Detection of Rift Valley Fever Virus RNA in Formalin-Fixed Mosquitoes by In Situ Hybridization (RNAscope®)
Abstract
1. Introduction
2. Materials and Methods
2.1. Mosquitoes, Viruses and Infection Protocol
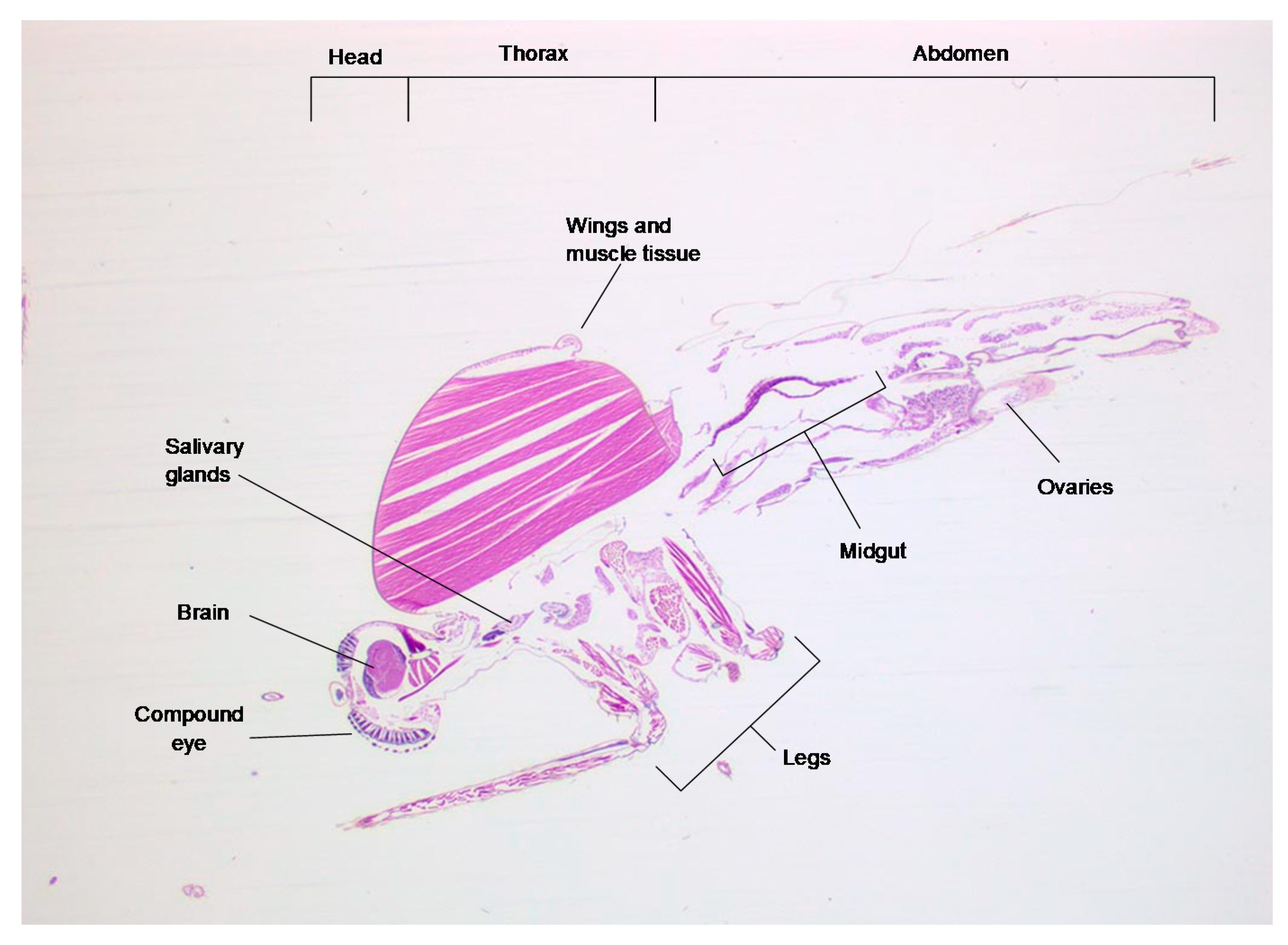
2.2. In Situ Hybridization: RNAscope®
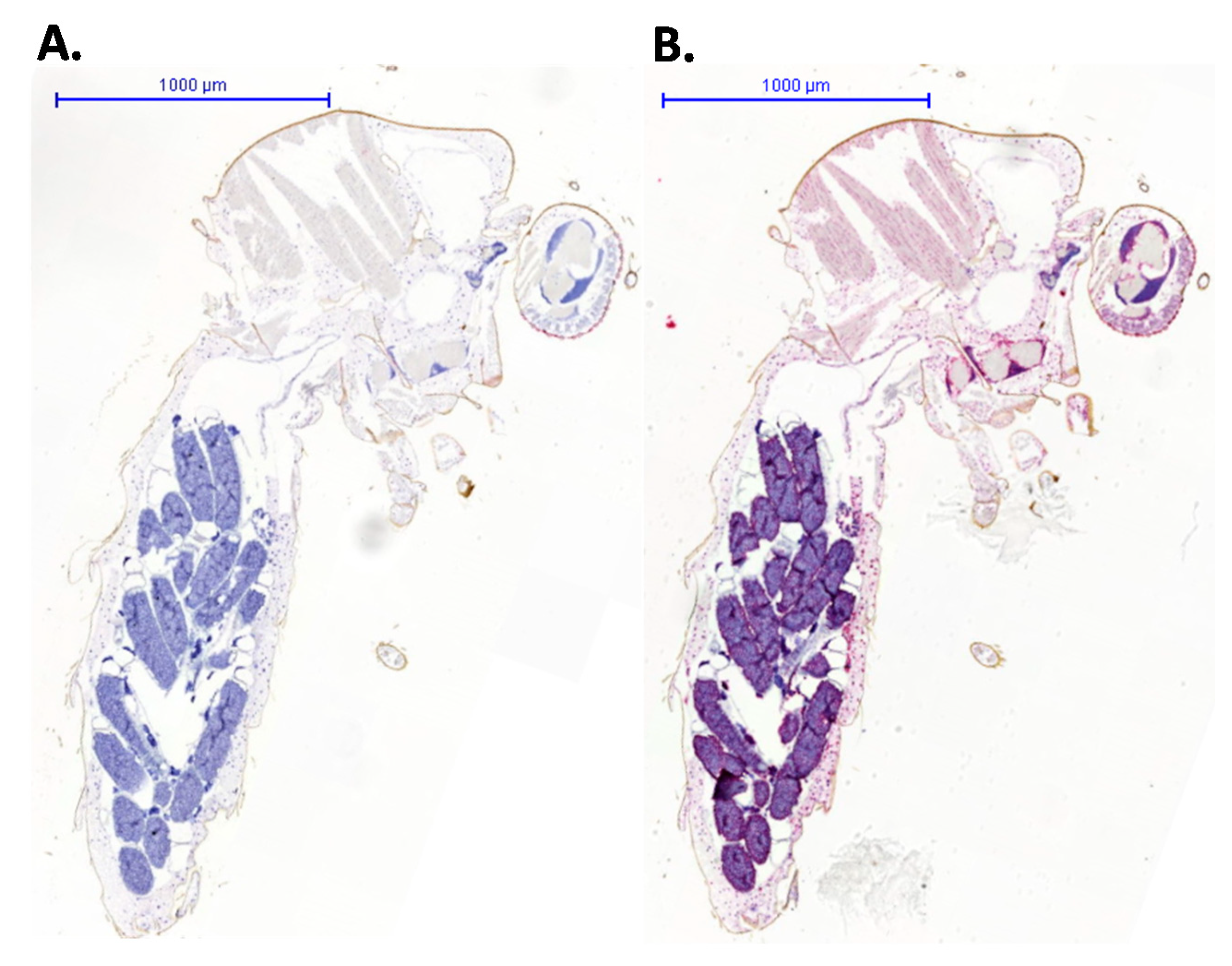
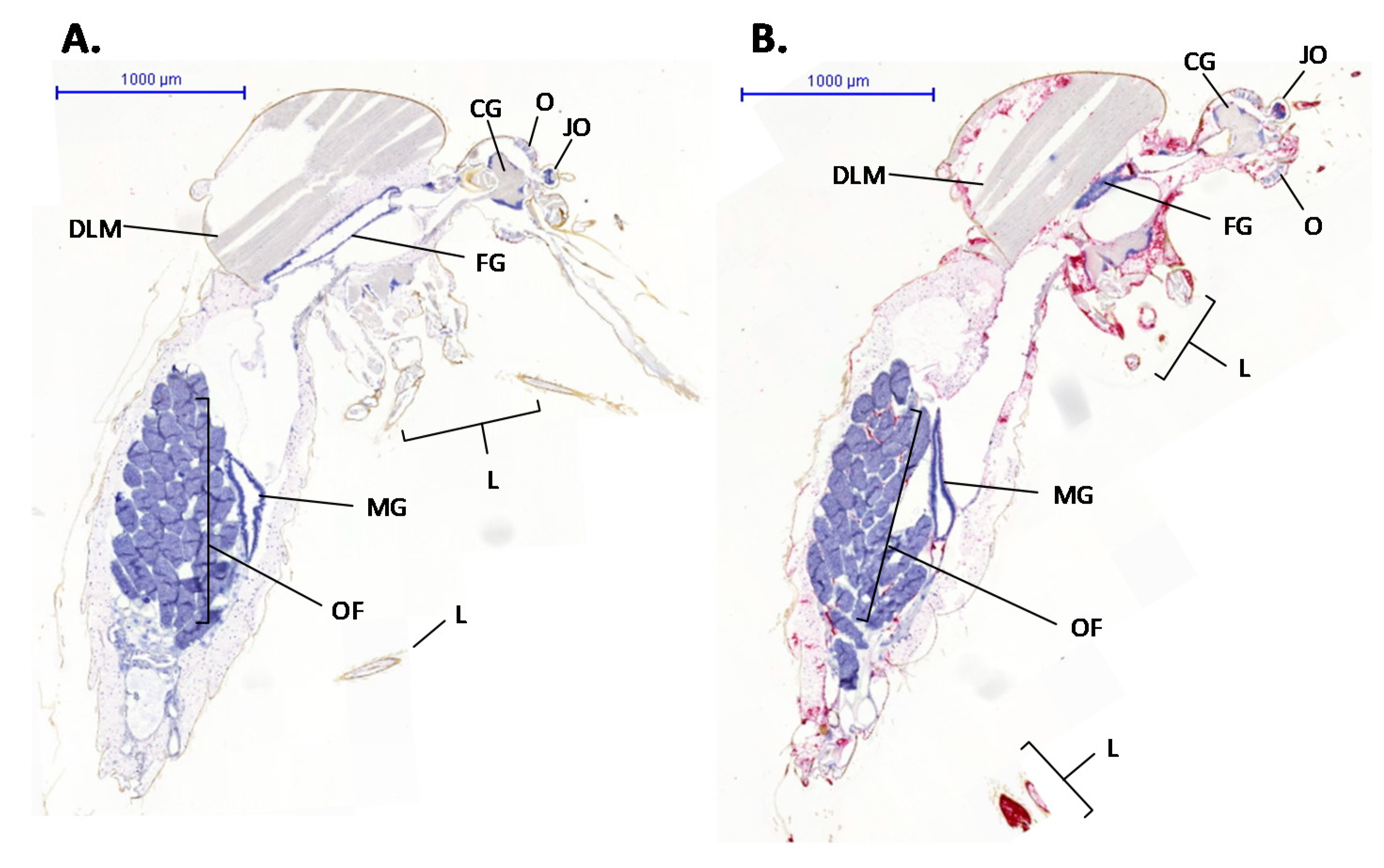
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mansfield, K.L.; Banyard, A.C.; McElhinney, L.M.; Johnson, N.; Horton, D.L.; Hernández-Triana, L.M.; Fooks, A.R. Rift Valley fever virus: A review of diagnosis and vaccination, and implications for emergence in Europe. Vaccine 2015, 33, 5520–5531. [Google Scholar] [CrossRef] [PubMed]
- Madani, T.A.; Al-Mazrou, Y.Y.; Al-Jeffri, M.H.; Mishkhas, A.A.; Al-Rebeah, A.M.; Turkistani, A.M.; Al-Sayed, M.O.; Abodahish, A.A.; Khan, A.S.; Ksiazek, T.G.; et al. Rift Valley fever epidemic in Saudi Arabia: Epidemiological, clinical and laboratory characteristics. Clin. Infect. Dis. 2003, 37, 1084–1092. [Google Scholar] [CrossRef] [PubMed]
- Tantely, L.M.; Boyer, S.; Fontenille, D. A review of mosquitoes associated with Rift Valley fever virus in Madagascar. Am. J. Trop. Med. Hyg. 2015, 92, 722–729. [Google Scholar] [CrossRef] [PubMed]
- Métras, R.; Cavalerie, L.; Dommergues, L.; Mérot, P.; Edmunds, W.J.; Keeling, M.J.; Cêtre-Sossah, C.; Cardinale, E. The epidemiology of Rift Valley fever in Mayotte: Insights and perspectives from 11 years of data. PLoS Negl. Trop. Dis. 2016, 10, e0004783. [Google Scholar] [CrossRef] [PubMed]
- Turell, M.J.; Linthicum, K.J.; Patrican, L.A.; Davies, F.G.; Kairo, A.; Bailey, C.L. Vector competence of selected African mosquito (Diptera: Culicidae) species from Rift Valley fever virus. J. Med. Entomol. 2008, 45, 102–108. [Google Scholar] [CrossRef] [PubMed]
- Moutailler, S.; Krida, G.; Schaffner, F.; Vazeille, M.; Failloux, A.B. Potential vectors of Rift Valley fever virus in the Mediterranean region. Vector Borne Zoonotic Dis. 2008, 8, 749–754. [Google Scholar] [CrossRef] [PubMed]
- Romoser, W.S.; Faran, M.E.; Bailey, C.L.; Ledthusnee, K. An immunocytochemical study of the distribution of Rift Valley fever virus in the mosquito Culex pipiens. Am. J. Trop. Med. Hyg. 1992, 46, 489–501. [Google Scholar] [CrossRef] [PubMed]
- Folly, A.J.; Dorey-Robinson, D.; Hernández-Triana, L.M.; Ackroyd, S.; Vidana, B.; Lean, F.Z.X.; Hicks, D.; Nuñez, A.; Johnson, N. Temperate conditions restrict Japanese encephalitis virus infection to the mid-gut and prevents systemic dissemination in Culex pipiens mosquitoes. Sci. Rep. 2020, 11, 6133. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Flanagan, J.; Su, N.; Wang, L.-C.; Bui, S.; Nielson, A.; Wu, X.; Vo, H.-T.; Ma, X.-J.; Lui, Y. RNAscope: A novel in situ RNA analysis platform for formalin-fixed, paraffin-embedded tissue. J. Mol. Diagn. 2012, 14, 22–29. [Google Scholar] [CrossRef] [PubMed]
- Ragan, I.K.; Schuk, K.N.; Upreti, D.; Odendaal, L.; Richt, J.A.; Trujilo, J.D.; Wilson, W.C.; Davis, A.S. Rift Valley fever viral RNA detection by in situ hybridization in formalin-fixed, paraffin-embedded tissues. Vector Borne Zoonotic Dis. 2019, 19, 553–556. [Google Scholar] [CrossRef] [PubMed]
- Manley, R.; Harrup, L.E.; Veronesi, E.; Stubbins, F.; Stoner, J.; Gubbins, S.; Wilson, A.; Batten, C.; Koenraadt, C.J.; Henstock, M.; et al. Testing of UK populations of Culex pipiens L. for Schmallenberg virus vector competence and their colonization. PLoS ONE 2015, 10, e0134453. [Google Scholar] [CrossRef] [PubMed]
- Lumley, S.; Hernández-Triana, L.M.; Horton, D.L.; Fernández de Marco, M.D.M.; Medlock, J.M.; Hewson, R.; Fooks, A.R.; Johnson, N. Competence of mosquitoes native to the United Kingdom to support replication and transmission of Rift Valley fever virus. Parasites Vectors 2018, 11, 308. [Google Scholar] [CrossRef] [PubMed]
- Franz, A.E.; Kantor, A.M.; Passarelli, A.L.; Clem, R.J. Tissue barriers to arbovirus infection in mosquitoes. Viruses 2015, 7, 3741–3767. [Google Scholar] [CrossRef] [PubMed]
- Romoser, W.S.; Wasieloski, L.P.; Pushko, P.; Kondig, J.P.; Ledthusnee, K.; Keira, M.; Ludwig, G.V. Evidence for arbovirus dissemination conduits from the mosquito (Diptera: Culicidae) midgut. J. Med. Entomol. 2004, 41, 467–475. [Google Scholar] [CrossRef] [PubMed]
- Higgs, S. How do mosquito vectors live with their viruses? In Microbe-Vector Interactions in Vector-Borne Diseases; Gillespie, S.H., Smith, G.L., Eds.; Cambridge University Press: Cambridge, UK, 2004; pp. 103–138. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lumley, S.; Hunter, L.; Emery, K.; Hewson, R.; Fooks, A.R.; Horton, D.L.; Johnson, N. Detection of Rift Valley Fever Virus RNA in Formalin-Fixed Mosquitoes by In Situ Hybridization (RNAscope®). Viruses 2021, 13, 1079. https://doi.org/10.3390/v13061079
Lumley S, Hunter L, Emery K, Hewson R, Fooks AR, Horton DL, Johnson N. Detection of Rift Valley Fever Virus RNA in Formalin-Fixed Mosquitoes by In Situ Hybridization (RNAscope®). Viruses. 2021; 13(6):1079. https://doi.org/10.3390/v13061079
Chicago/Turabian StyleLumley, Sarah, Laura Hunter, Kirsty Emery, Roger Hewson, Anthony R. Fooks, Daniel L. Horton, and Nicholas Johnson. 2021. "Detection of Rift Valley Fever Virus RNA in Formalin-Fixed Mosquitoes by In Situ Hybridization (RNAscope®)" Viruses 13, no. 6: 1079. https://doi.org/10.3390/v13061079
APA StyleLumley, S., Hunter, L., Emery, K., Hewson, R., Fooks, A. R., Horton, D. L., & Johnson, N. (2021). Detection of Rift Valley Fever Virus RNA in Formalin-Fixed Mosquitoes by In Situ Hybridization (RNAscope®). Viruses, 13(6), 1079. https://doi.org/10.3390/v13061079





