and those shown on the left are at an earlier stage of decoding than those on the right. Correspondingly, on the left the proportion of the mRNA (in red) that has passed through the ribosomes is small in contrast to that shown on the right side, and the nascent peptide emerging from the ribosome (blue) is longer on the right side than on the left. Gag is represented in ochre and Pol in green. These images are from a band of recoding tiles positioned around the middle of the outside walls of ‘a house’ in S.W. Cork, Ireland. Panel (b) shows the decoding seat component of the sculpture in the National Botanic Gardens, Dublin entitled ‘What is Life’. The title follows that used by Erwin Schrödinger of the Dublin Institute of Advanced Studies for his 1944 book (and previous year lectures). Both Watson and Crick independently credited the book ‘What is Life?’ as an early source of inspiration for them. A description of the components of the sculpture, including a hammerhead ribozyme and a ribosome can be found at http://whatislife.ie/ (accessed on 1 May 2021). The decoding seat is on a mound overlooking an iconic Charles Jencks 5.5 m high DNA double helix similar to those at Clare College Cambridge and near Cold Spring Harbor Laboratory beach. Each seat panel represents a codon. Three bars below and above each panel reflect its 3nt composition. Starting from the left, or 5′ end, the initial panels reflect all zero frame reading. A proportion of ribosomes shifting to the -1 frame is represented by the first split panel in which part of the panel is offset to the left by one third of a panel length. Continued triplet decoding by frameshifted ribosomes, and by zero frame ribosomes is represented by the panel at the right end.
5. More Recently Identified Viral Recoding Points to Greater Mechanistic Diversity
Following his Ph.D. in astrophysics, development of a new software program by post-doc Firth, allowed him, along with other members of our lab and collaborators in the 2008–2012 period, to identify recoding utilization by several viruses including influenza A, several mammalian and insect flaviviruses, a second occurrence in arteriviruses, cardioviruses, alphaviruses, Potyviridae plant viruses and a novel dicistrovirus ORF (and as considered above 3′ stimulators for alphavirus stop codon readthrough; in addition to overlapping ORFs accessed by leaky scanning, etc.).
Ribosome profiling is also proving valuable in identifying and characterizing frameshifting and stop codon readthrough [99,100], but will not be considered here.
Influenza A: +1 frameshifting: While shifts to the −1 reading frame are well known in virus gene expression, shifts to the other reading frame, by either by +1 or −2 frameshifting, are much less common (though the +1 frameshifting utilized in the decoding of a few chromosomal genes has been intensively studied). However influenza A, a single-stranded, negative-sense, segmented RNA virus, utilizes a low level of +1 frameshifting to express a protein designated PA-X. PA-X has the same N-terminal endonuclease domain as the canonical PA protein which is a subunit of the viral polymerase [101,102]. PA-X is involved in suppressing the adaptive immune response and in host cell shut off [101,103,104,105] for which the key amino acids have been identified [104]. The frameshift site is UCC UUU CGU [101,102]. Though no 3′ recoding signal is known, the A-site codon, CGU is one of the most seldom used codons in mammals and birds. However, while this is relevant for the sparseness of the corresponding aminoacyl tRNA and so for frameshift efficiency, several questions about significance of the identity of the A- and E-site codons remain unresolved [102]. Identification of a frameshift site in influenza virus RNA has pointed to the probable +1 frameshift sites for other viruses including chronic bee paralysis virus and Lake Sinai viruses [102], as well as two new strong, and related, candidates for mammalian cellular gene +1 frameshifting [106].
Two approaches have been taken to study the degree of significance of the free energy difference of tRNA pairing in the original, or 0- frame, and in the new frame, i.e., the thermodynamics of frameshifting [107,108]. One of the studies focuses in part on downstream structural stimulator buffering frameshift efficiency with respect to temperature variation [108]. Its results imply that unless relevant unidentified stimulators for influenza A virus frameshifting exist, the frameshifting level should be slightly higher during fever conditions compared to normal body temperature.
Flaviviruses: The Japanese encephalitis virus serogroup of flaviviruses, which includes West Nile virus, utilize −1 frameshifting to synthesize a protein, NS1’ that is important for viral invasiveness [109,110]. NS1’ induces expression of micro RNA-22 (miR-22) which antagonizes host mitochondrial antiviral-signaling protein (MAVS), and so inhibits IFN-I production thereby facilitating viral replication [111]. High levels of NS1’ generating flavivirus frameshifting are dependent on an RNA structural stimulator, a pseudoknot, 3′ of the frameshift site [109,110]. Japanese encephalitis virus infects about 68,000 people per year in the Asia Pacific region with about 20–30% ending in patient death. The live attenuated strain, JEV SA14-14-2 used for vaccination has a synonymous mutation of the pseudoknot that inactivates frameshift stimulation [112,113]. Consequently, after vaccination NS1’ is absent in sera, in contrast to the situation upon infection with most virulent viral strains, thereby creating a distinguishing diagnostic biomarker opportunity [114].
A study of the West Nile virus frameshifting 3′ stimulator used optical tweezers to apply tension to single mRNA molecules, mimicking the tension applied by the ribosome during frameshifting. It led to support for a hypothesis that conformational heterogeneity plays a key role in frameshifting and suggests that transitions between different conformers under tension are linked to efficient frameshifting [115].
Several insect flaviviruses also utilize frameshifting in their expression [116].
Arteriviruses and cardioviruses: Proteins binding 3′ of shift sites Partly because of their distance from shift sites, until 2010, structural RNAs 3′ of shift sites that stimulated frameshifting were generally thought to exert their effects close to or at, the unwinding site within the ribosome’s mRNA entrance tunnel, rather than transiently blocking entrance from the outside. They were only known to involve RNA. However, the 5′ end of the counterpart frameshift modulatory sequence involved in two subsequently discovered occurrences were several nucleotides further 3′ raising the possibility that they acted at the entrance to the mRNA entrance channel. However, as described next their action requires protein binding and given the currently unknown level of steric occupancy, location inference awaits structural studies. As already demonstrated in several bacterial frameshifting investigations, smFRET studies provide invaluable information about frameshifting (we are fortunate to have participated in one such study led by J. Puglisi [108]). Such information and structural insights from cryoEM are complimentary.
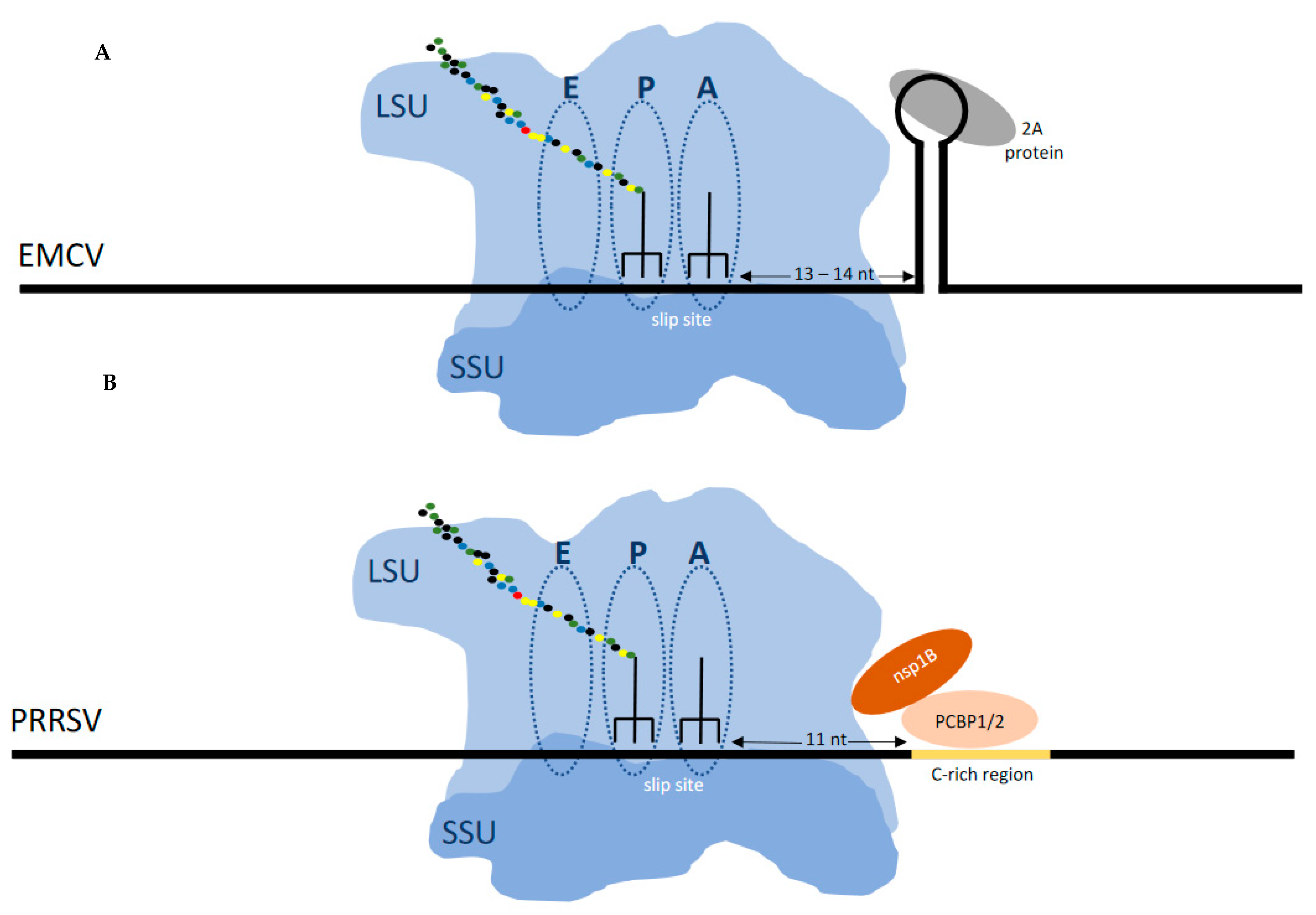
The family Arteriviridae, like the Coronaviridae, are in the order Nidovirales, and includes porcine reproductive and respiratory syndrome virus (PRRSV) which is perhaps the most important pig pathogen. PRRSV, whose genome is ca.15kb, productively utilizes three different frameshift-derived products. Programmed −1 frameshifting occurs just 5′ of the junction of its long ORF1a with its long ORF1b to synthesize its replicase polyprotein [117,118]. However, within the nsp coding sequence of ORF1a, PRRSV also employs 20% efficient −2 frameshifting, to generate a multifunctional product, nsp2TF (Trans-Frame), consisting of the N-terminal of nsp2 and a unique C-terminal domain [119,120]. Immediately 3′ of the shift site, there is a stop codon in the -1 frame. The −1 frameshifting also occurs at the same shift site and generates a product, nsp2N, that is a truncated form of nsp2 [121].
The 3′ stimulator for the frameshifting occurring at that shift site is not an intra-mRNA structure but instead 10nt 3′ of the shift site there is a protein binding site (CCCANCUCC). The viral protein nsp1 together with host poly(C) binding proteins bind at this site generating a RNA:protein complex that is at the leading edge of the ribosome when its decoding center is at the shift site [121,122,123] (Figure 4). A role for the frameshifting products is described below.
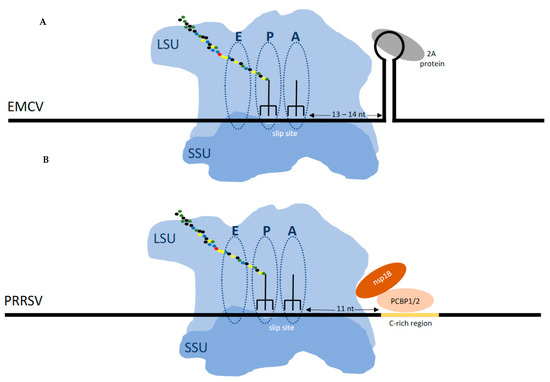
Figure 4.
(A) Frameshifting in EMCV (and TMEV) is stimulated by a protein:RNA complex positioned at the leading edge of the ribosome when the decoding center is on the shift site. The viral encoded 2A protein, which is encoded 5′ of the shift site, binds to the 3‘ stem loop to stimulate frameshifting. (B) Frameshifting in PRRSV is stimulated by a protein:RNA complex that requires dimerization of the virally encoded nsp1B protein and the chromosomally encoded polyC binding protein which bind to the 3‘ stem loop to stimulate frameshifting.
In contrast the cardioviruses (Picornaviridae) encephalomyocarditis virus and Theiler’s murine encephalomyelitis virus, feature a 3′ stem–loop RNA structure 13 or 14nt 3′ of the shift site. It is to this structure that a viral encoded protein, 2A, which is encoded 5′ of the shift site, binds to cause frameshifting. Early in infection there is no detectable frameshifting, but late in infection with increasing 2A synthesis frameshifting efficiency reaches 70–80% depending on the virus [124,125,126,127].
Alphaviruses: In addition to viral encoded proteins binding to sequence 3′ of frameshift sites and interacting with the leading edge of the ribosome to link frameshift efficiency to intracellular life cycle stage, other types of viral recoding signals are emerging. Alphavirus frameshifting is providing likely candidates. This frameshifting permits expression of a short −1 frame ORF which is embedded wholly within sequence encoding the overlapping protein [128].
The 3′ structural stimulators for the frameshifting utilized by some alphaviruses, e.g., Middelburg virus, are pseudodoknots [128,129]. For Semliki Forest virus despite bioinformatic [128,129] and structural probing [59] evidence for a single stem loop, a relatively short sequence without apparent intra-RNA stem loop potential on its own can mediate a significant proportion of the expected level of frameshifting [129]. How that sequence operates has not yet been reported, but analogous features are known elsewhere. (Single stem loops are known for other animal viruses, e.g., human T-lymphotropic virus type 2 [130].) The frameshift site for essentially all alphaviruses with the exception of Bebaru virus is the same as for HIV-1 and the majority also have G 3′ to the shift site as does HIV-1 [128]. For at least Sindbis virus, as for HIV-1, there are two frameshift products that differ by a single amino acid. Relevant tRNA availability determines the product ratio [59].
Given the efficiency of frameshifting seen from alphavirus frameshift cassettes with little alphavirus sequence 5′ of the shift site, it has come as a surprise that a nascent peptide feature is an important component of alphavirus frameshifting. This will be presented next though the inter-relationship of the nascent peptide influence and the 3′ stimulators has not yet been reported, and there is currently a dilemma regarding comparisons with levels in viral infected cells. The part of the product protein encoded 5′ of the frameshift site can cotranslationally form either of two topologies. The minor topology, which forms 20% of the time, has an extra trans-membrane domain compared to the more abundant topology. It has been reported that the force generated by the translocon-mediated membrane integration of the extra trans-membrane domain within the minor topomer, leads to a tension on the ribosome. In conjunction with the frameshift stimulators 3′ of the shift site [129], this tension has been proposed to be a stimulator for the -1 frameshifting [131] that results in synthesis of the trans-frame encoded virulence factor protein. In other words, mechanical force by translocon-mediated nascent protein insertion into membranes influences frameshifting efficiency [131], likely by modifying anticodon:codon interactions. (For frameshifting involving a particular pairing between mRNA 5′ of the shift site to rRNA [132], stimulation of −1 frameshifting was shown to occur without lengthening of the ribosomal rotated state lifetime, providing evidence that this enhances frameshifting by inducing mechanical force [108].)
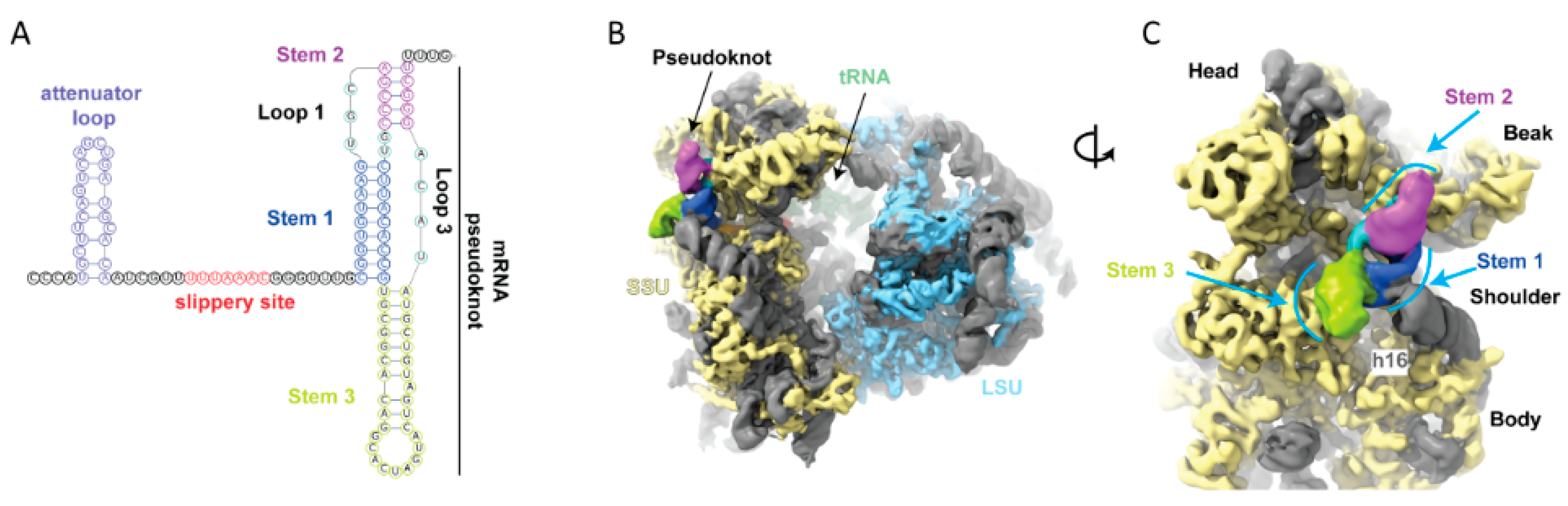
Further diversity of viral frameshifting signals associated with different ribosome interactions may await discovery. mRNA:rRNA pairing in the ribosome mRNA exit channel is known to be important for a substantial proportion of functionally utilized bacterial frameshifting occurrences, and suspected for one mammalian chromosomal gene family [6]. In relation to 5′ stimulators acting at the RNA level, unresolved issues relating to 5′ stem loops in both SARS CoV1 and CoV2 mRNAs and elsewhere also merit additional investigation [133,134].
Dicistroviruses: Despite the two cistron implication from their Dicistroviridae family name, Israeli acute paralysis and related dicistrovirus have a third ORF 3′ of the intergenic IRES that, in the +1 frame, overlaps the 5′ region of ORF2 [135]. In addition to directing ORF2 translation, the IRES also directs translation of the overlapping ORF and does so by forming a UG base pair adjacent to the IRES’ P-site tRNA mimicking domain [136]. The reason to mention this is its relationship to the intriguing situation with a different dicistrovirus, cricket paralysis virus, where recoding may be involved. In that virus, a subset of ribosomes recruited to the IRES bypasses 37 nucleotides downstream to resume translation at the +1-frame 13th codon—it is not AUG [137]. This is different from the translational bypassing known for a bacterial virus and will have much to reveal about decoding versatility.
7. Implications for Synthetic Manipulation of Frameshifting, Readthrough and StopGo for the Successful Delivery of Nanoparticle-Complexed Nucleoside-Modified RNA and of DNA Vaccines
The vaccine triumphs recently achieved with synthetic structured mRNA containing naturally occurring modified uridines, nanoparticle complexed for delivery, raise interesting mechanistic issues and open up important future opportunities. The modified uridines minimize recognition by RNA binding proteins including endosomal Toll-like receptors that are involved in the innate immunity reaction to foreign RNA as well as increasing its translatability, while the lipid nanoparticle facilitates delivery [151,152,153,154]. (CureVac does not use RNA base modification [155], but does utilize other features such as a histone mRNA stem loop that also stabilizes the mRNA.) Further developments including incorporating alphavirus-derived RNA polymerase encoding ‘self amplifying’ features are extending the capabilities. While the potential of these technologies for vaccines for other viruses (e.g., flaviviruses [156]) and certain forms of cancer is actively being explored, there is likely also potential for specific human genetic disease amelioration with a study on liver repair providing an indicator [157].
Of relevance here is the implications of these technologies, and of adenovirus backbone-based DNA vaccine technology, for independent studies to artificially modulate frameshifting or readthrough efficiencies.
Some early studies explored whether frameshifting structural stimulators could involve inter-RNA pairing instead of intra-mRNA pairing. Synthetic RNA oligos, several involving modified bases, complementary to sequence 3′ of a shift-prone site functioned as trans-acting components of an active stimulatory structure [158,159,160,161]. In theory this approach could be used to create compensatory frameshifting that might ameliorate the symptoms of genetic disease due to specific frameshift mutants. However, there were no illusions about the impractability at the time due to limitations of oligonucleotide delivery, and that even though major efforts were being made to solve the oligonucleotide delivery issue, this potential use was way down the priority list and not an incentive for the intense delivery efforts undertaken. The new technologies used in the COVID vaccines have the potential, for several genetic diseases, to circumvent consideration of such indirect schemes. However, for at least the initial immune response, short term expression is adequate, whereas for other purposes synthetically delivered RNA with the additional capability of self-replication, is being actively explored.
Nevertheless, in contrast to viruses such as measles and polio that are disseminated from the initial infection site via lymph or secondarily blood, for mucosae disseminated viruses the length of immunity following an initial infection can be short with consequent implications for herd immunity [162].
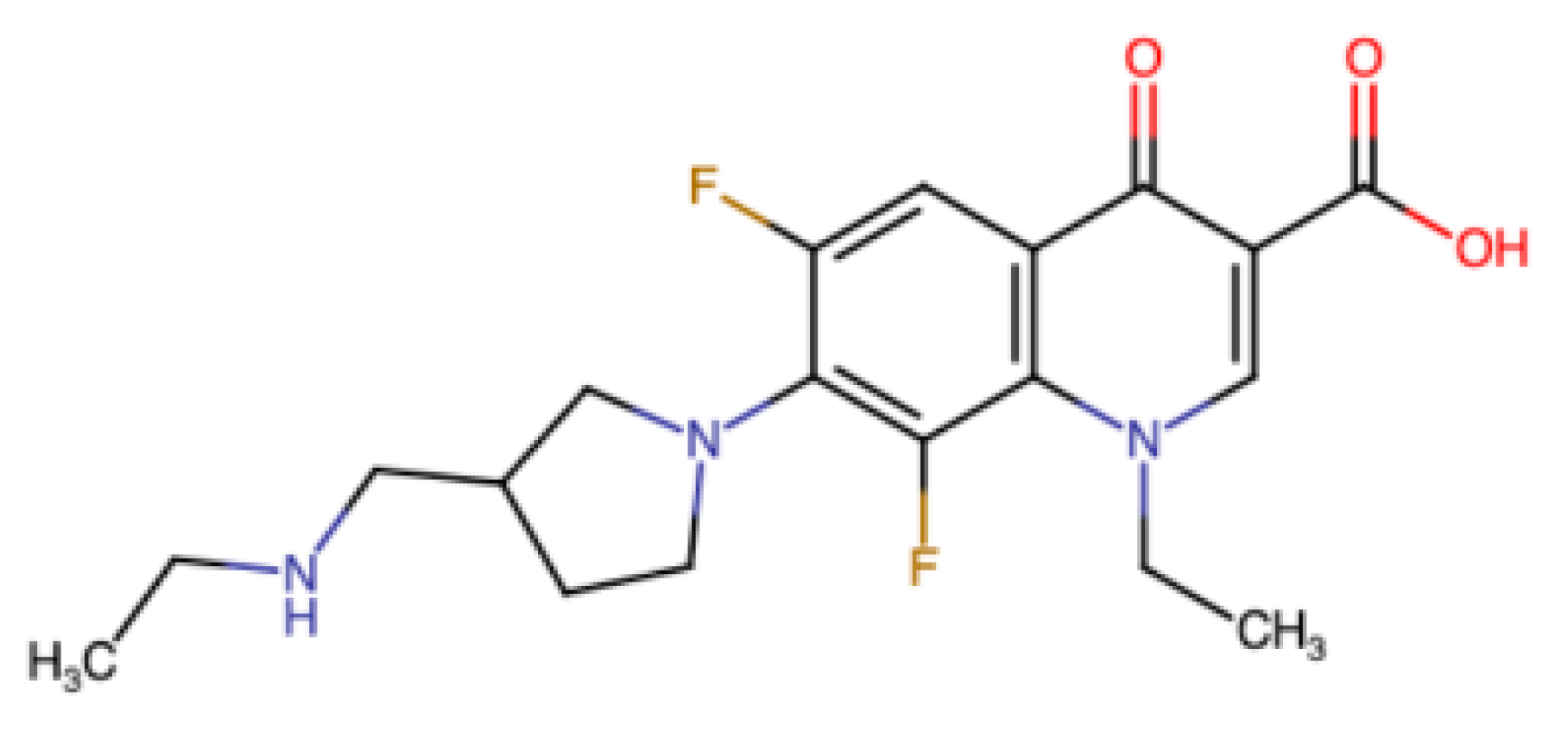
For this and other reasons, the new vaccine technologies do not displace the desirability of identifying good viral targets and, specific for them, cheap stable safe inhibitory drugs. Such compounds have potential benefit not only for those living in areas with few vaccination opportunities or low vaccine uptake. To avoid resistant mutants, a combination of drugs is desirable, and has potential to inhibit a wide variety of even currently unknown SARS-type viruses. Though further development is needed, for the COVID causative virus there have already been numerous reports of protease inhibitors, including [163,164]. The single essential ribosomal frameshifting event required for decoding that virus is also an appealing target and some inhibitors of it have been reported e.g., [98,165]. The SARS CoV-2 frameshifting inhibitory compound studied in those reports is merafloxacin (Figure 5). It inhibits viral propagation in tissue culture cells.
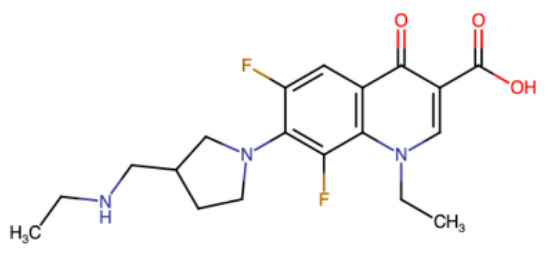
Figure 5.
Cartoon structure of merafloxacin [165], an inhibitor of SARS-CoV-2 frameshifting [98,165].
Is the new RNA vaccine technology relevant to the multiple serotypes of foot and mouth disease virus which, as is widely known, is highly infectious? Due to the cost and time needed for immunological protection it does not detract from the appeal of a drug that would target the form of recoding essential for that virus. As described in the section immediately above, the recoding involved is StopGo. In part as StopGo, at least involving the known sequence characteristics, does not occur in mammalian gene expression, when anti StopGo compounds are identified, they have significant potential to be important antiviral drugs [166].
8. Finding Framing Imperfection, or Rather Its ‘Trade-Off’ Occurrence, Despite Crick’s ‘Half-Right’ Reason for Thinking It Would Not Exist
The 70–80% efficiency of cardiovirus frameshifting is quite a contrast to titin synthesis where successful decoding involves the avoidance of frameshifting for >30,000 codons. Actually, for years it was thought that the selective pressure to avoid frameshifting was so great that even frameshifting errors would not be detectable [167,168] and it was not until 1972 that this perception changed. Among a set of mutants induced with a frameshift specific mutagen, the ‘leaky’ ones were discarded as it was thought they could not be ‘real’ frameshift mutants, and the others were considered to be ‘real’ frameshift mutants [169]. However, scepticism about decoding rigidity led to an examination of the ‘real’ frameshift mutants, and identification of small amounts of full-length protein, i.e., they were also ‘leaky but at a lower level [170]. If real frameshift mutants were involved, and compensatory errors at the transcription or translation level allowed synthesis of some full length product, how could it be distinguished at what level the errors occurred? A collaboration with Gorini’s lab, which had isolated single fidelity mutants, permitted the identification of error frameshifting by WT translation components [170]. This followed earlier work on mutants of tRNA and other translation components that perturbed framing and whose identity were later elucidated [171]. Crick was right about a central aspect of readout, translocation, being fundamental as it involves subunit ratcheting [172,173]. However, as we celebrate what is now the 60th anniversary [174] of ‘his’ discovery of the mode of readout [175], 3 years after predicting the existence of the molecule that later became known as tRNA [176], he did not foresee [168] that anticodons could temporarily ‘loose their grip’ of mRNA and so the potential for re-pairing to mRNA in a new frame. Relevant to primordial decoding, translocation can occur at a very low level in the absence of EF-G and GTP due to intrinsic tRNA:ribosomal features [177,178,179]. However, not only is EF-G (and EF2) mediated GTP hydrolysis [180] very important for speed, though its initial binding may contribute to some relevant loss of contacts, in part by stabilizing tRNA:mRNA pairing in the A-site, it is overall hugely significant for restraining unselected frameshifting and enabling selected 3′ frameshift stimulators to function [108,181,182,183]. The extent of importance raises the issues not just of proto-anticodon:codon dissociation and framing in primordial protein synthesis but also of mRNA diffusion. The possibility that one type of recoding stimulator involving mRNA:rRNA pairing may be a remnant of one relevant component is discussed elsewhere [184]. However, while the sophistication of recoding is a natural focus of attention, it needs to be balanced by the long existence of erroneous frameshifting and non-canonical initiation being exploited as two of many facets of the ‘arms race’ between viruses and their hosts.
9. Utilizing Imperfections, Including of Framing, to Inhibit Viruses (and Cancer): DRiPS
While the ubiquitin/proteasome pathway generation of viral peptides is important for immunity, a different defensive strategy for obtaining viral peptides to display for antiviral targeting will be considered next. At least for the frame maintenance component of genetic readout, for viruses and cancer cells, the optimal balance between translation speed and accuracy, is likely greatly different than that for normal organismal gene expression, where cellular economy is one of the relevant considerations (synthesis of the extra long protein, titin requires ribosomes maintaining frame for over 30,000 codons). Might the selective advantage for many viruses and cancers of high speed translation have an associated weakness that is exploited defensively? Following viral infection there is an extremely rapid immune system display of peptides derived from viral genes including from those whose products are generally highly stable proteins. At least partial independence from peptides derived from proteins at the end of their normal lifespan, led Yewdell to formulate a hypothesis in which Defective Ribosomal ProductS, DRiPS, that do not achieve functional integration into the proteome, are a major source of antigenic peptides [185]. The peptides are short (ca. 8–11 residues) and are quickly degraded unless presented in the MHC class I cells for TCD8+ immunosurveillance [186,187,188]. Unlike class II [189], the class I immunopeptidome is tightly linked to translation, highly dynamic and sensitive to infection, as well as neoplastic transformation and metabolic perturbation [190,191,192].
An illustration of the relevance of frameshifting is provided by a recent study of the effects of treating melanoma cells with interferon-γ ((IFNγ) [193]. IFNγ induces an enzyme involved in the breakdown of tryptophan along the kynurenine pathway and leads to tryptophan starvation. Ribosome profiling and other techniques led to the identification of frameshift-derived products and their origin [193]—‘hungry’ tryptophan codons in the ribosomal A-site leading to peptidyl-tRNA dissociation and re-pairing to mRNA in a new frame. The presentation of aberrant trans-frame peptides was detected, including from patient samples, with implications for immune recognition [193]. Limitation of specific fully functional tRNA is known in various other human diseases [194,195], and there is reduced supply in certain brain cells [196]. In some cases, there is known or suspected relevance to the generation of frameshift-derived peptides.
Despite this study, to date peptides derived from non-programmed frameshifting and stop codon readthrough are a modest minority of the non-canonical peptides destined for immune display. The repertoire of functionally important cellular proteins synthesized is now known to be significantly greater than apparent even a decade ago only in part due to the extent of utilization of non-AUG codons for initiation then being seriously underestimated [197,198,199,200,201,202,203,204,205,206,207,208,209,210,211].
The cellular response to infection by viruses such as cytomegalovirus (a herpes virus) and influenza viral infections, leads to non-canonical initiation by tRNALeu on CUG codons [212]. Without viral infection treatment of cells with the inflammatory cytokines, Tumor Necrosis Factor (TNF)-α or type I interferon has the same effect. As a virus is shutting down host protein synthesis, the host response of activating non-canonical decoding, and immune display of derived DRiPs, facilitates curtailment of viral spread via recognition by cytolytic T cells [212].
More generally, as described in several of the publications just cited, stress generates novel products that tend to be unstable and which should not be considered ‘noise’ since their peptides are preferentially displayed on the MHC class I peptidome—i.e., the DRiPs hypothesis has now gained wide support [213], with very recent work further elucidating it [214].
Though non-canonical translation is a major source of antigenic peptides, it constitutes a small fraction of total cellular translation, and so selectivity is key; the immunopeptidome poorly reflects either the transcriptome or the proteome [215,216]. Numerous studies have been undertaken to explore the important issues raised, but as also mentioned in the next section below, among them are model system studies [217]. It is exciting that technical advances including in mass spectrometry and analysis of its data, ribosomal profiling and other developments are facilitating furtherance of the conceptual advances, so that important understanding is in prospect.
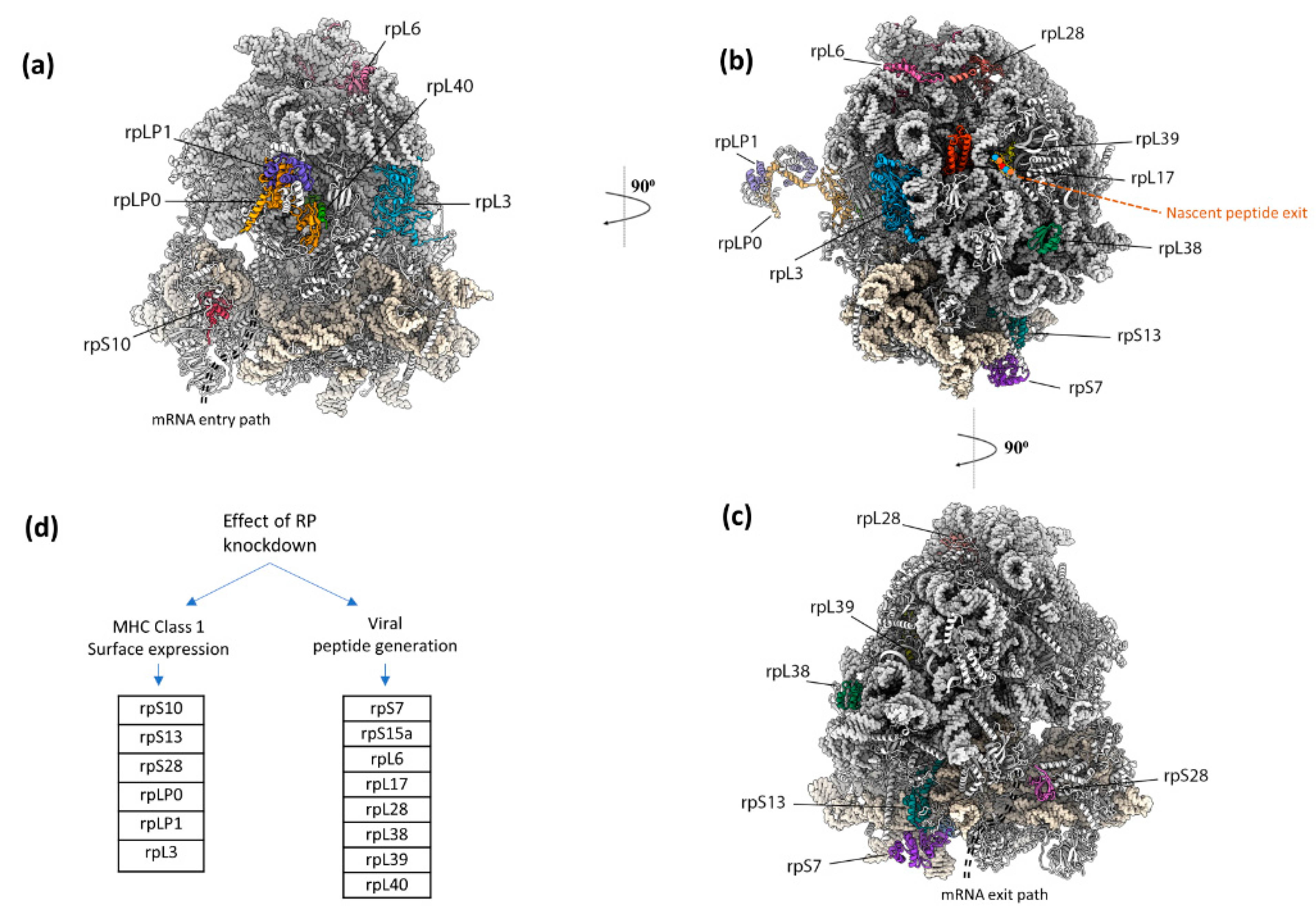
Eukaryotic ribosomes have numerous RNA segments and proteins not required for core protein synthesis as evident from comparison with minimal effectively functioning ribosomes. In addition to ribosome functioning to interact with the translocon being vital for the destination of a subset of newly synthesized proteins, the evolutionary selective pressure for some components may have been related to immune system peptide display, including of DRiPs. Experimental knockdown of expression of each of 80 ribosomal protein genes individually led to the identification of 14 ribosomal protein genes whose products modulate the presentation of specific peptides with at most only minor alteration of the synthesis of the products of standard decoding [192]. The 60S subunit proteins RPL6 and RPL28 which are adjacent on the ribosome and highlighted in Figure 6, perform opposite roles in generating an influenza A virus-encoded peptide. Depleting RPL6 decreases ubiquitin-dependent peptide presentation, whereas depleting RPL28 increases ubiquitin-dependent and -independent peptide presentation. Small ribosomal subunit protein S28 is also highly relevant [192] since its absence increases non-AUG initiation. These findings complement a genome-wide CRISPR screen that identified RPL23 as a negative regulator of CD8+ T cell killing of melanoma cells [218]. Whether there are specialized ‘immunoribosomes’ that perform roles in peptide display distinct from other ribosomes is unclear. (Potential experimental pitfalls relevant to resolving this general topic have been described [219].)
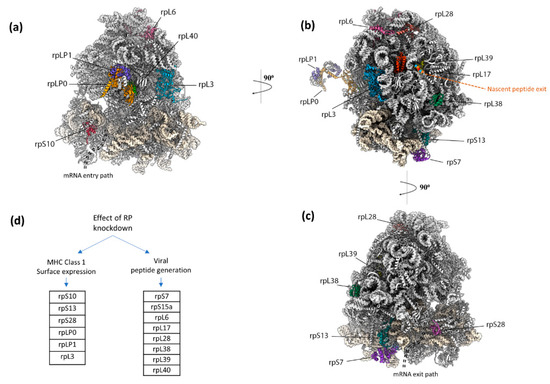
Figure 6.
Ribosomal proteins that impact MHC Class 1 peptide generation for immunosurveillance, according to Wei et al. 2019 [192]. (a–c) Depiction of ribosomal proteins that impact MHC Class 1 peptide generation for immunosurveillance [192]. rpL6 and rpL28, which are adjacent to each other on the large subunit, have opposing effects on viral peptide generation. rpL6 depletion decreases ubiquitin-dependent peptide presentation, whereas rpL28 depletion increases ubiquitin-dependent and -independent peptide presentation. Figure was generated with a human ribosome (PDB 4V6X) in ChimeraX. (d) Individual ribosomal protein knockdowns affect immunosurveillance by impacting either MHC Class 1 surface expression or viral peptide generation, and are grouped as reported.
10. Herpes Virus: DNA Viruses Can Use Adventitious Frameshifting to Evade Drugs and Programmed Frameshifting to Perhaps Facilitate Avoidance of Immune Detection
Drug evasion: While ribosomal frameshift utilization is well known for bacterial DNA viruses such as T7, λ and Listeria phage PSA, the relatively few eukaryotic DNA viruses known to avail of frameshifting is balanced by its importance. For many years the only known relevance of any type of frameshifting to herpes virus gene expression was as a result of D Coen’s lab in-depth study of utilization of non-programmed ‘error’ frameshifting that permits specific thymidine kinase mutants to become resistant to acyclovir and related drugs that are more orally available. These mutants are a serious problem for immunocompromised patients, with many patients worldwide becoming blind. Commonly the mutations are indels in homopolymeric stretches of guanines or cytosines that despite altering the reading frame, do not obliterate thymidine kinase activity. The most common mutant characterized has a single G inserted in a run of 7Gs with net +1 ribosomal frameshifting yielding ∼0.1% of WT activity [220] which is sufficient to permit reactivation from latency [221], and expression of an epitope recognized by T cells [222]. However, it is not enough to activate acyclovir and confer drug resistance.
Discovery of a new type of 3′ stimulator: Another mutation, which is present in 5–10% of clinical isolates, has one cytosine deleted from a run of six cytosines. Though this sequence was suspected to cause frameshifting, an early experiment with the then available dual luciferase assay system [223], yielded no detectable frameshifting. Though later shown to be unrepresentative of the natural situation, it is noteworthy as it illustrates that all such fused dual reporters are incapable of detecting a subset of at least frameshifting and can be misleading. In reality, even though it appears that the frameshifting only involves a single tRNA re-pairing to mRNA in the new frame, the real efficiency of frameshifting is 3–5% due to a 100-fold effect of a then novel stimulator [224]. Due to the absence of downstream stop codons in the sequence brought into the zero frame by the frameshift mutation, ribosomes that do not undergo frameshifting continue translating to the 3′ polyA-tail, i.e., the frameshift mutation creates a non-stop mRNA. The model proposed was that ribosomes stall when reaching the poly(A) tail causing reduced movement of trailing ribosomes. This leads to enhanced frameshifting on the shift site but with subsequent reduced synthesis of the frameshift derived product [224]. The results of several experiments were consistent with this model, and despite the level of net −1 frameshifting, the amount of frameshifting-derived thymidine kinase activity was again 0.1% of WT activity [224]; the same level as for that from the +G mutant. Stalling at Poly(A) is well known [225,226], and there are now other studies on enhanced frameshifting by a trailing ribosome that collides with a stalled ribosome [227], and paused elongating ribosomes can even cause a queue of ribosomes such that initiation can occur at a weak start site [211]. The obvious thought is to regard the non-stop stimulation of frameshifting discovered in the mutant herpes thymidine kinase coding sequence as adventitious frameshifting since it only occurs in mutants. However, might there have been selective pressure to have runs of repeat sequences that are prone to frameshift mutations, and no stop codons in at least one alternative frame to permit ribosomes to reach the poly(A) tail? That could permit ribosome pile-up and enhance the possibility of several nonstandard events. There is no evidence for such ‘bet hedging’ in this case, though, for instance [6,228,229], it has been widely considered elsewhere. Finally, when the run of 5 Cs in a non-stop mRNA is replaced by an HIV gag pol frameshift cassette, frameshifting is also enhanced compared to a derivative with an in-frame stop codon, raising questions as to where else such frameshifting may occur [224].
11. Immune Evasion
The innate immune response provides the first line of defense against infecting viruses and other intruding pathogens. This ancient-origin response is very important for the initial control of infection and allows time for launching an adequate adaptive immune response. It involves the coordinated activation of several transcription factors that are followed by the activation of type I interferons (IFN), their secretion from infected cells and binding to the surface of adjacent cells. This results in activation of a signaling pathway that in turn leads to the expression of interferon stimulated genes whose products counteract infection. However, host development of relevant IFNγ-secreting cells in response to infection by the economically important arterivirus, PRRSV, introduced above, is relatively slow [230]. The −2/−1 frameshift products encoded by PRRSV function to suppress the type I IFN response and delay an effective innate immune response [231].
All three nsp2 variants (nsp2, nsp2TF and nsp2N) include an N-terminal papain-like proteinase domain involved in replicase polyprotein cleavage and have deubiquitinase activity. In vitro transfection experiments showed that their deubiquitinase activity suppresses the innate immune response [232,233,234]. Nsp2TF has been directly shown to function as a deubiquitinase that antagonizes ubiquitination of host cell proteins [120,231]. However, in infected cells nsp2 localizes to the virus replication complex for polyprotein processing. Nsp2TF localizes to the endoplasmic-reticulum–golgi intermediate compartment secretory pathway to stabilize the major structural protein plus facilitate the virus assembly, and it is difficult to determine the location of nsp2N since it contains no amino acid sequence not in nsp2 [119,121,231,235]. In part due to the different locations, the exact mechanism of host innate immune suppression remains to be elucidated.
Furthermore, frameshifting can also be relevant to avoidance of the adaptive immune response. While many cancers develop immune evasion by inhibiting MHC class I expression in a rather brief period, selection over a long time has refined viral strategies for the same end result and may be especially significant for persistent viruses such as the arterivirus, PRRS and herpes viruses. With respect to PRRS, swine MHC class I molecules are essential components of antigen presentation for the subsequent activation of CD8+ T cells, as well as for the detection of virally infected cells by cytotoxic T cells [236]. The C-terminal region of the TF-domain of the −2 frameshift derived Nsp2TF reduces expression of these molecules in alveolar macrophages, PK15-CD163 cells and monocyte-derived dendritic cells [237]. Nsp2TF promotes arterivirus assembly by interacting with the major viral envelope proteins during their transport along the exocytic pathway and antagonizing host defensive ubiquitination-dependent proteasomal degradation [120]. While this is just one of the ubiquitin-related strategies viruses have evolved [238], unlike the many viral encoded deubiquinases that act on relevant cellular substrates, Nsp2TF acts on other viral encoded proteins [120]. Nsp2TF is targeted to the same compartments of the secretory pathway (ERGIC and Golgi), in which the two major envelope proteins of PRRSV, GP5 and M, accumulate.
Persistence of herpes viruses is more widely known and aspects of two long diverged oncogenic viruses, the gammaherpesviruses, Kaposi’s sarcoma-associated herpesvirus (KSHV) and Epstein–Barr virus (EBV), will be considered. These viruses have a short productive phase and a predominant latent infection though expression of a limited number of proteins some of which perform an immunomodulatory role to prevent recognition by the host immunosurveillance system. The most prominent of these is the major latency-associated nuclear antigen 1, that for KSHV is known as LANA1, and its EBV counterpart is EBNA1. Each has a central domain that features repeat sequence. EBNA1 and LANA1 repeat sequences function in part to inhibit major histocompatibility complex (MHC) peptide presentation [239,240,241], and for this specific peptide sequences within the repeat have been identified [242].
However, in 2014, the Chang and Moore group discovered that decoding of the ORFs encoding nuclear antigens of the long diverged tumor viruses, Kaposi’s sarcoma-associated herpesvirus (KSHV) and Epstein–Barr virus (EBV), LANA1 and EBNA1, respectively, is associated with efficient +1/−2 frameshifting [5]. LANA1 and EBNA1 are multifunctional proteins involved in episome maintenance, latency, regulation of transcription, cell cycle, and immune surveillance. The frameshift derived proteins, derive in part from decoding alternative frames in these viral repeat regions [5]. At the nucleotide level their repeat sequences are nearly identical to each other, but their framing is offset so that the zero frame products are quite different (EBNA1′s is glycine-alanine rich). The frameshift derived product from the LANA1 coding sequence has a serine/arginine-rich repeat sequence protein [5]. Similar sequences are found in some of the neurodegenerative associated proteins derived from frameshifting at repeat sequences [196,243,244,245].
The frameshift-derived product from the EBNA1 coding sequence is LANA1-like in having a glutamine and glutamic-rich sequence, implicating a crucial role for these sequences in both viruses (Figure 7). EBNA1 and LANA1 repeat sequences function in part to inhibit major histocompatibility complex (MHC) peptide presentation [239,240,241], with specific peptide sequences within the repeat being identified [242]. Within the mRNA repeat region there are G-quadruplex structures whose destabilization increases translation and antigen presentation [246,247] and to which nucleolin binds [248], with consequences for antigen presentation [248,249]. In addition to the well known productive utilization of polymerase slippage at specific linear sequences, for instance by paramyxoviruses, G-rich structures can cause polymerase slippage [250]. While this has not been studied for EBV and KSHV, it has potential to also contribute to decoding of parts of their repeat sequence in what would in WT be an alternative reading frame. Much remains to be elucidated and, only partly because of the nuclear dimension involved, it is challenging.
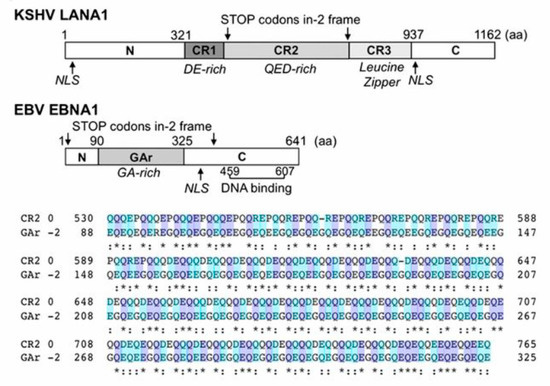
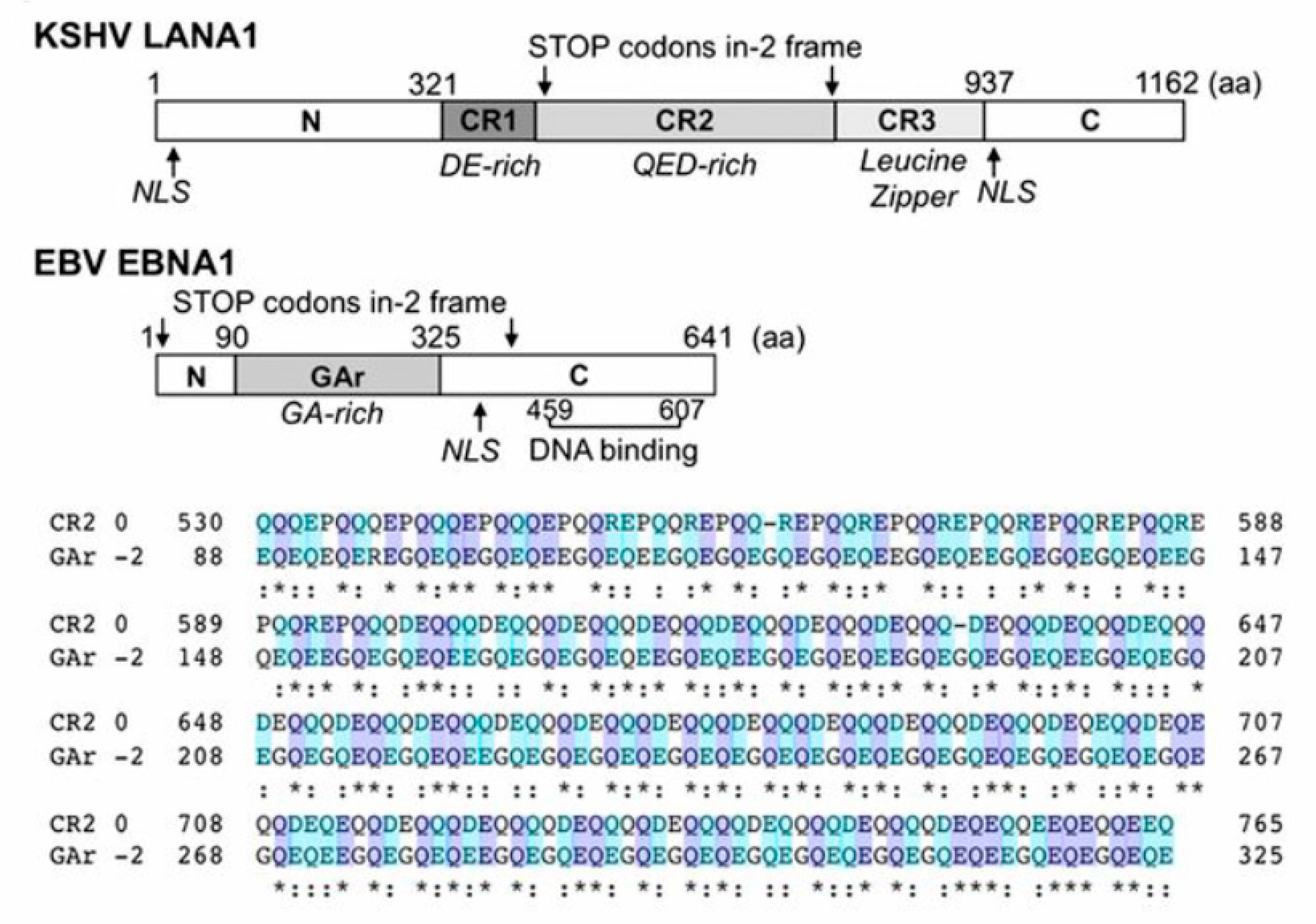
Figure 7.
Map of KSHV LANA1 and EBV EBNA1 and amino acid sequence alignment comparing LANA1.CR2 and EBNA1.GAr in the −2 frame (EBNA1ARF). EBNA1 comprises the N terminus, GA-rich central domain, and C-terminal DNA binding domain. Stop codons of the −2 frame are indicated with arrows. Although the EBNA1 GAr (0 frame) has no amino acid similarity to the LANA1 CR, EBNA1ARF has ∼35% similarity to the 0 frame of the LANA1 CR2 domain, and both sequences contain highly acidic QE-rich repeats. From Ref. [5].
12. Perspective
Animal viruses have impressively exploited selection to beneficially manipulate even the readout within internal coding regions of their own mRNAs. Though occurring without foresight, the level of sophistication gives the appearance of wiliness. What types of unknown animal viruses might feature the most extreme examples of recoding in their expression? At our current stage of ignorance, the best guess would seem to be Drosophila neuronal viruses given exceptional readthrough occurring in those cells [251,252,253]. Stretching the definition to include ciliate viruses, then whether Condylostoma has been successful in restricting its infecting viruses to those that encode long polyproteins by the expedient of reassigning UAG, UAA and UGA to be sense codons and only recoding them to specify termination by proximity to Poly-A tails [254,255] is of interest (also see [256,257]). Further, features of putative single stranded RNA viruses for the ciliate Euplotes, where frameshifting is rampant in chromosomal gene expression [258], are also pertinent. While further understanding of these cases is of great interest, the discovery of new “unknown unknowns” would be even more exciting. At the other extreme, there is no hint of recoding in adenovirus expression (Ulf Pettersson, pers comm, 2021). Perhaps this is not surprising since it generates over 900 alternatively spliced transcripts [259]. Fortunately, when mapping of its protein coding genes was initiated, splicing was an ‘unknown unknown’ and its complexity in that case did not inhibit an approach taken to map its protein coding genes [260].
Finally, in advance of the next Olympic games, perhaps the title of an earlier review “Degree of Difficulty 9.5, Style 10.0” [261] reflects what we know so far about recoding—even though much of even this type of decoding versatility remains to be discovered.
Author Contributions
J.F.A. drafted the text, P.R.B. generated Figure 6, all authors participated in generating the other components and finalization of the MS. All authors have read and agreed to the published version of the manuscript.
Funding
Our work is supported by Irish Research Council Advanced Laureate Award IRCLA/2019/74 to J.F.A. who thanks Prof. G.F. Fitzgerald for ‘bridge’ funds from the Carbery group.
Acknowledgments
Warm thanks to Ray Gesteland for great and extensive interactions at an earlier period and to P.V. Baranov for ongoing invaluable exchanges and collaborations. We are especially grateful to Nenad Ban and Jody Puglisi for collaborative opportunities on biophysical analyses of frameshifting. Interactions with graphic artist Kerry Matz in 2004 for recoding illustrations including those shown in Figure 1A, and in 2012 with the late Charles Jencks about the decoding seat (Figure 1B) and other components of the ‘What is Life’ sculpture, were stimulating and highly appreciated. We are indebted to Steve Oroszlan’s colleagues, Dolph Hatfield for earlier collaborative tRNA work plus a Nirenberg symposium and Alan Rein for the invitation to contribute this review. We also dedicate this review to the memory of collaborator and friend, Brookhaven virologist, Carl W. Anderson. The authors apologize to the numerous scientists whose relevant work could not be cited for space reasons.
Conflicts of Interest
The authors declare no conflict of interest or ethical issues.
References
- Gesteland, R.F.; Weiss, R.B.; Atkins, J.F. Recoding: Reprogrammed Genetic Decoding. Science 1992, 257, 1640–1641. [Google Scholar] [CrossRef]
- Atkins, J.F.; Gesteland, R.F. Recoding: Expansion of Decoding Rules Enriches Gene Expression; Springer: New York, NY, USA, 2010; p. 466. [Google Scholar]
- Choi, J.; Grosely, R.; Prabhakar, A.; Lapointe, C.P.; Wang, J.; Puglisi, J.D. How Messenger RNA and Nascent Chain Sequences Regulate Translation Elongation. Annu. Rev. Biochem. 2018, 87, 421–449. [Google Scholar] [CrossRef]
- Rodnina, M.V.; Korniy, N.; Klimova, M.; Karki, P.; Peng, B.Z.; Senyushkina, T.; Belardinelli, R.; Maracci, C.; Wohlgemuth, I.; Samatova, E.; et al. Translational Recoding: Canonical Translation Mechanisms Reinterpreted. Nucleic Acids Res. 2020, 48, 1056–1067. [Google Scholar] [CrossRef] [Green Version]
- Kwun, H.J.; Toptan, T.; Da Silva, S.R.; Atkins, J.F.; Moore, P.S.; Chang, Y. Human DNA Tumor Viruses Generate Alternative Reading Frame Proteins through Repeat Sequence Recoding. Proc. Natl. Acad. Sci. USA 2014, 111, E4342–E4349. [Google Scholar] [CrossRef] [Green Version]
- Atkins, J.F.; Loughran, G.; Bhatt, P.R.; Firth, A.E.; Baranov, P.V. Ribosomal Frameshifting and Transcriptional Slippage: From Genetic Steganography and Cryptography to Adventitious Use. Nucleic Acids Res. 2016, 44, 7007–7078. [Google Scholar] [CrossRef] [Green Version]
- Weiner, A.M.; Weber, K. A Single UGA Codon Functions as a Natural Termination Signal in the Coliphage Qβ Coat Protein Cistron. J. Mol. Biol. 1973, 80, 837–855. [Google Scholar] [CrossRef]
- Barrell, B.G.; Air, G.M.; Hutchison, C.A. Overlapping Genes in Bacteriophage ΦX174. Nature 1976, 264, 34–41. [Google Scholar] [CrossRef]
- Atkins, J.F.; Steitz, J.A.; Anderson, C.W.; Model, P. Binding of Mammalian Ribosomes to MS2 Phage RNA Reveals an Overlapping Gene Encoding a Lysis Function. Cell 1979, 18, 247–256. [Google Scholar] [CrossRef]
- Gamarnik, A.V.; Andino, R. Switch from Translation to RNA Replication in a Positive-Stranded RNA Virus. Genes Dev. 1998, 12, 2293–2304. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miller, W.A.; Jackson, J.; Feng, Y. Cis- and Trans-Regulation of Luteovirus Gene Expression by the 3′ End of the Viral Genome. Virus Res. 2015, 206, 37–45. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiang, H.; Franz, C.J.; Wu, G.; Renshaw, H.; Zhao, G.; Firth, A.E.; Wang, D. Orsay Virus Utilizes Ribosomal Frameshifting to Express a Novel Protein That Is Incorporated into Virions. Virology 2014, 450, 213–221. [Google Scholar] [CrossRef] [Green Version]
- Molteni, C.G.; Principi, N.; Esposito, S. Reactive Oxygen and Nitrogen Species during Viral Infections. Free Radic. Res. 2014, 48, 1163–1169. [Google Scholar] [CrossRef]
- Khomich, O.A.; Kochetkov, S.N.; Bartosch, B.; Ivanov, A.V. Redox Biology of Respiratory Viral Infections. Viruses 2018, 10, 392. [Google Scholar] [CrossRef] [Green Version]
- Guillin, O.M.; Vindry, C.; Ohlmann, T.; Chavatte, L. Selenium, Selenoproteins and Viral Infection. Nutrients 2019, 11, 2101. [Google Scholar] [CrossRef] [Green Version]
- Brigelius-Flohé, R.; Flohé, L. Regulatory Phenomena in the Glutathione Peroxidase Superfamily. Antioxid. Redox Signal. 2020, 33, 498–516. [Google Scholar] [CrossRef]
- Jiang, X.; Stockwell, B.R.; Conrad, M. Ferroptosis: Mechanisms, Biology and Role in Disease. Nat. Rev. Mol. Cell Biol. 2021, 22, 266–282. [Google Scholar] [CrossRef] [PubMed]
- Avery, J.C.; Hoffmann, P.R. Selenium, Selenoproteins, and Immunity. Nutrients 2018, 10, 1203. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Korwar, A.M.; Hossain, A.; Lee, T.-J.; Shay, A.E.; Basrur, V.; Conlon, K.; Smith, P.B.; Carlson, B.A.; Salis, H.M.; Patterson, A.D.; et al. Selenium-Dependent Metabolic Reprogramming during Inflammation and Resolution. J. Biol. Chem. 2021, 296, 100410. [Google Scholar] [CrossRef]
- Sheridan, P.A.; Zhong, N.; Carlson, B.A.; Perella, C.M.; Hatfield, D.L.; Beck, M.A. Decreased Selenoprotein Expression Alters the Immune Response during Influenza Virus Infection in Mice. J. Nutr. 2007, 137, 1466–1471. [Google Scholar] [CrossRef] [PubMed]
- Shisler, J.L.; Senkevich, T.G.; Berry, M.J.; Moss, B. Ultraviolet-Induced Cell Death Blocked by a Selenoprotein from a Human Dermatotropic Poxvirus. Science 1998, 279, 102–105. [Google Scholar] [CrossRef] [PubMed]
- Mix, H.; Lobanov, A.V.; Gladyshev, V.N. SECIS Elements in the Coding Regions of Selenoprotein Transcripts Are Functional in Higher Eukaryotes. Nucleic Acids Res. 2007, 35, 414–423. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McFadden, G. Even Viruses Can Learn to Cope with Stress. Science 1998, 279, 40. [Google Scholar] [CrossRef] [PubMed]
- Fradejas-Villar, N.; Seeher, S.; Anderson, C.B.; Doengi, M.; Carlson, B.A.; Hatfield, D.L.; Schweizer, U.; Howard, M.T. The RNA-Binding Protein Secisbp2 Differentially Modulates UGA Codon Reassignment and RNA Decay. Nucleic Acids Res. 2017, 45, 4094–4107. [Google Scholar] [CrossRef] [Green Version]
- Lin, H.C.; Ho, S.C.; Chen, Y.Y.; Khoo, K.H.; Hsu, P.H.; Yen, H.C.S. CRL2 Aids Elimination of Truncated Selenoproteins Produced by Failed UGA/Sec Decoding. Science 2015, 349, 91–95. [Google Scholar] [CrossRef] [Green Version]
- Baclaocos, J.; Santesmasses, D.; Mariotti, M.; Bierła, K.; Vetick, M.B.; Lynch, S.; McAllen, R.; Mackrill, J.J.; Loughran, G.; Guigó, R.; et al. Processive Recoding and Metazoan Evolution of Selenoprotein P: Up to 132 UGAs in Molluscs. J. Mol. Biol. 2019, 431, 4381–4407. [Google Scholar] [CrossRef]
- Santesmasses, D.; Mariotti, M.; Gladyshev, V.N. Bioinformatics of Selenoproteins. Antioxid. Redox Signal. 2020, 33, 525–536. [Google Scholar] [CrossRef]
- Berry, M.J.; Banu, L.; Chen, Y.; Mandel, S.J.; Kieffer, J.D.; Harney, J.W.; Larsen, P.R. Recognition of UGA as a Selenocysteine Codon in Type I Deiodinase Requires Sequences in the 3′ Untranslated Region. Nature 1991, 353, 273–276. [Google Scholar] [CrossRef]
- Wu, S.; Mariotti, M.; Santesmasses, D.; Hill, K.E.; Baclaocos, J.; Aparicio-Prat, E.; Li, S.; Mackrill, J.; Wu, Y.; Howard, M.T.; et al. Human Selenoprotein P and S Variant mRNAs with Different Numbers of SECIS Elements and Inferences from Mutant Mice of the Roles of Multiple SECIS Elements. Open Biol. 2016, 6, 160241. [Google Scholar] [CrossRef] [PubMed]
- Budiman, M.E.; Bubenik, J.L.; Miniard, A.C.; Middleton, L.M.; Gerber, C.A.; Cash, A.; Driscoll, D.M. Eukaryotic Initiation Factor 4a3 Is a Selenium-Regulated RNA-Binding Protein That Selectively Inhibits Selenocysteine Incorporation. Mol. Cell 2009, 35, 479–489. [Google Scholar] [CrossRef] [Green Version]
- Howard, M.T.; Copeland, P.R. New Directions for Understanding the Codon Redefinition Required for Selenocysteine Incorporation. Biol. Trace Elem. Res. 2019, 192, 18–25. [Google Scholar] [CrossRef]
- Jedrychowski, M.P.; Lu, G.Z.; Szpyt, J.; Mariotti, M.; Garrity, R.; Paulo, J.A.; Schweppe, D.K.; Laznik-Bogoslavski, D.; Kazak, L.; Murphy, M.P.; et al. Facultative Protein Selenation Regulates Redox Sensitivity, Adipose Tissue Thermogenesis, and Obesity. Proc. Natl. Acad. Sci. USA 2020, 117, 10789–10796. [Google Scholar] [CrossRef]
- Firth, A.E.; Wills, N.M.; Gesteland, R.F.; Atkins, J.F. Stimulation of Stop Codon Readthrough: Frequent Presence of an Extended 3′ RNA Structural Element. Nucleic Acids Res. 2011, 39, 6679–6691. [Google Scholar] [CrossRef] [Green Version]
- Brown, A.; Shao, S.; Murray, J.; Hegde, R.S.; Ramakrishnan, V. Structural Basis for Stop Codon Recognition in Eukaryotes. Nature 2015, 524, 493–496. [Google Scholar] [CrossRef]
- Matheisl, S.; Berninghausen, O.; Becker, T.; Beckmann, R. Structure of a Human Translation Termination Complex. Nucleic Acids Res. 2015, 43, 8615–8626. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Philipson, L.; Andersson, P.; Olshevsky, U.; Weinberg, R.; Baltimore, D.; Gesteland, R. Translation of MuLV and MSV RNAs in Nuclease-Treated Reticulocyte Extracts: Enhancement of the gag-pol Polypeptide with Yeast Suppressor tRNA. Cell 1978, 13, 189–199. [Google Scholar] [CrossRef]
- Yoshinaka, Y.; Katoh, I.; Copeland, T.D.; Oroszlan, S. Murine Leukemia Virus Protease Is Encoded by the gag-pol Gene and Is Synthesized through Suppression of an Amber Termination Codon. Proc. Natl. Acad. Sci. USA 1985, 82, 1618–1622. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Feng, Y.X.; Hatfield, D.L.; Rein, A.; Levin, J.G. Translational Readthrough of the Murine Leukemia Virus gag Gene Amber Codon Does Not Require Virus-Induced Alteration of tRNA. J. Virol. 1989, 63, 2405–2410. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wills, N.M.; Gesteland, R.F.; Atkins, J.F. Evidence That a Downstream Pseudoknot Is Required for Translational Read-through of the Moloney Murine Leukemia Virus gag Stop Codon. Proc. Natl. Acad. Sci. USA 1991, 88, 6991–6995. [Google Scholar] [CrossRef] [Green Version]
- Wills, N.M.; Gesteland, R.F.; Atkins, J.F. Pseudoknot-Dependent Read-through of Retroviral gag Termination Codons: Importance of Sequences in the Spacer and Loop 2. EMBO J. 1994, 13, 4137–4144. [Google Scholar] [CrossRef]
- Feng, Y.X.; Yuan, H.; Rein, A.; Levin, J.G. Bipartite Signal for Read-through Suppression in Murine Leukemia Virus mRNA: An Eight-Nucleotide Purine-Rich Sequence Immediately Downstream of the gag Termination Codon Followed by an RNA Pseudoknot. J. Virol. 1992, 66, 5127–5132. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Felsenstein, K.M.; Goff, S.P. Mutational Analysis of the gag-pol Junction of Moloney Murine Leukemia Virus: Requirements for Expression of the gag-pol Fusion Protein. J. Virol. 1992, 66, 6601–6608. [Google Scholar] [CrossRef] [Green Version]
- Alam, S.L.; Wills, N.M.; Ingram, J.A.; Atkins, J.F.; Gesteland, R.F. Structural Studies of the RNA Pseudoknot Required for Readthrough of the gag-Termination Codon of Murine Leukemia Virus. J. Mol. Biol. 1999, 288, 837–852. [Google Scholar] [CrossRef]
- Houck-Loomis, B.; Durney, M.A.; Salguero, C.; Shankar, N.; Nagle, J.M.; Goff, S.P.; D’Souza, V.M. An Equilibrium-Dependent Retroviral mRNA Switch Regulates Translational Recoding. Nature 2011, 480, 561–564. [Google Scholar] [CrossRef]
- Orlova, M.; Yueh, A.; Leung, J.; Goff, S.P. Reverse Transcriptase of Moloney Murine Leukemia Virus Binds to Eukaryotic Release Factor 1 to Modulate Suppression of Translational Termination. Cell 2003, 115, 319–331. [Google Scholar] [CrossRef] [Green Version]
- Tang, X.; Zhu, Y.; Baker, S.L.; Bowler, M.W.; Chen, B.J.; Chen, C.; Hogg, J.R.; Goff, S.P.; Song, H. Structural Basis of Suppression of Host Translation Termination by Moloney Murine Leukemia Virus. Nat. Commun. 2016, 7, 12070. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Irigoyen, N.; Dinan, A.M.; Brierley, I.; Firth, A.E. Ribosome Profiling of the Retrovirus Murine Leukemia Virus. Retrovirology 2018, 15, 10. [Google Scholar] [CrossRef] [Green Version]
- Lyon, K.; Aguilera, L.U.; Morisaki, T.; Munsky, B.; Stasevich, T.J. Live-Cell Single RNA Imaging Reveals Bursts of Translational Frameshifting. Mol. Cell 2019, 75, 172–183.e9. [Google Scholar] [CrossRef]
- Jacks, T.; Varmus, H.E. Expression of the Rous Sarcoma Virus pol Gene by Ribosomal Frameshifting. Science 1985, 230, 1237–1242. [Google Scholar] [CrossRef]
- Jacks, T.; Townsley, K.; Varmus, H.E.; Majors, J. Two Efficient Ribosomal Frameshifting Events Are Required for Synthesis of Mouse Mammary Tumor Virus gag-Related Polyproteins. Proc. Natl. Acad. Sci. USA 1987, 84, 4298–4302. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jacks, T.; Power, M.D.; Masiarz, F.R.; Luciw, P.A.; Barr, P.J.; Varmus, H.E. Characterization of Ribosomal Frameshifting in HIV-1 gag-pol Expression. Nature 1988, 331, 280–283. [Google Scholar] [CrossRef] [PubMed]
- Jacks, T.; Madhani, H.D.; Masiarz, F.R.; Varmus, H.E. Signals for Ribosomal Frameshifting in the Rous Sarcoma Virus gag-pol Region. Cell 1988, 55, 447–458. [Google Scholar] [CrossRef]
- Hizi, A.; Henderson, L.E.; Copeland, T.D.; Sowder, R.C.; Hixson, C.V.; Oroszlan, S. Characterization of Mouse Mammary Tumor Virus gag-pro Gene Products and the Ribosomal Frameshift Site by Protein Sequencing. Proc. Natl. Acad. Sci. USA 1987, 84, 7041–7045. [Google Scholar] [CrossRef] [Green Version]
- Yoshinaka, Y.; Katoh, I.; Copeland, T.D.; Smythers, G.W.; Oroszlan, S. Bovine Leukemia Virus Protease: Purification, Chemical Analysis, and in Vitro Processing of gag Precursor Polyproteins. J. Virol. 1986, 57, 826–832. [Google Scholar] [CrossRef] [Green Version]
- Marczinke, B.; Fisher, R.; Vidakovic, M.; Bloys, A.J.; Brierley, I. Secondary Structure and Mutational Analysis of the Ribosomal Frameshift Signal of Rous Sarcoma Virus. J. Mol. Biol. 1998, 284, 205–225. [Google Scholar] [CrossRef] [PubMed]
- Weiss, R.B.; Dunn, D.M.; Shuh, M.; Atkins, J.F.; Gesteland, R.F. E. Coli Ribosomes Re-Phase on Retroviral Frameshift Signals at Rates Ranging from 2 to 50 Percent. New Biol. 1989, 1, 159–169. [Google Scholar]
- Yelverton, E.; Lindsley, D.; Yamauchi, P.; Gallant, J.A. The Function of a Ribosomal Frameshifting Signal from Human Immunodeficiency Virus-1 in Escherichia coli. Mol. Microbiol. 1994, 11, 303–313. [Google Scholar] [CrossRef]
- Korniy, N.; Goyal, A.; Hoffmann, M.; Samatova, E.; Peske, F.; Pöhlmann, S.; Rodnina, M.V. Modulation of HIV-1 gag/gag-pol Frameshifting by tRNA Abundance. Nucleic Acids Res. 2019, 47, 5210–5222. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Korniy, N.; Samatova, E.; Anokhina, M.M.; Peske, F.; Rodnina, M.V. Mechanisms and Biomedical Implications of –1 Programmed Ribosome Frameshifting on Viral and Bacterial mRNAs. FEBS Lett. 2019, 593, 1468–1482. [Google Scholar] [CrossRef]
- Charbonneau, J.; Gendron, K.; Ferbeyre, G.; Brakier-Gingras, L. The 5′ UTR of HIV-1 Full-Length mRNA and the Tat Viral Protein Modulate the Programmed -1 Ribosomal Frameshift That Generates HIV-1 Enzymes. RNA 2012, 18, 519–529. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Garcia-Miranda, P.; Becker, J.T.; Benner, B.E.; Blume, A.; Sherer, N.M.; Butcher, S.E. Stability of HIV Frameshift Site RNA Correlates with Frameshift Efficiency and Decreased Virus Infectivity. J. Virol. 2016, 90, 6906–6917. [Google Scholar] [CrossRef] [Green Version]
- Penno, C.; Kumari, R.; Baranov, P.V.; Van Sinderen, D.; Atkins, J.F. Specific Reverse Transcriptase Slippage at the HIV Ribosomal Frameshift Sequence: Potential Implications for Modulation of GagPol Synthesis. Nucleic Acids Res. 2017, 45, 10156–10167. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Penno, C.; Sharma, V.; Coakley, A.; Motherway, M.O.C.; Van Sinderen, D.; Lubkowska, L.; Kireeva, M.L.; Kashlev, M.; Baranov, P.V.; Atkins, J.F. Productive mRNA Stem Loop-Mediated Transcriptional Slippage: Crucial Features in Common with Intrinsic Terminators. Proc. Natl. Acad. Sci. USA 2015, 112, 1984–1993. [Google Scholar] [CrossRef] [Green Version]
- Chung, B.Y.W.; Miller, W.A.; Atkins, J.F.; Firth, A.E. An Overlapping Essential Gene in the Potyviridae. Proc. Natl. Acad. Sci. USA 2008, 105, 5897–5902. [Google Scholar] [CrossRef] [Green Version]
- Olspert, A.; Chung, B.Y.; Atkins, J.F.; Carr, J.P.; Firth, A.E. Transcriptional Slippage in the Positive-sense RNA Virus Family Potyviridae. EMBO Rep. 2015, 16, 995–1004. [Google Scholar] [CrossRef] [PubMed]
- Rodamilans, B.; Valli, A.; Mingot, A.; San León, D.; Baulcombe, D.; López-Moya, J.J.; García, J.A. RNA Polymerase Slippage as a Mechanism for the Production of Frameshift Gene Products in Plant Viruses of the Potyviridae Family. J. Virol. 2015, 89, 6965–6967. [Google Scholar] [CrossRef] [Green Version]
- Weiss, R.; Gallant, J. Mechanism of Ribosome Frameshifting during Translation of the Genetic Code. Nature 1983, 302, 389–393. [Google Scholar] [CrossRef] [PubMed]
- Atkins, J.F.; Nichols, B.P.; Thompson, S. The Nucleotide Sequence of the First Externally Suppressible-1 Frameshift Mutant, and of Some Nearby Leaky Frameshift Mutants. EMBO J. 1983, 2, 1345–1350. [Google Scholar] [CrossRef]
- Weiss, R.B.; Dunn, D.M.; Atkins, J.F.; Gesteland, R.F. Slippery Runs, Shifty Stops, Backward Steps, and Forward Hops: -2, -1, +1, +2, +5, and +6 Ribosomal Frameshifting. Cold Spring Harb. Symp. Quant. Biol. 1987, 52, 687–693. [Google Scholar] [CrossRef]
- Dunn, J.J.; Studier, F.W.; Gottesman, M. Complete Nucleotide Sequence of Bacteriophage T7 DNA and the Locations of T7 Genetic Elements. J. Mol. Biol. 1983, 166, 477–535. [Google Scholar] [CrossRef]
- Condron, B.G.; Gesteland, R.F.; Atkins, J.F. An Analysis of Sequences Stimulating Frameshifting in the Decoding of Gene 10 of Bacteriophage T7. Nucleic Acids Res. 1991, 19, 5607–5612. [Google Scholar] [CrossRef]
- Atkins, J.F.; Gesteland, R.F.; Reid, B.R.; Anderson, C.W. Normal tRNAs Promote Ribosomal Frameshifting. Cell 1979, 18, 1119–1131. [Google Scholar] [CrossRef]
- Dayhuff, T.J.; Atkins, J.F.; Gesteland, R.F. Characterization of Ribosomal Frameshift Events by Protein Sequence Analysis. J. Biol. Chem. 1986, 261, 7491–7500. [Google Scholar] [CrossRef]
- Atkins, J.F.; Herr, A.J.; Massire, C.; O’Connor, M.; Ivanov, I.; Gesteland, R.F. Poking a Hole in the Sanctity of the Triplet Code: Inferences for Framing. In The Ribosome. Structure, Function, Antibiotics and Cellular Interactions; Garret, R.A., Douthwaite, S.R., Liljas, A., Matheson, A.T., Moore, P.B., Noller, H.F., Eds.; ASM Press: Washington, DC, USA, 2000; pp. 367–383. [Google Scholar]
- Caulfield, T.; Coban, M.; Tek, A.; Flores, S.C. Molecular Dynamics Simulations Suggest a Non-Doublet Decoding Model of –1 Frameshifting by tRNAser3. Biomolecules 2019, 9, 745. [Google Scholar] [CrossRef] [Green Version]
- Brierley, I.; Boursnell, M.E.; Binns, M.M.; Bilimoria, B.; Blok, V.C.; Brown, T.D.; Inglis, S.C. An Efficient Ribosomal Frame-Shifting Signal in the Polymerase-Encoding Region of the Coronavirus IBV. EMBO J. 1987, 6, 3779–3785. [Google Scholar] [CrossRef] [PubMed]
- Brierley, I.; Jenner, A.J.; Inglis, S.C. Mutational Analysis of the “Slippery-Sequence” Component of a Coronavirus Ribosomal Frameshifting Signal. J. Mol. Biol. 1992, 227, 463–479. [Google Scholar] [CrossRef]
- Dinan, A.M.; Keep, S.; Bickerton, E.; Britton, P.; Firth, A.E.; Brierley, I. Comparative Analysis of Gene Expression in Virulent and Attenuated Strains of Infectious Bronchitis Virus at Subcodon Resolution. J. Virol. 2019, 93, 714–719. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Licznar, P.; Mejlhede, N.; Prère, M.F.; Wills, N.; Gesteland, R.F.; Atkins, J.F.; Fayet, O. Programmed Translational -1 Frameshifting on Hexanucleotide Motifs and the Wobble Properties of tRNAs. EMBO J. 2003, 22, 4770–4778. [Google Scholar] [CrossRef] [Green Version]
- Pleij, C.W.A.; Rietveld, K.; Bosch, L. A New Principle of RNA Folding Based on Pseudoknotting. Nucleic Acids Res. 1985, 13, 1717–1731. [Google Scholar] [CrossRef]
- ten Dam, E.B.; Pleij, C.W.A.; Bosch, L. RNA Pseudoknots: Translational Frameshifting and Readthrough on Viral RNAs. Virus Genes 1990, 4, 121–136. [Google Scholar] [CrossRef] [PubMed]
- Brierley, I.; Digard, P.; Inglis, S.C. Characterization of an Efficient Coronavirus Ribosomal Frameshifting Signal: Requirement for an RNA Pseudoknot. Cell 1989, 57, 537–547. [Google Scholar] [CrossRef]
- Brierley, I.; Rolley, N.J.; Jenner, A.J.; Inglis, S.C. Mutational Analysis of the RNA Pseudoknot Component of a Coronavirus Ribosomal Frameshifting Signal. J. Mol. Biol. 1991, 220, 889–902. [Google Scholar] [CrossRef]
- Napthine, S.; Liphardt, J.; Bloys, A.; Routledge, S.; Brierley, I. The Role of RNA Pseudoknot Stem 1 Length in the Promotion of Efficient -1 Ribosomal Frameshifting. J. Mol. Biol. 1999, 288, 305–320. [Google Scholar] [CrossRef]
- Jungreis, I.; Sealfon, R.; Kellis, M. SARS-CoV-2 Gene Content and COVID-19 Mutation Impact by Comparing 44 Sarbecovirus Genomes. Nat. Commun. 2021, 12, 2642. [Google Scholar] [CrossRef] [PubMed]
- Ziv, O.; Price, J.; Shalamova, L.; Kamenova, T.; Goodfellow, I.; Weber, F.; Miska, E.A. The Short- and Long-Range RNA-RNA Interactome of SARS-CoV-2. Mol. Cell 2020, 80, 1067–1077. [Google Scholar] [CrossRef] [PubMed]
- Baranov, P.V.; Henderson, C.M.; Anderson, C.B.; Gesteland, R.F.; Atkins, J.F.; Howard, M.T. Programmed Ribosomal Frameshifting in Decoding the SARS-CoV Genome. Virology 2005, 332, 498–510. [Google Scholar] [CrossRef] [Green Version]
- Plant, E.P.; Pérez-Alvarado, G.C.; Jacobs, J.L.; Mukhopadhyay, B.; Hennig, M.; Dinman, J.D. A Three-Stemmed mRNA Pseudoknot in the SARS Coronavirus Frameshift Signal. PLoS Biol. 2005, 3, 1012–1023. [Google Scholar] [CrossRef] [Green Version]
- Su, M.C.; Chang, C.T.; Chu, C.H.; Tsai, C.H.; Chang, K.Y. An Atypical RNA Pseudoknot Stimulator and an Upstream Attenuation Signal for -1 Ribosomal Frameshifting of SARS Coronavirus. Nucleic Acids Res. 2005, 33, 4265–4275. [Google Scholar] [CrossRef] [Green Version]
- Dos Ramos, F.; Carrasco, M.; Doyle, T.; Brierley, I. Programmed -1 Ribosomal Frameshifting in the SARS Coronavirus. Biochem Soc. Trans. 2004, 32, 1081–1083. [Google Scholar] [CrossRef] [Green Version]
- Ishimaru, D.; Plant, E.P.; Sims, A.C.; Yount, B.L.; Roth, B.M.; Eldho, N.V.; Pérez-Alvarado, G.C.; Armbruster, D.W.; Baric, R.S.; Dinman, J.D.; et al. RNA Dimerization Plays a Role in Ribosomal Frameshifting of the SARS Coronavirus. Nucleic Acids Res. 2013, 41, 2594–2608. [Google Scholar] [CrossRef]
- Plant, E.P.; Sims, A.C.; Baric, R.S.; Dinman, J.D.; Taylor, D.R. Altering SARS Coronavirus Frameshift Efficiency Affects Genomic and Subgenomic RNA Production. Viruses 2013, 5, 279–294. [Google Scholar] [CrossRef] [Green Version]
- Shehu-Xhilaga, M.; Crowe, S.M.; Mak, J. Maintenance of the Gag/Gag-Pol Ratio Is Important for Human Immunodeficiency Virus Type 1 RNA Dimerization and Viral Infectivity. J. Virol. 2001, 75, 1834–1841. [Google Scholar] [CrossRef] [Green Version]
- Suzuki, Y.; Chin, W.X.; Han, Q.; Ichiyama, K.; Lee, C.H.; Eyo, Z.W.; Ebina, H.; Takahashi, H.; Takahashi, C.; Tan, B.H.; et al. Characterization of RyDEN (C19orf66) as an Interferon-Stimulated Cellular Inhibitor against Dengue Virus Replication. PLoS Pathog. 2016, 12, e1005357. [Google Scholar] [CrossRef] [Green Version]
- Wu, Y.; Yang, X.; Yao, Z.; Dong, X.; Zhang, D.; Hu, Y.; Zhang, S.; Lin, J.; Chen, J.; An, S.; et al. C19orf66 Interrupts Zika Virus Replication by Inducing Lysosomal Degradation of Viral NS3. PLoS Negl. Trop. Dis. 2020, 14, e0008083. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kinast, V.; Plociennikowska, A.; Anggakusuma; Bracht, T.; Todt, D.; Brown, R.J.P.; Boldanova, T.; Zhang, Y.; Brüggemann, Y.; Friesland, M.; et al. C19orf66 Is an Interferon-Induced Inhibitor of HCV Replication That Restricts Formation of the Viral Replication Organelle. J. Hepatol. 2020, 73, 549–558. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Xuan, Y.; Han, Y.; Ding, X.; Ye, K.; Yang, F.; Gao, P.; Goff, S.P.; Gao, G. Regulation of HIV-1 Gag-Pol Expression by Shiftless, an Inhibitor of Programmed -1 Ribosomal Frameshifting. Cell 2019, 176, 625–635.e14. [Google Scholar] [CrossRef] [Green Version]
- Bhatt, P.R.; Scaiola, A.; Loughran, G.; Leibundgut, M.; Kratzel, A.; Meurs, R.; Dreos, R.; O’Connor, K.M.; McMillan, A.; Bode, J.W.; et al. Structural Basis of Ribosomal Frameshifting during Translation of the SARS-CoV-2 RNA Genome. Science 2021, 372, 1306–1313. [Google Scholar] [CrossRef] [PubMed]
- Baranov, P.V.; Atkins, J.F.; Yordanova, M.M. Augmented Genetic Decoding: Global, Local and Temporal Alterations of Decoding Processes and Codon Meaning. Nat. Rev. Genet. 2015, 16, 517–529. [Google Scholar] [CrossRef]
- Meydan, S.; Klepacki, D.; Karthikeyan, S.; Margus, T.; Thomas, P.; Jones, J.E.; Khan, Y.; Briggs, J.; Dinman, J.D.; Vázquez-Laslop, N.; et al. Programmed Ribosomal Frameshifting Generates a Copper Transporter and a Copper Chaperone from the Same Gene. Mol. Cell 2017, 65, 207–219. [Google Scholar] [CrossRef] [Green Version]
- Jagger, B.W.; Wise, H.M.; Kash, J.C.; Walters, K.A.; Wills, N.M.; Xiao, Y.L.; Dunfee, R.L.; Schwartzman, L.M.; Ozinsky, A.; Bell, G.L.; et al. An Overlapping Protein-Coding Region in Influenza A Virus Segment 3 Modulates the Host Response. Science 2012, 337, 199–204. [Google Scholar] [CrossRef] [Green Version]
- Firth, A.E.; Jagger, B.W.; Wise, H.M.; Nelson, C.C.; Parsawar, K.; Wills, N.M.; Napthine, S.; Taubenberger, J.K.; Digard, P.; Atkins, J.F. Ribosomal Frameshifting Used in Influenza A Virus Expression Occurs within the Sequence UCC-UUU-CGU and Is in the +1 Direction. Open Biol. 2012, 2, 120109. [Google Scholar] [CrossRef] [Green Version]
- Hayashi, T.; MacDonald, L.A.; Takimoto, T. Influenza A Virus Protein PA-X Contributes to Viral Growth and Suppression of the Host Antiviral and Immune Responses. J. Virol. 2015, 89, 6442–6452. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oishi, K.; Yamayoshi, S.; Kawaoka, Y. Identification of Amino Acid Residues in Influenza A Virus PA-X That Contribute to Enhanced Shutoff Activity. Front. Microbiol. 2019, 10, 432. [Google Scholar] [CrossRef] [Green Version]
- Oishi, K.; Yamayoshi, S.; Kawaoka, Y. Mapping of a Region of the PA-X Protein of Influenza A Virus That Is Important for Its Shutoff Activity. J. Virol. 2015, 89, 8661–8665. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dinan, A.M.; Atkins, J.F.; Firth, A.E. ASXL Gain-of-Function Truncation Mutants: Defective and Dysregulated Forms of a Natural Ribosomal Frameshifting Product? Biol. Direct 2017, 12, 24. [Google Scholar] [CrossRef]
- Bock, L.V.; Caliskan, N.; Korniy, N.; Peske, F.; Rodnina, M.V.; Grubmüller, H. Thermodynamic Control of −1 Programmed Ribosomal Frameshifting. Nat. Commun. 2019, 10, 4598. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.; O’Loughlin, S.; Atkins, J.F.; Puglisi, J.D. The Energy Landscape of −1 Ribosomal Frameshifting. Sci. Adv. 2020, 6, eaax6969. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Firth, A.E.; Atkins, J.F. A Conserved Predicted Pseudoknot in the NS2A-Encoding Sequence of West Nile and Japanese Encephalitis Flaviviruses Suggests NS1′ May Derive from Ribosomal Frameshifting. Virol. J. 2009, 6, 14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Melian, E.B.; Hinzman, E.; Nagasaki, T.; Firth, A.E.; Wills, N.M.; Nouwens, A.S.; Blitvich, B.J.; Leung, J.; Funk, A.; Atkins, J.F.; et al. NS1′ of Flaviviruses in the Japanese Encephalitis Virus Serogroup Is a Product of Ribosomal Frameshifting and Plays a Role in Viral Neuroinvasiveness. J. Virol. 2010, 84, 1641–1647. [Google Scholar] [CrossRef] [Green Version]
- Zhou, D.; Li, Q.; Jia, F.; Zhang, L.; Wan, S.; Li, Y.; Song, Y.; Chen, H.; Cao, S.; Ye, J. The Japanese Encephalitis Virus NS1′ Protein Inhibits Type I IFN Production by Targeting MAVS. J. Immunol. 2020, 204, 1287–1298. [Google Scholar] [CrossRef]
- Sun, J.; Yu, Y.; Deubel, V. Japanese Encephalitis Virus NS1′ Protein Depends on Pseudoknot Secondary Structure and Is Cleaved by Caspase during Virus Infection and Cell Apoptosis. Microbes Infect. 2012, 14, 930–940. [Google Scholar] [CrossRef]
- Ye, Q.; Li, X.F.; Zhao, H.; Li, S.H.; Deng, Y.Q.; Cao, R.Y.; Song, K.Y.; Wang, H.J.; Hua, R.H.; Yu, Y.X.; et al. A Single Nucleotide Mutation in NS2A of Japanese Encephalitis-Live Vaccine Virus (SA14-14-2) Ablates NS1′ Formation and Contributes to Attenuation. J. Gen. Virol. 2012, 93, 1959–1964. [Google Scholar] [CrossRef]
- Wang, J.; Li, X.; Gu, J.; Fan, Y.; Zhao, P.; Cao, R.; Chen, P. The A66G Back Mutation in NS2A of JEV SA14-14-2 Strain Contributes to Production of NS1′ Protein and the Secreted NS1′ Can Be Used for Diagnostic Biomarker for Virulent Virus Infection. Infect. Genet. Evol. 2015, 36, 116–125. [Google Scholar] [CrossRef]
- Halma, M.T.J.; Ritchie, D.B.; Cappellano, T.R.; Neupane, K.; Woodside, M.T. Complex Dynamics under Tension in a High-Efficiency Frameshift Stimulatory Structure. Proc. Natl. Acad. Sci. USA 2019, 116, 19500–19505. [Google Scholar] [CrossRef] [PubMed]
- Firth, A.E.; Blitvich, B.J.; Wills, N.M.; Miller, C.L.; Atkins, J.F. Evidence for Ribosomal Frameshifting and a Novel Overlapping Gene in the Genomes of Insect-Specific Flaviviruses. Virology 2010, 399, 153–166. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- den Boon, J.A.; Snijder, E.J.; Chirnside, E.D.; de Vries, A.A.; Horzinek, M.C.; Spaan, W.J. Equine Arteritis Virus Is Not a Togavirus but Belongs to the Coronaviruslike Superfamily. J. Virol. 1991, 65, 2910–2920. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fang, Y.; Snijder, E.J. The PRRSV Replicase: Exploring the Multifunctionality of an Intriguing Set of Nonstructural Proteins. Virus Res. 2010, 154, 61–76. [Google Scholar] [CrossRef]
- Fang, Y.; Treffers, E.E.; Li, Y.; Tas, A.; Sun, Z.; Van Der Meer, Y.; De Ru, A.H.; Van Veelen, P.A.; Atkins, J.F.; Snijder, E.J.; et al. Efficient—2 Frameshifting by Mammalian Ribosomes to Synthesize an Additional Arterivirus Protein. Proc. Natl. Acad. Sci. USA 2012, 109, 2920–2928. [Google Scholar] [CrossRef] [Green Version]
- Guo, R.; Yan, X.; Li, Y.; Cui, J.; Misra, S.; Firth, A.E.; Snijder, E.J.; Fang, Y. A Swine Arterivirus Deubiquitinase Stabilizes Two Major Envelope Proteins and Promotes Production of Viral Progeny. PLoS Pathog. 2021, 17, e1009403. [Google Scholar] [CrossRef]
- Li, Y.; Treffers, E.E.; Napthine, S.; Tas, A.; Zhu, L.; Sun, Z.; Bell, S.; Mark, B.L.; Van Veelen, P.A.; Van Hemert, M.J.; et al. Transactivation of Programmed Ribosomal Frameshifting by a Viral Protein. Proc. Natl. Acad. Sci. USA 2014, 111, 2172–2181. [Google Scholar] [CrossRef] [Green Version]
- Napthine, S.; Treffers, E.E.; Bell, S.; Goodfellow, I.; Fang, Y.; Firth, A.E.; Snijder, E.J.; Brierley, I. A Novel Role for Poly(C) Binding Proteins in Programmed Ribosomal Frameshifting. Nucleic Acids Res. 2016, 44, 5491–5503. [Google Scholar] [CrossRef] [Green Version]
- Patel, A.; Treffers, E.E.; Meier, M.; Patel, T.R.; Stetefeld, J.; Snijder, E.J.; Mark, B.L. Molecular Characterization of the RNA-Protein Complex Directing 22/21 Programmed Ribosomal Frameshifting during Arterivirus Replicase Expression. J. Biol. Chem. 2020, 295, 17904–17921. [Google Scholar] [CrossRef]
- Loughran, G.; Firth, A.E.; Atkins, J.F. Ribosomal Frameshifting into an Overlapping Gene in the 2B-Encoding Region of the Cardiovirus Genome. Proc. Natl. Acad. Sci. USA 2011, 108, 1111–1119. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Finch, L.K.; Ling, R.; Napthine, S.; Olspert, A.; Michiels, T.; Lardinois, C.; Bell, S.; Loughran, G.; Brierley, I.; Firth, A.E. Characterization of Ribosomal Frameshifting in Theiler’s Murine Encephalomyelitis Virus. J. Virol. 2015, 89, 8580–8589. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Napthine, S.; Ling, R.; Finch, L.K.; Jones, J.D.; Bell, S.; Brierley, I.; Firth, A.E. Protein-Directed Ribosomal Frameshifting Temporally Regulates Gene Expression. Nat. Commun. 2017, 8, 15582. [Google Scholar] [CrossRef] [Green Version]
- Napthine, S.; Bell, S.; Hil, C.H.; Brierley, I.; Firt, A.E. Characterization of the Stimulators of Protein-Directed Ribosomal Frameshifting in Theiler’s Murine Encephalomyelitis Virus. Nucleic Acids Res. 2019, 47, 8207–8223. [Google Scholar] [CrossRef]
- Firth, A.E.; Chung, B.Y.W.; Fleeton, M.N.; Atkins, J.F. Discovery of Frameshifting in Alphavirus 6K Resolves a 20-Year Enigma. Virol. J. 2008, 5, 108. [Google Scholar] [CrossRef] [Green Version]
- Chung, B.Y.W.; Firth, A.E.; Atkins, J.F. Frameshifting in Alphaviruses: A Diversity of 3′ Stimulatory Structures. J. Mol. Biol. 2010, 397, 448–456. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.G.; Maas, S.; Rich, A. Comparative Mutational Analysis of Cis-Acting RNA Signals for Translational Frameshifting in HIV-1 and HTLV-2. Nucleic Acids Res. 2001, 29, 1125–1131. [Google Scholar] [CrossRef] [Green Version]
- Harrington, H.R.; Zimmer, M.H.; Chamness, L.M.; Nash, V.; Penn, W.D.; Miller, T.F.; Mukhopadhyay, S.; Schlebach, J.P. Cotranslational Folding Stimulates Programmed Ribosomal Frameshifting in the Alphavirus Structural Polyprotein. J. Biol. Chem. 2020, 295, 6798–6808. [Google Scholar] [CrossRef] [Green Version]
- Larsen, B.; Wills, N.M.; Gesteland, R.F.; Atkins, J.F. rRNA-mRNA Base Pairing Stimulates a Programmed -1 Ribosomal Frameshift. J. Bacteriol. 1994, 176, 6842–6851. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hu, H.T.; Cho, C.P.; Lin, Y.H.; Chang, K.Y. A General Strategy to Inhibiting Viral -1 Frameshifting Based on Upstream Attenuation Duplex Formation. Nucleic Acids Res. 2016, 44, 256–266. [Google Scholar] [CrossRef] [Green Version]
- Huang, W.P.; Cho, C.P.; Chang, K.Y. mRNA-Mediated Duplexes Play Dual Roles in the Regulation of Bidirectional Ribosomal Frameshifting. Int. J. Mol. Sci. 2018, 19, 3867. [Google Scholar] [CrossRef] [Green Version]
- Firth, A.E.; Wang, Q.S.; Jan, E.; Atkins, J.F. Bioinformatic Evidence for a Stem-Loop Structure 5′-Adjacent to the IGR-IRES and for an Overlapping Gene in the Bee Paralysis Dicistroviruses. Virol. J. 2009, 6, 193. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ren, Q.; Wang, Q.S.; Firth, A.E.; Chan, M.M.Y.; Gouw, J.W.; Guarna, M.M.; Foster, L.J.; Atkins, J.F.; Jan, E. Alternative Reading Frame Selection Mediated by a tRNA-like Domain of an Internal Ribosome Entry Site. Proc. Natl. Acad. Sci. USA 2012, 109, 630–639. [Google Scholar] [CrossRef] [Green Version]
- Kerr, C.H.; Wang, Q.S.; Moon, K.M.; Keatings, K.; Allan, D.W.; Foster, L.J.; Jan, E. IRES-Dependent Ribosome Repositioning Directs Translation of a +1 Overlapping ORF That Enhances Viral Infection. Nucleic Acids Res. 2018, 46, 11952–11967. [Google Scholar] [CrossRef] [Green Version]
- Donnelly, M.L.L.; Luke, G.; Mehrotra, A.; Li, X.; Hughes, L.E.; Gani, D.; Ryan, M.D. Analysis of the Aphthovirus 2A/2B Polyprotein “cleavage” Mechanism Indicates Not a Proteolytic Reaction, but a Novel Translational Effect: A Putative Ribosomal “Skip”. J. Gen. Virol. 2001, 82, 1013–1025. [Google Scholar] [CrossRef] [PubMed]
- Atkins, J.F.; Wills, N.M.; Loughran, G.; Wu, C.-Y.; Parsawar, K.; Ryan, M.D.; Wang, C.-H.; Nelson, C.C. A Case for “StopGo”: Reprogramming Translation to Augment Codon Meaning of GGN by Promoting Unconventional Termination (Stop) after Addition of Glycine and Then Allowing Continued Translation (Go). RNA 2007, 13, 803–810. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Doronina, V.A.; Wu, C.; de Felipe, P.; Sachs, M.S.; Ryan, M.D.; Brown, J.D. Site-Specific Release of Nascent Chains from Ribosomes at a Sense Codon. Mol. Cell. Biol. 2008, 28, 4227–4239. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brown, J.D.; Ryan, M.D. Ribosome “Skipping”: “Stop-Carry On” or “StopGo” Translation. In Recoding: Expansion of Decoding Rules Enriches Gene Expression; Atkins, J.F., Gesteland, R.F., Eds.; Springer: New York, NY, USA, 2010; pp. 101–121. [Google Scholar]
- Luke, G.A.; de Felipe, P.; Lukashev, A.; Kallioinen, S.E.; Bruno, E.A.; Ryan, M.D. Occurrence, Function and Evolutionary Origins of “2A-like” Sequences in Virus Genomes. J. Gen. Virol. 2008, 89, 1036–1042. [Google Scholar] [CrossRef]
- Loughran, G.; Libbey, J.E.; Uddowla, S.; Scallan, M.F.; Ryan, M.D.; Fujinami, R.S.; Rieder, E.; Atkins, J.F. Theiler’s Murine Encephalomyelitis Virus Contrasts with Encephalomyocarditis and Foot-and-Mouth Disease Viruses in Its Functional Utilization of the StopGo Non-Standard Translation Mechanism. J. Gen. Virol. 2013, 94, 348–353. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kjær, J.; Belsham, G.J. Modifications to the Foot-and-Mouth Disease Virus 2A Peptide: Influence on Polyprotein Processing and Virus Replication. J. Virol. 2018, 92, e02218-17. [Google Scholar] [CrossRef] [Green Version]
- Kjær, J.; Belsham, G.J. Selection of Functional 2A Sequences within Foot-and-Mouth Disease Virus; Requirements for the NPGP Motif with a Distinct Codon Bias. RNA 2018, 24, 12–17. [Google Scholar] [CrossRef] [Green Version]
- Luke, G.A.; Ryan, M.D. Therapeutic Applications of the ‘NPGP’ Family of Viral 2As. Rev. Med. Virol. 2018, 28, 2001. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Loughran, G.; Howard, M.T.; Firth, A.E.; Atkins, J.F. Avoidance of Reporter Assay Distortions from Fused Dual Reporters. Rna 2017, 23, 1285–1289. [Google Scholar] [CrossRef]
- Khan, Y.A.; Loughran, G.; Steckelberg, A.L.; Brown, K.; Kiniry, S.J.; Stewart, H.; Baranov, P.V.; Kieft, J.S.; Firth, A.E.; Atkins, J.F. Evaluating Programmed Frameshifting in CCR5 mRNA Decoding. Nature 2021, in press. [Google Scholar]
- Yu, F.H.; Huang, K.J.; Wang, C.T. HIV-1 Mutant Assembly, Processing and Infectivity Expresses Pol Independent of Gag. Viruses 2020, 12, 54. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Witte, O.N.; Baltimore, D. Relationship of Retrovirus Polyprotein Cleavages to Virion Maturation Studied with Temperature-Sensitive Murine Leukemia Virus Mutants. J. Virol. 1978, 26, 750–761. [Google Scholar] [CrossRef] [Green Version]
- Zhao, B.S.; He, C. Pseudouridine in a New Era of RNA Modifications. Cell Res. 2015, 25, 153–154. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pardi, N.; Tuyishime, S.; Muramatsu, H.; Kariko, K.; Mui, B.L.; Tam, Y.K.; Madden, T.D.; Hope, M.J.; Weissman, D. Expression Kinetics of Nucleoside-Modified mRNA Delivered in Lipid Nanoparticles to Mice by Various Routes. J. Control. Release 2015, 217, 345–351. [Google Scholar] [CrossRef] [Green Version]
- Mauger, D.M.; Joseph Cabral, B.; Presnyak, V.; Su, S.V.; Reid, D.W.; Goodman, B.; Link, K.; Khatwani, N.; Reynders, J.; Moore, M.J.; et al. mRNA Structure Regulates Protein Expression through Changes in Functional Half-Life. Proc. Natl. Acad. Sci. USA 2019, 116, 24075–24083. [Google Scholar] [CrossRef] [Green Version]
- Karikó, K. In Vitro-Transcribed mRNA Therapeutics: Out of the Shadows and Into the Spotlight. Mol. Ther. 2019, 27, 691–692. [Google Scholar] [CrossRef] [Green Version]
- Thess, A.; Grund, S.; Mui, B.L.; Hope, M.J.; Baumhof, P.; Fotin-Mleczek, M.; Schlake, T. Sequence-Engineered mRNA Without Chemical Nucleoside Modifications Enables an Effective Protein Therapy in Large Animals. Mol. Ther. 2015, 23, 1456–1464. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wollner, C.J.; Richner, J.M. mRNA Vaccines against Flaviviruses. Vaccines 2021, 9, 148. [Google Scholar] [CrossRef] [PubMed]
- Rizvi, F.; Everton, E.; Smith, A.R.; Liu, H.; Osota, E.; Beattie, M.; Tam, Y.; Pardi, N.; Weissman, D.; Gouon-Evans, V. Murine Liver Repair via Transient Activation of Regenerative Pathways in Hepatocytes Using Lipid Nanoparticle-Complexed Nucleoside-Modified mRNA. Nat. Commun. 2021, 12, 613. [Google Scholar] [CrossRef] [PubMed]
- Howard, M.T.; Gesteland, R.F.; Atkins, J.F. Efficient Stimulation of Site-Specific Ribosome Frameshifting by Antisense Oligonucleotides. RNA 2004, 10, 1653–1661. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Olsthoorn, R.C.L.; Laurs, M.; Sohet, F.; Hilbers, C.W.; Heus, H.A.; Pleij, C.W.A. Novel Application of sRNA: Stimulation of Ribosomal Frameshifting. RNA 2004, 10, 1702–1703. [Google Scholar] [CrossRef] [Green Version]
- Henderson, C.M.; Anderson, C.B.; Howard, M.T. Antisense-Induced Ribosomal Frameshifting. Nucleic Acids Res. 2006, 34, 4302–4310. [Google Scholar] [CrossRef] [Green Version]
- Yu, C.H.; Noteborn, M.H.M.; Olsthoorn, R.C.L. Stimulation of Ribosomal Frameshifting by Antisense LNA. Nucleic Acids Res. 2010, 38, 8277–8283. [Google Scholar] [CrossRef]
- Yewdell, J.W. Individuals Cannot Rely on COVID-19 Herd Immunity: Durable Immunity to Viral Disease Is Limited to Viruses with Obligate Viremic Spread. PLoS Pathog. 2021, 17, e1009509. [Google Scholar] [CrossRef]
- Qiao, J.; Li, Y.-S.; Zeng, R.; Liu, F.-L.; Luo, R.-H.; Huang, C.; Wang, Y.-F.; Zhang, J.; Quan, B.; Shen, C.; et al. SARS-CoV-2 M pro Inhibitors with Antiviral Activity in a Transgenic Mouse Model. Science 2021, 371, 1374–1378. [Google Scholar] [CrossRef]
- Günther, S.; Reinke, P.Y.A.; Fernández-Garciá, Y.; Lieske, J.; Lane, T.J.; Ginn, H.M.; Koua, F.H.M.; Ehrt, C.; Ewert, W.; Oberthuer, D.; et al. X-Ray Screening Identifies Active Site and Allosteric Inhibitors of SARS-CoV-2 Main Protease. Science 2021, 372, 642–646. [Google Scholar] [CrossRef]
- Sun, Y.; Abriola, L.; Surovtseva, Y.V.; Lindenbach, B.D.; Guo, J.U. Restriction of SARS-CoV-2 Replication by Targeting Programmed −1 Ribosomal Frameshifting In Vitro. Proc. Natl. Acad. Sci. USA 2021, in press. [Google Scholar] [CrossRef]
- Atkins, J.F.; Ryan, M.D. Foot and Mouth’s Achilles’ Heel? Nat. Biotechnol. 2008, 26, 1335–1336. [Google Scholar] [CrossRef] [PubMed]
- Whitfield, H.J.; Martin, R.G.; Ames, B.N. Classification of Aminotransferase (C Gene) Mutants in the Histidine Operon. J. Mol. Biol. 1966, 21, 335–355. [Google Scholar] [CrossRef]
- Crick, F.H. What Mad Pursuit: A Personal View of Scientific Discovery; Basic Books: New York, NY, USA, 1988; p. 208. [Google Scholar]
- Newton, A. Isolation and Characterization of Frameshift Mutations in the lac Operon. J. Mol. Biol. 1970, 49, 589–601. [Google Scholar] [CrossRef]
- Atkins, J.F.; Elseviers, D.; Gorini, L. Low Activity of β-Galactosidase in Frameshift Mutants of Escherichia coli. Proc. Natl. Acad. Sci. USA 1972, 69, 1192–1195. [Google Scholar] [CrossRef] [Green Version]
- Atkins, J.F.; Björk, G.R. A Gripping Tale of Ribosomal Frameshifting: Extragenic Suppressors of Frameshift Mutations Spotlight P-Site Realignment. Microbiol. Mol. Biol. Rev. 2009, 73, 178–210. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bretscher, M.S. Translocation in Protein Synthesis: A Hybrid Structure Model. Nature 1968, 218, 675–677. [Google Scholar] [CrossRef] [PubMed]
- Moazed, D.; Noller, H.F. Intermediate States in the Movement of Transfer RNA in the Ribosome. Nature 1989, 342, 142–148. [Google Scholar] [CrossRef]
- Atkins, J.F.; Gesteland, R.F. mRNA Readout at 40. Nature 2001, 414, 693. [Google Scholar] [CrossRef]
- Crick, F.H.C.; Barnett, L.; Brenner, S.; Watts-Tobin, R.J. General Nature of the Genetic Code for Proteins. Nature 1961, 192, 1227–1232. [Google Scholar] [CrossRef]
- Ibba, M.; Becker, H.D.; Stathopoulos, C.; Tumbula, D.L.; Söll, D. The Adaptor Hypothesis Revisited. Trends Biochem. Sci. 2000, 25, 311–316. [Google Scholar] [CrossRef]
- Spirin, A.S. Ribosomal Translocation: Facts and Models. Prog. Nucleic Acid Res. Mol. Biol. 1985, 32, 75–114. [Google Scholar] [PubMed]
- Southworth, D.R.; Brunelle, J.L.; Green, R. EFG-Independent Translocation of the mRNA:tRNA Complex Is Promoted by Modification of the Ribosome with Thiol-Specific Reagents. J. Mol. Biol. 2002, 324, 611–623. [Google Scholar] [CrossRef]
- Geggier, P.; Dave, R.; Feldman, M.B.; Terry, D.S.; Altman, R.B.; Munro, J.B.; Blanchard, S.C. Conformational Sampling of Aminoacyl-tRNA during Selection on the Bacterial Ribosome. J. Mol. Biol. 2010, 399, 576–595. [Google Scholar] [CrossRef] [Green Version]
- Rodnina, M.V.; Peske, F.; Peng, B.Z.; Belardinelli, R.; Wintermeyer, W. Converting GTP Hydrolysis into Motion: Versatile Translational Elongation Factor G. Biol. Chem. 2019, 401, 131–142. [Google Scholar] [CrossRef] [PubMed]
- Peng, B.Z.; Bock, L.V.; Belardinelli, R.; Peske, F.; Grubmüller, H.; Rodnina, M.V. Active Role of Elongation Factor G in Maintaining the mRNA Reading Frame during Translation. Sci. Adv. 2019, 5, eaax8030. [Google Scholar] [CrossRef] [Green Version]
- Zhou, J.; Lancaster, L.; Donohue, J.P.; Noller, H.F. Spontaneous Ribosomal Translocation of mRNA and tRNAs into a Chimeric Hybrid State. Proc. Natl. Acad. Sci. USA 2019, 116, 7813–7818. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Niblett, D.; Nelson, C.; Leung, C.S.; Rexroad, G.; Cosy, J.; Zhou, J.; Lancaster, L.; Noller, H.F. Mutations in Domain IV of Elongation Factor EF-G Confer -1 Frameshifting. RNA 2021, 27, 40–53. [Google Scholar] [CrossRef]
- Atkins, J.F.; Böck, A.; Matsufuji, S.; Gesteland, R.F. Dynamics of the genetic code. In The RNA World: The Nature of Modern RNA Suggest a Prebiotic RNA World; Gesteland, R.F., Cech, T.R., Atkins, J.F., Eds.; Cold Spring Harbor Laboratory Press: New York, NY, USA, 1999; pp. 637–673. [Google Scholar]
- Yewdell, J.W. Hide and Seek in the Peptidome. Science 2003, 301, 1334–1335. [Google Scholar] [CrossRef]
- Bourdetsky, D.; Schmelzer, C.E.H.; Admon, A. The Nature and Extent of Contributions by Defective Ribosome Products to the HLA Peptidome. Proc. Natl. Acad. Sci. USA 2014, 111, 1591–1599. [Google Scholar] [CrossRef] [Green Version]
- Qian, S.-B.; Reits, E.; Neefjes, J.; Deslich, J.M.; Bennink, J.R.; Yewdell, J.W. Tight Linkage between Translation and MHC Class I Peptide Ligand Generation Implies Specialized Antigen Processing for Defective Ribosomal Products. J. Immunol. 2006, 177, 227–233. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dersh, D.; Hollý, J.; Yewdell, J.W. A Few Good Peptides: MHC Class I-Based Cancer Immunosurveillance and Immunoevasion. Nat. Rev. Immunol. 2021, 21, 116–128. [Google Scholar] [CrossRef] [PubMed]
- Chong, C.; Müller, M.; Pak, H.S.; Harnett, D.; Huber, F.; Grun, D.; Leleu, M.; Auger, A.; Arnaud, M.; Stevenson, B.J.; et al. Integrated Proteogenomic Deep Sequencing and Analytics Accurately Identify Non-Canonical Peptides in Tumor Immunopeptidomes. Nat. Commun. 2020, 11, 1293. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Caron, E.; Vincent, K.; Fortier, M.H.; Laverdure, J.P.; Bramoullé, A.; Hardy, M.P.; Voisin, G.; Roux, P.P.; Lemieux, S.; Thibault, P.; et al. The MHC I Immunopeptidome Conveys to the Cell Surface an Integrative View of Cellular Regulation. Mol. Syst. Biol. 2011, 7, 533. [Google Scholar] [CrossRef] [PubMed]
- Laumont, C.M.; Vincent, K.; Hesnard, L.; Audemard, É.; Bonneil, É.; Laverdure, J.P.; Gendron, P.; Courcelles, M.; Hardy, M.P.; Côté, C.; et al. Noncoding Regions Are the Main Source of Targetable Tumor-Specific Antigens. Sci. Transl. Med. 2018, 10, eaau5516. [Google Scholar] [CrossRef] [Green Version]
- Wei, J.; Kishton, R.J.; Angel, M.; Conn, C.S.; Dalla-Venezia, N.; Marcel, V.; Vincent, A.; Catez, F.; Ferré, S.; Ayadi, L.; et al. Ribosomal Proteins Regulate MHC Class I Peptide Generation for Immunosurveillance. Mol. Cell 2019, 73, 1162–1173.e5. [Google Scholar] [CrossRef] [Green Version]
- Bartok, O.; Pataskar, A.; Nagel, R.; Laos, M.; Goldfarb, E.; Hayoun, D.; Levy, R.; Körner, P.R.; Kreuger, I.Z.M.; Champagne, J.; et al. Anti-Tumour Immunity Induces Aberrant Peptide Presentation in Melanoma. Nature 2020, 590, 332–337. [Google Scholar] [CrossRef]
- De Crécy-Lagard, V.; Boccaletto, P.; Mangleburg, C.G.; Sharma, P.; Lowe, T.M.; Leidel, S.A.; Bujnicki, J.M. Matching tRNA Modifications in Humans to Their Known and Predicted Enzymes. Nucleic Acids Res. 2019, 47, 2143–2159. [Google Scholar] [CrossRef]
- Nagayoshi, Y.; Chujo, T.; Hirata, S.; Nakatsuka, H.; Chen, C.W.; Takakura, M.; Miyauchi, K.; Ikeuchi, Y.; Carlyle, B.C.; Kitchen, R.R.; et al. Loss of Ftsj1 Perturbs Codon-Specific Translation Efficiency in the Brain and Is Associated with X-Linked Intellectual Disability. Sci. Adv. 2021, 7, eabf3072. [Google Scholar] [CrossRef]
- Girstmair, H.; Saffert, P.; Rode, S.; Czech, A.; Holland, G.; Bannert, N.; Ignatova, Z. Depletion of Cognate Charged Transfer RNA Causes Translational Frameshifting within the Expanded CAG Stretch in Huntingtin. Cell Rep. 2013, 3, 148–159. [Google Scholar] [CrossRef] [Green Version]
- Chen, J.; Brunner, A.D.; Cogan, J.Z.; Nunez, J.K.; Fields, A.P.; Adamson, B.; Itzhak, D.N.; Li, J.Y.; Mann, M.; Leonetti, M.D.; et al. Pervasive Functional Translation Of Noncanonical Human Open Reading Frames. Science 2020, 367, 1140–1146. [Google Scholar] [CrossRef] [PubMed]
- van Heesch, S.; Witte, F.; Schneider-Lunitz, V.; Schulz, J.F.; Adami, E.; Faber, A.B.; Kirchner, M.; Maatz, H.; Blachut, S.; Sandmann, C.L.; et al. The Translational Landscape of the Human Heart. Cell 2019, 178, 242–260.e29. [Google Scholar] [CrossRef] [Green Version]
- Ivanov, I.P.; Wei, J.; Caster, S.Z.; Smith, K.M.; Michel, A.M.; Zhang, Y.; Firth, A.E.; Freitag, M.; Dunlap, J.C.; Bell-Pedersen, D.; et al. Translation Initiation from Conserved Non-AUG Codons Provides Additional Layers of Regulation and Coding Capacity. MBio 2017, 8, e00844-17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tzani, I.; Ivanov, I.P.; Andreev, D.E.; Dmitriev, R.I.; Dean, K.A.; Baranov, P.V.; Atkins, J.F.; Loughran, G. Systematic Analysis of the PTEN 5′ Leader Identifies a Major AUU Initiated Proteoform. Open Biol. 2016, 6, 150203. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Starck, S.R.; Shastri, N. Nowhere to Hide: Unconventional Translation Yields Cryptic Peptides for Immune Surveillance. Immunol. Rev. 2016, 272, 8–16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Damme, P.; Gawron, D.; Van Criekinge, W.; Menschaert, G. N-Terminal Proteomics and Ribosome Profiling Provide a Comprehensive View of the Alternative Translation Initiation Landscape in Mice and Men. Mol. Cell. Proteomics 2014, 13, 1245–1261. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ivanov, I.P.; Firth, A.E.; Michel, A.M.; Atkins, J.F.; Baranov, P.V. Identification of Evolutionarily Conserved Non-AUG-Initiated N-Terminal Extensions in Human Coding Sequences. Nucleic Acids Res. 2011, 39, 4220–4234. [Google Scholar] [CrossRef] [PubMed]
- Eisenberg, A.R.; Higdon, A.L.; Hollerer, I.; Fields, A.P.; Jungreis, I.; Diamond, P.D.; Kellis, M.; Jovanovic, M.; Brar, G.A. Translation Initiation Site Profiling Reveals Widespread Synthesis of Non-AUG-Initiated Protein Isoforms in Yeast. Cell Syst. 2020, 11, 145–160.e5. [Google Scholar] [CrossRef]
- Khan, Y.A.; Jungreis, I.; Wright, J.C.; Mudge, J.M.; Choudhary, J.S.; Firth, A.E.; Kellis, M. Evidence for a Novel Overlapping Coding Sequence in POLG Initiated at a CUG Start Codon. BMC Genet. 2020, 21, 25. [Google Scholar] [CrossRef] [Green Version]
- Loughran, G.; Zhdanov, A.V.; Mikhaylova, M.S.; Rozov, F.N.; Datskevich, P.N.; Kovalchuk, S.I.; Serebryakova, M.V.; Kiniry, S.J.; Michel, A.M.; O’Connor, P.B.F.; et al. Unusually Efficient CUG Initiation of an Overlapping Reading Frame in POLG mRNA Yields Novel Protein POLGARF. Proc. Natl. Acad. Sci. USA 2020, 117, 24936–24946. [Google Scholar] [CrossRef]
- Erhard, F.; Dölken, L.; Schilling, B.; Schlosser, A. Identification of the Cryptic HLA-I Immunopeptidome. Cancer Immunol. Res. 2020, 8, 1018–1026. [Google Scholar] [CrossRef] [PubMed]
- Lu, S.; Zhang, J.; Lian, X.; Sun, L.; Meng, K.; Chen, Y.; Sun, Z.; Yin, X.; Li, Y.; Zhao, J.; et al. A Hidden Human Proteome Encoded by “non-Coding” Genes. Nucleic Acids Res. 2019, 47, 8111–8125. [Google Scholar] [CrossRef] [PubMed]
- Brunet, M.A.; Brunelle, M.; Lucier, J.F.; Delcourt, V.; Levesque, M.; Grenier, F.; Samandi, S.; Leblanc, S.; Aguilar, J.D.; Dufour, P.; et al. OpenProt: A More Comprehensive Guide to Explore Eukaryotic Coding Potential and Proteomes. Nucleic Acids Res. 2019, 47, 403–410. [Google Scholar] [CrossRef] [Green Version]
- Na, C.H.; Barbhuiya, M.A.; Kim, M.S.; Verbruggen, S.; Eacker, S.M.; Pletnikova, O.; Troncoso, J.C.; Halushka, M.K.; Menschaert, G.; Overall, C.M.; et al. Discovery of Noncanonical Translation Initiation Sites through Mass Spectrometric Analysis of Protein N Termini. Genome Res. 2018, 28, 25–36. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ivanov, I.P.; Shin, B.S.; Loughran, G.; Tzani, I.; Young-Baird, S.K.; Cao, C.; Atkins, J.F.; Dever, T.E. Polyamine Control of Translation Elongation Regulates Start Site Selection on Antizyme Inhibitor mRNA via Ribosome Queuing. Mol. Cell 2018, 70, 254–264. [Google Scholar] [CrossRef] [Green Version]
- Prasad, S.; Starck, S.R.; Shastri, N. Presentation of Cryptic Peptides by MHC Class I Is Enhanced by Inflammatory Stimuli. J. Immunol. 2016, 197, 2981–2991. [Google Scholar] [CrossRef] [Green Version]
- Yewdell, J.W.; Hollý, J. DRiPs Get Molecular. Curr. Opin. Immunol. 2020, 64, 130–136. [Google Scholar] [CrossRef]
- Ruiz Cuevas, M.V.; Hardy, M.P.; Hollý, J.; Bonneil, É.; Durette, C.; Courcelles, M.; Lanoix, J.; Côté, C.; Staudt, L.M.; Lemieux, S.; et al. Most Non-Canonical Proteins Uniquely Populate the Proteome or Immunopeptidome. Cell Rep. 2021, 34, 108815. [Google Scholar] [CrossRef]
- Hukelmann, J.L.; Anderson, K.E.; Sinclair, L.V.; Grzes, K.M.; Murillo, A.B.; Hawkins, P.T.; Stephens, L.R.; Lamond, A.I.; Cantrell, D.A. The Cytotoxic T Cell Proteome and Its Shaping by the Kinase mTOR. Nat. Immunol. 2016, 17, 104–112. [Google Scholar] [CrossRef] [Green Version]
- Yewdell, J.W.; Dersh, D.; Fåhraeus, R. Peptide Channeling: The Key to MHC Class I Immunosurveillance? Trends Cell Biol. 2019, 29, 929–939. [Google Scholar] [CrossRef]
- Goodenough, E.; Robinson, T.M.; Zook, M.B.; Flanigan, K.M.; Atkins, J.F.; Howard, M.T.; Eisenlohr, L.C. Cryptic MHC Class I-Binding Peptides Are Revealed by Aminoglycoside-Induced Stop Codon Read-through into the 3′ UTR. Proc. Natl. Acad. Sci. USA 2014, 111, 5670–5675. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Patel, S.J.; Sanjana, N.E.; Kishton, R.J.; Eidizadeh, A.; Vodnala, S.K.; Cam, M.; Gartner, J.J.; Jia, L.; Steinberg, S.M.; Yamamoto, T.N.; et al. Identification of Essential Genes for Cancer Immunotherapy. Nature 2017, 548, 537–542. [Google Scholar] [CrossRef] [Green Version]
- Ferretti, M.B.; Karbstein, K. Does Functional Specialization of Ribosomes Really Exist? RNA 2019, 25, 521–538. [Google Scholar] [CrossRef]
- Horsburgh, B.C.; Kollmus, H.; Hauser, H.; Coen, D.M. Translational Recoding Induced by G-Rich mRNA Sequences That Form Unusual Structures. Cell 1996, 86, 949–959. [Google Scholar] [CrossRef] [Green Version]
- Griffiths, A.; Chen, S.-H.; Horsburgh, B.C.; Coen, D.M. Translational Compensation of a Frameshift Mutation Affecting Herpes Simplex Virus Thymidine Kinase Is Sufficient To Permit Reactivation from Latency. J. Virol. 2003, 77, 4703–4709. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zook, M.B.; Howard, M.T.; Sinnathamby, G.; Atkins, J.F.; Eisenlohr, L.C. Epitopes Derived by Incidental Translational Frameshifting Give Rise to a Protective CTL Response. J. Immunol. 2006, 176, 6928–6934. [Google Scholar] [CrossRef] [Green Version]
- Grentzmann, G.; Ingram, J.A.; Kelly, P.J.; Gesteland, R.F.; Atkins, J.F. A Dual-Luciferase Reporter System for Studying Recoding Signals. RNA 1998, 4, 479–486. [Google Scholar]
- Pan, D.; Coen, D.M. Net -1 Frameshifting on a Noncanonical Sequence in a Herpes Simplex Virus Drug-Resistant Mutant Is Stimulated by Nonstop mRNA. Proc. Natl. Acad. Sci. USA 2012, 109, 14852–14857. [Google Scholar] [CrossRef] [Green Version]
- Chandrasekaran, V.; Juszkiewicz, S.; Choi, J.; Puglisi, J.D.; Brown, A.; Shao, S.; Ramakrishnan, V.; Hegde, R.S. Mechanism of Ribosome Stalling during Translation of a Poly(A) Tail. Nat. Struct. Mol. Biol. 2019, 26, 1132–1140. [Google Scholar] [CrossRef] [PubMed]
- Tesina, P.; Lessen, L.N.; Buschauer, R.; Cheng, J.; Wu, C.C.; Berninghausen, O.; Buskirk, A.R.; Becker, T.; Beckmann, R.; Green, R. Molecular Mechanism of Translational Stalling by Inhibitory Codon Combinations and Poly(A) Tracts. EMBO J. 2020, 39, e103365. [Google Scholar] [CrossRef]
- Smith, A.M.; Costello, M.S.; Kettring, A.H.; Wingo, R.J.; Moore, S.D. Ribosome Collisions Alter Frameshifting at Translational Reprogramming Motifs in Bacterial mRNAs. Proc. Natl. Acad. Sci. USA 2019, 116, 21769–21779. [Google Scholar] [CrossRef] [Green Version]
- Rocha, E.P.C.; Matic, I.; Taddei, F. Over-Representation of Repeats in Stress Response Genes: A Strategy to Increase Versatility under Stressful Conditions? Nucleic Acids Res. 2002, 30, 1886–1894. [Google Scholar] [CrossRef] [Green Version]
- Ramsay, J.P.; Ronson, C.W. Silencing Quorum Sensing and Ice Mobility through Antiactivation and Ribosomal Frameshifting. Mob. Genet. Elements 2015, 5, 103–108. [Google Scholar] [CrossRef] [Green Version]
- Loving, C.L.; Osorio, F.A.; Murtaugh, M.P.; Zuckermann, F.A. Innate and Adaptive Immunity against Porcine Reproductive and Respiratory Syndrome Virus. Vet. Immunol. Immunopathol. 2015, 167, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Shang, P.; Shyu, D.; Carrillo, C.; Naraghi-Arani, P.; Jaing, C.J.; Renukaradhya, G.J.; Firth, A.E.; Snijder, E.J.; Fang, Y. Nonstructural Proteins Nsp2TF and Nsp2N of Porcine Reproductive and Respiratory Syndrome Virus (PRRSV) Play Important Roles in Suppressing Host Innate Immune Responses. Virology 2018, 517, 164–176. [Google Scholar] [CrossRef]
- Sun, Z.; Chen, Z.; Lawson, S.R.; Fang, Y. The Cysteine Protease Domain of Porcine Reproductive and Respiratory Syndrome Virus Nonstructural Protein 2 Possesses Deubiquitinating and Interferon Antagonism Functions. J. Virol. 2010, 84, 7832–7846. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, Z.; Li, Y.; Ransburgh, R.; Snijder, E.J.; Fang, Y. Nonstructural Protein 2 of Porcine Reproductive and Respiratory Syndrome Virus Inhibits the Antiviral Function of Interferon-Stimulated Gene 15. J. Virol. 2012, 86, 3839–3850. [Google Scholar] [CrossRef] [Green Version]
- Van Kasteren, P.B.; Bailey-Elkin, B.A.; James, T.W.; Ninaber, D.K.; Beugeling, C.; Khajehpour, M.; Snijder, E.J.; Mark, B.L.; Kikkert, M. Deubiquitinase Function of Arterivirus Papain-like Protease 2 Suppresses the Innate Immune Response in Infected Host Cells. Proc. Natl. Acad. Sci. USA 2013, 110, 838–847. [Google Scholar] [CrossRef] [Green Version]
- Knoops, K.; Barcena, M.; Limpens, R.W.A.L.; Koster, A.J.; Mommaas, A.M.; Snijder, E.J. Ultrastructural Characterization of Arterivirus Replication Structures: Reshaping the Endoplasmic Reticulum To Accommodate Viral RNA Synthesis. J. Virol. 2012, 86, 2474–2487. [Google Scholar] [CrossRef] [Green Version]
- Schmidt, J.; Dojcinovic, D.; Guillaume, P.; Luescher, I. Analysis, Isolation, and Activation of Antigen-Specific CD4+ and CD8+ T Cells by Soluble MHC-Peptide Complexes. Front. Immunol. 2013, 4, 218. [Google Scholar] [CrossRef] [Green Version]
- Cao, Q.M.; Subramaniam, S.; Ni, Y.Y.; Cao, D.; Meng, X.J. The Non-Structural Protein Nsp2TF of Porcine Reproductive and Respiratory Syndrome Virus down-Regulates the Expression of Swine Leukocyte Antigen Class I. Virology 2016, 491, 115–124. [Google Scholar] [CrossRef]
- Randow, F.; Lehner, P.J. Viral Avoidance and Exploitation of the Ubiquitin System. Nat. Cell Biol. 2009, 11, 527–534. [Google Scholar] [CrossRef]
- Levitskaya, J.; Coram, M.; Levitsky, V.; Imreh, S.; Steigerwald-Mullen, P.M.; Klein, G.; Kurilla, M.G.; Masucci, M.G. Inhibition of Antigen Processing by the Internal Repeat Region of the Epstein-Barr Virus Nuclear Antigen-1. Nature 1995, 375, 685–688. [Google Scholar] [CrossRef]
- Yin, Y.; Manoury, B.; Fåhraeus, R. Self-Inhibition of Synthesis and Antigen Presentation by Epstein-Barr Virus-Encoded EBNA1. Science 2003, 301, 1371–1374. [Google Scholar] [CrossRef]
- Kwun, H.J.; da Silva, S.R.; Shah, I.M.; Blake, N.; Moore, P.S.; Chang, Y. Kaposi’s Sarcoma-Associated Herpesvirus Latency-Associated Nuclear Antigen 1 Mimics Epstein-Barr Virus EBNA1 Immune Evasion through Central Repeat Domain Effects on Protein Processing. J. Virol. 2007, 81, 8225–8235. [Google Scholar] [CrossRef] [Green Version]
- Kwun, H.J.; da Silva, S.R.; Qin, H.; Ferris, R.L.; Tan, R.; Chang, Y.; Moore, P.S. The Central Repeat Domain 1 of Kaposi’s Sarcoma-Associated Herpesvirus (KSHV) Latency Associated-Nuclear Antigen 1 (LANA1) Prevents Cis MHC Class I Peptide Presentation. Virology 2011, 412, 357–365. [Google Scholar] [CrossRef]
- Stochmanski, S.J.; Therrien, M.; Laganière, J.; Rochefort, D.; Laurent, S.; Karemera, L.; Gaudet, R.; Vyboh, K.; Van Meyel, D.J.; Di Cristo, G.; et al. Expanded ATXN3 Frameshifting Events Are Toxic in Drosophila and Mammalian Neuron Models. Hum. Mol. Genet. 2012, 21, 2211–2218. [Google Scholar] [CrossRef] [Green Version]
- Saffert, P.; Adamla, F.; Schieweck, R.; Atkins, J.F.; Ignatova, Z. An Expanded CAG Repeat in Huntingtin Causes +1 Frameshifting. J. Biol. Chem. 2016, 291, 18505–18513. [Google Scholar] [CrossRef] [Green Version]
- Tabet, R.; Schaeffer, L.; Freyermuth, F.; Jambeau, M.; Workman, M.; Lee, C.Z.; Lin, C.C.; Jiang, J.; Jansen-West, K.; Abou-Hamdan, H.; et al. CUG Initiation and Frameshifting Enable Production of Dipeptide Repeat Proteins from ALS/FTD C9ORF72 Transcripts. Nat. Commun. 2018, 9, 152. [Google Scholar] [CrossRef]
- Murat, P.; Zhong, J.; Lekieffre, L.; Cowieson, N.P.; Clancy, J.L.; Preiss, T.; Balasubramanian, S.; Khanna, R.; Tellam, J. G-Quadruplexes Regulate Epstein-Barr Virus-Encoded Nuclear Antigen 1 mRNA Translation. Nat. Chem. Biol. 2014, 10, 358–364. [Google Scholar] [CrossRef]
- Dabral, P.; Babu, J.; Zareie, A.; Verma, S.C. LANA and HnRNP A1 Regulate the Translation of LANA mRNA through G-Quadruplexes. J. Virol. 2019, 94, e01508–e01519. [Google Scholar] [CrossRef]
- Lista, M.J.; Martins, R.P.; Billant, O.; Contesse, M.A.; Findakly, S.; Pochard, P.; Daskalogianni, C.; Beauvineau, C.; Guetta, C.; Jamin, C.; et al. Nucleolin Directly Mediates Epstein-Barr Virus Immune Evasion through Binding to G-Quadruplexes of EBNA1 mRNA. Nat. Commun. 2017, 8, 16043. [Google Scholar] [CrossRef] [PubMed]
- Martins, R.P.; Malbert-Colas, L.; Lista, M.J.; Daskalogianni, C.; Apcher, S.; Pla, M.; Findakly, S.; Blondel, M.; Fåhraeus, R. Nuclear Processing of Nascent Transcripts Determines Synthesis of Full-Length Proteins and Antigenic Peptides. Nucleic Acids Res. 2019, 47, 3086–3100. [Google Scholar] [CrossRef]
- Penno, C.; Kumari, R.; Baranov, P.V.; Van Sinderen, D.; Atkins, J.F. Stimulation of Reverse Transcriptase Generated CDNAs with Specific Indels by Template RNA Structure: Retrotransposon, DNTP Balance, RT-Reagent Usage. Nucleic Acids Res. 2017, 45, 10143–10155. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, Y.; Sun, T.; Bi, Z.; Ni, J.Q.; Pastor-Pareja, J.C.; Javid, B. Premature Termination Codon Readthrough in Drosophila Varies in a Developmental and Tissue-Specific Manner. Sci. Rep. 2020, 10, 8485. [Google Scholar] [CrossRef]
- Hudson, A.M.; Szabo, N.L.; Loughran, G.; Wills, N.M.; Atkins, J.F.; Cooley, L. Tissue-Specific Dynamic Codon Redefinition in Drosophila. Proc. Natl. Acad. Sci. USA 2021, 118, 5. [Google Scholar] [CrossRef] [PubMed]
- Karki, P.; Carney, T.D.; Maracci, C.; Yatsenko, A.S.; Shcherbata, H.R.; Rodnina, M.V. Tissue-Specific Regulation of Translational Readthrough Tunes Functions of the Traffic Jam Transcription Factor. bioRxiv 2020. [Google Scholar] [CrossRef]
- Swart, E.C.; Serra, V.; Petroni, G.; Nowacki, M. Genetic Codes with No Dedicated Stop Codon: Context-Dependent Translation Termination. Cell 2016, 166, 691–702. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heaphy, S.M.; Mariotti, M.; Gladyshev, V.N.; Atkins, J.F.; Baranov, P.V. Novel Ciliate Genetic Code Variants Including the Reassignment of All Three Stop Codons to Sense Codons in Condylostoma magnum. Mol. Biol. Evol. 2016, 33, 2885–2889. [Google Scholar] [CrossRef] [Green Version]
- Záhonová, K.; Kostygov, A.Y.; Ševčíková, T.; Yurchenko, V.; Eliáš, M. An Unprecedented Non-Canonical Nuclear Genetic Code with All Three Termination Codons Reassigned as Sense Codons. Curr. Biol. 2016, 26, 2364–2369. [Google Scholar] [CrossRef] [Green Version]
- Bachvaroffi, T.R. A Precedented Nuclear Genetic Code with All Three Termination Codons Reassigned as Sense Codons in the Syndinean Amoebophrya Sp. Ex Karlodinium veneficum. PLoS ONE 2019, 14, e0212912. [Google Scholar] [CrossRef]
- Lobanov, A.V.; Heaphy, S.M.; Turanov, A.A.; Gerashchenko, M.V.; Pucciarelli, S.; Devaraj, R.R.; Xie, F.; Petyuk, V.A.; Smith, R.D.; Klobutcher, L.A.; et al. Position-Dependent Termination and Widespread Obligatory Frameshifting in Euplotes Translation. Nat. Struct. Mol. Biol. 2017, 24, 61–68. [Google Scholar] [CrossRef] [Green Version]
- Westergren Jakobsson, A.; Segerman, B.; Wallerman, O.; Bergström Lind, S.; Zhao, H.; Rubin, C.-J.; Pettersson, U.; Akusjärvi, G. The Human Adenovirus 2 Transcriptome: An Amazing Complexity of Alternatively Spliced mRNAs. J. Virol. 2020, 95, e01869-20. [Google Scholar]
- Lewis, J.B.; Anderson, C.W.; Atkins, J.F. Further Mapping of Late Adenovirus Genes by Cell-Free Translation of RNA Selected by Hybridization to Specific DNA Fragments. Cell 1977, 12, 37–44. [Google Scholar] [CrossRef]
- Atkins, J.F.; Weiss, R.B.; Gesteland, R.F. Ribosome Gymnastics-Degree of Difficulty 9.5, Style 10.0. Cell 1990, 62, 413–423. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).