Abstract
Japanese encephalitis virus (JEV) is a zoonotic pathogen mainly found in East and Southeast Asia and transmitted by mosquitoes. The objective of this review is to summarize the knowledge on the diversity of JEV mosquito vector species. Therefore, we systematically analyzed reports of JEV found in field-caught mosquitoes as well as experimental vector competence studies. Based on the investigated publications, we classified 14 species as confirmed vectors for JEV due to their documented experimental vector competence and evidence of JEV found in wild mosquitoes. Additionally, we identified 11 mosquito species, belonging to five genera, with an experimentally confirmed vector competence for JEV but lacking evidence on their JEV transmission capacity from field-caught mosquitoes. Our study highlights the diversity of confirmed and potential JEV vector species. We also emphasize the variety in the study design of vector competence investigations. To account for the diversity of the vector species and regional circumstances, JEV vector competence should be studied in the local context, using local mosquitoes with local virus strains under local climate conditions to achieve reliable data. In addition, harmonization of the design of vector competence experiments would lead to better comparable data, informing vector and disease control measures.
1. Introduction
Japanese encephalitis (JE) is a vector borne zoonosis and one of the world’s leading encephalitic diseases, particularly in the Asia-Pacific region [1]. The disease is endemic in 24 countries in South and Southeast Asia from Pakistan to Japan, northern Australia and Oceania [2], putting more than three billion people at risk of infection. The annual incidence of JE is estimated to be around 69,000 cases [3] but this is likely to be underestimated due to insufficient surveillance systems and the lack of precise diagnostic tools. Based on an estimated annual loss of 709,000 disability-adjusted life years [4], JE has even a higher disease burden than dengue.
JE is caused by the Japanese encephalitis virus (JEV) belonging to the Flaviviridae family [5]. The main epidemiological pattern is an enzootic cycle where the virus is transmitted between birds and/or pigs by mosquitoes [6]. Humans and other mammal species like horses serve as dead-end hosts. Recently, direct pig-to-pig transmission by oronasal infection was demonstrated under laboratory conditions [7]. The importance of this vector-free infection route for the maintenance of the JEV epidemiological cycle is substantiated by mathematical modelling using serological data from field investigations [8]. However, mosquitoes are still considered the key players in terms of virus transmission and therefore investigations of their capacity to efficiently pass on JEV are vital for risk assessments and public health recommendations. In contrast to other known flaviviruses, a broad range of mosquito species can theoretically transmit JEV, including several genera such as Aedes (Ae.), Anopheles (An.), Culex (Cx.) and Mansonia (Ma.) [9]. The main considered JEV vector species are Cx. tritaeniorhynchus, Cx. vishnui and Cx. gelidus [10,11,12].
The distribution of Cx. tritaeniorhynchus is widespread across Southeast Asia and adjacent tropical areas. It extends to Australia and from the Middle East to Africa [13,14,15]. It also has recently been reported in Greece. This species is considered the main JEV vector and its distribution area coincides with the epidemiological risk area of JE [16]. Cx. vishnui is also widely distributed in Southeast Asia, and can be found from India in the west, to Japan in the east, South Korea to the north and Indonesia to the south. This species has long been recognized as an important vector for JEV because the females feed primarily on pigs and birds, but also opportunistically bite humans [17]. The reports on the involvement of Cx. gelidus in JEV transmission are historic [18,19]. Depending on the country, this species is considered a primary or secondary vector of JEV [12].
The work of Oliveira and colleagues [9,20,21] confirmed that Cx. tritaeniorhynchus and Cx. gelidus should be considered primary JEV vectors. Additionally, the authors emphasize the important role of Cx. quinquefasciatus for JEV transmission. Generally, many species are also considered secondary vectors, particularly Ae. japonicus, Cx. fuscocephala and Cx. vishnui [9].
Vector competence in the context of arbovirus transmission describes the ability of a particular insect to become infected, maintain viral replication and transmit a specific virus. The transmission of the pathogen to the vector occurs during a blood meal on an infectious host, establishing a systemic infection leading to an infection of the salivary glands, enabling the virus to be transmitted by blood feeding to another host. To establish a systemic infection, the virus has to pass several physiological barriers, especially in the midgut and the salivary glands of the mosquito. The period from acquiring the virus to becoming infectious is the so-called extrinsic incubation period (EIP). In contrast to vector competence, which describes only the ability for viral transmission, vector capacity expresses the efficiency of the transmission in a specific vector-host relationship in a given environment. Therefore it includes the vector competence itself but also mosquito parameters (density of vectors in relation to the density of the host, proportion of vectors feeding on the host relative to the gonotrophic cycle, survival rate of the vector and EIP) [22].
Besides the infection of female mosquitoes through the bite of an infected host, JEV can also be transmitted horizontally (veneral) [23] or vertically in mosquitoes (transovarial or during oviposition) [24]. However, we exclude a detailed analysis of these findings, as often the transmission capacity of the offspring was not further investigated.
The objective of this study is to perform a systematic review of JEV competence surveys in mosquitoes and categorize the JEV vector species into confirmed and potential vector species. Hence, confirmed vectors are species with proven vector competence and JEV found in field-caught mosquitoes. Potential vectors are species with demonstrated experimental vector competence but missing evidence of JEV presence in field-caught mosquitoes. Additionally, we describe the diversity of JEV vectors, and compare the methods used to assess vector competence of JEV vectors, and the consequences in terms of interpretation of results.
2. Materials and Methods
2.1. Literature Search
This study was conducted by using the PubMed database (cutoff date 19 April 2021). We included the search entries “(Japanese encephalitis [Title/Abstract]) AND (virus isolation [Title/Abstract])”, “(Japanese encephalitis [Title/Abstract]) AND (vector competence [Title/Abstract])”, “(Japanese encephalitis [Title/Abstract]) AND transmission [Title/Abstract] AND (mosquito [Title/Abstract])”, “(Japanese encephalitis [Title]) AND (vector [Title])”, “(Japanese encephalitis [Title]) AND (mosquitoes [Title])”, and “(Japanese encephalitis [Title]) AND (mosquito [Title])”. The results were analyzed according to the PRISMA guidelines [25]. We only included studies describing JEV detection and/or isolation from field-caught mosquitoes, and experimental vector competence studies.
2.2. Mosquito Classification and Taxonomy
According to the classification provided by the Mosquito Taxonomic Inventory [26] some mosquito names were not valid anymore (i.e., declared as synonyms) and had to be updated. Consequently, when this was the case, the name as it appears in the original article is given in parentheses after the updated name. As a matter of clarity, regarding members of the very large and composite Aedes genus we followed the classification of Wilkerson et al. [27].
2.3. Classification in Confirmed and Potential Vector Species
A mosquito species was considered a confirmed vector if (i) studies demonstrated the successful isolation of JEV from field-caught mosquitoes, and (ii) if artificial infection experiments showed successful transmission. Whereas potential vectors where mosquito species with proven vector competence but evidence of JEV in field-caught mosquitoes was missing. In this review we only discussed the vector competence as the intrinsic ability of a mosquito species (or population) to transmit JEV.
The herein discussed papers used the following terms: infection rate, defined as proportion of mosquitoes with JEV detected in their bodies among all tested/blood-fed mosquitoes including mosquitoes naturally blood fed, experimentally infected or infected on animals; dissemination rate, related to the proportion of mosquitoes with JEV detected in their legs, wings and/or head among all infected/blood-fed mosquitoes; transmission rate, defined as the proportion of mosquitoes with JEV detected in their saliva or salivary glands among all infected mosquitoes. Additionally, the transmission rate in studies demonstrating the transmission to another animal is defined as proportion of JEV positive animals among those exposed to infected mosquitoes, and does not consider the extent of exposure (i.e., time of exposure and number of infected mosquitoes). The period between infection of the mosquitoes and outcome measures is described in days post infection (dpi).
3. Results
To describe the diversity of JEV mosquito vectors, we screened 650 publications and included 158 of them in this review (Figure 1) demonstrating JEV detection and/or isolation from field-caught mosquitoes, and/or investigations of the vector competence for JEV. We split the results accordingly into (i) confirmed vector species (Table 1, Figure 2) when their vector competence was proven by experimental infection and transmission, and JEV was found in field-caught mosquitoes, (ii) potential vector species with proven experimental vector competence but no documented JEV in field mosquitoes.

Figure 1.
PRISMA flow chart [25] for systematic search of relevant publications.

Table 1.
Confirmed JEV mosquito vector species.
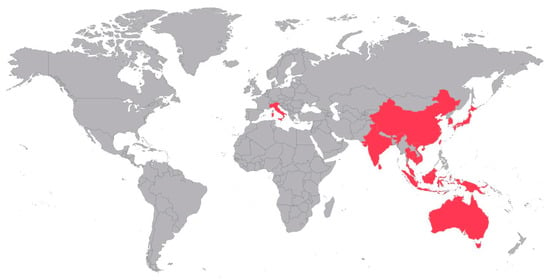
Figure 2.
Distribution of reports on JEV detection and isolations from field-caught mosquitoes of the confirmed vector species Ae. albopictus, Ae. vexans, Ae. vigilax, Ar. subalbatus, Cx. annulirostris, Cx. bitaeniorhynchus, Cx. fuscocephala, Cx. gelidus, Cx. pipiens, Cx. pseudovishnui, Cx. quinquefasciatus, Cx. sitiens, Cx. tritaeniorhyncus and/or Cx. vishnui.
We identified 14 species as confirmed vectors of JEV (Table 1): Ae. albopictus, Ae. vexans, Ae. vigilax, Armigeres (Ar.) subalbatus, Cx. annulirostris, Cx. bitaeniorhynchus, Cx. fuscocephala, Cx. gelidus, Cx. pipiens, Cx. pseudovishnui, Cx. quinquefasciatus, Cx. sitiens, Cx. tritaeniorhyncus and Cx. vishnui, based on reports stating JEV in field-caught mosquitoes and the confirmation of the vector competence by experimental infection (Table S1).
We classified 11 mosquito species as potential JEV vector based on experimental vector competence studies: Ae. detritus, Ae. dorsalis, Ae. japonicus, Ae. kochi, Ae. nigromaculis, Ae. notosciptus, An. tessellatus, Cx. tarsalis, Cs. annulata, Cs. inornata and Ve. funerea.
Furthermore, we found reports for 26 species from five different mosquito genera where JEV was detected and/or isolated from field-caught mosquitoes. This includes Aedes (4 species), Anopheles (9 species), Coquilettidia (1 species), Culex (7 species) and Mansonia (5 species) mosquitoes. However, for these species the vector competence was not investigated so far.
3.1. Confirmed Vectors
Aedes (Stegomyia) albopictus (Skuse, 1894)
Ae. albopictus was found JEV positive and the virus was isolated from these mosquitoes in Malaysia and in Taiwan [28,30]. An early investigation of the vector competence of a Taiwanese laboratory colony of Ae. albopictus was not able to detect infection in mosquitoes fed on viremic pigs [161]. However, later investigations with Ae. albopictus from Taiwan [32] and France [34] were both able to detect virus in saliva of 47% (14 dpi) and in 20–63% of the mosquitoes at 11–13 dpi, respectively. Both studies infected the mosquitoes by feeding them an artificial, infectious blood meal with a high viral load (Taiwan: 107 pfu/mL; France: 106 ffu/mL). A study on the replication capacity of the JEV chimeric vaccine confirmed replication of JEV (wildtype SA-14) in Ae. albopictus infected either by intrathoracic injection or by feeding an infectious blood meal [162]. A study with Australian Ae. albopictus was also successful at demonstrating transmission even by using a lower dose of 103.5 TCID50/mL (approx. 103.3 pfu/mL) for infection by feeding an infectious blood meal [33]. Interestingly, an earlier Taiwanese study [31] using several laboratory colonies of Ae. albopictus originating from different provinces found only one of the three colonies able to transmit JEV to weanling mice (27–39% transmission rate, depending on mosquito infection via intrathoracic injection or artificial blood meal). Also, a study infecting a Chinese laboratory colony of Ae. albopictus with JEV strains isolated from different bat species was able to detect JEV in the mosquitoes 4–20 dpi but did not investigate the transmission capacity [163].
Aedes (Ochlerotatus) vexans (Meigen, 1830)
JEV was isolated from field Ae. vexans mosquitoes in Taiwan [28]. Early experimental infections in 1946 on Guam [35] observed successful transmission to infant mice but neither the mosquito infection procedure nor the transmission rate was described in further detail. Another investigation from the same time performed with US mosquitoes was not able to detect any infection and transmission [36].
Aedes (Ochlerotatus) vigilax (Skuse, 1889)
During an investigation of a JE outbreak in northern Queensland, Australia, JEV was isolated from a pooled sample of Ae. vigilax mosquitoes from the Torres Strait island region [37]. Also, a laboratory colony and field-caught mosquitoes of Ae. vigilax (mentioned as Ochlerotatus vigilax) from North Queensland, were analyzed for their competence to transmit JEV [38]. This study showed that both mosquito populations could get infected with JEV when fed an artificial, infectious blood meal, but surprisingly transmission to mice was only observed with the field-caught mosquitoes and not with the females from the laboratory colony.
Armigeres (Armigeres) subalbatus (Coquillett, 1898)
JEV was isolated from several pooled samples of Ar. subalbatus caught in Taiwan in 1997 [28], and several times in Yunnan province in China [40,41]. The virus was found in a pool of mosquitoes collected in India in 2011–2013 [39]. A vector competence study with Taiwanese mosquitoes detected virus in the salivary glands in 40% to 88% of the mosquitoes depending on the JEV strain and the time point after infection [32]. The same group also determined that Wolbachia infection has no influence on the JEV transmission capacity of Ar. subalbatus [42].
Culex (Culex) annulirostris Skuse, 1889
During a JE outbreak in 1995 on Badu Island, located north of the Australia mainland and south of Papua New-Guinea, JEV was isolated from several Cx. annulirostris [43] and later also on other islands of the Torres Strait [37]. Early experiments with mosquitoes from Guam (then named Cx. jepsoni) were able to demonstrate transmission to mice [35]. An extensive vector competence study with Australian mosquitoes demonstrated JEV transmission to mice via Cx. annulirostris from a laboratory colony and via two populations of field collected Cx. annulirostris from Queensland [38]. Van den Hurk and colleagues later also used a laboratory colony of Australian Cx. annulirostris to prove JEV transmission to flying fox (Pteropus alecta Temminck, 1837) as 60% of the exposed flying foxes seroconverted after exposure to infected mosquitoes [44].
Culex (Culex) bitaeniorhynchus Giles, 1901
JEV positive Cx. bitaeniorhynchus were found in India [39], South Korea [45,46,47,48] and Malaysia [29], and in the latter several isolates were attained from field-caught mosquitoes. Several studies with mosquitoes from India demonstrated the vector competence of this species [49,50,51,164]. All these studies infected the mosquitoes by feeding on viremic young ducks or chickens, and afterwards demonstrated further transmission from blood-fed mosquitoes to naïve ducklings or chicks.
Culex (Culex) fuscocephala Theobald, 1907
JEV was first isolated from Cx. fuscocephala in Thailand in 1970 [55], and later also detected and/or isolated in Indonesia [57,165], in Sri Lanka in 1987–1988 [52], in Malaysia in 1993 [59], in Taiwan [54,56] and throughout several surveys in India in mosquitoes collected 1985–1987 [58], 1991–1994 [53] and 2011–2013 [39]. Vector competence studies in the 1970s showed successful transmission to young chickens by mosquitoes from Thailand [60] and Taiwan [61] infected under laboratory conditions by feeding on viremic chicks or pigs, respectively.
Culex (Culex) gelidus Theobald, 1901
JEV was detected in Cx. gelidus collected between 1987–1988 in Sri Lanka [52], Australia [67] and several times in mosquitoes from India [53,62,63,64,65,66,68,69,70]. The role of this species as JEV vector was further confirmed by several studies describing successful virus isolation from field-caught mosquitoes in India [53,58,81], Indonesia [57,76,165], Vietnam [75], Thailand [71,77], Malaysia [19,59,72,73,74,78], Sri Lanka [52] and Australia [79,80]. Gould and colleagues also performed transmission experiments with a Malaysian Cx. gelidus laboratory colony and observed a transmission rate to young chickens of up to 85% when mosquitoes were infected by biting viremic chicks [19]. However, when mosquitoes were allowed to bite infected horses no further transmission to chickens could be detected [151], indicating early the role of horses as dead-end host in the JEV transmission cycle producing not a high enough viremia to be infectious for mosquitoes. Later, a small amount of Australian field-caught mosquitoes were infected and a single JEV transmission event to a suckling mouse was observed [38]. A study on a laboratory colony of Cx. gelidus from India investigated viral growth kinetics and found JEV in the saliva at 10 dpi and 14 dpi [82].
Culex (Culex) pipiens Linnaeus, 1758
JEV was detected in Cx. pipiens during recent outbreaks in South Korea [46,48,84], and Italy [83], and during the re-instated surveillance activities in Shanghai, China [85]. Reports also exist about early isolations in Japan [86], South Korea [87] and China [88]. Several studies investigated the vector competence of Cx. pipiens, including the subspecies Cx. pipiens pipiens, Cx. pipiens molestus and Cx. pipiens pallens (Table S1).
A recent study from New Zealand [166] noted successful infection but no transmission. However, this study was not able to show JEV transmission for any of the investigated mosquito species, even for the well-described JEV vector Cx. quinquefasciatus, making these results questionable. In contrast, others were able to observe JEV transmission from Cx. pipiens to mice [90], and determine possible transmission by detecting JEV in the saliva of the mosquitoes as early as 7 dpi for a certain JEV isolate from China [94], and at 21 dpi in Cx. pipiens from the UK [95]. The virus was also found in the saliva of Cx. pipiens when infected by intrathoracic injection [97]. In this study, the infected mosquitoes were also able to infect newly hatched ducklings at 10 dpi.
For the subtype Cx. pipiens molestus efficient transmission to mice was shown with a laboratory colony from the US [36] and one from Taiwan [92]. Transmission to young chickens was also obtained with field-caught Cx. pipiens molestus from Uzbekistan [93].
In addition to the vector competence for Cx. pipiens molestus, Reeves & Hammon also observed transmission to mice and a chicken with a US laboratory colony of Cx. pipiens pipiens [36]. This was confirmed by a recent study using recombinant JEV strains and Cx. pipiens pipiens from France, measuring JEV in the saliva of up to 41% of the infected mosquitoes [34]. A recent study examined the vector competence of a laboratory colony of Cx. pipiens pipiens from the UK at different temperatures [98]. At 20 °C, no JEV was detected in saliva, whereas at 25 °C transmission seems possible because 90% of the mosquitoes had JEV in their saliva.
JEV was also found in mosquito pools of Cx. pipiens pallens collected in 2015 in Shandong province, China [89]. Female Cx. pipiens pallens were used to investigate the role of mosquito defensins [96,167] and of a C-type lectin protein [168] on the JEV infection documenting substantial amounts of JEV in the salivary glands (10 dpi). Doi and colleagues showed that the subtype Cx. pipiens pallens could be successfully infected with JEV but only when a sufficiently high virus concentration (at least 104 LD50) was fed via an artificial blood meal [169]. They also demonstrated JEV transmission via Cx. pipiens pallens to lizards and then further to mice [91]. Another study from Japan demonstrated that this subspecies could become infected when fed on young JEV-infected chicken during the peak of the viremic phase [23]. However, a more recent investigation with field-caught mosquitoes from South Korea was not able to observe transmission to young chickens [156], even though the experimental procedure was proven with Cx. pipiens molestus [93].
Culex (Culex) pseudovishnui Colless, 1957
JEV was isolated in India from Cx. pseudovishnui from Karnata [102], from Goa [103], and was also detected in several pooled mosquito samples from other areas in India [99,100,101]. Several vector competence studies were performed with Cx. pseudovishnui (Table S1). Mosquitoes from a laboratory colony in Japan were successfully infected when fed on chicken with a viremia of at least 103 LD50, whereas a lower viremia was not sufficient to establish an infection in these mosquitoes 10–14 dpi [169]. Later investigations with mosquitoes from India found JEV in the salivary glands when the mosquitoes were infected via feeding on viremic chicks [104], via intrathoracic injection and feeding of an artificial, infectious blood meal [105].
Culex (Culex) quinquefasciatus Say, 1823
JEV was detected in Cx. quinquefasciatus mosquitoes from India [39] and Vietnam [106]. Isolations were successful from mosquitoes in India [58], Vietnam [75], Thailand [107] and Taiwan [28]. Early vector competence studies with Cx. quinquefasciatus (sometimes called Cx. (pipiens) fatigans or Cx. pipiens quinquefasciatus) laboratory colonies from Japan [169] and India [51,110] were able to observe concentration-dependent infection rates, and confirm transmission to young chickens, respectively. Similar to the experiments with Cx. pipiens pallens, Doi and colleagues also demonstrated JEV transmission via Cx. pipiens fatigans to lizards and then further to mice [91]. Several studies in the 1940s on laboratory colonies and mosquitoes from Guam [35,108,109,170] demonstrated transmission of a human JEV isolate from Okinawa to infant mice when the mosquitoes were infected by feeding on infectious blood presented on cotton. Reeves and Hammon also used this technique for successfully infecting Cx. quinquefasciatus and demonstrating further transmission to mice, whereas they were not able to infect mosquitoes successfully by feeding them on viremic chicken [36]. More recent vector competence studies detected JEV in salivary glands two weeks after infection of Cx. quinquefasciatus from Taiwan [32], and in the saliva of mosquitoes from USA [112,113]. Laboratory colony mosquitoes from China were also successfully infected with JEV isolates from bats [163]. In experiments with Indian Cx. quinquefasciatus it was also documented that colonization with certain bacteria (Pseudomonas sp. and Acinetobacter junii) slightly increased their susceptibly for infection with JEV [171], and that simultaneous or sequential infection with Bagaza virus (Flaviviridae, Ntaya Flavivirus serocomplex) reduced replication of JEV [172]. An investigation of van den Hurk and colleagues revealed higher infection rates and JEV transmission to mice via laboratory colony mosquitoes, whereas the field-caught mosquitoes were not able to transmit JEV [38]. Not only do the microbiota and origin of the mosquitoes influence their transmission capacity, but also the ambient temperature, as shown for Cx. quinquefasciatus held either at 23 °C or 28 °C with mosquitoes from Brazil [111]. Infection and dissemination rate were similar but the transmission rate 14 and 21 dpi was elevated at the higher temperature. An extensive study using several JEV strains revealed that viruses belonging to genotype I had higher infection, dissemination and transmission rates in Cx. quinquefasciatus than viruses from genotype III [114]. Additionally, the EIP was shorter for infections with genotype I strains. However, one study performed in New Zealand with endemic Cx. quinquefasciatus, as well as mosquitoes from a US laboratory colony, was not able to detect transmission [166].
Culex (Culex) sitiens Wiedemann, 1828
JEV was found in Cx. sitiens in East and South Asia, as well as in northern Oceania. The virus was found and/or isolated from mosquitoes in Malaysia [29,115], Taiwan [28,116], Papua New Guinea [118] and Australia [67,117,119,120]. In Australia, Cx. sitiens was identified as a competent JEV vector using a virus strain isolated from Australian Och. vigilax and mosquitoes from a laboratory colony from Queensland [38].
Culex (Culex) tritaeniorhynchus Giles, 1901
Cx. tritaeniorhynchus is considered the primary JEV vector in a lot of countries. It was first isolated from this mosquito species in the 1930s in Japan [173] as well as later through several decades of Japanese surveillance [130,136,145]. Virus isolation was also successful with mosquitoes from Indonesia [57,132,133,165], India [53,58,70,102,129,137,143], Malaysia [59,72,74], Thailand [71,135], Taiwan [28,56,131,134], South Korea [139], Vietnam [75,141], China [40,41,89,138,140,142,147], Cambodia [146] and Singapore [144]. In addition, JEV was detected over the last few decades in Cx. tritaeniorhynchus in Sri Lanka [52], India [39,53,62,63,64,65,66,68,69,99,100,101,121,122,126], Malaysia [29], Taiwan [116], Vietnam [106], South Korea [45,46,47,48,123], Taiwan [54,124], Japan [125] and China [85,127,128]. Simultaneously to the JEV isolation, Mitamura and colleagues also used Cx. tritaeniorhynchus mosquitoes naturally infected with JEV to demonstrate transmission of JEV to mice [148]. A study on the replication capacity of the JEV chimeric vaccine confirmed replication of JEV (wildtype SA-14) in Cx. tritaeniorhynchus infected either by intrathoracic injection or by feeding an infectious blood meal [162]. Others were also able to successfully infect mosquitoes from Japan by feeding them on viremic pigs [161] or infecting them via an artificial, infectious blood meal [174], and infect mosquitoes from a laboratory colony in Taiwan [31]. Vector competence studies showed successful JEV transmission to mice [23,90,92,148,152], horses [151], pigs [150,152], young chickens [51,60,61,110,149,151,154,156], young ducks [49] as well as several ardeid birds like Black-crowned Night Herons, Plumed Egrets, Great Egrets [150] and Indian pond herons [154]. Most of the early studies infected the respective mosquitoes by feeding them on viremic pigs or chickens [49,51,60,61,110,150,151,152,154,156,161,169]. Nowadays vector competence studies use mostly artificial blood meals to infect mosquitoes because the virus titer can be easier modified then in viremic animals used for feeding. This technique was used to demonstrate the vector competence of Cx. tritaeniorhynchus from Japan [23,153,157], Singapore [149] and Taiwan [92,155]. Another route for infection is the direct injection of JEV into the thorax of a mosquito. This is rarely used for vector competence, as results from this kind of studies cannot elusively document vector competence as the virus is not ingested and therefore has not crossed the mosquito midgut barrier. Several studies using Cx. tritaeniorhynchus showed that with intrathoracic injection high infection rates can be reached, also leading to high transmission rates [105,155]. The investigation of Chen and colleagues also showed that the JEV vaccine strain J 2-8 is not able to replicate in mosquitoes whereas its parent viral strain SA-14 establishes a disseminated infection resulting in successful JEV transmission to mice [155]. Cx. tritaeniorhynchus mosquitoes were also used to investigate the influence of Bagaza virus on the replication of JEV [172]. The replication was impaired but the study did not look into the effect on transmission. A recent investigation of a Cx. tritaeniorhynchus laboratory colony in Japan showed transmission of three different JEV genotypes. Similar to the results obtained with Cx. quinquefasciatus [114], JEV genotype I had a shorter EIP in Cx. tritaeniorhynchus compared to genotypes III and V, whereas the transmission rates where similar for all three tested genotypes [157].
Culex (Culex) vishnui Theobald, 1901
JEV was detected in Cx. vishnui in Malaysia [29], Vietnam [141], and several times in India [53,62,63,100,101,143,158,159]. Additionally, the virus was isolated from mosquitoes in India [53,160], Thailand [135] and Indonesia [57]. A laboratory colony of Indian Cx. vishnui was also used to prove the vector competence of this species with a JEV strain isolated in India [105].
3.2. Potential Vectors
Eleven mosquito species, belonging to five genera, were successfully tested under laboratory conditions to transmit JEV, but the virus was neither detected nor isolated from field-caught mosquitoes. The species fulfilling these criteria are Ae. detritus, Ae. dorsalis, Ae. japonicus, Ae. kochi, Ae. nigromaculis, Ae. notosciptus, An. tessellatus, Cx. tarsalis, Cs. annulata, Cs. inornata and Ve. funerea.
Aedes (Ochlerotatus) detritus (Haliday, 1833)
Ae. detritus (Ochlerotatus detritus) mosquitoes from England, were investigated for their potential to transmit JEV at 23 °C and 28 °C [111]. Infection and dissemination rates were similar, but the transmission was more efficient at the lower temperature (67% for 23 °C vs. 33% for 28 °C at 21 dpi). However, the number of investigated mosquitoes was also very low. This finding is lower than Cx. quinquefasciatus transmission rates observed in the same study at elevated temperatures (70% for 28 °C vs. 50% for 23 °C at 21 dpi).
Aedes (Ochlerotatus) dorsalis (Meigen, 1830)
In 1946, Reeves and Hammon published their findings on the JEV transmission by North American Ae. dorsalis [36]. Mosquitoes were infected by feeding them with an artificial, infectious blood meal. Sixteen days later, they were allowed to bite young mice. One of the six mice used in this experiment was successfully infected with JEV from the bite of Ae. dorsalis.
Aedes (Hulecoeteomyia) japonicus (Theobald, 1901)
Ae. japonicus was tested several times for its capacity to transmit JEV. Experiments with Ae. japonicus from Japan [23] were able to document transmission to mice when they infected the mosquitoes by feeding on viremic chicken displaying a low viral titer (103.7 pfu/mL) as well as by feeding them an artificial blood meal with a high viral dose (106.2 pfu/mL). Huber and colleagues showed that Ae. japonicus collected in Germany can be successfully infected with JEV [175]. A recent study with a laboratory colony of Ae. japonicus from Japan demonstrated the successful infection, dissemination and transmission of three different JEV genotypes [157].
Aedes (Ochlerotatus) kochi (Dönitz, 1901)
A large study investigating the JEV vector capacity of 16 mosquito species from Australia included Ae. kochi and showed that one of the two field-collected mosquito populations was able to transmit JEV [38]. Although the number of investigated mosquitoes was very low, the transmission to mice was observed only with a single mosquito.
Aedes (Ochlerotatus) nigromaculis (Ludlow, 1906)
In the early 1940s, Ae. nigromaculis mosquitoes from the US were orally infected via an artificial blood meal [36]. Successful infection was proven by recovering the virus from blood-fed mosquitoes 16 days after infection. Additionally, this study demonstrated JEV transmission to mice as several mice developed encephalitis and the virus was cultivated from brain samples of these mice.
Aedes (Rampamyia) notoscriptus (Skuse, 1889)
Van den Hurk and colleagues also investigated an Ae. notoscriptus (mentioned as Ochlerotatus notoscriptus) laboratory colony, and field-caught mosquitoes from Queensland, Australiafor their JEV vector competence [38]. From laboratory colony mosquitoes, they detected transmission to three of the eleven mice in their experiment. As seen with other field-caught populations in their study, the initial number of infected mosquitoes and their survival rate was very low, and the field mosquitoes were not probing on the infant mice therefore the transmission could not be tested but only infection and dissemination of JEV. Similar problems were seen with field-caught Ae. notoscriptus from New Zealand [166]. This study was not even able to detect infection in their mosquitoes two weeks after the infectious blood meal. Therefore, the vector competence status of Ae. notoscriptus remains questionable.
Anopheles (Anopheles) tessellatus Theobald, 1901
A laboratory strain of An. tessellatus was shown to be able to transmit JEV to young chickens, the mosquitoes being infected also by feeding on viremic chicks [110]. This is the only study proving JEV vector competence for an Anopheles species. Other studies on An. hyrcanus [176] and An. freeborni [36] were not able to demonstrate transmission to chicks or mice.
Culex (Culex) tarsalis Coquillett, 1896
A study using North American Cx. tarsalis infected with JEV via an artificial blood meal was performed [36]. The infection of the mosquitoes was proven by detecting the virus from blood-fed mosquitoes more than two weeks after the infectious blood meal. Furthermore, transmission to young mice was also demonstrated.
Culiseta (Culiseta) annulata Schrank, 1776
In a lab survey performed in the UK [95], Cs. annulata was shown to be able to transmit JEV, but only when the mosquitoes were kept at 21 °C, whereas when held at 24 °C no virus was detectable in their saliva.
Culiseta (Culiseta) inornata Williston, 1893
As with other US mosquitoes, Reeves and Hammon also tested Cs. inornata for its JEV vector competence [36]. Infection was demonstrated by recovering the virus from blood-fed mosquitoes after artificial blood meal, and the transmission to young mice was shown too. In contrast, Cs. incidens could also get infected with JEV but was not able to transmit the virus in return [36].
Verrallina (Verrallina) funerea Theobald, 1903
Besides other Australian mosquito species, field-caught Ve. funereal mosquitoes from North Queensland were shown to be able to transmit JEV to young mice when infected via an artificial blood meal [38].
3.3. Mosquito Species with JEV Isolation in the Field
Additionally, to confirmed and potential vectors there are mosquito species with documented JEV detection and/or isolation from field mosquitoes (Table 2).
Seven other mosquito species were demonstrated either to be unable to transmit the virus or their JEV vector competence was not tested so far. However, reports on JEV isolations from field-caught mosquitoes of these species imply their possible role as JEV vectors (Table 2). JEV strains were isolated from Ae. butleri and Ae. lineatopennis in Malaysia [29,59]. Also the isolation of JEV from various Anopheles species was several times successful: from An. annularis and An. vagus in Indonesia [57,133], from An. sinensis in Yunnan province in China [40,41,142], from An. subpictus [102], and from An. peditaeniatus [58] in India, and from diverse Anopheles as well as Mansonia species in Malaysia [74]. Finally, the virus was also isolated from Cx. annulus and Cx. fuscanus in Taiwan [28,56,131,177,178], and from Coquillettiddia ochracea from Shandong province, China [89].

Table 2.
Mosquito species with evidence for JEV from field-caught mosquitoes.
Table 2.
Mosquito species with evidence for JEV from field-caught mosquitoes.
| Mosquito Species | Virus Detection in Field-Caught Mosquitoes | Virus Isolation from Field-Caught Mosquitoes |
|---|---|---|
| Aedes butleri | 1992 in Malaysia [29]; 1992–1993 in Malaysia [59] | |
| Aedes curtipes | 1968 in Malaysia [74] | |
| Aedes lineatopennis | 1992–1993 in Malaysia [59] | |
| Aedes vexans nocturnus | 2000–2004 in Taiwan [116] | |
| Anopheles ssp | 1969 in Malaysia [74] | |
| Anopheles annularis | 1978–1980 in Indonesia [57]; 1979 in Indonesia [133] | |
| Anopheles barbirostris | 1973 in India [158]; 2011–2013 in India [39] | |
| Anopheles hyrcanus | 1973 in India [158]; 1974–1975 in India [179] | |
| Anopheles pallidus | 2011–2013 in India [39] | |
| Anopheles peditaeniatus | 1985–1987 in India [58] | |
| Anopheles subpictus | 1996 in India [121]; 1997–1999 in India [180]; 2011–2013 in India [39] | 1977–1979 in India [102] |
| Anopheles sinensis | 2007 in China [40]; 2007–2009 in China [142]; 2009–2010 in China [41] | |
| Anopheles vagus | 1978–1980 in Indonesia [57]; 1979 in Indonesia [133] | |
| Coquillettidia ochracea | 2015 in China [89] | |
| Culex annulus | 1967 in Taiwan [177]; 1969 in Taiwan [131,178]; 1974–1976 in Taiwan [56]; 1995–1996 in Taiwan [28] | |
| Culex epidesmus | 1974–1975 in India [179] | |
| Culex fuscanus | 1995–1996 in Taiwan [28] | |
| Culex infula | 2011–2013 in India [39] | |
| Culex orientalis | 2012 in South Korea [84] | |
| Culex rubithoracis | 2002–2004 in Taiwan [116] | |
| Culex whitmorei | 1962–1966 in India [129]; 1987–1988 in Sri Lanka [52] | |
| Mansonia ssp | 1969 in Malaysia | |
| Mansonia bonneae/dives | 1969 in Malaysia [74] | |
| Mansonia annulifera | 1999–2000 in India [65]; 2011–2013 in India [39] | |
| Mansonia indiana | 1996 in India [121]; 1999–2000 in India [65] | |
| Mansonia uniformis | 1987–1988 in Sri Lanka [52]; 1996 in India [121]; 1999–2000 in India [65]; 2011–2013 in India [39] | 1969 in Malaysia [74] |
4. Discussion
This article mainly highlights the diversity of mosquito species able to transmit JEV and the diversity of methodology used for vector competence experiments.
4.1. Diversity of Mosquito Vector Species and Consequence in Terms of Public Health
Based on our literature search we found 14 mosquito species as confirmed vectors and eleven species we considered as potential vectors. An earlier meta-analysis on JEV infection on vectors and hosts [20] highlighted the importance of the Culex genus as important JEV vectors. However, the highest susceptibility (measured as minimum infection rate) was found for An. subpictus. Overall, the highest JEV infection rates were reported in Cx. annulirostris, Cx. sitiens, Cx. fuscocephala and Ae. japonicus [21]. A recent study highlighted the importance of Cx. tritaeniorhynchus, Cx. gelidus, Cx. sitiens and Cx. fuscocephala as JEV vector species [9,21], which is in accordance with these species categorized as confirmed vector species. Generally, Cx. tritaeniorhynchus is acknowledged as an important vector species, and is certainly studied the most (Table S1) [11,38,181]. In contrast, Cx. vishnui was found positive in the field several times in different countries (Table 1), but only one study confirmed its capability to transmit the virus [105]. This could be partially explained by the difficulty to rear this species and subsequently the issues to perform vector competence studies.
All 14 mosquito species described here as competent vectors are also known to bite pigs and humans, and are generally well adapted to live in close proximity to humans and human settlements. The biting behavior is an important component of the vectorial capacity of a vector species. For Ae. albopictus, Ae. vexans and Ar. subalbatus [182,183,184] it is well documented that they bite birds, mammals (including pigs) and humans. Culex mosquitoes are generally described as ornithophilic species meaning that they prefer to bite birds. The confirmed JEV vectors, Cx. annulirostris, Cx. bitaeniorhynchus, Cx. gelidus, Cx. fuscocephala, Cx. pipiens, Cx. quinquefasciatus, Cx. sitiens, Cx. tritaeniorhyncus and Cx. vishnui are mainly opportunistic feeders and were reported to bite both birds and mammals [12,185,186,187,188,189,190]. Moreover, Cx. pipiens and Cx. quinquefasciatus seem to prefer feeding on humans rather than birds [189,191,192,193]. Also, Cx. gelidus, Cx. tritaeniorhynchus and Cx. vishnui prefer large mammals (pigs and cows) over birds as shown in a large field study investigating the trophic behavior of Cambodian mosquitoes under natural conditions [191]. The host feeding behavior combined with the competence for JEV transmission of these vectors should be issued in risk assessment studies.
One of the first public health issues is how well the combination virus-vector can adapt to weather conditions different from its current geographic range. Indeed, the increasingly frequent and rapid population movements often participate in emergence or re-emergence of viruses. In particular, with species such as Ae. vexans or Cx. pipiens, well adapted to temperate climates, there is a real risk of seeing JE emerging in temperate countries. In the Southern hemisphere, Cx. annulirostris is now spreading further and further South [194] or Ae. albopictus invades habitats in Southern and Central Europe, as well as temperate regions in the United States and recently in Canada [195,196,197]. In addition, the main JEV vector Cx. tritaeniorhynchus is present across South, East and Southeast Asia, demonstrating its high adaptability among different habitats and climate zones [198,199,200].
This review also highlights one of the recurring issues related to the discipline of medical entomology, and to taxonomy in general. In fact, on several occasions, we have come up against the difficulty of finding valid species names (Cx. molestus and Och. vivax for Cx. pipiens and Ae. vexans, respectively). Even in a recent meta-analysis [20], discrepancies can be found. Mosquito taxonomy is always changing and keeping up with actual and valid names can be a daunting task. As an example, the name of Aedes albopictus is widely known by a large part of the scientific and public communities and stakeholders, while its recent change to Stegomyia albopicta creates communication problems since people might think that it is another species. While adapting and updating taxonomical names is important to try fitting a phylogenetic reality, taxonomists also have to bear in mind that changing names can potentially alter public health communication.
4.2. Diversity of Vector Competence Experiments: Problems and Solutions
For some vector species, it is clearly established that they are competent vectors for JEV as the virus was detected and/or isolated several times form this species and transmission was observed in various experimental infection experiments. In this review, we identified Ae. albopictus, Ae. vexans, Ae. vigilax, Ar. subalbatus, Cx. annulirostris, Cx. bitaeniorhynchus, Cx. fuscophala, Cx. gelidus, Cx. pipiens, Cx. pseudovishnui, Cx. quinquefasciatus, Cx. sitiens, Cx. tritaeniorhynchus and Cx. vishnui as confirmed vectors. However, for some species there are contradictory results regarding their potential of JEV transmission. Discrepancies in the outcome of vector competence studies might be caused by differences in the infection method, the mosquito population or the virus used for infection. Vector competence studies have several practical restrictions, leading to a broad variety of experimental designs that were used over the past decades. Therefore, the experimental determination of vector competence poses several challenges, and a broad range of mosquitoes, JEV strains and infection methods can be used in the different studies investigating the vector competence.
4.2.1. Influence of Mosquito Origin and Rearing on Vector Competence
The mosquitoes that can be used for the studies range from established colonies, long adapted to laboratory conditions, to early generations (F1–F5) of field-caught mosquitoes. The origin of the mosquitoes itself can influence the outcome of the vector competence studies as was nicely demonstrated with a laboratory colony of Cx. annulirostris and Cx. quinquefasciatus able to transmit JEV, whereas field-caught mosquitoes of the same species showed limited or no transmission [38]. However, mosquitoes always have to be reared at least for one generation in the laboratory, as wild adult mosquitoes can be collected to get eggs from females or by directly collecting eggs and larvae in breeding sites. Therefore, even if F1 mosquitoes are often used for vector competence studies, the rearing under laboratory condition might influence their potential for virus transmission. Several studies have shown that the environmental conditions [153,201], and therefore also the rearing in the laboratory, can influence the transmission of JEV in certain mosquito species. The temperature is an especially important parameter as seen in comparative vector competence studies [95,98,111].
4.2.2. Influence of Virus Strain on Vector Competence
The virus used for the vector competence study can dramatically influence their outcome. In the early days of JEV research, virus strains were isolated by inoculating suckling mice intracranial. This method favors the isolation of neurotropic virus strains. The isolates were then passaged several times in mice, which might lead to further adaptation of the virus strains. Nowadays, arboviruses are mostly isolated in cell culture using mosquito cell lines. However, since decades, the most commonly used cell line for arbovirus isolation is C6/36 [202], which is a cell line adapted from larvae of Ae. albopictus. This could also introduce a bias, especially for viruses where Aedes species are not the primary vectors as it is the case for JEV. It was shown that JEV attenuation through several cell culture passages can lead to loss of infectivity in mosquitoes [203]. Additionally, the viral titer used for infecting mosquitoes is a crucial bottleneck for proving vector competence. Many studies use a rather high viral load (above 105 infectious units/mL; see Table S1). It is questionable if high infection titers represent the biological situation properly as peak viremia titers are reported for pigs with 103 to 105 TCID50/mL [150,204,205] and for young poultry with 104 to 106 pfu/mL [49,206] in experimental studies. This issue is also documented by the work of Gould and colleagues on Cx. gelidus showing that mosquitoes fed on viremic young chicken could successfully transmit JEV but not if the mosquitoes fed on viremic horses [151]: horses are dead-end-hosts and therefore do not develop sufficiently high viremia to infect mosquitoes. The influence of the virus strain used for vector competence studies was also observed, showing higher infection, dissemination and transmission rates, as well as a shorter EIP for JEV genotype I strains than for genotype III isolates [114]. A shorter EIP for JEV genotype I was also observed with Cx. tritaeniorhynchus [157].
4.2.3. Influence of Applied Techniques on Vector Competence
The infection methodology and the outcome measurement are of importance. The first experimental infection and transmission study was published in 1936 in Japan with naturally JEV-infected Cx. tritaeniorhynchus [148]. Besides this initial study, later investigations infected mosquitoes under laboratory conditions. Nowadays, infection by feeding mosquitoes with artificial, infectious blood meals is the most common method, whereas early studies often let the mosquitoes feed on viremic animals like pigs or young chickens or ducks. The latter mimics the natural infection process; however, it creates the need to handle both viremic animal(s) and mosquitoes under special containment conditions (often biosafety level 3, BSL3). These restricted conditions drastically limit the number of institutions able to perform these experiments. Mosquitoes might show preferences for feeding on the blood of certain species (e.g., rodent blood often used due to availability and feeding preference of mosquitoes). All these factors can influence the outcome of the vector competence study. A standardization of the infection method appears a very challenging task, especially due to the varying feeding and host preferences of different mosquito species. Besides differences in the infection method, the outcome of infection is determined in various ways. Three different parameters can be measured by classical vector competence studies: infection, dissemination and transmission. For novel or unknown vector species, the EIP is an important parameter that should be determined by sampling the infected mosquitoes over a broad range of time points (from 7 dpi up to the death of the mosquitoes). However, most studies used 14 and/or 21 dpi as preferred time point(s).
4.2.4. Other Factors Influencing Vector Competence
Additionally, the vector competence might be influenced by local adaption mechanisms between vector and virus [205]. Despite the challenges of these experimental studies, the investigation of the vector competence of local mosquitoes to local arbovirus strains taking local conditions into account, as shown for other arboviruses with e.g., temperature fluctuations [207,208,209], is important, as it provides valuable data for risk assessments concerning the spread and (re)emergence of JEV [210,211,212]. The influence of the microbiome [213] of the local mosquito populations on the vector competence should be investigated. Increased focus on the influence of insect-specific viruses revealed their often impairing effect on virus transmission [214]. For instance, it was shown that co-infection with the Banna virus M14 (Reoviridae) decreased the infectivity of JEV in Cx. tritaeniorhynchus dramatically [215]. In addition, the effect of infection with Wolbachia endobacteria should be considered, as they are widespread in several JEV vector species like Ae. albopictus, Cx. quinquefasciatus and Ar. subalbatus [42,216,217]. This is of particular interest as the impact of trans-infection of JEV vector species with non-naïve Wolbachia strains is currently under investigation as a measure for vector control [218].
The broad diversity in the experimental design, as well as used mosquitoes and JEV strains, can be easily seen for mosquito species with many studies such as Cx. quinquefasciatus and Cx. tritaeniorhynchus (Table S1). Whereas the studies nearly always come to the conclusion that these species are competent JEV vectors, the level of transmission varies greatly between the studies. As the studies used very different methods to investigate the vector competence, the detailed outcome of these experiments are hardly comparable.
In addition, the transmission capacity on specific ecological niches should not be neglected. Some studies investigated JEV transmission, including bats in its epidemiological cycle. It was shown that Ae. albopictus and Cx. quinquefasciatus can be infected with JEV strains found in bats [163], and that Cx. annulirostris can transmit the virus to flying foxes (Pteropus alecto) [44]. Lizards were also demonstrated to be competent hosts for JEV and supporting JEV transmission via Cx. pipiens pallens and Cx. quinquefasciatus [91].
Many studies were performed in countries that have the necessary resources (laboratory capacity, financial support, trained personnel) like Japan, Australia, USA, Taiwan, India or South Korea (Table S1). This is dangerous as there is a lack of data for many of the developing countries where JEV is endemic leading to neglected JEV awareness, preparedness and control.
Finally, survival rate upon JEV infection should also be carefully monitored, as a certain amount of mosquitoes dies from JEV infection [157]. This effect of JEV on the lifespan of the mosquitoes is important to consider when studying the vector competence and estimating the risk of transmission.
5. Conclusions
We described the variety of species able to transmit JEV successfully and discussed the broad variety of vector competence studies and the challenges that come with them. Overall, the risk assessment on potential vectors should always include information on the abundance, spatial and temporal distribution of the mosquito species, as well as surveillance of wild mosquitoes for the presence of JEV, and not only be based on vector competence experiments performed under controlled conditions in the laboratory. The JEV vector competence should preferably be studied in the local context, infecting local mosquitoes with local viral strains under local climate conditions to achieve reliable data. In addition, harmonization of the design of vector competence investigations would lead to better comparable data, informing vector and disease control measures.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/v13061154/s1, Table S1: Mosquito vector competence studies for JEV.
Author Contributions
Conceptualization, S.B.; methodology, H.A.; formal analysis, H.A., P.-O.M., S.B.; writing—original draft preparation, H.A., S.B.; writing—review and editing, P.-O.M., V.C. All authors have read and agreed to the published version of the manuscript.
Funding
H.A. is supported by the German Centre for International Migration and Development. The postdoctoral fellowship of P.-O.M. is supported by the Calmette and Yersin Programme of the Institut Pasteur Department of International Affairs.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Lindahl, J.F. Japanese Encephalitis Virus in Pigs and Vectors in the Mekong Delta. With Special Reference to Urban Farming. Ph.D. Thesis, Swedish University of Agricultural Sciences, Upsalla, Sweden, 2012. [Google Scholar]
- Mackenzie, J.S. Emerging Zoonotic Encephalitis Viruses: Lessons from Southeast Asia and Oceania. J. Neurovirol. 2005, 11, 434–440. [Google Scholar] [CrossRef]
- Campbell, G.L.; Hills, S.L.; Fischer, M.; Jacobson, J.A.; Hoke, C.H.; Hombach, J.M.; Marfin, A.A.; Solomon, T.; Tsai, T.F.; Tsu, V.D.; et al. Estimated Global Incidence of Japanese Encephalitis: A Systematic Review. Bull. World Health Organ. 2011, 89, 766–774, 774A–774E. [Google Scholar] [CrossRef] [PubMed]
- Mathers, C.D.; Ezzati, M.; Lopez, A.D. Measuring the Burden of Neglected Tropical Diseases: The Global Burden of Disease Framework. PLoS Negl. Trop. Dis. 2007, 1, e114. [Google Scholar] [CrossRef] [PubMed]
- Solomon, T.; Ni, H.; Beasley, D.W.C.; Ekkelenkamp, M.; Cardosa, M.J.; Barrett, A.D.T. Origin and Evolution of Japanese Encephalitis Virus in Southeast Asia. J. Virol. 2003, 77, 3091–3098. [Google Scholar] [CrossRef] [PubMed]
- Ladreyt, H.; Durand, B.; Dussart, P.; Chevalier, V. How Central Is the Domestic Pig in the Epidemiological Cycle of Japanese Encephalitis Virus? A Review of Scientific Evidence and Implications for Disease Control. Viruses 2019, 11, 949. [Google Scholar] [CrossRef]
- Ricklin, M.E.; García-Nicolás, O.; Brechbühl, D.; Python, S.; Zumkehr, B.; Nougairede, A.; Charrel, R.N.; Posthaus, H.; Oevermann, A.; Summerfield, A. Vector-Free Transmission and Persistence of Japanese Encephalitis Virus in Pigs. Nat. Commun. 2016, 7, 10832. [Google Scholar] [CrossRef] [PubMed]
- Diallo, A.O.I.; Chevalier, V.; Cappelle, J.; Duong, V.; Fontenille, D.; Duboz, R. How Much Does Direct Transmission between Pigs Contribute to Japanese Encephalitis Virus Circulation? A Modelling Approach in Cambodia. PLoS ONE 2018, 13, e0201209. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, A.R.S.; Strathe, E.; Etcheverry, L.; Cohnstaedt, L.W.; McVey, D.S.; Piaggio, J.; Cernicchiaro, N. Assessment of Data on Vector and Host Competence for Japanese Encephalitis Virus: A Systematic Review of the Literature. Prev. Vet. Med. 2018, 154, 71–89. [Google Scholar] [CrossRef]
- Oliveira, A.R.S.; Cohnstaedt, L.W.; Cernicchiaro, N. Japanese Encephalitis Virus: Placing Disease Vectors in the Epidemiologic Triad. Ann. Entomol. Soc. Am. 2018, 111, 295–303. [Google Scholar] [CrossRef]
- Pearce, J.C.; Learoyd, T.P.; Langendorf, B.J.; Logan, J.G. Japanese Encephalitis: The Vectors, Ecology and Potential for Expansion. J. Travel Med. 2018, 25, S16–S26. [Google Scholar] [CrossRef]
- Sudeep, A.B. Culex Gelidus: An Emerging Mosquito Vector with Potential to Transmit Multiple Virus Infections. J. Vector Borne Dis. 2014, 51, 251–258. [Google Scholar] [PubMed]
- Boussès, P.; Dehecq, J.S.; Brengues, C.; Fontenille, D. Inventaire actualisé des moustiques (Diptera: Culicidae) de l’île de La Réunion, océan Indien. Bull. Soc. Pathol. Exot. 2013, 106, 113–125. [Google Scholar] [CrossRef] [PubMed]
- Alves, J.; de Pina, A.; Diallo, M.; Dia, I. First Report of Culex (Culex) Tritaeniorhynchus Giles, 1901 (Diptera: Culicidae) in the Cape Verde Islands. Zool. Caboverdiana 2014, 5, 14–19. [Google Scholar]
- Fall, A.; Diaïté, A.; Seck, M.; Bouyer, J.; Lefrançois, T.; Vachiéry, N.; Aprelon, R.; Faye, O.; Konaté, L.; Lancelot, R. West Nile Virus Transmission in Sentinel Chickens and Potential Mosquito Vectors, Senegal River Delta, 2008–2009. Int. J. Environ. Res. Public Health 2013, 10, 4718–4727. [Google Scholar] [CrossRef] [PubMed]
- Lytra, I.; Emmanouel, N. Study of Culex Tritaeniorhynchus and Species Composition of Mosquitoes in a Rice Field in Greece. Acta Trop. 2014, 134, 66–71. [Google Scholar] [CrossRef] [PubMed]
- Sirivanakarn, S. Medical Entomology Studies—III. A Revision of the Subgenus Culex in the Oriental Region (Diptera: Culicidae). Cont. Am. Entomol. Inst. 1976, 12, 1–271. [Google Scholar]
- Barnett, H.C.; Gould, D.J. Colonization of Culex Gelidus Theobald and Some Resultant Effects on Its Biology. Trans. R Soc. Trop. Med. Hyg. 1962, 56, 423–428. [Google Scholar] [CrossRef]
- Gould, D.J.; Barnett, H.C.; Suyemoto, W. Transmission of Japanese Encephalitis Virus by Culex Gelidus Theobald. Trans. R Soc. Trop. Med. Hyg. 1962, 56, 429–435. [Google Scholar] [CrossRef]
- Oliveira, A.R.S.; Cohnstaedt, L.W.; Strathe, E.; Hernández, L.E.; McVey, D.S.; Piaggio, J.; Cernicchiaro, N. Meta-Analyses of the Proportion of Japanese Encephalitis Virus Infection in Vectors and Vertebrate Hosts. Parasites Vectors 2017, 10, 418. [Google Scholar] [CrossRef]
- Oliveira, A.R.S.; Cohnstaedt, L.W.; Strathe, E.; Etcheverry, L.; McVey, D.S.; Piaggio, J.; Cernicchiaro, N. Meta-Analyses of Japanese Encephalitis Virus Infection, Dissemination, and Transmission Rates in Vectors. Am. J. Trop. Med. Hyg. 2018, 98, 883–890. [Google Scholar] [CrossRef] [PubMed]
- Rückert, C.; Ebel, G.D. How Do Virus–Mosquito Interactions Lead to Viral Emergence? Trends Parasitol. 2018, 34, 310–321. [Google Scholar] [CrossRef]
- Takashima, I.; Rosen, L. Horizontal and Vertical Transmission of Japanese Encephalitis Virus by Aedes Japonicus (Diptera: Culicidae). J. Med. Entomol. 1989, 26, 454–458. [Google Scholar] [CrossRef] [PubMed]
- Rosen, L.; Tesh, R.; Lien, J.; Cross, J. Transovarial Transmission of Japanese Encephalitis Virus by Mosquitoes. Science 1978, 199, 909–911. [Google Scholar] [CrossRef] [PubMed]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; Group, T.P. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef]
- Harbach, R. Mosquito Taxonomic Inventory—Updated 22 January 2021. Available online: http://mosquito-taxonomic-inventory.info/sites/mosquito-taxonomic-inventory.info/files/Valid%20Species%20List_92.pdf (accessed on 28 January 2021).
- Wilkerson, R.C.; Linton, Y.-M.; Fonseca, D.M.; Schultz, T.R.; Price, D.C.; Strickman, D.A. Making Mosquito Taxonomy Useful: A Stable Classification of Tribe Aedini That Balances Utility with Current Knowledge of Evolutionary Relationships. PLoS ONE 2015, 10, e0133602. [Google Scholar] [CrossRef] [PubMed]
- Weng, M.H.; Lien, J.C.; Wang, Y.M.; Lin, C.C.; Lin, H.C.; Chin, C. Isolation of Japanese Encephalitis Virus from Mosquitoes Collected in Northern Taiwan between 1995 and 1996. J. Microbiol. Immunol. Infect. 1999, 32, 9–13. [Google Scholar]
- Vythilingam, I.; Oda, K.; Chew, T.K.; Mahadevan, S.; Vijayamalar, B.; Morita, K.; Tsuchie, H.; Igarashi, A. Isolation of Japanese Encephalitis Virus from Mosquitoes Collected in Sabak Bernam, Selangor, Malaysia in 1992. J. Am. Mosq. Control Assoc. 1995, 11, 94–98. [Google Scholar]
- Su, C.-L.; Yang, C.-F.; Teng, H.-J.; Lu, L.-C.; Lin, C.; Tsai, K.-H.; Chen, Y.-Y.; Chen, L.-Y.; Chang, S.-F.; Shu, P.-Y. Molecular Epidemiology of Japanese Encephalitis Virus in Mosquitoes in Taiwan during 2005–2012. PLoS Negl. Trop. Dis. 2014, 8, e3122. [Google Scholar] [CrossRef]
- Weng, M.H.; Lien, J.C.; Wang, Y.M.; Wu, H.L.; Chin, C. Susceptibility of Three Laboratory Strains of Aedes Albopictus (Diptera: Culicidae) to Japanese Encephalitis Virus from Taiwan. J. Med. Entomol. 1997, 34, 745–747. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.J.; Dong, C.F.; Chiou, L.Y.; Chuang, W.L. Potential Role of Armigeres Subalbatus (Diptera: Culicidae) in the Transmission of Japanese Encephalitis Virus in the Absence of Rice Culture on Liu-Chiu Islet, Taiwan. J. Med. Entomol. 2000, 37, 108–113. [Google Scholar] [CrossRef]
- Nicholson, J.; Ritchie, S.A.; van den Hurk, A.F. Aedes Albopictus (Diptera: Culicidae) as a Potential Vector of Endemic and Exotic Arboviruses in Australia. J. Med. Entomol. 2014, 51, 661–669. [Google Scholar] [CrossRef]
- de Wispelaere, M.; Desprès, P.; Choumet, V. European Aedes Albopictus and Culex Pipiens Are Competent Vectors for Japanese Encephalitis Virus. PLoS Negl. Trop. Dis. 2017, 11, e0005294. [Google Scholar] [CrossRef] [PubMed]
- Hodes, H.L. Experimental Transmission of Japanese B. Encephalitis by Mosquitoes and Mosquito Larvae. Bull. Johns Hopkins Hosp. 1946, 79, 358. [Google Scholar]
- Reeves, W.C.; Hammon, W.M. Laboratory Transmission of Japanese B Encephalitis Virus by Seven Species (Three Genera) of North American Mosquitoes. J. Exp. Med. 1946, 83, 185–194. [Google Scholar] [CrossRef] [PubMed]
- Hanna, J.N.; Ritchie, S.A.; Phillips, D.A.; Lee, J.M.; Hills, S.L.; van den Hurk, A.F.; Pyke, A.T.; Johansen, C.A.; Mackenzie, J.S. Japanese Encephalitis in North Queensland, Australia, 1998. Med. J. Aust. 1999, 170, 533–536. [Google Scholar] [CrossRef]
- van den Hurk, A.F.; Nisbet, D.J.; Hall, R.A.; Kay, B.H.; Mackenzie, J.S.; Ritchie, S.A. Vector Competence of Australian Mosquitoes (Diptera: Culicidae) for Japanese Encephalitis Virus. J. Med. Entomol. 2003, 40, 82–90. [Google Scholar] [CrossRef] [PubMed]
- Thenmozhi, V.; Balaji, T.; Selvam, A.; Venkatasubramani, K.; Dhananjeyan, K.J. A Longitudinal Study on Abundance and Infection Frequency of Japanese Encephalitis Vectors in Tirunelveli District, Tamil Nadu, India. Int. J. Mol. Res. 2015, 2, 166–169. [Google Scholar]
- Feng, Y.; Fu, S.; Zhang, H.; Li, M.; Zhou, T.; Wang, J.; Zhang, Y.; Wang, H.; Tang, Q.; Liang, G. Distribution of Mosquitoes and Mosquito-Borne Viruses along the China-Myanmar Border in Yunnan Province. Jpn. J. Infect. Dis. 2012, 65, 215–221. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Lu, H.-J.; Liu, Z.-J.; Jing, J.; Ren, J.-Q.; Liu, Y.-Y.; Lu, F.; Jin, N.-Y. Japanese Encephalitis Virus in Mosquitoes and Swine in Yunnan Province, China 2009-2010. Vector-Borne Zoonotic Dis. 2013, 13, 41–49. [Google Scholar] [CrossRef] [PubMed]
- Tsai, K.-H.; Huang, C.-G.; Wu, W.-J.; Chuang, C.-K.; Lin, C.-C.; Chen, W.-J. Parallel Infection of Japanese Encephalitis Virus and Wolbachia within Cells of Mosquito Salivary Glands. J. Med. Entomol. 2006, 43, 752–756. [Google Scholar] [CrossRef]
- Ritchie, S.A.; Phillips, D.; Broom, A.; Mackenzie, J.; Poidinger, M.; van den Hurk, A. Isolation of Japanese Encephalitis Virus from Culex Annulirostris in Australia. Am. J. Trop. Med. Hyg. 1997, 56, 80–84. [Google Scholar] [CrossRef]
- van den Hurk, A.F.; Smith, C.S.; Field, H.E.; Smith, I.L.; Northill, J.A.; Taylor, C.T.; Jansen, C.C.; Smith, G.A.; Mackenzie, J.S. Transmission of Japanese Encephalitis Virus from the Black Flying Fox, Pteropus Alecto, to Culex Annulirostris Mosquitoes, despite the Absence of Detectable Viremia. Am. J. Trop. Med. Hyg. 2009, 81, 457–462. [Google Scholar] [CrossRef] [PubMed]
- Takhampunya, R.; Kim, H.-C.; Tippayachai, B.; Kengluecha, A.; Klein, T.A.; Lee, W.-J.; Grieco, J.; Evans, B.P. Emergence of Japanese Encephalitis Virus Genotype V in the Republic of Korea. Virol. J. 2011, 8, 449. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.C.; Takhampunya, R.; Tippayachai, B.; Chong, S.-T.; Park, J.-Y.; Kim, M.-S.; Seo, H.-J.; Yeh, J.-Y.; Lee, W.-J.; Lee, D.-K.; et al. Japanese Encephalitis Virus in Culicine Mosquitoes (Diptera: Culicidae) of the Republic of Korea, 2008–2010. Mil. Med. 2015, 180, 158–167. [Google Scholar] [CrossRef]
- Kim, H.C.; Klein, T.A.; Takhampunya, R.; Evans, B.P.; Mingmongkolchai, S.; Kengluecha, A.; Grieco, J.; Masuoka, P.; Kim, M.-S.; Chong, S.-T.; et al. Japanese Encephalitis Virus in Culicine Mosquitoes (Diptera: Culicidae) Collected at Daeseongdong, a Village in the Demilitarized Zone of the Republic of Korea. J. Med. Entomol. 2011, 48, 1250–1256. [Google Scholar] [CrossRef] [PubMed]
- Seo, H.-J.; Kim, H.C.; Klein, T.A.; Ramey, A.M.; Lee, J.-H.; Kyung, S.-G.; Park, J.-Y.; Cho, Y.S.; Cho, I.-S.; Yeh, J.-Y. Molecular Detection and Genotyping of Japanese Encephalitis Virus in Mosquitoes during a 2010 Outbreak in the Republic of Korea. PLoS ONE 2013, 8, e55165. [Google Scholar] [CrossRef]
- Dhanda, V.; Banerjee, K.; Deshmukh, P.K.; Ilkal, M.A. Experimental Viraemia and Transmission of Japanese Encephalitis Virus by Mosquitoes in Domestic Ducks. Indian J. Med. Res. 1977, 66, 881–888. [Google Scholar]
- Banerjee, K.; Deshmukh, P.K.; Ilkal, M.A.; Dhanda, V. Transmission of Japanese Encephalitis Virus by Culex Bitaeniorhynchus Giles. Indian J. Med. Res. 1978, 67, 889–893. [Google Scholar] [PubMed]
- Banerjee, K.; Deshmukh, P.K.; Ilkal, M.A.; Dhanda, V. Comparative Susceptibility of Three Species of Mosquitoes to Infection with Japanese Encephalitis Virus. Indian J. Med. Res. 1983, 78, 603–606. [Google Scholar] [PubMed]
- Peiris, J.S.M.; Amerasinghe, F.P.; Amerasinghe, P.H.; Ratnayake, C.B.; Karunaratne, S.H.P.P.; Tsai, T.F. Japanese Encephalitis in Sri Lanka—The Study of an Epidemic: Vector Incrimination, Porcine Infection and Human Disease. Trans. R Soc. Trop. Med. Hyg. 1992, 86, 307–313. [Google Scholar] [CrossRef]
- Gajanana, A.; Rajendran, R.; Samuel, P.P.; Thenmozhi, V.; Tsai, T.F.; Kimura-Kuroda, J.; Reuben, R. Japanese Encephalitis in South Arcot District, Tamil Nadu, India: A Three-Year Longitudinal Study of Vector Abundance and Infection Frequency. J. Med. Entomol. 1997, 34, 651–659. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.-C.; Wang, C.-Y.; Teng, H.-J.; Chen, C.-F.; Chang, M.-C.; Lu, L.-C.; Lin, C.; Jian, S.-W.; Wu, H.-S. Comparison of the Efficacy of CO2-Baited and Unbaited Light Traps, Gravid Traps, Backpack Aspirators, and Sweep Net Collections for Sampling Mosquitoes Infected with Japanese Encephalitis Virus. J. Vector Ecol. 2011, 36, 68–74. [Google Scholar] [CrossRef] [PubMed]
- Gould, D.J.; Edelman, R.; Grossman, R.A.; Nisalak, A.; Sullivan, M.F. Study of Japanese Encephalitis Virus in Chiangmai Valley, Thailand. IV. Vector Studies. Am. J. Epidemiol. 1974, 100, 49–56. [Google Scholar] [CrossRef] [PubMed]
- Hsu, S.H.; Huang, W.C.; Cross, J.H. The Isolation of Japanese Encephalitis Virus from Taiwan Mosquitoes by Mosquito Cell Cultures and Mouse Inoculation. J. Med. Entomol. 1978, 14, 698–701. [Google Scholar] [CrossRef] [PubMed]
- Olson, J.G.; Ksiazek, T.G.; Tan, R.; Atmosoedjono, S.; Lee, V.H.; Converse, J.D. Correlation of Population Indices of Female Culex Tritaeniorhynchus with Japanese Encephalitis Viral Activity in Kapuk, Indonesia. Southeast Asian J. Trop. Med. Public Health 1985, 16, 337–342. [Google Scholar]
- Mourya, D.T.; Ilkal, M.A.; Mishra, A.C.; Jacob, P.G.; Pant, U.; Ramanujam, S.; Mavale, M.S.; Bhat, H.R.; Dhanda, V. Isolation of Japanese Encephalitis Virus from Mosquitoes Collected in Karnataka State, India from 1985 to 1987. Trans. R Soc. Trop. Med. Hyg. 1989, 83, 550–552. [Google Scholar] [CrossRef]
- Vythilingam, I.; Oda, K.; Mahadevan, S.; Abdullah, G.; Thim, C.S.; Hong, C.C.; Vijayamalar, B.; Sinniah, M.; Igarashi, A. Abundance, Parity, and Japanese Encephalitis Virus Infection of Mosquitoes (Diptera:Culicidae) in Sepang District, Malaysia. J. Med. Entomol. 1997, 34, 257–262. [Google Scholar] [CrossRef] [PubMed]
- Muangman, D.; Edelman, R.; Sullivan, M.J.; Gould, D.J. Experimental Transmission of Japanese Encephalitis Virus by Culex Fuscocephala. Am. J. Trop. Med. Hyg. 1972, 21, 482–486. [Google Scholar] [CrossRef] [PubMed]
- Okuno, T.; Mitchell, C.J.; Chen, P.S.; Hsu, S.; Ryu, E. Experimental Transmission of Japanese Encephalitis Virus by Culex Tritaeniorhynchus and C. Fuscocephalus. Ann. Trop. Med. Parasitol. 1975, 69, 203–206. [Google Scholar] [CrossRef] [PubMed]
- Tewari, S.C.; Thenmozhi, V.; Rajendran, R.; Appavoo, N.C.; Gajanana, A. Detection of Japanese Encephalitis Virus Antigen in Desiccated Mosquitoes: An Improved Surveillance System. Trans. R Soc. Trop. Med. Hyg. 1999, 93, 525–526. [Google Scholar] [CrossRef]
- Tewari, S.C.; Thenmozhi, V.; Arunachalam, N.; Philip Samuel, P.; Tyagi, B.K. Desiccated Vector Mosquitoes Used for the Surveillance of Japanese Encephalitis Virus Activity in Endemic Southern India. Trop. Med. Int. Health 2008, 13, 286–290. [Google Scholar] [CrossRef]
- Rajendran, R.; Thenmozhi, V.; Tewari, S.C.; Balasubramanian, A.; Ayanar, K.; Manavalan, R.; Gajanana, A.; Kabilan, L.; Thakare, J.P.; Satyanarayana, K. Longitudinal Studies in South Indian Villages on Japanese Encephalitis Virus Infection in Mosquitoes and Seroconversion in Goats. Trop. Med. Int. Health 2003, 8, 174–181. [Google Scholar] [CrossRef]
- Arunachalam, N.; Philip Samuel, P.; Hiriyan, J.; Thenmozhi, V.; Gajanana, A. Japanese Encephalitis in Kerala, South India: Can Mansonia (Diptera: Culicidae) Play a Supplemental Role in Transmission? J. Med. Entomol. 2004, 41, 456–461. [Google Scholar] [CrossRef]
- Upadhyayula, S.M.; Rao Mutheneni, S.; Nayanoori, H.K.; Natarajan, A.; Goswami, P. Impact of Weather Variables on Mosquitoes Infected with Japanese Encephalitis Virus in Kurnool District, Andhra Pradesh. Asian Pac. J. Trop. Med. 2012, 5, 337–341. [Google Scholar] [CrossRef]
- Ritchie, S.A.; van den Hurk, A.F.; Zborowski, P.; Kerlin, T.J.; Banks, D.; Walker, J.A.; Lee, J.M.; Montgomery, B.L.; Smith, G.A.; Pyke, A.T.; et al. Operational Trials of Remote Mosquito Trap Systems for Japanese Encephalitis Virus Surveillance in the Torres Strait, Australia. Vector-Borne Zoonotic Dis. 2007, 7, 497–506. [Google Scholar] [CrossRef] [PubMed]
- Ramesh, D.; Muniaraj, M.; Samuel, P.P.; Thenmozhi, V.; Venkatesh, A.; Nagaraj, J.; Tyagi, B.K. Seasonal Abundance & Role of Predominant Japanese Encephalitis Vectors Culex Tritaeniorhynchus & Cx. Gelidus Theobald in Cuddalore District, Tamil Nadu. Indian J. Med. Res. 2015, 142, S23–S29. [Google Scholar] [CrossRef] [PubMed]
- Thenmozhi, V.; Paramasivan, R.; Philip Samuel, P.; Kamaraj, T.; Balaji, T.; Dhananjeyan, K.J.; Venkatasubramani, K.; Leo, S.V.J.; Babu, R.S.; Govindarajan, R.; et al. Japanese Encephalitis Virus Isolation from Mosquitoes during an Outbreak in 2011 in Alappuzha District, Kerala. J. Vector Borne Dis. 2013, 50, 229–231. [Google Scholar]
- Pantawane, P.B.; Dhanze, H.; Ravi Kumar, G.V.P.P.S.; Grace, M.R.; Dudhe, N.C.; Bhilegaonkar, K.N. TaqMan Real-Time RT-PCR Assay for Detecting Japanese Encephalitis Virus in Swine Blood Samples and Mosquitoes. Anim. Biotechnol. 2019, 30, 267–272. [Google Scholar] [CrossRef] [PubMed]
- Simasathien, P.; Rohitayodhin, S.; Nisalak, A.; Singharaj, P.; Halstead, S.B.; Russell, P.K. Recovery of Japanese Encephalitis Virus from Wild Caught Mosquitoes in Thailand. Southeast Asian J. Trop Med. Public Health 1972, 3, 52–54. [Google Scholar] [PubMed]
- Simpson, D.I.; Bowen, E.T.; Platt, G.S.; Way, H.; Smith, C.E.; Peto, S.; Kamath, S.; Lim, B.L.; Lim, T.W. Japanese Encephalitis in Sarawak: Virus Isolation and Serology in a Land Dyak Village. Trans. R Soc. Trop. Med. Hyg. 1970, 64, 503–510. [Google Scholar] [CrossRef]
- Heathcote, O.H.U. Japanese Encephalitis in Sarawak: Studies on Juvenile Mosquito Populations. Trans. R Soc. Trop. Med. Hyg. 1970, 64, 483–488. [Google Scholar] [CrossRef]
- Simpson, D.I.; Bowen, E.T.; Way, H.J.; Platt, G.S.; Hill, M.N.; Kamath, S.; Lim, T.W.; Bendell, P.J.; Heathcote, O.H. Arbovirus Infections in Sarawak, October 1968--February 1970: Japanese Encephalitis Virus Isolations from Mosquitoes. Ann. Trop. Med. Parasitol. 1974, 68, 393–404. [Google Scholar] [CrossRef] [PubMed]
- Thoa, N.T.K.; Vien, N.T.; Mai, T.T.; Xuan, N.T.N. Japanese Encephalitis Vectors: Isolation of Virus from Culicine Mosquitoes in the Saigon Area. Southeast Asian J. Trop. Med. Public Health 1974, 5, 408–412. [Google Scholar]
- Van Peenen, P.F.D.; Joseph, P.L.; Atmosoedjono, S.; Irsiana, R.; Saroso, J.S. Japanese Encephalitis Virus from Pigs and Mosquitoes in Jakarta, Indonesia. Trans. R Soc. Trop. Med. Hyg. 1975, 69, 477–479. [Google Scholar] [CrossRef]
- Gingrich, J.B.; Nisalak, A.; Latendresse, J.R.; Sattabongkot, J.; Hoke, C.H.; Pomsdhit, J.; Chantalakana, C.; Satayaphanta, C.; Uechiewcharnkit, K.; Innis, B.L. Japanese Encephalitis Virus in Bangkok: Factors Influencing Vector Infections in Three Suburban Communities. J. Med. Entomol. 1992, 29, 436–444. [Google Scholar] [CrossRef]
- Simpson, D.I.; Smith, C.E.G.; Marshall, T.F.; Platt, G.S.; Way, H.J.; Bowen, E.T.W.; Bright, W.F.; Day, J.; McMahon, D.A.; Hill, M.N.; et al. Arbovirus Infections in Sarawak: The Role of the Domestic Pig. Trans. R Soc. Trop. Med. Hyg. 1976, 70, 66–72. [Google Scholar] [CrossRef]
- Pyke, A.T.; Williams, D.T.; Nisbet, D.J.; van den Hurk, A.F.; Taylor, C.T.; Johansen, C.A.; Macdonald, J.; Hall, R.A.; Simmons, R.J.; Mason, R.J.; et al. The Appearance of a Second Genotype of Japanese Encephalitis Virus in the Australasian Region. Am. J. Trop. Med. Hyg. 2001, 65, 747–753. [Google Scholar] [CrossRef] [PubMed]
- van den Hurk, A.F.; Nisbet, D.J.; Johansen, C.A.; Foley, P.N.; Ritchie, S.A.; Mackenzie, J.S. Japanese Encephalitis on Badu Island, Australia: The First Isojation of Japanese Encephalitis Virus from Culex Gelidus in the Australasian Region and the Role of Mosquito Host-Feeding Patterns in Virus Transmission Cycles. Trans. R Soc. Trop. Med. Hyg. 2001, 95, 595–600. [Google Scholar] [CrossRef]
- Arunachalam, N.; Murty, U.S.N.; Narahari, D.; Balasubramanian, A.; Philip Samuel, P.; Thenmozhi, V.; Paramasivan, R.; Rajendran, R.; Tyagi, B.K. Longitudinal Studies of Japanese Encephalitis Virus Infection in Vector Mosquitoes in Kurnool District, Andhra Pradesh, South India. J. Med. Entomol. 2009, 46, 633–639. [Google Scholar] [CrossRef]
- Sudeep, A.B.; Ghodke, Y.S.; George, R.P.; Ingale, V.S.; Dhaigude, S.D.; Gokhale, M.D. Vectorial Capacity of Culex Gelidus (Theobald) Mosquitoes to Certain Viruses of Public Health Importance in India. J. Vector Borne Dis. 2015, 52, 153–158. [Google Scholar] [PubMed]
- Ravanini, P.; Huhtamo, E.; Ilaria, V.; Crobu, M.G.; Nicosia, A.M.; Servino, L.; Rivasi, F.; Allegrini, S.; Miglio, U.; Magri, A.; et al. Japanese Encephalitis Virus RNA Detected in Culex Pipiens Mosquitoes in Italy. Eurosurveillance 2012, 17. [Google Scholar] [CrossRef]
- Kim, H.; Cha, G.-W.; Jeong, Y.E.; Lee, W.-G.; Chang, K.S.; Roh, J.Y.; Yang, S.C.; Park, M.Y.; Park, C.; Shin, E.-H. Detection of Japanese Encephalitis Virus Genotype V in Culex Orientalis and Culex Pipiens (Diptera: Culicidae) in Korea. PLoS ONE 2015, 10, e0116547. [Google Scholar] [CrossRef] [PubMed]
- Fang, Y.; Zhang, Y.; Zhou, Z.-B.; Xia, S.; Shi, W.-Q.; Xue, J.-B.; Li, Y.-Y.; Wu, J.-T. New Strains of Japanese Encephalitis Virus Circulating in Shanghai, China after a Ten-Year Hiatus in Local Mosquito Surveillance. Parasites Vectors 2019, 12, 22. [Google Scholar] [CrossRef] [PubMed]
- Buescher, E.L.; Scherer, W.F.; Rosenberg, M.Z.; Gresser, I.; Hardy, J.L.; Bullock, H.R. Ecologic Studies of Japanese Encephalitis Virus in Japan. II. Mosquito Infection. Am. J. Trop. Med. Hyg. 1959, 8, 651664. [Google Scholar] [CrossRef]
- Lee, H.W.; Min, B.W.; Lee, Y.W. Japanese Encephalitis Virus Isolation from Mosquitoes of Korea. J. Korean Med. Assoc. 1969, 12, 429–440. [Google Scholar]
- Huang, C.H. Studies of Japanese Encephalitis in China. In Advances in Virus Research; Elsevier: Amsterdam, The Netherlands, 1982; Volume 27, pp. 71–101. ISBN 978-0-12-039827-0. [Google Scholar]
- Shi, Q.; Song, X.; Lv, Y.; Huang, X.; Kou, J.; Wang, H.W.; Zhang, H.; Cheng, P.; Gong, M. Potential Risks Associated with Japanese Encephalitis Prevalence in Shandong Province, China. Vector-Borne Zoonotic Dis. 2019, 19, 640–645. [Google Scholar] [CrossRef] [PubMed]
- Hammon, W.M.; Rees, D.M.; Casals, J.; Meiklejohn, G. Experimental Transmission of Japanese B Encephalitis Virus by Culex Tritaeniorhynchus and Culex Pipiens Var. Pallens, Suspected Natural Vectors. Am. J. Hyg. 1949, 50, 46–50. [Google Scholar] [CrossRef] [PubMed]
- Doi, R.; Oya, A.; Shirasaka, A.; Yabe, S.; Sasa, M. Studies on Japanese Encephalitis Virus Infection of Reptiles. II. Role of Lizards on Hibernation of Japanese Encephalitis Virus. Jpn. J. Exp. Med. 1983, 53, 125–134. [Google Scholar]
- Weng, M.H.; Lien, J.C.; Lin, C.C.; Yao, C.W. Vector Competence of Culex Pipiens Molestus (Diptera: Culicidae) from Taiwan for a Sympatric Strain of Japanese Encephalitis Virus. J. Med. Entomol. 2000, 37, 780–783. [Google Scholar] [CrossRef]
- Turell, M.J.; Mores, C.N.; Dohm, D.J.; Komilov, N.; Paragas, J.; Lee, J.S.; Shermuhemedova, D.; Endy, T.P.; Kodirov, A.; Khodjaev, S. Laboratory Transmission of Japanese Encephalitis and West Nile Viruses by Molestus Form of Culex Pipiens (Diptera: Culicidae) Collected in Uzbekistan in 2004. J. Med. Entomol. 2006, 43, 296–300. [Google Scholar] [CrossRef]
- Hameed, M.; Liu, K.; Anwar, M.N.; Wahaab, A.; Safdar, A.; Di, D.; Boruah, P.; Xu, J.; Wang, X.; Li, B.; et al. The Emerged Genotype I of Japanese Encephalitis Virus Shows an Infectivity Similar to Genotype III in Culex Pipiens Mosquitoes from China. PLoS Negl. Trop. Dis. 2019, 13, e0007716. [Google Scholar] [CrossRef] [PubMed]
- Chapman, G.E.; Sherlock, K.; Hesson, J.C.; Blagrove, M.S.C.; Lycett, G.J.; Archer, D.; Solomon, T.; Baylis, M. Laboratory Transmission Potential of British Mosquitoes for Equine Arboviruses. Parasites Vectors 2020, 13, 413. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.; Xiao, C.; Xi, S.; Hameed, M.; Wahaab, A.; Shao, D.; Li, Z.; Li, B.; Wei, J.; Qiu, Y.; et al. Mosquito Defensins Enhance Japanese Encephalitis Virus Infection by Facilitating Virus Adsorption and Entry within the Mosquito. J. Virol. 2020, 94. [Google Scholar] [CrossRef] [PubMed]
- Di, D.; Li, C.; Zhang, J.; Hameed, M.; Wang, X.; Xia, Q.; Li, H.; Xi, S.; Li, Z.; Liu, K.; et al. Experimental Infection of Newly Hatched Domestic Ducklings via Japanese Encephalitis Virus-Infected Mosquitoes. Pathogens 2020, 9, 371. [Google Scholar] [CrossRef]
- Folly, A.J.; Dorey-Robinson, D.; Hernández-Triana, L.M.; Ackroyd, S.; Vidana, B.; Lean, F.Z.X.; Hicks, D.; Nuñez, A.; Johnson, N. Temperate Conditions Restrict Japanese Encephalitis Virus Infection to the Mid-Gut and Prevents Systemic Dissemination in Culex Pipiens Mosquitoes. Sci. Rep. 2021, 11, 6133. [Google Scholar] [CrossRef]
- Dhanda, V.; Mourya, D.T.; Mishra, A.C.; Ilkal, M.A.; Pant, U.; Jacob, P.G.; Bhat, H.R. Japanese Encephalitis Virus Infection in Mosquitoes Reared from Field-Collected Immatures and in Wild-Caught Males. Am. J. Trop. Med. Hyg. 1989, 41, 732–736. [Google Scholar] [CrossRef]
- Borah, J.; Dutta, P.; Khan, S.A.; Mahanta, J. Epidemiological Concordance of Japanese Encephalitis Virus Infection among Mosquito Vectors, Amplifying Hosts and Humans in India. Epidemiol. Infect. 2013, 141, 74–80. [Google Scholar] [CrossRef]
- Nyari, N.; Singh, D.; Kakkar, K.; Sharma, S.; Pandey, S.N.; Dhole, T.N. Entomological and Serological Investigation of Japanese Encephalitis in Endemic Area of Eastern Uttar Pradesh, India. J. Vector Borne Dis. 2015, 52, 321–328. [Google Scholar]
- George, S.; Jacob, P.G.; Rao, J.A. Isolation of Japanese Encephalitis & West Nile Viruses from Mosquitoes Collected in Kolar District of Karnataka State during 1977-79. Indian J. Med. Res. 1987, 85, 235–238. [Google Scholar]
- Naik, P.S.; Ilkal, M.A.; Pant, U.; Kulkarni, S.M.; Dhanda, V. Isolation of Japanese Encephalitis Virus from Culex Pseudovishnui Colless, 1957 (Diptera: Culicidae) in Goa. Indian J. Med. Res. 1990, 91, 331–333. [Google Scholar]
- Mourya, D.T.; Mishra, A.C.; Soman, R.S. Transmission of Japanese Encephalitis Virus in Culex Pseudovishnui & C. Tritaeniorhynchus Mosquitoes. Indian J. Med. Res. 1991, 93, 250–252. [Google Scholar] [PubMed]
- Mourya, D.T.; Mishra, A.C. Antigen Distribution Pattern of Japanese Encephalitis Virus in Culex Tritaeniorhynchus, C. Vishnui & C. Pseudovishnui. Indian J. Med. Res. 2000, 111, 157–161. [Google Scholar] [PubMed]
- Lindahl, J.F.; Ståhl, K.; Chirico, J.; Boqvist, S.; Thu, H.T.V.; Magnusson, U. Circulation of Japanese Encephalitis Virus in Pigs and Mosquito Vectors within Can Tho City, Vietnam. PLoS Negl. Trop. Dis. 2013, 7, e2153. [Google Scholar] [CrossRef] [PubMed]
- Nitatpattana, N.; Apiwathnasorn, C.; Barbazan, P.; Leemingsawat, S.; Yoksan, S.; Gonzalez, J.-P. First Isolation of Japanese Encephalitis from Culex Quinquefasciatus in Thailand. Southeast Asian J. Trop. Med. Public Health 2005, 36, 875–878. [Google Scholar]
- Hurlbut, H.S.; Thomas, J.I. Potential Vectors of Japanese Encephalitis in the Caroline Islands. Am. J. Trop. Med. Hyg. 1949, s1-29, 215–217. [Google Scholar] [CrossRef] [PubMed]
- Hurlbut, H.S.; Thomas, J.I. Observations on the Experimental Transmission of Japanese Encephalitis by Mosquitoes. Am. J. Trop. Med. Hyg. 1950, s1-30, 683–688. [Google Scholar] [CrossRef]
- Banerjee, K.; Deshmukh, P.K.; Ilkal, M.A.; Dhanda, V. Experimental Transmission of Japanese Encephalitis Virus through Anopheles Tessellatus and Culex Fatigans Mosquitoes. Indian J. Med. Res. 1977, 65, 746–752. [Google Scholar]
- Mackenzie-Impoinvil, L.; Impoinvil, D.E.; Galbraith, S.E.; Dillon, R.J.; Ranson, H.; Johnson, N.; Fooks, A.R.; Solomon, T.; Baylis, M. Evaluation of a Temperate Climate Mosquito, Ochlerotatus Detritus (=Aedes Detritus), as a Potential Vector of Japanese Encephalitis Virus. Med. Vet. Entomol. 2015, 29, 1–9. [Google Scholar] [CrossRef]
- Huang, Y.-J.S.; Harbin, J.N.; Hettenbach, S.M.; Maki, E.; Cohnstaedt, L.W.; Barrett, A.D.T.; Higgs, S.; Vanlandingham, D.L. Susceptibility of a North American Culex Quinquefasciatus to Japanese Encephalitis Virus. Vector-Borne Zoonotic Dis. 2015, 15, 709–711. [Google Scholar] [CrossRef]
- Huang, Y.-J.S.; Hettenbach, S.M.; Park, S.L.; Higgs, S.; Barrett, A.D.T.; Hsu, W.-W.; Harbin, J.N.; Cohnstaedt, L.W.; Vanlandingham, D.L. Differential Infectivities among Different Japanese Encephalitis Virus Genotypes in Culex Quinquefasciatus Mosquitoes. PLoS Negl. Trop. Dis. 2016, 10, e0005038. [Google Scholar] [CrossRef]
- Karna, A.K.; Bowen, R.A. Experimental Evaluation of the Role of Ecologically-Relevant Hosts and Vectors in Japanese Encephalitis Virus Genotype Displacement. Viruses 2019, 11, 32. [Google Scholar] [CrossRef] [PubMed]
- Vythilingam, I.; Oda, K.; Tsuchie, H.; Mahadevan, S.; Vijayamalar, B. Isolation of Japanese Encephalitis Virus from Culex Sitiens Mosquitoes in Selangor, Malaysia. J. Am. Mosq. Control Assoc. 1994, 10, 228–229. [Google Scholar]
- Weng, M.H.; Lien, J.C.; Ji, D.D. Monitoring of Japanese Encephalitis Virus Infection in Mosquitoes (Diptera: Culicidae) at Guandu Nature Park, Taipei, 2002–2004. J. Med. Entomol. 2005, 42, 1085–1088. [Google Scholar] [CrossRef]
- Van Den Hurk, A.F.; Montgomery, B.L.; Northill, J.A.; Smith, I.L.; Zborowski, P.; Ritchie, S.A.; Mackenzie, J.S.; Smith, G.A. Short Report: The First Isolation of Japanese Encephalitis Virus from Mosquitoes Collected from Mainland Australia. Am. J. Trop. Med. Hyg. 2006, 75, 21–25. [Google Scholar] [CrossRef] [PubMed]
- Johansen, C.A.; van den Hurk, A.F.; Ritchie, S.A.; Zborowski, P.; Nisbet, D.J.; Paru, R.; Bockarie, M.J.; Macdonald, J.; Drew, A.C.; Khromykh, T.I.; et al. Isolation of Japanese Encephalitis Virus from Mosquitoes (Diptera: Culicidae) Collected in the Western Province of Papua New Guinea, 1997–1998. Am. J. Trop. Med. Hyg. 2000, 62, 631–638. [Google Scholar] [CrossRef] [PubMed]
- Johansen, C.A.; van den Hurk, A.F.; Pyke, A.T.; Zborowski, P.; Phillips, D.A.; Mackenzie, J.S.; Ritchie, S.A. Entomological Investigations of an Outbreak of Japanese Encephalitis Virus in the Torres Strait, Australia, in 1998. J. Med. Entomol. 2001, 38, 581–588. [Google Scholar] [CrossRef] [PubMed]
- Johansen, C.A.; Nisbet, D.J.; Foley, P.N.; Van Den Hurk, A.F.; Hall, R.A.; Mackenzie, J.S.; Ritchie, S.A. Flavivirus Isolations from Mosquitoes Collected from Saibai Island in the Torres Strait, Australia, during an Incursion of Japanese Encephalitis Virus. Med. Vet. Entomol. 2004, 18, 281–287. [Google Scholar] [CrossRef] [PubMed]
- Dhanda, V.; Thenmozhi, V.; Kumar, N.P.; Hiriyan, J.; Arunachalam, N.; Balasubramanian, A.; Ilango, A.; Gajanana, A. Virus Isolation from Wild-Caught Mosquitoes during a Japanese Encephalitis Outbreak in Kerala in 1996. Indian J. Med. Res. 1997, 106, 4–6. [Google Scholar]
- Das, B.P.; Sharma, S.N.; Kabilan, L.; Lal, S.; Saxena, V.K. First Time Detection of Japanese Encephalitis Virus Antigen in Dry and Unpreserved Mosquito Culex Tritaeniorhynchus Giles, 1901, from Karnal District of Haryana State of India. J. Commun. Dis. 2005, 37, 131–133. [Google Scholar] [PubMed]
- Jeong, Y.E.; Jeon, M.J.; Cho, J.E.; Han, M.G.; Choi, H.J.; Shin, M.Y.; Park, H.J.; Kim, W.; Moon, B.C.; Park, J.-S.; et al. Development and Field Evaluation of a Nested RT-PCR Kit for Detecting Japanese Encephalitis Virus in Mosquitoes. J. Virol. Methods 2011, 171, 248–252. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.-Y.; Fan, Y.-C.; Tu, W.-C.; Chang, R.-Y.; Shih, C.-C.; Lu, I.-H.; Chien, M.-S.; Lee, W.-C.; Chen, T.-H.; Chang, G.-J.; et al. Japanese Encephalitis Virus Genotype Replacement, Taiwan, 2009–2010. Emerg. Infect. Dis. 2011, 17, 2354–2356. [Google Scholar] [CrossRef] [PubMed]
- Murakami, M.; Hori, K.; Kitagawa, Y.; Oikawa, Y.; Kamimura, K.; Takegami, T. An Ecological Survey of Mosquitoes and the Distribution of Japanese Encephalitis Virus in Ishikawa Prefecture, Japan, between 2010 and 2014. Jpn. J. Infect. Dis. 2017, 70, 362–367. [Google Scholar] [CrossRef] [PubMed]
- Philip Samuel, P.; Thenmozhi, V.; Muniaraj, M.; Ramesh, D.; Victor Jerald Leo, S.; Balaji, T.; Venkatasubramani, K.; Nagaraj, J.; Paramasivan, R. Changing Paradigm in the Epidemiology of Japanese Encephalitis in a Non-Endemic Region. J. Vector Borne Dis. 2018, 55, 203–207. [Google Scholar] [CrossRef] [PubMed]
- Tao, Z.; Liu, G.; Wang, M.; Wang, H.; Lin, X.; Song, L.; Wang, S.; Wang, H.; Liu, X.; Cui, N.; et al. Molecular Epidemiology of Japanese Encephalitis Virus in Mosquitoes during an Outbreak in China, 2013. Sci. Rep. 2014, 4, 4908. [Google Scholar] [CrossRef] [PubMed]
- Fang, Y.; Zhang, W.; Xue, J.-B.; Zhang, Y. Monitoring Mosquito-Borne Arbovirus in Various Insect Regions in China in 2018. Front. Cell Infect. Microbiol. 2021, 11, 640993. [Google Scholar] [CrossRef] [PubMed]
- Carey, D.E.; Reuben, R.; Myers, R.M. Japanese Encephalitis Studies in Vellore, South India. I. Virus Isolation from Mosquitoes. Indian J. Med. Res. 1968, 56, 1309–1318. [Google Scholar]
- Yamamoto, H.; Ishida, N.; Akiyama, K.; Shiratori, T.; Konno, J. Epidemiological Analyses of Japanese Encephalitis Virus Spread from Mosquitoes to Pigs through 5 Years. Jpn. J. Med. Sci. Biol. 1970, 23, 75–85. [Google Scholar] [CrossRef]
- Okuno, T.; Tseng, P.T.; Liu, S.Y.; Hsu, S.T.; Huang, C.T. Rates of Infection with Japanese Encephalitis Virus of Two Culicine Species of Mosquito in Taiwan. Bull. World Health Organ. 1971, 44, 599–604. [Google Scholar] [PubMed]
- Van Peenen, P.F.D.; Irsiana, R.; Saroso, J.S.; Joseph, S.W.; Shope, R.E.; Joseph, P.L. First Isolation of Japanese Encephalitis Virus from Java. Mil. Med. 1974, 139, 821–823. [Google Scholar] [CrossRef]
- Olson, J.G.; Ksiazek, T.G.; Lee, V.H.; Tan, R.; Shope, R.E. Isolation of Japanese Encephalitis Virus from Anopheles Annularis and Anopheles Vagus in Lombok, Indonesia. Trans. R. Soc. Trop. Med. Hyg. 1985, 79, 845–847. [Google Scholar] [CrossRef]
- Rosen, L.; Lien, J.C.; Lu, L.C. A Longitudinal Study of the Prevalence of Japanese Encephalitis Virus in Adult and Larval Culex Tritaeniorhynchus Mosquitoes in Northern Taiwan. Am. J. Trop. Med. Hyg. 1989, 40, 557–560. [Google Scholar] [CrossRef] [PubMed]
- Leake, C.J.; Ussery, M.A.; Nisalak, A.; Hoke, C.H.; Andre, R.G.; Burke, D.S. Virus Isolations from Mosquitoes Collected during the 1982 Japanese Encephalitis Epidemic in Northern Thailand. Trans. R. Soc. Trop. Med. Hyg. 1986, 80, 831–837. [Google Scholar] [CrossRef]
- Tadano, M.; Kanemura, K.; Hasegawa, H.; Makino, Y.; Fukunaga, T. Epidemiological and Ecological Studies of Japanese Encephalitis in Okinawa, Subtropical Area in Japan. I. Investigations on Antibody Levels to Japanese Encephalitis Virus in Swine Sera and Vector Mosquito in Okinawa, Miyako and Ishigaki Islands. Microbiol. Immunol. 1994, 38, 117–122. [Google Scholar] [CrossRef] [PubMed]
- Pant, U.; Ilkal, M.A.; Soman, R.S.; Shetty, P.S.; Kanojia, P.C.; Kaul, H.N. First Isolation of Japanese Encephalitis Virus from the Mosquito, Culex Tritaeniorhynchus Giles, 1901 (Diptera: Culicidae) in Gorakhpur District, Uttar Pradesh. Indian J. Med. Res. 1994, 99, 149–151. [Google Scholar] [PubMed]
- Liu, H.; Liu, Z.-J.; Jing, J.; Ren, J.-Q.; Liu, Y.-Y.; Guo, H.-H.; Fan, M.; Lu, H.-J.; Jin, N.-Y. Reverse Transcription Loop-Mediated Isothermal Amplification for Rapid Detection of Japanese Encephalitis Virus in Swine and Mosquitoes. Vector-Borne Zoonotic Dis. 2012, 12, 1042–1052. [Google Scholar] [CrossRef] [PubMed]
- Turell, M.J.; O’Guinn, M.L.; Wasieloski, L.P.; Dohm, D.J.; Lee, W.-J.; Cho, H.-W.; Kim, H.-C.; Burkett, D.A.; Mores, C.N.; Coleman, R.E.; et al. Isolation of Japanese Encephalitis and Getah Viruses from Mosquitoes (Diptera: Culicidae) Collected near Camp Greaves, Gyonggi Province, Republic of Korea, 2000. J. Med. Entomol. 2003, 40, 580–584. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Fu, S.; Gong, Z.; Ge, J.; Meng, W.; Feng, Y.; Wang, J.; Zhai, Y.; Wang, H.; Nasci, R.; et al. Distribution of Arboviruses and Mosquitoes in Northwestern Yunnan Province, China. Vector-Borne Zoonotic Dis. 2009, 9, 623–630. [Google Scholar] [CrossRef]
- Kuwata, R.; Nga, P.T.; Yen, N.T.; Hoshino, K.; Isawa, H.; Higa, Y.; Hoang, N.V.; Trang, B.M.; Loan, D.P.; Phong, T.V.; et al. Surveillance of Japanese Encephalitis Virus Infection in Mosquitoes in Vietnam from 2006 to 2008. Am. J. Trop. Med. Hyg. 2013, 88, 681–688. [Google Scholar] [CrossRef]
- Li, L.; Guo, X.; Zhao, Q.; Tong, Y.; Fan, H.; Sun, Q.; Xing, S.; Zhou, H.; Zhang, J. Investigation on Mosquito-Borne Viruses at Lancang River and Nu River Watersheds in Southwestern China. Vector-Borne Zoonotic Dis. 2017. [Google Scholar] [CrossRef]
- Kumari, R.; Kumar, K.; Rawat, A.; Singh, G.; Yadav, N.K.; Chauhan, L.S. First Indigenous Transmission of Japanese Encephalitis in Urban Areas of National Capital Territory of Delhi, India. Trop. Med. Int. Health 2013, 18, 743–749. [Google Scholar] [CrossRef] [PubMed]
- Yap, G.; Mailepessov, D.; Lim, X.F.; Chan, S.; How, C.B.; Humaidi, M.; Yeo, G.; Chong, C.S.; Lam-Phua, S.G.; Lee, R.; et al. Detection of Japanese Encephalitis Virus in Culex Mosquitoes in Singapore. Am. J. Trop. Med. Hyg. 2020, 103, 1234–1240. [Google Scholar] [CrossRef] [PubMed]
- Kuwata, R.; Sugiyama, H.; Yonemitsu, K.; Van Dung, N.; Terada, Y.; Taniguchi, M.; Shimoda, H.; Takano, A.; Maeda, K. Isolation of Japanese Encephalitis Virus and a Novel Insect-Specific Flavivirus from Mosquitoes Collected in a Cowshed in Japan. Arch. Virol. 2015, 160, 2151–2159. [Google Scholar] [CrossRef]
- Duong, V.; Choeung, R.; Gorman, C.; Laurent, D.; Crabol, Y.; Mey, C.; Peng, B.; Di Francesco, J.; Hul, V.; Sothy, H.; et al. Isolation and Full-Genome Sequences of Japanese Encephalitis Virus Genotype I Strains from Cambodian Human Patients, Mosquitoes and Pigs. J. Gen. Virol. 2017, 98, 2287–2296. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Rehman, M.U.; Li, K.; Luo, H.; Lan, Y.; Nabi, F.; Zhang, L.; Iqbal, M.K.; Zhu, S.; Javed, M.T.; et al. Epidemiologic Survey of Japanese Encephalitis Virus Infection, Tibet, China, 2015. Emerg. Infect. Dis. 2017, 23, 1023–1024. [Google Scholar] [CrossRef] [PubMed]
- Mitamura, T.; Kitaoka, M.; Watanabe, M.; Okuba, K.; Tnejin, S.; Yamada, S.; Mori, K.; Asada, J. Study on Japanese Encephalitis Virus. Animal Experiments and Mosquito Transmission Experiments. Kansai Iji 1936, 1, 260–261. [Google Scholar]
- Hale, J.H.; Colless, D.H.; Lim, K.A. Investigation of the Malaysian Form of Culex Tritaeniorhynchus as a Potential Vector of Japanese B Encephalitis Virus on Singapore Island. Ann. Trop. Med. Parasitol. 1957, 51, 17–25. [Google Scholar] [CrossRef]
- Gresser, I.; Hardy, J.L.; Hu, S.M.K.; Scherer, W.F. Factors Influencing Transmission of Japanese B Encephalitis Virus by a Colonized Strain of Culex Tritaeniorhynchus Giles, from Infected Pigs and Chicks to Susceptible Pigs and Birds. Am. J. Trop. Med. Hyg. 1958, 7, 365–373. [Google Scholar] [CrossRef]
- Gould, D.J.; Byrne, R.J.; Hayes, D.E. Experimental Infection of Horses with Japanese Encephalitis Virus by Mosquito Bite. Am. J. Trop. Med. Hyg. 1964, 13, 742–746. [Google Scholar] [CrossRef] [PubMed]
- Carey, D.E.; Reuben, R.; Myers, R.M. Japanese Encephalitis Studies in Vellore, South India. V. Experimental Infection and Transmission. Indian J. Med. Res. 1969, 57, 282–289. [Google Scholar] [PubMed]
- Takahashi, M. The Effects of Environmental and Physiological Conditions of Culex Tritaeniorhynchus on the Pattern of Transmission of Japanese Encephalitis Virus. J. Med. Entomol. 1976, 13, 275–284. [Google Scholar] [CrossRef]
- Soman, R.S.; Rodrigues, F.M.; Guttikar, S.N.; Guru, P.Y. Experimental Viraemia and Transmission of Japanese Encephalitis Virus by Mosquitoes in Ardeid Birds. Indian J. Med. Res. 1977, 66, 709–718. [Google Scholar]
- Chen, B.Q.; Beaty, B.J. Japanese Encephalitis Vaccine (2-8 Strain) and Parent (SA 14 Strain) Viruses in Culex Tritaeniorhynchus Mosquitoes. Am. J. Trop. Med. Hyg. 1982, 31, 403–407. [Google Scholar] [CrossRef]
- Turell, M.J.; Mores, C.N.; Dohm, D.J.; Lee, W.-J.; Kim, H.-C.; Klein, T.A. Laboratory Transmission of Japanese Encephalitis, West Nile, and Getah Viruses by Mosquitoes (Diptera: Culicidae) Collected near Camp Greaves, Gyeonggi Province, Republic of Korea 2003. J. Med. Entomol. 2006, 43, 1076–1081. [Google Scholar] [CrossRef]
- Faizah, A.N.; Kobayashi, D.; Amoa-Bosompem, M.; Higa, Y.; Tsuda, Y.; Itokawa, K.; Miura, K.; Hirayama, K.; Sawabe, K.; Isawa, H. Evaluating the Competence of the Primary Vector, Culex Tritaeniorhynchus, and the Invasive Mosquito Species, Aedes Japonicus Japonicus, in Transmitting Three Japanese Encephalitis Virus Genotypes. PLoS Negl. Trop. Dis. 2020, 14, e0008986. [Google Scholar] [CrossRef] [PubMed]
- Chakravarty, S.K.; Sarkar, J.K.; Chakravarty, M.S.; Mukherjee, M.K.; Mukherjee, K.K.; Das, B.C.; Hati, A.K. The First Epidemic of Japanese Encephalitis Studied in India—Virological Studies. Indian J. Med. Res. 1975, 63, 77–82. [Google Scholar]
- Sahu, S.S.; Dash, S.; Sonia, T.; Muthukumaravel, S.; Sankari, T.; Gunasekaran, K.; Jambulingam, P. Entomological Investigation of Japanese Encephalitis Outbreak in Malkangiri District of Odisha State, India. Mem. Inst. Oswaldo Cruz 2018, 113, e170499. [Google Scholar] [CrossRef]
- Sarkar, A.; Taraphdar, D.; Mukhopadhyay, S.K.; Chakrabarti, S.; Chatterjee, S. Molecular Evidence for the Occurrence of Japanese Encephalitis Virus Genotype I and III Infection Associated with Acute Encephalitis in Patients of West Bengal, India, 2010. Virol. J. 2012, 9, 271. [Google Scholar] [CrossRef]
- Hurlbut, H.S. The Pig-Mosquito Cycle of Japanese Encephalitis Virus in Taiwan. J. Med. Entomol. 1964, 1, 301–307. [Google Scholar] [CrossRef]
- Bhatt, T.R.; Crabtree, M.B.; Guirakhoo, F.; Monath, T.P.; Miller, B.R. Growth Characteristics of the Chimeric Japanese Encephalitis Virus Vaccine Candidate, ChimeriVax-JE (YF/JE SA14--14--2), in Culex Tritaeniorhynchus, Aedes Albopictus, and Aedes Aegypti Mosquitoes. Am. J. Trop. Med. Hyg. 2000, 62, 480–484. [Google Scholar] [CrossRef]
- Liu, S.; Zhang, Q.; Zhou, J.; Yu, S.; Zheng, X.; Chen, Q. Susceptibility of Aedes albopictus and Culex pipiens quinquefasciatus to infection with bat Japanese encephalitis virus isolates. Nan Fang Yi Ke Da Xue Xue Bao 2012, 32, 515–518. [Google Scholar]
- Banerjee, K.; Deshmukh, P.K. Transmission of Japanese Encephalitis Virus to Chicks by Individual Culex Bitaeniorhynchus Mosquitoes. Indian J. Med. Res. 1987, 86, 726–727. [Google Scholar]
- Van Peenen, P.F.D.; Joseph, P.L.; Atmosoedjono, S.; Irsiana, R.; Saroso, J.S. Isolation of Japanese Encephalitis Virus from Mosquitoes near Bogor, West Java, Indonesia. J. Med. Entomol. 1975, 12, 573–574. [Google Scholar] [CrossRef]
- Kramer, L.D.; Chin, P.; Cane, R.P.; Kauffman, E.B.; Mackereth, G. Vector Competence of New Zealand Mosquitoes for Selected Arboviruses. Am. J. Trop. Med. Hyg. 2011, 85, 182–189. [Google Scholar] [CrossRef]
- Liu, K.; Hou, F.; Wahaab, A.; Kang, L.; Xie, F.; Ma, X.; Xia, Q.; Xiao, C.; Shao, D.; Li, B.; et al. Mosquito Defensin Facilitates Japanese Encephalitis Virus Infection by Downregulating the C6/36 Cell-Surface Antiviral Protein HSC70B. Vet. Microbiol. 2021, 253, 108971. [Google Scholar] [CrossRef]
- Liu, K.; Qian, Y.; Jung, Y.-S.; Zhou, B.; Cao, R.; Shen, T.; Shao, D.; Wei, J.; Ma, Z.; Chen, P.; et al. MosGCTL-7, a C-Type Lectin Protein, Mediates Japanese Encephalitis Virus Infection in Mosquitoes. J. Virol. 2017, 91. [Google Scholar] [CrossRef] [PubMed]
- Doi, R.; Shirasaka, A.; Sasa, M.; Oya, A. Studies on the Susceptibility of Three Species of Mosquitoes to Japanese Encephalitis Virus. J. Med. Entomol. 1977, 13, 591–594. [Google Scholar] [CrossRef]
- Hurlbut, H.S. The Transmission of Japanese B Encephalitis by Mosquitoes after Experimental Hibernation. Am. J. Hyg. 1950, 51, 265–268. [Google Scholar] [CrossRef] [PubMed]
- Mourya, D.T.; Gokhale, M.D.; Pidiyar, V.; Barde, P.V.; Patole, M.; Mishra, A.C.; Shouche, Y. Study of the Effect of the Midgut Bacterial Flora of Culex Quinquefasciatus on the Susceptibility of Mosquitoes to Japanese Encephalitis Virus. Acta Virol. 2002, 46, 257–260. [Google Scholar] [PubMed]
- Sudeep, A.B.; Bondre, V.P.; George, R.; Ghodke, Y.S.; Aher, R.V.; Gokhale, M.D. Bagaza Virus Inhibits Japanese Encephalitis & West Nile Virus Replication in Culex Tritaeniorhynchus & Cx. Quinquefasciatus Mosquitoes. Indian J. Med. Res. 2015, 142 (Suppl. S1), S44–S51. [Google Scholar] [CrossRef] [PubMed]
- Mitamura, T.; Kitaoka, M.; Mori, K.; Okuba, K. Isolation of the Virus of Japanese Epidemic Encephalitis from Mosquitoes Caught in Nature. Tokyi Iji Shinshi 1938, 62, 820–831. [Google Scholar]
- Takahashi, M. Variation in Susceptibility among Colony Strains of Culex Tritaeniorhynchus to Japanese Encephalitis Virus Infection. Jpn. J. Med. Sci. Biol. 1980, 33, 321–329. [Google Scholar] [CrossRef] [PubMed]
- Huber, K.; Jansen, S.; Leggewie, M.; Badusche, M.; Schmidt-Chanasit, J.; Becker, N.; Tannich, E.; Becker, S.C. Aedes Japonicus Japonicus (Diptera: Culicidae) from Germany Have Vector Competence for Japan Encephalitis Virus but Are Refractory to Infection with West Nile Virus. Parasitol. Res. 2014, 113, 3195–3199. [Google Scholar] [CrossRef]
- Dhanda, V.; Kaul, H.N. Mosquito Vectors of Japanese Encephalitis Virus and Their Bionomics in India. Proc. Indian Natl. Sci. Acad. 1980, 6, 759–768. [Google Scholar]
- Cates, M.D.; Detels, R. Japanese Encephalitis Virus in Taiwan: Preliminary Evidence for Culex Annulus Theob. as a Vector. J. Med. Entomol. 1969, 6, 327–328. [Google Scholar] [CrossRef] [PubMed]
- Cross, J.H.; Lien, J.C.; Huang, W.C.; Lien, S.C.; Chiu, S.F. Japanese Encephalitis Virus Surveillance in Taiwan. II. Isolations from Mosquitoes and Bats in Taipei Area 1969-1970. Taiwan Yi Xue Hui Za Zhi 1971, 70, 681–686. [Google Scholar] [PubMed]
- Banerjee, K.; Mahadev, P.V.; Ilkal, M.A.; Mishra, A.C.; Dhanda, V.; Modi, G.B.; Geevarghese, G.; Kaul, H.N.; Shetty, P.S.; George, P.J. Isolation of Japanese Encephalitis Virus from Mosquitoes Collected in Bankura District (West Bengal) during October 1974 to December 1975. Indian J. Med. Res. 1979, 69, 201–205. [Google Scholar] [PubMed]
- Thenmozhi, V.; Rajendran, R.; Ayanar, K.; Manavalan, R.; Tyagi, B.K. Long-Term Study of Japanese Encephalitis Virus Infection in Anopheles Subpictus in Cuddalore District, Tamil Nadu, South India. Trop. Med. Int. Health 2006, 11, 288–293. [Google Scholar] [CrossRef]
- Hemmerter, S.; Šlapeta, J.; van den Hurk, A.F.; Cooper, R.D.; Whelan, P.I.; Russell, R.C.; Johansen, C.A.; Beebe, N.W. A Curious Coincidence: Mosquito Biodiversity and the Limits of the Japanese Encephalitis Virus in Australasia. BMC Evol. Biol. 2007, 7, 100. [Google Scholar] [CrossRef]
- Delatte, H.; Desvars, A.; Bouétard, A.; Bord, S.; Gimonneau, G.; Vourc’h, G.; Fontenille, D. Blood-Feeding Behavior of Aedes Albopictus, a Vector of Chikungunya on La Réunion. Vector-Borne Zoonotic Dis. 2010, 10, 249–258. [Google Scholar] [CrossRef]
- Srinivas, S.D.; Pandian, R.S.; Dwarakanath, S.K. Biting Behaviour of Armigeres Subalbatus (Coquillett) with Reference to Host Selection and Landing. Indian J. Exp. Biol. 1994, 32, 348–350. [Google Scholar] [PubMed]
- Lien, J.C.; Huang, W.C.; Cross, J.H. Japanese Encephalitis Virus Surveillance in the Taipei Area, Taiwan in 1978. Southeast Asian J. Trop. Med. Public Health 1980, 11, 177–183. [Google Scholar] [PubMed]
- Prummongkol, S.; Panasoponkul, C.; Apiwathnasorn, C.; Lek-Uthai, U. Biology of Culex Sitiens, a Predominant Mosquito in Phang Nga, Thailand after a Tsunami. J. Insect Sci. 2012, 12, 1–8. [Google Scholar] [CrossRef]
- Hall-Mendelin, S.; Jansen, C.C.; Cheah, W.Y.; Montgomery, B.L.; Hall, R.A.; Ritchie, S.A.; van den Hurk, A.F. Culex Annulirostris (Diptera: Culicidae) Host Feeding Patterns and Japanese Encephalitis Virus Ecology in Northern Australia. J. Med. Entomol. 2012, 49, 371–377. [Google Scholar] [CrossRef] [PubMed]
- Murty, U.S.; Rao, M.S.; Arunachalam, N. The Effects of Climatic Factors on the Distribution and Abundance of Japanese Encephalitis Vectors in Kurnool District of Andhra Pradesh, India. J. Vector Borne Dis. 2010, 47, 26–32. [Google Scholar] [PubMed]
- Reuben, R.; Thenmozhi, V.; Samuel, P.P.; Gajanana, A.; Mani, T.R. Mosquito Blood Feeding Patterns as a Factor in the Epidemiology of Japanese Encephalitis in Southern India. Am. J. Trop. Med. Hyg. 1992, 46, 654–663. [Google Scholar] [CrossRef] [PubMed]
- Zinser, M.; Ramberg, F.; Willott, E. Scientific Note Culex Quinquefasciatus (Diptera: Culicidae) as a Potential West Nile Virus Vector in Tucson, Arizona: Blood Meal Analysis Indicates Feeding on Both Humans and Birds. J. Insect Sci. 2004, 4, 20. [Google Scholar] [CrossRef]
- Hasegawa, M.; Tuno, N.; Yen, N.T.; Nam, V.S.; Takagi, M. Influence of the Distribution of Host Species on Adult Abundance of Japanese Encephalitis Vectors Culex Vishnui Subgroup and Culex Gelidus in a Rice-Cultivating Village in Northern Vietnam. Am. J. Trop. Med. Hyg. 2008, 78, 159–168. [Google Scholar] [CrossRef] [PubMed]
- Boyer, S.; Durand, B.; Yean, S.; Brengues, C.; Maquart, P.-O.; Fontenille, D.; Chevalier, V. Host-Feeding Preference and Diel Activity of Mosquito Vectors of the Japanese Encephalitis Virus in Rural Cambodia. Pathogens 2021, 10, 376. [Google Scholar] [CrossRef] [PubMed]
- Farajollahi, A.; Fonseca, D.M.; Kramer, L.D.; Marm Kilpatrick, A. “Bird Biting” Mosquitoes and Human Disease: A Review of the Role of Culex Pipiens Complex Mosquitoes in Epidemiology. Infect. Genet. Evol. 2011, 11, 1577–1585. [Google Scholar] [CrossRef] [PubMed]
- Molaei, G.; Andreadis, T.G.; Armstrong, P.M.; Bueno, R.; Dennett, J.A.; Real, S.V.; Sargent, C.; Bala, A.; Randle, Y.; Guzman, H.; et al. Host Feeding Pattern of Culex Quinquefasciatus (Diptera: Culicidae) and Its Role in Transmission of West Nile Virus in Harris County, Texas. Am. J. Trop. Med. Hyg. 2007, 77, 73–81. [Google Scholar] [CrossRef]
- Mottram, P.; Kettle, D.S. Development and Survival of Immature Culex Annulirostris Mosquitoes in Southeast Queensland. Med. Vet. Entomol. 1997, 11, 181–186. [Google Scholar] [CrossRef]
- Medlock, J.M.; Avenell, D.; Barrass, I.; Leach, S. Analysis of the Potential for Survival and Seasonal Activity of Aedes Albopictus (Diptera: Culicidae) in the United Kingdom. J. Vector Ecol. 2006, 31, 292–304. [Google Scholar] [CrossRef]
- Rochlin, I.; Ninivaggi, D.V.; Hutchinson, M.L.; Farajollahi, A. Climate Change and Range Expansion of the Asian Tiger Mosquito (Aedes Albopictus) in Northeastern USA: Implications for Public Health Practitioners. PLoS ONE 2013, 8, e60874. [Google Scholar] [CrossRef] [PubMed]
- Giordano, B.V.; Gasparotto, A.; Liang, P.; Nelder, M.P.; Russell, C.; Hunter, F.F. Discovery of an Aedes (Stegomyia) Albopictus Population and First Records of Aedes (Stegomyia) Aegypti in Canada. Med. Vet. Entomol. 2020, 34, 10–16. [Google Scholar] [CrossRef] [PubMed]
- Ree, H.I.; Hong, H.K.; Lee, J.S.; Wada, Y.; Lolivet, P. Dispersal Experiment on Culex Tritaeniorhynchus in Korea. Korean J. Zool. 1978, 21, 59–66. [Google Scholar]
- Masuoka, P.; Klein, T.A.; Kim, H.-C.; Claborn, D.M.; Achee, N.; Andre, R.; Chamberlin, J.; Taylor, K.; Small, J.; Anyamba, A.; et al. Modeling and Analysis of Mosquito and Environmental Data to Predict the Risk of Japanese Encephalitis. In Proceedings of the ASPRS 2009 Annual Conference, Baltimore, MD, USA, 9–13 March 2009. [Google Scholar]
- Longbottom, J.; Browne, A.J.; Pigott, D.M.; Sinka, M.E.; Golding, N.; Hay, S.I.; Moyes, C.L.; Shearer, F.M. Mapping the Spatial Distribution of the Japanese Encephalitis Vector, Culex Tritaeniorhynchus Giles, 1901 (Diptera: Culicidae) within Areas of Japanese Encephalitis Risk. Parasites Vectors 2017, 10, 148. [Google Scholar] [CrossRef] [PubMed]
- Dickson, L.B.; Jiolle, D.; Minard, G.; Moltini-Conclois, I.; Volant, S.; Ghozlane, A.; Bouchier, C.; Ayala, D.; Paupy, C.; Moro, C.V.; et al. Carryover Effects of Larval Exposure to Different Environmental Bacteria Drive Adult Trait Variation in a Mosquito Vector. Sci. Adv. 2017, 3, e1700585. [Google Scholar] [CrossRef] [PubMed]
- White, L.A. Susceptibility of Aedes Albopictus C6/36 Cells to Viral Infection. J. Clin. Microbiol. 1987, 25, 1221–1224. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, M.; Yabe, S.; Okada, T. Effects of Various Passages on Some Properties of an Attenuated Strain of Japanese Encephalitis Virus with Special Regard to Mosquito Infectivity. Jpn. J. Med. Sci. Biol. 1969, 22, 163–174. [Google Scholar] [CrossRef]
- Park, S.L.; Huang, Y.-J.S.; Lyons, A.C.; Ayers, V.B.; Hettenbach, S.M.; McVey, D.S.; Burton, K.R.; Higgs, S.; Vanlandingham, D.L. North American Domestic Pigs Are Susceptible to Experimental Infection with Japanese Encephalitis Virus. Sci. Rep. 2018, 8, 7951. [Google Scholar] [CrossRef] [PubMed]
- Scherer, W.F.; Moyer, J.T.; Izumi, T. Immunologic Studies of Japanese Encephalitis Virus in Japan. V. Maternal Antibodies, Antibody Responses and Viremia Following Infection of Swine. J. Immunol. 1959, 83, 620–626. [Google Scholar] [PubMed]
- Cleton, N.B.; Bosco-Lauth, A.; Page, M.J.; Bowen, R.A. Age-Related Susceptibility to Japanese Encephalitis Virus in Domestic Ducklings and Chicks. Am. J. Trop. Med. Hyg. 2014, 90, 242–246. [Google Scholar] [CrossRef] [PubMed]
- Carrington, L.B.; Seifert, S.N.; Armijos, M.V.; Lambrechts, L.; Scott, T.W. Reduction of Aedes Aegypti Vector Competence for Dengue Virus under Large Temperature Fluctuations. Am. J. Trop. Med. Hyg. 2013, 88, 689–697. [Google Scholar] [CrossRef] [PubMed]
- Lambrechts, L.; Chevillon, C.; Albright, R.G.; Thaisomboonsuk, B.; Richardson, J.H.; Jarman, R.G.; Scott, T.W. Genetic Specificity and Potential for Local Adaptation between Dengue Viruses and Mosquito Vectors. BMC Evol. Biol. 2009, 9, 160. [Google Scholar] [CrossRef] [PubMed]
- Zouache, K.; Fontaine, A.; Vega-Rua, A.; Mousson, L.; Thiberge, J.-M.; Lourenco-De-Oliveira, R.; Caro, V.; Lambrechts, L.; Failloux, A.-B. Three-Way Interactions between Mosquito Population, Viral Strain and Temperature Underlying Chikungunya Virus Transmission Potential. Proc. Biol. Sci. 2014, 281, 20141078. [Google Scholar] [CrossRef] [PubMed]
- Miot, E.F.; Calvez, E.; Aubry, F.; Dabo, S.; Grandadam, M.; Marcombe, S.; Oke, C.; Logan, J.G.; Brey, P.T.; Lambrechts, L. Risk of Arbovirus Emergence via Bridge Vectors: Case Study of the Sylvatic Mosquito Aedes Malayensis in the Nakai District, Laos. Sci. Rep. 2020, 10, 7750. [Google Scholar] [CrossRef] [PubMed]
- Pongsiri, A.; Ponlawat, A.; Thaisomboonsuk, B.; Jarman, R.G.; Scott, T.W.; Lambrechts, L. Differential Susceptibility of Two Field Aedes Aegypti Populations to a Low Infectious Dose of Dengue Virus. PLoS ONE 2014, 9, e92971. [Google Scholar] [CrossRef] [PubMed]
- Fontaine, A.; Lequime, S.; Moltini-Conclois, I.; Jiolle, D.; Leparc-Goffart, I.; Reiner, R.C.; Lambrechts, L. Epidemiological Significance of Dengue Virus Genetic Variation in Mosquito Infection Dynamics. PLoS Pathog. 2018, 14, e1007187. [Google Scholar] [CrossRef] [PubMed]
- Dickson, L.B.; Ghozlane, A.; Volant, S.; Bouchier, C.; Ma, L.; Vega-Rúa, A.; Dusfour, I.; Jiolle, D.; Paupy, C.; Mayanja, M.N.; et al. Diverse Laboratory Colonies of Aedes Aegypti Harbor the Same Adult Midgut Bacterial Microbiome. Parasites Vectors 2018, 11, 207. [Google Scholar] [CrossRef]
- Baidaliuk, A.; Miot, E.F.; Lequime, S.; Moltini-Conclois, I.; Delaigue, F.; Dabo, S.; Dickson, L.B.; Aubry, F.; Merkling, S.H.; Cao-Lormeau, V.-M.; et al. Cell-Fusing Agent Virus Reduces Arbovirus Dissemination in Aedes Aegypti Mosquitoes In Vivo. J. Virol. 2019, 93. [Google Scholar] [CrossRef]
- Huang, C.H.; Liang, H.C.; Jia, F.L. Beneficial Role of a Nonpathogenic Orbi-like Virus: Studies on the Interfering Effect of M14 Virus in Mice and Mosquitoes Infected with Japanese Encephalitis Virus. Intervirology 1985, 24, 147–153. [Google Scholar] [CrossRef] [PubMed]
- Carvajal, T.M.; Capistrano, J.D.R.; Hashimoto, K.; Go, K.J.D.; Cruz, M.A.I.J.; Martinez, M.J.L.B.; Tiopianco, V.S.P.; Amalin, D.M.; Watanabe, K. Detection and Distribution of Wolbachia Endobacteria in Culex Quinquefasciatus Populations (Diptera: Culicidae) from Metropolitan Manila, Philippines. J. Vector Borne Dis. 2018, 55, 265–270. [Google Scholar] [CrossRef] [PubMed]
- Nugapola, N.W.N.P.; De Silva, W.A.P.P.; Karunaratne, S.H.P.P. Distribution and Phylogeny of Wolbachia Strains in Wild Mosquito Populations in Sri Lanka. Parasites Vectors 2017, 10, 230. [Google Scholar] [CrossRef] [PubMed]
- Jeffries, C.L.; Walker, T. The Potential Use of Wolbachia-Based Mosquito Biocontrol Strategies for Japanese Encephalitis. PLoS Negl. Trop. Dis. 2015, 9, e0003576. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).