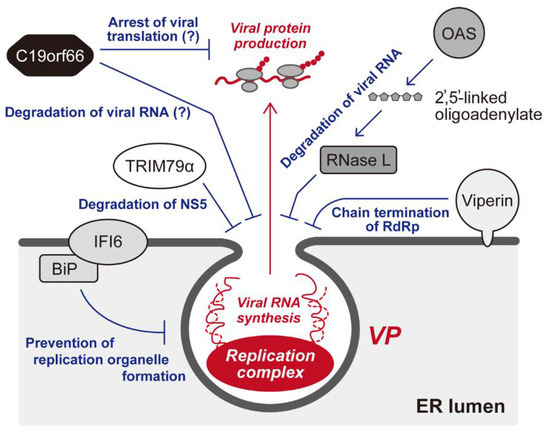
Abstract
Flavivirus consists of a large number of arthropod-borne viruses, many of which cause life-threatening diseases in humans. A characteristic feature of flavivirus infection is to induce the rearrangement of intracellular membrane structure in the cytoplasm. This unique membranous structure called replication organelle is considered as a microenvironment that provides factors required for the activity of the flaviviral replication complex. The replication organelle serves as a place to coordinate viral RNA amplification, protein translation, and virion assembly and also to protect the viral replication complex from the cellular immune defense system. In this review, we summarize the current understanding of how the formation and function of membrane-associated flaviviral replication organelle are regulated by cellular factors.
1. Introduction
The genus Flavivirus of the Flaviviridae family is a large virus group comprised of many emerging arthropod-borne pathogens. Flaviviruses are predominantly transmitted by mosquitoes and ticks and include clinically important viruses: Japanese encephalitis virus (JEV), dengue virus (DENV), yellow fever virus (YFV), West Nile virus (WNV), and tick-borne encephalitis virus (TBEV), which cause life-threatening diseases such as hemorrhagic fever, encephalitis, and meningitis in humans [1]. Furthermore, the recent explosive outbreak of Zika virus (ZIKV), mainly in South and Central America, has revealed that infection with this flavivirus is responsible for congenital microcephaly [2]. Therefore, flavivirus infections are continuing global threats to public health. However, there are no specific antivirals available for most flavivirus infections.
Flavivirus is a lipid-enveloped RNA virus. Its ~11,000-nucleotide-long single-stranded positive-sense RNA (ssRNA(+)) viral genome contains a single long open reading frame (ORF) encoding three structural (capsid [C], pre-membrane [prM], and envelope [E]) and seven non-structural (NS1, NS2A, NS2B, NS3, NS4A, NS4B, and NS5) proteins, flanked by highly structured 5′ and 3′ untranslated regions (UTRs). Structural proteins are required for the formation of infectious viral particles, whereas NS proteins play important roles in viral RNA replication, protein processing, and virion assembly [1]. Additionally, some of the flaviviral NS proteins have been reported to counteract the interferon (IFN)-mediated antiviral response in virus-infected cells [3].
Flavivirus infection begins with the binding of E glycoproteins with cell-surface entry receptors. A number of cellular molecules have been shown to be entry receptors for flavivirus infection, including heparan sulfate, C-type lectins, and phosphatidylserine receptors [4]. Internalization of the attached virion is mediated by clathrin-dependent endocytosis. Subsequently, a fusion between viral and endosomal membranes occurs, which is triggered by the structural rearrangements of the E protein induced in the acidic environment of the endosomal vesicles. After penetration of the nucleocapsid into the cytoplasm, the single ORF is translated from viral RNA, which is disassociated from C proteins, and a precursor polyprotein is co- and post-translationally cleaved by viral and host-encoded proteases. The viral protein involved in this polyprotein processing step is NS3, which possesses helicase, RNA triphosphatase, and serine protease activities [5,6].
The viral ssRNA(+) also serves as a template for the synthesis of new copies of genomic RNA, in which negative-sense RNA is first generated and then directs the amplification of new positive-sense RNAs. This viral RNA synthesis is catalyzed by the RNA-dependent RNA polymerase (RdRp) activity of NS5. During the viral RNA synthesis process, the N-terminal portion of NS5, which is reported to contain guanylyltransferase (GTPase) and methyltransferase (MTase), involves the formation of a type 1 cap (m7GpppAmp) structure at the 5′ end of the viral RNA [5,7].
This viral RNA amplification process takes place on the endoplasmic reticulum (ER) membrane, where the so-called “replication complex” is formed. Subsequently, the nucleocapsids, composed of C proteins and synthesized ssRNA(+), are assembled with prM and E heterodimers and cellular lipid bilayers to form immature particles in the ER lumen. At this budding step, the prM makes the immature particles non-infectious by repressing the fusogenic activity of the E protein. However, during transport through the secretory pathway, the conformational change of glycoproteins takes place in the acidic environment of the trans-Golgi network (TGN), resulting in processing of the prM by the cellular protease, and thereby creating a mature virion [8,9]. Then, the infectious virion egress from the infected cell occurs through exocytosis.
The process of the replication complex formation requires many viral and cellular factors [10]. For instance, some trans-membrane NS proteins function as the scaffold to retain the replication complex to ER. Among them, NS2B serves as a cofactor of NS3 protease activity [5]. Additionally, DNAJC14, a cellular protein chaperone, is shown to facilitate the viral protein processing step, resulting in the formation of a functional replication complex [10,11]. Of particular interest is that the intracellular membrane structure within the ER is rearranged by a virus infection, which is closely associated with the activity of the flaviviral replication complex, including viral RNA amplification and protein translation [12,13,14,15,16,17,18,19,20]. The establishment of this membranous microenvironment is thought to be necessary to promote flavivirus replication. Additionally, this architecture wrapped by the ER membrane is presumed to contribute to the protection of the viral replication machinery from cellular immune defense systems [21,22,23,24]. In this review, we will focus our attention on the biogenesis and regulation of the flavivirus-induced organelle-like membranous structures by viral and cellular factors.
2. Flavivirus Infection Rearranges Intracellular Membrane System and Creates Organelle-Like Structures
Flaviviruses induce a unique organelle-like structure, called a “viral replication organelle,” in the cytoplasm of infected cells [25,26]. Since the viral proteins and double-strand RNA (dsRNA) are detected within the viral replication organelle [26], this could be the location of viral genome replication, viral protein synthesis, and viral particle assembly. Although the size of this structure varies in each flavivirus species and host cell type, several ER marker proteins have been detected in this structure, suggesting that the membrane of the viral replication organelle is derived from the ER in all cases. The imaging analysis using probes for high-mannose glycans, such as concanavalin A (CoA) and wheat germ agglutinin (WGA), revealed that the structure could originate from the pre-Golgi area of the ER [15]. The viral replication organelle has been considered to be composed of two distinct compartments: a vesicle packet (VP) and a convoluted membrane (CM) (Figure 1).
Several electron tomography analyses of flavivirus-infected cells showed that a VP approximately 50–90 nm in diameter and containing a few dozen small membrane vesicles appears [27]. Further, 3D imaging analysis revealed that the membranes of luminal small vesicles are not separated from the packet membranes, and the inner areas of these vesicles are connected to the cytosol through small pores. Since dsRNA, which is an intermediate product of the viral genome replication, is detected in the VP area of the viral replication organelle via immuno-fluorescent imaging analysis, VPs have been considered to be the location of viral genome replication. Previously, a model was proposed in which the progeny positive-stranded RNAs are synthesized in the VP vesicles by the viral replication complexes, and the newly synthesized genomic RNAs are exported to the outside of the vesicles to be assembled into infectious particles at a proximal area of the vesicle pore (Figure 1) [28].
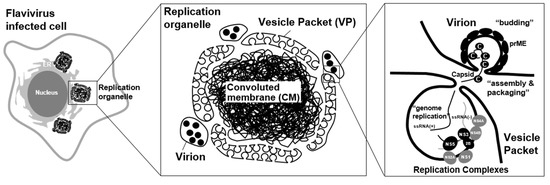
Figure 1.
Flavivirus replication organelle. Virus antigen-positive structure was observed in flavivirus infected cells. These structures, called viral replication organelle, are composed of two distinct compartments: a vesicle packet (VP) and a convoluted membrane (CM). VP contains a few dozen small membrane vesicles, and the inner areas of these vesicles are connected to the cytosol through small pores. VPs have been considered to be the location of viral genome replication. The progeny positive-sense RNAs are synthesized in the VP vesicles by the viral replication complexes. The newly synthesized genomic RNAs are exported to the outside of the vesicles to be assembled into infectious particles at a proximal area of the vesicle pore [28]. A CM is forming a reticulovesicular network of membranes. Since it contains abundant viral non-structural membrane proteins, the role of the CM was previously proposed to be as the location for the processing or storage of viral polyproteins [8].
Unlike a VP structure, a CM is observed as an electron-dense structure in EM analysis, probably forming a reticulovesicular network of membranes. Since it contains abundant viral non-structural membrane proteins, the role of the CM was previously proposed to be as the location for the processing or storage of viral polyproteins [14]. However, its morphology seems to resemble a smooth ER, and no ribosome is detected in the CM, so it is now considered to be a place for lipid storage rather than polyprotein synthesis [14]. Considering that CM formation disrupts the mitochondrion-associated membranes (MAMs), which serve as a platform for MAVS (mitochondrial antiviral signaling)-mediated innate immune signaling, this structure may play a role in the suppression of innate immune signaling [22]. Interestingly, the CM is not always detected in flavivirus-infected cells. For instance, CM structures sometimes could be hardly observed in DENV-infected mosquito cells [29] and ZIKV-infected human neural progenitor cells (hNPCs) [30], but the condition for this structure to appear has not yet been defined. Besides the CM, a zippered ER (zER) membrane, which is a closely aligned ER cisterna, was observed in the ZIKV-infected hepatoma cells [30]; however, the role of this structure is unknown. In TEBV-infected tick cells, the formation of the VP was less abundant compared to infected mammalian cells. Instead, membrane-associated end-closed tubular structures, which were arranged in fascicle-like bundles and reached up 800 nm in length, were often found. Since the tubules were more abundant in the tick cells persistently infected with TBEV, this unique structure may play an essential role in maintaining persistent infection of flaviviruses in arthropod cells [31].
3. Viral Proteins Involved in the Formation of a Viral Replication Organelle
The viral replication organelle biogenesis is initiated by the production of viral proteins from the first ssRNA(+) viral genome. The polyprotein of DENV contains proteins NS1 to NS5, which induce the formation of ER membrane invaginations. These induced structures are very similar to the VPs observed in the DENV-infected cells, which indicates that these NS proteins are sufficient to generate a viral replication organelle [32]. Among them, the membrane-anchored NS1, NS2A, NS4A, and NS4B proteins play central roles in the ER shape deformation to create a viral replication organelle [1,33]. The membrane structures observed in the individual protein-expressing cells are different from those observed in the virus-infected cells, suggesting that the cooperation of these proteins and/or host factors is required for the formation of a viral replication organelle [1].
The expression of a polyprotein lacking the NS1 coding region does not induce VP structure formation, which indicates the essential role of NS1 in this process [32]. NS1 is post-translationally modified via N-linked glycosylation and has six invariant intramolecular disulfide bonds to form a dimer in the ER lumen. NS1 consists of three domains, a core “β-ladder” domain, a small “β-roll” dimerization domain, and a “wing” domain [34]. In the NS1 dimer structure, the NS1 dimer directly binds to the ER membrane via the hydrophobic surface of the protrusion structure created by the β-roll and connector subdomain of the wing, and it plays an important role in membrane bending from the inside of the ER luminal space [34]. Bending of the membrane has been considered to be related to the formation of vesicles during VP production [35] and the production of infectious particles [36].
NS2A, NS4A, and NS4B proteins do not constitute virus particles and do not exhibit enzymatic activities. Among them, NS4A and NS4B have been shown to play an essential role on the membrane deformation during viral replication-organelle formation [37]. NS4A consists of three regions, a hydrophilic N-terminal region in the cytoplasm, a hydrophobic region associated with endoplasmic reticulum (ER) membranes (pTMS1–pTMS3), and a C-terminal region named 2K. The 2K region functions as the signal sequence for translocation of the NS4B protein into the lumen and is cleaved off by NS2B-NS3 viral protease [38]. The NS4A N-terminal region plays role in homo-oligomerization and is supposed to be the region interacting with cellular factor, vimentin [39]. On the other hand, NS4B contains five hydrophobic segments. Two N-terminal segments (pTMD1 and pTMD2) are considered to be associated with the ER luminal side of the membrane, and three C-terminal segments (pTMD3–pTMD5) are anchored into the ER membrane as transmembrane regions [40,41]. NS4B also forms a homodimer in virus-infected cells. The expression of both NS4A and NS4B was able to induce an ER membrane rearrangement that was similar to that observed in infected cells. Therefore, these proteins are considered to be the main driving factors for viral replication-organelle formation [38,41,42]. Both proteins have been demonstrated to be able to interact with other viral proteins, including NS3 localized in the cytoplasm and NS1 positioned in the ER lumen to modulate viral replication [43,44,45].
4. Host ER Shaping Proteins Are Involved in the Formation of a Viral Replication Organelle
Some host ER shaping proteins, such as reticulon (RTN) and atlastin (ATL) family proteins, are also involved in membrane deformation during viral replication organelle formation. These proteins are highly conserved in eukaryotes with short, hairpin-like transmembrane domains, which are considered to cause membrane deformation by forming a wedge-like structure that is embedded into the ER membrane at the cytoplasmic leaflet of the lipid bilayer. Aktepe et al. reported that RTN 3.1A, one of the RTN family proteins, is required for flavivirus genome replication. The depletion of RTN 3.1A in the ZIKV-infected or WNV-infected cells decreased membrane curvature formation and replication vesicle production in the VPs. On the other hand, RTN3.1A associates with NS4A, and this interaction probably mediates membrane remodeling to promote vesicle formation [46].
ATL family proteins are also involved in the formation of a flavivirus replication organelle [1]. These proteins are localized at the ER membrane and are considered to be involved in the fusion of ER tubules to maintain ER mesh networks [47,48]. Neufeldt et al. reported that the depletions of ATL2 and ATL3 impair the production of infectious DENV and ZIKV particles [1]. ATL2 depletion also suppresses the viral genome replication. Furthermore, the vesicles in the infected cells were condensed within a small perinuclear region, and the vesicle shapes were distorted in the ATL2-depleted cells, suggesting that ATL2 may also be required for the formation of replication vesicles in the viral replication organelle. The requirement of the ATL family for viral genome replication was also reported by Monel et al. [49]. They found that ATL3 is recruited to the viral replication organelle along with the viral NS2A and NS2B3 proteins.
5. The Endosomal Sorting Complex Required for Transport (ESCRT) Proteins Is Potentially Involved in Membrane Invagination and Fission
The proteome analysis for the JEV-infected cells revealed that host ESCRT proteins were recruited to the viral replication organelle [50]. ESCRT proteins are involved in membrane invagination toward the luminal side from the cytoplasm and in membrane sealing from the cytoplasmic phase [51]. In the flavivirus-infected cells, depletion of the CMHP2 or CHMP4 family proteins significantly impairs infectious particle production, suggesting that ESCRT-mediated membrane sealing is mainly involved in the viral budding process, rather than VP formation [50]. However, studies using other ssRNA(+) viruses that can infect Saccharomyces cerevisiae have suggested a different model. Brome mosaic virus (BMV) and tomato bushy stunt virus (TBSV) infection have been shown to remodel ER membrane and create spherules [52]. This structure is similar to the vesicles in VPs observed in Flavivirus infected cells, because the requirement of host factors, such as RTN family proteins, is the same to that for VPs formation [53]. In a study using BMV and BSV, the genetic analysis of yeast for non-essential genes has clarified that several ESCRT-encoding genes are required for viral RNA genome replication. Furthermore, a lack of ESCRT factors affects the formation of spherules [54,55], suggesting that ESCRT factors play important roles in the viral replication organelle. Studies of flavivirus infection by Tabata et al. also indicated that the depletion of ESCRT factors slightly affects virus genome replication, suggesting that the roles of ESCRT factors may commonly be involved in the VP formation of ssRNA(+) virus replication organelles.
6. Cellular Autophagic Machinery Is Activated in the Flavivirus-Infected Cells
Autophagy is a major bulk-degradation process that involves sequestering the cytoplasmic target with the isolation membrane and delivering its contents to the lysosome. A series of autophagy-related gene (ATG) proteins is known to be required for the autophagosome formations [56]. Autophagy has been identified as a process that supplies nutrients by degrading the cells under starvation conditions, but it is now understood as an important process for the elimination of unnecessary cellular components, such as damaged organelles, protein aggregates, and pathogens [56]. This process is known as “selective autophagy”.
JEV [57], WNV [58], DENV [59], and ZIKV [60] infections activate the autophagic machinery. The accumulation of LC3-II has been observed in these flavivirus-infected cells [61,62]. Pharmacologic inhibition of PI3 kinases associated with autophagy suppresses flavivirus propagation [63]. Furthermore, autophagy activation by treatment with an mTOR inhibitor, such as Torin1 or rapamycin, subsequently increases ZIKV propagation [60,61,64,65], suggesting that autophagic machinery positively regulates flavivirus propagation in the cells. Several indicators, such as that the autophagic isolation membrane could be derived from the ER membrane and some viral proteins co-localize with LC3-II expressed on the isolation membrane, led to the hypothesis that the virus induces autophagy to utilize the autophagic isolation membrane for the materials needed for a viral replication organelle. Recently, Evans et al. have reported that BPIFB3 (BPI fold-containing family B member 3), a regulator of autophagy, is involved in the formation of DENV and ZIKV replication organelles [66]. In this report, the author proposed the model that BPIFB3 has a role in impairing the ER-phagy, selective autophagic degradation of ER, which may negatively regulate viral replication. So far, many different conclusions have been reported; however, the involvement of autophagic machinery in the formation of a flavivirus replication organelle remains controversial [67,68].
Mertz et al., reported that the change of autophagy status after the DENV infection by image-based flow cytometry approach. Interestingly, early phase of infection, basal and activated autophagic flux was enhanced, but in the late stage of infection, autophagosome formation was blocked, and autophagic-degradation capacity was reduced. These results suggest that the requirement for autophagy is different in each replication step [62].
Recently, genome-wide CRISPR-Cas9 screening has identified TMEM41B (trans-membrane protein 41B) as a novel host factor required for flavivirus replication [69]. This protein is involved in the VP formation to protect against the host innate immune response and the induction of apoptosis. TMEM41B has been shown to have roles in the early step of autophagosome formation [70]. Interestingly, the role of TMEM41B in flavivirus replication is independent of autophagy because the phenotype of TMEM41B knockout cells is different from that of Beclin1, ATG5, or ATG7 knockout cells [69]. Its phospholipid scramblases activity, rather than autophagic machinery, may be involved in the viral replication organelle formation [71].
7. Flavivirus Infection Changes the Intracellular Membrane Lipid Composition
WNV and ZIKV infection are known to affect the intracellular membrane lipid composition [34,72]. These changes in the membrane lipid composition potentially influence the curvature and thickness of the local membranes [35]. The size balance between the head group and hydrophobic tails affects the shape of the lipid and the spontaneous curvature of the membrane. Specifically, phosphatidylcholine (PC), sphingolipid, and ceramide are enriched in the ER compartments around the flavivirus replication site. The yeast genetics analyses identified choline requiring 2 (Cho2p), a PC synthase, as a host factor required for ssRNA(+) virus replication [36]. PC enrichment in the viral replication organelle is associated with the recruitment of Cho2p by interacting with the viral protein. Treatment with nSMase2 (neutral sphingomyelinase 2) inhibitors, such as GW4869, spiroepoxide, or glutathione, or siRNA depletion of nSMase2 decreases the production of flavivirus subviral particles and leads to the accumulation of viral structural proteins in the cells, indicating that nSMase is required for viral particle secretion [72]. Enzymes involved in the major phospholipid metabolism may play an important role in viral replication organelle biogenesis.
Phosphatidylinositol is also a key molecule in viral replication organelle formation. siRNA knockdown screening identified phosphatidylinositol-4-phosphate (PI4P) kinase (PI4K) as the host factor required for virus replication [73]. PI4P, a product of PI4K, is present in the ER and Golgi apparatus, as well as the viral replication site of other ssRNA(+) viruses, such as picornaviruses [74]. The production of PI4P at the viral replication site can serve as a signal to recruit proteins that have a PH domain, such as the oxysterol-binding protein (OSBP) [75].
Cholesterol is also involved in flavivirus replication. Treatment with a reagent that alters the distribution of membrane cholesterol reduces viral genome replication [76]. The distribution of fatty acid synthases (FASNs) and 3-hydroxy-3-methylglutaryl-coenzyme A reductases (HMGCRs), cholesterol-synthesizing enzymes involved in the production of the cholesterol precursor, is correlated to flaviviral NS proteins [77]. DENV NS3 directly interacts with the FASN and plays a role in recruiting the FASN to the virus replication site. This recruitment facilitates the upregulation of fatty acid synthesis at the viral replication site [78]. HMGCR activities are also increased at viral replication sites. These events affect the local membrane phospholipid composition and are required for the formation of a viral replication organelle [77].
9. Conclusions
The induction of specialized membranous compartments, where genome amplification, protein translation, and particle assembly take place, is a common feature of a variety of viruses. In this respect, flavivirus is a well-studied virus that generates a unique organelle-like structure in the cytoplasm to facilitate virus propagation. As introduced in this review, many cellular factors have been shown to participate in the formation of the replication organelle. On another front, it is becoming apparent that the membrane-associated flaviviral replication complex can be a target of cellular restriction factors as a critical aspect of the IFN-mediated antiviral response. Therefore, further research into the virus–host relationship in the functional regulation of replication organelles will provide a new clue for the development of anti-flavivirus drugs.
Author Contributions
Conceptualization, writing—review and editing, E.M. and Y.S.; funding acquisition, Y.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by JSPS KAKENHI Grant Number JP19K07596.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Neufeldt, C.J.; Cortese, M.; Acosta, E.G.; Bartenschlager, R. Rewiring cellular networks by members of the Flaviviridae family. Nat. Rev. Genet. 2018, 16, 125–142. [Google Scholar] [CrossRef] [PubMed]
- Rasmussen, S.A.; Jamieson, D.; Honein, M.A.; Petersen, L.R. Zika Virus and Birth Defects—Reviewing the Evidence for Causality. New Engl. J. Med. 2016, 374, 1981–1987. [Google Scholar] [CrossRef]
- García-Sastre, A. Ten Strategies of Interferon Evasion by Viruses. Cell Host Microbe 2017, 22, 176–184. [Google Scholar] [CrossRef]
- Perera-Lecoin, M.; Meertens, L.; Carnec, X.; Amara, A. Flavivirus Entry Receptors: An Update. Viruses 2013, 6, 69–88. [Google Scholar] [CrossRef] [PubMed]
- Bollati, M.; Alvarez, K.; Assenberg, R.; Baronti, C.; Canard, B.; Cook, S.; Coutard, B.; Decroly, E.; de Lamballerie, X.; Gould, E.A.; et al. Structure and functionality in flavivirus NS-proteins: Perspectives for drug design. Antivir. Res. 2010, 87, 125–148. [Google Scholar] [CrossRef]
- Murray, C.L.; Jones, C.T.; Rice, C.M. Architects of assembly: Roles of Flaviviridae non-structural proteins in virion morphogenesis. Nat. Rev. Genet. 2008, 6, 699–708. [Google Scholar] [CrossRef]
- Egloff, M.-P.; Decroly, E.; Malet, H.; Selisko, B.; Benarroch, D.; Ferron, F.; Canard, B. Structural and Functional Analysis of Methylation and 5′-RNA Sequence Requirements of Short Capped RNAs by the Methyltransferase Domain of Dengue Virus NS5. J. Mol. Biol. 2007, 372, 723–736. [Google Scholar] [CrossRef]
- Yu, I.-M.; Zhang, W.; Holdaway, H.A.; Li, L.; Kostyuchenko, V.A.; Chipman, P.R.; Kuhn, R.J.; Rossmann, M.G.; Chen, J. Structure of the Immature Dengue Virus at Low pH Primes Proteolytic Maturation. Science 2008, 319, 1834–1837. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Lok, S.-M.; Yu, I.-M.; Zhang, Y.; Kuhn, R.J.; Chen, J.; Rossmann, M.G. The Flavivirus Precursor Membrane-Envelope Protein Complex: Structure and Maturation. Science 2008, 319, 1830–1834. [Google Scholar] [CrossRef] [PubMed]
- Aktepe, T.E.; MacKenzie, J.M. Shaping the flavivirus replication complex: It is curvaceous! Cell. Microbiol. 2018, 20, e12884. [Google Scholar] [CrossRef]
- Yi, Z.; Sperzel, L.; Nürnberger, C.; Bredenbeek, P.J.; Lubick, K.J.; Best, S.M.; Stoyanov, C.T.; Law, L.M.J.; Yuan, Z.; Rice, C.M.; et al. Identification and Characterization of the Host Protein DNAJC14 as a Broadly Active Flavivirus Replication Modulator. PLoS Pathog. 2011, 7, e1001255. [Google Scholar] [CrossRef]
- Rothan, H.A.; Kumar, M. Role of Endoplasmic Reticulum-Associated Proteins in Flavivirus Replication and Assembly Complexes. Pathogens 2019, 8, 148. [Google Scholar] [CrossRef] [PubMed]
- Mackenzie, J.M.; Khromykh, A.A.; Jones, M.; Westaway, E.G. Subcellular Localization and Some Biochemical Properties of the Flavivirus Kunjin Nonstructural Proteins NS2A and NS4A. Virology 1998, 245, 203–215. [Google Scholar] [CrossRef] [PubMed]
- Welsch, S.; Miller, S.; Romero-Brey, I.; Merz, A.; Bleck, C.K.; Walther, P.; Fuller, S.D.; Antony, C.; Krijnse-Locker, J.; Bartenschlager, R. Composition and Three-Dimensional Architecture of the Dengue Virus Replication and Assembly Sites. Cell Host Microbe 2009, 5, 365–375. [Google Scholar] [CrossRef]
- Gillespie, L.K.; Hoenen, A.; Morgan, G.; MacKenzie, J.M. The Endoplasmic Reticulum Provides the Membrane Platform for Biogenesis of the Flavivirus Replication Complex. J. Virol. 2010, 84, 10438–10447. [Google Scholar] [CrossRef]
- MacKenzie, J. Wrapping Things up about Virus RNA Replication. Traffic 2005, 6, 967–977. [Google Scholar] [CrossRef]
- Miller, S.; Locker, J.K. Modification of intracellular membrane structures for virus replication. Nat. Rev. Microbiol. 2008, 6, 363–374. [Google Scholar] [CrossRef]
- Salonen, A.; Ahola, T.; Kääriäinen, L. Viral RNA Replication in Association with Cellular Membranes. Curr. Top. Microbiol. Immunol. 2004, 285, 139–173. [Google Scholar] [CrossRef]
- Boon, J.A.D.; Diaz, A.; Ahlquist, P. Cytoplasmic Viral Replication Complexes. Cell Host Microbe 2010, 8, 77–85. [Google Scholar] [CrossRef]
- Nazmi, A.; Dutta, K.; Basu, A. RIG-I Mediates Innate Immune Response in Mouse Neurons Following Japanese Encephalitis Virus Infection. PLoS ONE 2011, 6, e21761. [Google Scholar] [CrossRef]
- Netherton, C.; Moffat, K.; Brooks, E.; Wileman, T. A Guide to Viral Inclusions, Membrane Rearrangements, Factories, and Viroplasm Produced During Virus Replication. Int. Rev. Cytol. 2007, 70, 101–182. [Google Scholar] [CrossRef]
- Chatel-Chaix, L.; Cortese, M.; Romero-Brey, I.; Bender, S.; Neufeldt, C.J.; Fischl, W.; Scaturro, P.; Schieber, N.; Schwab, Y.; Fischer, B.; et al. Dengue Virus Perturbs Mitochondrial Morphodynamics to Dampen Innate Immune Responses. Cell Host Microbe 2016, 20, 342–356. [Google Scholar] [CrossRef]
- Överby, A.K.; Popov, V.L.; Niedrig, M.; Weber, F. Tick-Borne Encephalitis Virus Delays Interferon Induction and Hides Its Double-Stranded RNA in Intracellular Membrane Vesicles. J. Virol. 2010, 84, 8470–8483. [Google Scholar] [CrossRef] [PubMed]
- Hoenen, A.; Liu, W.; Kochs, G.; Khromykh, A.A.; MacKenzie, J.M. West Nile virus-induced cytoplasmic membrane structures provide partial protection against the interferon-induced antiviral MxA protein. J. Gen. Virol. 2007, 88, 3013–3017. [Google Scholar] [CrossRef] [PubMed]
- Romero-Brey, I.; Bartenschlager, R. Endoplasmic Reticulum: The Favorite Intracellular Niche for Viral Replication and Assembly. Viruses 2016, 8, 160. [Google Scholar] [CrossRef]
- Arakawa, M.; Morita, E. Flavivirus Replication Organelle Biogenesis in the Endoplasmic Reticulum: Comparison with Other Single-Stranded Positive-Sense RNA Viruses. Int. J. Mol. Sci. 2019, 20, 2336. [Google Scholar] [CrossRef]
- Romero-Brey, I.; Bartenschlager, R. Membranous Replication Factories Induced by Plus-Strand RNA Viruses. Viruses 2014, 6, 2826–2857. [Google Scholar] [CrossRef]
- Chatel-Chaix, L.; Bartenschlager, R. Dengue Virus- and Hepatitis C Virus-Induced Replication and Assembly Compartments: The Enemy Inside—Caught in the Web. J. Virol. 2014, 88, 5907–5911. [Google Scholar] [CrossRef]
- Junjhon, J.; Pennington, J.G.; Edwards, T.J.; Perera, R.; Lanman, J.; Kuhn, R.J. Ultrastructural Characterization and Three-Dimensional Architecture of Replication Sites in Dengue Virus-Infected Mosquito Cells. J. Virol. 2014, 88, 4687–4697. [Google Scholar] [CrossRef]
- Cortese, M.; Goellner, S.; Acosta, E.G.; Neufeldt, C.J.; Oleksiuk, O.; Lampe, M.; Haselmann, U.; Funaya, C.; Schieber, N.; Ronchi, P.; et al. Ultrastructural characterization of Zika virus replication factories. Cell Rep. 2017, 18, 2113–2123. [Google Scholar] [CrossRef]
- Offerdahl, D.K.; Dorward, D.W.; Hansen, B.T.; Bloom, M.E. A Three-Dimensional Comparison of Tick-Borne Flavivirus Infection in Mammalian and Tick Cell Lines. PLoS ONE 2012, 7, e47912. [Google Scholar] [CrossRef]
- Płaszczyca, A.; Scaturro, P.; Neufeldt, C.J.; Cortese, M.; Cerikan, B.; Ferla, S.; Brancale, A.; Pichlmair, A.; Bartenschlager, R. A novel interaction between dengue virus nonstructural protein 1 and the NS4A-2K-4B precursor is required for viral RNA replication but not for formation of the membranous replication organelle. PLoS Pathog. 2019, 15, e1007736. [Google Scholar] [CrossRef]
- Stern, O.; Hung, Y.-F.; Valdau, O.; Yaffe, Y.; Harris, E.; Hoffmann, S.; Willbold, D.; Sklan, E.H. An N-Terminal Amphipathic Helix in Dengue Virus Nonstructural Protein 4A Mediates Oligomerization and Is Essential for Replication. J. Virol. 2013, 87, 4080–4085. [Google Scholar] [CrossRef] [PubMed]
- Melo, C.; De Oliveira, D.N.; Lima, E.D.O.; Guerreiro, T.; Esteves, C.Z.; Beck, R.M.; Padilla, M.A.; Milanez, G.; Arns, C.W.; Proenca-Modena, J.L.; et al. A Lipidomics Approach in the Characterization of Zika-Infected Mosquito Cells: Potential Targets for Breaking the Transmission Cycle. PLoS ONE 2016, 11, e0164377. [Google Scholar] [CrossRef]
- Hannun, Y.A.; Obeid, L.M. Sphingolipids and their metabolism in physiology and disease. Nat. Rev. Mol. Cell Biol. 2018, 19, 175–191. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Zhang, Z.; Chukkapalli, V.; Nchoutmboube, J.; Li, J.; Randall, G.; Belov, G.A.; Wang, X. Positive-strand RNA viruses stimulate host phosphatidylcholine synthesis at viral replication sites. Proc. Natl. Acad. Sci. USA 2016, 113, E1064–E1073. [Google Scholar] [CrossRef]
- Li, X.-D.; Ye, H.-Q.; Deng, C.-L.; Liu, S.-Q.; Zhang, H.-L.; Shang, B.-D.; Shi, P.-Y.; Yuan, Z.-M.; Zhang, B. Genetic interaction between NS4A and NS4B for replication of Japanese encephalitis virus. J. Gen. Virol. 2015, 96, 1264–1275. [Google Scholar] [CrossRef]
- Miller, S.; Kastner, S.; Krijnse-Locker, J.; Bühler, S.; Bartenschlager, R. The Non-structural Protein 4A of Dengue Virus Is an Integral Membrane Protein Inducing Membrane Alterations in a 2K-regulated Manner. J. Biol. Chem. 2007, 282, 8873–8882. [Google Scholar] [CrossRef]
- Teo, C.S.H.; Chu, J.J.H. Cellular Vimentin Regulates Construction of Dengue Virus Replication Complexes through Interaction with NS4A Protein. J. Virol. 2014, 88, 1897–1913. [Google Scholar] [CrossRef]
- Kaufusi, P.H.; Kelley, J.F.; Yanagihara, R.; Nerurkar, V.R. Induction of Endoplasmic Reticulum-Derived Replication-Competent Membrane Structures by West Nile Virus Non-Structural Protein 4B. PLoS ONE 2014, 9, e84040. [Google Scholar] [CrossRef] [PubMed]
- Miller, S.; Sparacio, S.; Bartenschlager, R. Subcellular Localization and Membrane Topology of the Dengue Virus Type 2 Non-structural Protein 4B. J. Biol. Chem. 2006, 281, 8854–8863. [Google Scholar] [CrossRef] [PubMed]
- Roosendaal, J.; Westaway, E.G.; Khromykh, A.; MacKenzie, J.M. Regulated Cleavages at the West Nile Virus NS4A-2K-NS4B Junctions Play a Major Role in Rearranging Cytoplasmic Membranes and Golgi Trafficking of the NS4A Protein. J. Virol. 2006, 80, 4623–4632. [Google Scholar] [CrossRef]
- Shiryaev, S.A.; Chernov, A.V.; Aleshin, A.E.; Shiryaeva, T.N.; Strongin, A.Y. NS4A regulates the ATPase activity of the NS3 helicase: A novel cofactor role of the non-structural protein NS4A from West Nile virus. J. Gen. Virol. 2009, 90, 2081–2085. [Google Scholar] [CrossRef]
- Youn, S.; Li, T.; McCune, B.T.; Edeling, M.A.; Fremont, D.H.; Cristea, I.M.; Diamond, M.S. Evidence for a Genetic and Physical Interaction between Nonstructural Proteins NS1 and NS4B That Modulates Replication of West Nile Virus. J. Virol. 2012, 86, 7360–7371. [Google Scholar] [CrossRef]
- Lindenbach, B.D.; Rice, C.M. Genetic Interaction of Flavivirus Nonstructural Proteins NS1 and NS4A as a Determinant of Replicase Function. J. Virol. 1999, 73, 4611–4621. [Google Scholar] [CrossRef]
- Aktepe, T.E.; Liebscher, S.; Prier, J.; Simmons, C.P.; Mackenzie, J.M. The Host Protein Reticulon 3.1A Is Utilized by Flaviviruses to Facilitate Membrane Remodelling. Cell Rep. 2017, 21, 1639–1654. [Google Scholar] [CrossRef]
- Rismanchi, N.; Soderblom, C.; Stadler, J.; Zhu, P.-P.; Blackstone, C. Atlastin GTPases are required for Golgi apparatus and ER morphogenesis. Hum. Mol. Genet. 2008, 17, 1591–1604. [Google Scholar] [CrossRef]
- Hu, J.; Rapoport, T.A. Fusion of the endoplasmic reticulum by membrane-bound GTPases. Semin. Cell Dev. Biol. 2016, 60, 105–111. [Google Scholar] [CrossRef]
- Monel, B.; Rajah, M.M.; Hafirassou, M.L.; Ahmed, S.S.; Burlaud-Gaillard, J.; Zhu, P.-P.; Nevers, Q.; Buchrieser, J.; Porrot, F.; Meunier, C.; et al. Atlastin Endoplasmic Reticulum-Shaping Proteins Facilitate Zika Virus Replication. J. Virol. 2019, 93. [Google Scholar] [CrossRef] [PubMed]
- Tabata, K.; Arimoto, M.; Arakawa, M.; Nara, A.; Saito, K.; Omori, H.; Arai, A.; Ishikawa, T.; Konishi, E.; Suzuki, R.; et al. Unique Requirement for ESCRT Factors in Flavivirus Particle Formation on the Endoplasmic Reticulum. Cell Rep. 2016, 16, 2339–2347. [Google Scholar] [CrossRef]
- Hurley, J.H. ESCRTs are everywhere. EMBO J. 2015, 34, 2398–2407. [Google Scholar] [CrossRef]
- Schwartz, M.; Chen, J.; Janda, M.; Sullivan, M.; Boon, J.D.; Ahlquist, P. A Positive-Strand RNA Virus Replication Complex Parallels Form and Function of Retrovirus Capsids. Mol. Cell 2002, 9, 505–514. [Google Scholar] [CrossRef]
- Diaz, A.; Wang, X.; Ahlquist, P. Membrane-shaping host reticulon proteins play crucial roles in viral RNA replication compartment formation and function. Proc. Natl. Acad. Sci. USA 2010, 107, 16291–16296. [Google Scholar] [CrossRef] [PubMed]
- Diaz, A.; Zhang, J.; Ollwerther, A.; Wang, X.; Ahlquist, P. Host ESCRT proteins are required for bromovirus RNA replication compartment assembly and function. PLoS Pathog. 2015, 11, e1004742. [Google Scholar] [CrossRef] [PubMed]
- Barajas, D.; Jiang, Y.; Nagy, P.D. A Unique Role for the Host ESCRT Proteins in Replication of Tomato bushy stunt virus. PLoS Pathog. 2009, 5, e1000705. [Google Scholar] [CrossRef]
- Mizushima, N. A brief history of autophagy from cell biology to physiology and disease. Nat. Cell Biol. 2018, 20, 521–527. [Google Scholar] [CrossRef]
- Sharma, M.; Bhattacharyya, S.; Sharma, K.B.; Chauhan, S.; Asthana, S.; Abdin, M.Z.; Vrati, S.; Kalia, M. Japanese encephalitis virus activates autophagy through XBP1 and ATF6 ER stress sensors in neuronal cells. J. Gen. Virol. 2017, 98, 1027–1039. [Google Scholar] [CrossRef]
- Shives, K.D.; Beatman, E.L.; Chamanian, M.; O’Brien, C.; Hobson-Peters, J.; Beckham, J.D. West Nile Virus-Induced Activation of Mammalian Target of Rapamycin Complex 1 Supports Viral Growth and Viral Protein Expression. J. Virol. 2014, 88, 9458–9471. [Google Scholar] [CrossRef]
- Heaton, N.S.; Randall, G. Dengue Virus-Induced Autophagy Regulates Lipid Metabolism. Cell Host Microbe 2010, 8, 422–432. [Google Scholar] [CrossRef]
- Liang, Q.; Luo, Z.; Zeng, J.; Chen, W.; Foo, S.S.; Lee, S.A.; Ge, J.; Wang, S.; Goldman, S.A.; Zlokovic, B.V.; et al. Zika Virus NS4A and NS4B proteins deregulate Akt-mTOR signaling in human fetal neural stem cells to inhibit neurogenesis and induce autophagy. Cell Stem Cell 2016, 19, 663–671. [Google Scholar] [CrossRef]
- Chiramel, A.I.; Best, S.M. Role of autophagy in Zika virus infection and pathogenesis. Virus Res. 2018, 254, 34–40. [Google Scholar] [CrossRef]
- Metz, P.; Chiramel, A.; Chatel-Chaix, L.; Alvisi, G.; Bankhead, P.; Mora-Rodríguez, R.; Long, G.; Hamacher-Brady, A.; Brady, N.R.; Bartenschlager, R. Dengue Virus Inhibition of Autophagic Flux and Dependency of Viral Replication on Proteasomal Degradation of the Autophagy Receptor p62. J. Virol. 2015, 89, 8026–8041. [Google Scholar] [CrossRef]
- Beatman, E.; Oyer, R.; Shives, K.D.; Hedman, K.; Brault, A.C.; Tyler, K.L.; Beckham, J.D. West Nile virus growth is independent of autophagy activation. Virology 2012, 433, 262–272. [Google Scholar] [CrossRef] [PubMed]
- McLean, J.E.; Wudzinska, A.; Datan, E.; Quaglino, D.; Zakeri, Z. Flavivirus NS4A-induced Autophagy Protects Cells against Death and Enhances Virus Replication. J. Biol. Chem. 2011, 286, 22147–22159. [Google Scholar] [CrossRef]
- Hamel, R.; Dejarnac, O.; Wichit, S.; Ekchariyawat, P.; Neyret, A.; Luplertlop, N.; Perera-Lecoin, M.; Surasombatpattana, P.; Talignani, L.; Thomas, F.; et al. Biology of Zika Virus Infection in Human Skin Cells. J. Virol. 2015, 89, 8880–8896. [Google Scholar] [CrossRef] [PubMed]
- Evans, A.S.; Lennemann, N.J.; Coyne, C.B. BPIFB3 Regulates Endoplasmic Reticulum Morphology to Facilitate Flavivirus Replication. J. Virol. 2020, 94. [Google Scholar] [CrossRef]
- Liu, Y.; Gordesky-Gold, B.; Leney-Greene, M.; Weinbren, N.L.; Tudor, M.; Cherry, S. Inflammation-Induced, STING-Dependent Autophagy Restricts Zika Virus Infection in the Drosophila Brain. Cell Host Microbe 2018, 24, 57–68. [Google Scholar] [CrossRef] [PubMed]
- Lennemann, N.J.; Coyne, C.B. Dengue and Zika viruses subvert reticulophagy by NS2B3-mediated cleavage of FAM134B. Autophagy 2017, 13, 322–332. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, H.-H.; Schneider, W.M.; Rozen-Gagnon, K.; Miles, L.A.; Schuster, F.; Razooky, B.; Jacobson, E.; Wu, X.; Yi, S.; Rudin, C.M.; et al. TMEM41B Is a Pan-Flavivirus Host Factor. Cell 2021, 184, 133–148.e20. [Google Scholar] [CrossRef]
- Morita, K.; Hama, Y.; Izume, T.; Tamura, N.; Ueno, T.; Yamashita, Y.; Sakamaki, Y.; Mimura, K.; Morishita, H.; Shihoya, W.; et al. Genome-wide CRISPR screen identifies TMEM41B as a gene required for autophagosome formation. J. Cell Biol. 2018, 217, 3817–3828. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.E.; Wang, Y.; Du, X.; Zhang, T.; Mak, H.Y.; Hancock, S.E.; McEwen, H.; Pandzic, E.; Whan, R.M.; Aw, Y.C.; et al. TMEM41B and VMP1 are scramblases and regulate the distribution of cholesterol and phosphatidylserine. J. Cell Biol. 2021, 220. [Google Scholar] [CrossRef] [PubMed]
- Martín-Acebes, M.A.; Merino-Ramos, T.; Blázquez, A.-B.; Casas, J.; Escribano-Romero, E.; Sobrino, F.; Saiz, J.-C. The Composition of West Nile Virus Lipid Envelope Unveils a Role of Sphingolipid Metabolism in Flavivirus Biogenesis. J. Virol. 2014, 88, 12041–12054. [Google Scholar] [CrossRef]
- Reiss, S.; Rebhan, I.; Backes, P.; Romero-Brey, I.; Erfle, H.; Matula, P.; Kaderali, L.; Poenisch, M.; Blankenburg, H.; Hiet, M.-S.; et al. Recruitment and Activation of a Lipid Kinase by Hepatitis C Virus NS5A Is Essential for Integrity of the Membranous Replication Compartment. Cell Host Microbe 2011, 9, 32–45. [Google Scholar] [CrossRef]
- Hsu, N.-Y.; Ilnytska, O.; Belov, G.; Santiana, M.; Chen, Y.-H.; Takvorian, P.M.; Pau, C.; Van Der Schaar, H.; Kaushik-Basu, N.; Balla, T.; et al. Viral Reorganization of the Secretory Pathway Generates Distinct Organelles for RNA Replication. Cell 2010, 141, 799–811. [Google Scholar] [CrossRef] [PubMed]
- Amako, Y.; Sarkeshik, A.; Hotta, H.; Yates, J., III; Siddiqui, A. Role of oxysterol binding protein in hepatitis C virus infection. J. Virol. 2009, 83, 9237–9246. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Perry, J.W.; Lauring, A.S.; Neddermann, P.; De Francesco, R.; Tai, A.W. Oxysterol-Binding Protein Is a Phosphatidylinositol 4-Kinase Effector Required for HCV Replication Membrane Integrity and Cholesterol Trafficking. Gastroenterology 2014, 146, 1373–1385.e11. [Google Scholar] [CrossRef]
- Soto-Acosta, R.; Bautista-Carbajal, P.; Cervantes-Salazar, M.; Angel-Ambrocio, A.H.; Del Angel, R.M. DENV up-regulates the HMG-CoA reductase activity through the impairment of AMPK phosphorylation: A potential antiviral target. PLoS Pathog. 2017, 13, e1006257. [Google Scholar] [CrossRef] [PubMed]
- Heaton, N.S.; Perera, R.; Berger, K.L.; Khadka, S.; LaCount, D.J.; Kuhn, R.J.; Randall, G. Dengue virus nonstructural protein 3 redistributes fatty acid synthase to sites of viral replication and increases cellular fatty acid synthesis. Proc. Natl. Acad. Sci. USA 2010, 107, 17345–17350. [Google Scholar] [CrossRef] [PubMed]
- Berthoux, L. The Restrictome of Flaviviruses. Virol. Sin. 2020, 35, 363–377. [Google Scholar] [CrossRef]
- Schoggins, J.W. Interferon-Stimulated Genes: What Do They All Do? Annu. Rev. Virol. 2019, 6, 567–584. [Google Scholar] [CrossRef]
- Chan, Y.K.; Gack, M.U. Viral evasion of intracellular DNA and RNA sensing. Nat. Rev. Genet. 2016, 14, 360–373. [Google Scholar] [CrossRef] [PubMed]
- Takeuchi, O.; Akira, S. Pattern Recognition Receptors and Inflammation. Cell 2010, 140, 805–820. [Google Scholar] [CrossRef] [PubMed]
- Nazmi, A.; Dutta, K.; Hazra, B.; Basu, A. Role of pattern recognition receptors in flavivirus infections. Virus Res. 2014, 185, 32–40. [Google Scholar] [CrossRef]
- Loo, Y.-M.; Fornek, J.; Crochet, N.; Bajwa, G.; Perwitasari, O.; Martinez-Sobrido, L.; Akira, S.; Gill, M.A.; García-Sastre, A.; Katze, M.G.; et al. Distinct RIG-I and MDA5 Signaling by RNA Viruses in Innate Immunity. J. Virol. 2007, 82, 335–345. [Google Scholar] [CrossRef]
- Nasirudeen, A.M.A.; Wong, H.H.; Thien, P.; Xu, S.; Lam, K.-P.; Liu, D.X. RIG-I, MDA5 and TLR3 Synergistically Play an Important Role in Restriction of Dengue Virus Infection. PLoS Negl. Trop. Dis. 2011, 5, e926. [Google Scholar] [CrossRef]
- Fredericksen, B.L.; Keller, B.C.; Fornek, J.; Katze, M.G.; Gale, M. Establishment and Maintenance of the Innate Antiviral Response to West Nile Virus Involves both RIG-I and MDA5 Signaling through IPS-1. J. Virol. 2007, 82, 609–616. [Google Scholar] [CrossRef]
- Welte, T.; Reagan, K.; Fang, H.; Machain-Williams, C.; Zheng, X.; Mendell, N.; Chang, G.-J.J.; Wu, P.; Blair, C.D.; Wang, T. Toll-like receptor 7-induced immune response to cutaneous West Nile virus infection. J. Gen. Virol. 2009, 90, 2660–2668. [Google Scholar] [CrossRef]
- Maringer, K.; Fernandez-Sesma, A. Message in a bottle: Lessons learned from antagonism of STING signalling during RNA virus infection. Cytokine Growth Factor Rev. 2014, 25, 669–679. [Google Scholar] [CrossRef]
- Moretti, J.; Roy, S.; Bozec, D.; Martinez, J.; Chapman, J.R.; Ueberheide, B.; Lamming, D.W.; Chen, Z.; Horng, T.; Yeretssian, G.; et al. STING Senses Microbial Viability to Orchestrate Stress-Mediated Autophagy of the Endoplasmic Reticulum. Cell 2017, 171, 809–823. [Google Scholar] [CrossRef] [PubMed]
- Yu, C.-Y.; Chang, T.-H.; Liang, J.-J.; Chiang, R.-L.; Lee, Y.-L.; Liao, C.-L.; Lin, Y.-L. Dengue Virus Targets the Adaptor Protein MITA to Subvert Host Innate Immunity. PLoS Pathog. 2012, 8, e1002780. [Google Scholar] [CrossRef]
- Aguirre, S.; Maestre, A.M.; Pagni, S.; Patel, J.; Savage, T.; Gutman, D.; Maringer, K.; Bernal-Rubio, D.; Shabman, R.S.; Simon, V.; et al. DENV Inhibits Type I IFN Production in Infected Cells by Cleaving Human STING. PLoS Pathog. 2012, 8, e1002934. [Google Scholar] [CrossRef]
- Diamond, M.S. Mechanisms of Evasion of the Type I Interferon Antiviral Response by Flaviviruses. J. Interf. Cytokine Res. 2009, 29, 521–530. [Google Scholar] [CrossRef]
- Schneider, W.M.; Chevillotte, M.D.; Rice, C.M. Interferon-Stimulated Genes: A Complex Web of Host Defenses. Annu. Rev. Immunol. 2014, 32, 513–545. [Google Scholar] [CrossRef] [PubMed]
- Schoggins, J.W.; Wilson, S.J.; Panis, M.; Murphy, M.Y.; Jones, C.T.; Bieniasz, P.; Rice, C.M. A diverse range of gene products are effectors of the type I interferon antiviral response. Nature 2011, 472, 481–485. [Google Scholar] [CrossRef]
- Hornung, V.; Hartmann, R.; Ablasser, A.; Hopfner, K.-P. OAS proteins and cGAS: Unifying concepts in sensing and responding to cytosolic nucleic acids. Nat. Rev. Immunol. 2014, 14, 521–528. [Google Scholar] [CrossRef] [PubMed]
- Silverman, R.H. Viral Encounters with 2′,5′-Oligoadenylate Synthetase and RNase L during the Interferon Antiviral Response. J. Virol. 2007, 81, 12720–12729. [Google Scholar] [CrossRef]
- Perelygin, A.A.; Scherbik, S.V.; Zhulin, I.; Stockman, B.M.; Li, Y.; Brinton, M.A. Positional cloning of the murine flavivirus resistance gene. Proc. Natl. Acad. Sci. USA 2002, 99, 9322–9327. [Google Scholar] [CrossRef] [PubMed]
- Lin, R.-J.; Yu, H.-P.; Chang, B.-L.; Tang, W.-C.; Liao, C.-L.; Lin, Y.-L. Distinct Antiviral Roles for Human 2′,5′-Oligoadenylate Synthetase Family Members against Dengue Virus Infection. J. Immunol. 2009, 183, 8035–8043. [Google Scholar] [CrossRef]
- Whelan, J.N.; Li, Y.; Silverman, R.H.; Weiss, S.R. Zika Virus Production Is Resistant to RNase L Antiviral Activity. J. Virol. 2019, 93. [Google Scholar] [CrossRef]
- Helbig, K.J.; Beard, M.R. The Role of Viperin in the Innate Antiviral Response. J. Mol. Biol. 2014, 426, 1210–1219. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, S.; Marsh, E.N.G. Viperin: An ancient radical SAM enzyme finds its place in modern cellular metabolism and innate immunity. J. Biol. Chem. 2020, 295, 11513–11528. [Google Scholar] [CrossRef]
- Seo, J.-Y.; Yaneva, R.; Cresswell, P. Viperin: A Multifunctional, Interferon-Inducible Protein that Regulates Virus Replication. Cell Host Microbe 2011, 10, 534–539.e3. [Google Scholar] [CrossRef]
- Fenwick, M.K.; Li, Y.; Cresswell, P.; Modis, Y.; Ealick, S.E. Structural studies of viperin, an antiviral radical SAM enzyme. Proc. Natl. Acad. Sci. USA 2017, 114, 6806–6811. [Google Scholar] [CrossRef] [PubMed]
- Gizzi, A.S.; Grove, T.L.; Arnold, J.J.; Jose, J.; Jangra, R.K.; Garforth, S.J.; Du, Q.; Cahill, S.M.; Dulyaninova, N.G.; Love, J.D.; et al. A naturally occurring antiviral ribonucleotide encoded by the human genome. Nature 2018, 558, 610–614. [Google Scholar] [CrossRef] [PubMed]
- Helbig, K.J.; Carr, J.M.; Calvert, J.K.; Wati, S.; Clarke, J.N.; Eyre, N.S.; Narayana, S.K.; Fiches, G.N.; McCartney, E.M.; Beard, M.R. Viperin Is Induced following Dengue Virus Type-2 (DENV-2) Infection and Has Anti-viral Actions Requiring the C-terminal End of Viperin. PLoS Neglected Trop. Dis. 2013, 7, e2178. [Google Scholar] [CrossRef] [PubMed]
- Parker, N.; Porter, A.C.G. Identification of a novel gene family that includes the interferon-inducible human genes 6–16 and ISG12. BMC Genom. 2004, 5, 8. [Google Scholar] [CrossRef] [PubMed]
- Gjermandsen, I.M.; Justesen, J.; Martensen, P.M. The Interferon-Induced Gene ISG12 Is Regulated by Various Cytokines as the Gene 6-16 In Human Cell Lines. Cytokine 2000, 12, 233–238. [Google Scholar] [CrossRef]
- Qi, Y.; Li, Y.; Zhang, Y.; Zhang, L.; Wang, Z.; Zhang, X.; Gui, L.; Huang, J. IFI6 inhibits apoptosis via mitochondrial-dependent pathway in dengue virus 2 infected vascular endothelial cells. PLoS ONE 2015, 10, e0132743. [Google Scholar]
- Park, G.-H.; Kim, K.-Y.; Cho, S.W.; Cheong, J.Y.; Yu, G.I.; Shin, D.H.; Kwack, K.B. Association between Interferon-Inducible Protein 6 (IFI6) Polymorphisms and Hepatitis B Virus Clearance. Genom. Inform. 2013, 11, 15–23. [Google Scholar] [CrossRef] [PubMed]
- Szegedi, K.; Sonkoly, E.; Nagy, N.; Németh, I.B.; Bata-Csörgő, Z.; Kemény, L.; Dobozy, A.; Széll, M. The anti-apoptotic protein G1P3 is overexpressed in psoriasis and regulated by the non-coding RNA, PRINS. Exp. Dermatol. 2010, 19, 269–278. [Google Scholar] [CrossRef]
- Li, J.; Huang, K.; Hu, G.; Babarinde, I.A.; Li, Y.; Dong, X.; Chen, Y.-S.; Shang, L.; Guo, W.; Wang, J.; et al. An alternative CTCF isoform antagonizes canonical CTCF occupancy and changes chromatin architecture to promote apoptosis. Nat. Commun. 2019, 10, 1535. [Google Scholar] [CrossRef]
- Li, J.; Ding, S.C.; Cho, H.; Chung, B.C.; Gale, M.; Chanda, S.K.; Diamond, M.S. A Short Hairpin RNA Screen of Interferon-Stimulated Genes Identifies a Novel Negative Regulator of the Cellular Antiviral Response. mBio 2013, 4, e00385-13. [Google Scholar] [CrossRef] [PubMed]
- Dukhovny, A.; Lamkiewicz, K.; Chen, Q.; Fricke, M.; Jabrane-Ferrat, N.; Marz, M.; Jung, J.U.; Sklan, E.H. A CRISPR Activation Screen Identifies Genes That Protect against Zika Virus Infection. J. Virol. 2019, 93, e00211-19. [Google Scholar] [CrossRef] [PubMed]
- Schoggins, J.W.; Dorner, M.; Feulner, M.; Imanaka, N.; Murphy, M.Y.; Ploss, A.; Rice, C.M. Dengue reporter viruses reveal viral dynamics in interferon receptor-deficient mice and sensitivity to interferon effectors in vitro. Proc. Natl. Acad. Sci. USA 2012, 109, 14610–14615. [Google Scholar] [CrossRef] [PubMed]
- Richardson, R.B.; Ohlson, M.B.; Eitson, J.L.; Kumar, A.; McDougal, M.B.; Boys, I.N.; Mar, K.B.; De La Cruz-Rivera, P.C.; Douglas, C.; Konopka, G.; et al. A CRISPR screen identifies IFI6 as an ER-resident interferon effector that blocks flavivirus replication. Nat. Microbiol. 2018, 3, 1214–1223. [Google Scholar] [CrossRef] [PubMed]
- Van Gent, M.; Sparrer, K.M.; Gack, M.U. TRIM Proteins and Their Roles in Antiviral Host Defenses. Annu. Rev. Virol. 2018, 5, 385–405. [Google Scholar] [CrossRef]
- Taylor, R.T.; Lubick, K.J.; Robertson, S.J.; Broughton, J.P.; Bloom, M.E.; Bresnahan, W.A.; Best, S.M. TRIM79α, an Interferon-Stimulated Gene Product, Restricts Tick-Borne Encephalitis Virus Replication by Degrading the Viral RNA Polymerase. Cell Host Microbe 2011, 10, 185–196. [Google Scholar] [CrossRef]
- Suzuki, Y.; Chin, W.-X.; Han, Q.; Ichiyama, K.; Lee, C.H.; Eyo, Z.W.; Ebina, H.; Takahashi, H.; Takahashi, C.; Tan, B.H.; et al. Characterization of RyDEN (C19orf66) as an Interferon-Stimulated Cellular Inhibitor against Dengue Virus Replication. PLoS Pathog. 2016, 12, e1005357. [Google Scholar] [CrossRef]
- Balinsky, C.A.; Schmeisser, H.; Wells, A.; Ganesan, S.; Jin, T.; Singh, K.; Zoon, K.C. IRAV (FLJ11286), an Interferon-Stimulated Gene with Antiviral Activity against Dengue Virus, Interacts with MOV10. J. Virol. 2017, 91. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Yang, X.; Yao, Z.; Dong, X.; Zhang, D.; Hu, Y.; Zhang, S.; Lin, J.; Chen, J.; An, S.; et al. C19orf66 interrupts Zika virus replication by inducing lysosomal degradation of viral NS3. PLoS Negl. Trop. Dis. 2020, 14, e0008083. [Google Scholar] [CrossRef]
- Boys, I.N.; Xu, E.; Mar, K.B.; De La Cruz-Rivera, P.C.; Eitson, J.L.; Moon, B.; Schoggins, J.W. RTP4 Is a Potent IFN-Inducible Anti-flavivirus Effector Engaged in a Host-Virus Arms Race in Bats and Other Mammals. Cell Host Microbe 2020, 28, 712–723.e9. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Xuan, Y.; Han, Y.; Ding, X.; Ye, K.; Yang, F.; Gao, P.; Goff, S.P.; Gao, G. Regulation of HIV-1 Gag-Pol Expression by Shiftless, an Inhibitor of Programmed -1 Ribosomal Frameshifting. Cell 2019, 176, 625–635.e14. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, W.; Srivastav, K.; Muller, M. C19ORF66 Broadly Escapes Virus-Induced Endonuclease Cleavage and Restricts Kaposi’s Sarcoma-Associated Herpesvirus. J. Virol. 2019, 93, e00373-19. [Google Scholar] [CrossRef] [PubMed]
- Berman, A.J.; Thoreen, C.C.; Dedeic, Z.; Chettle, J.; Roux, P.P.; Blagden, S.P. Controversies around the function of LARP1. RNA Biol. 2021, 18, 207–217. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).