From Structural Studies to HCV Vaccine Design
Abstract
1. Introduction
2. HCV Env Glycoproteins
2.1. E1E2 Heterodimer
Structural Studies of E1E2
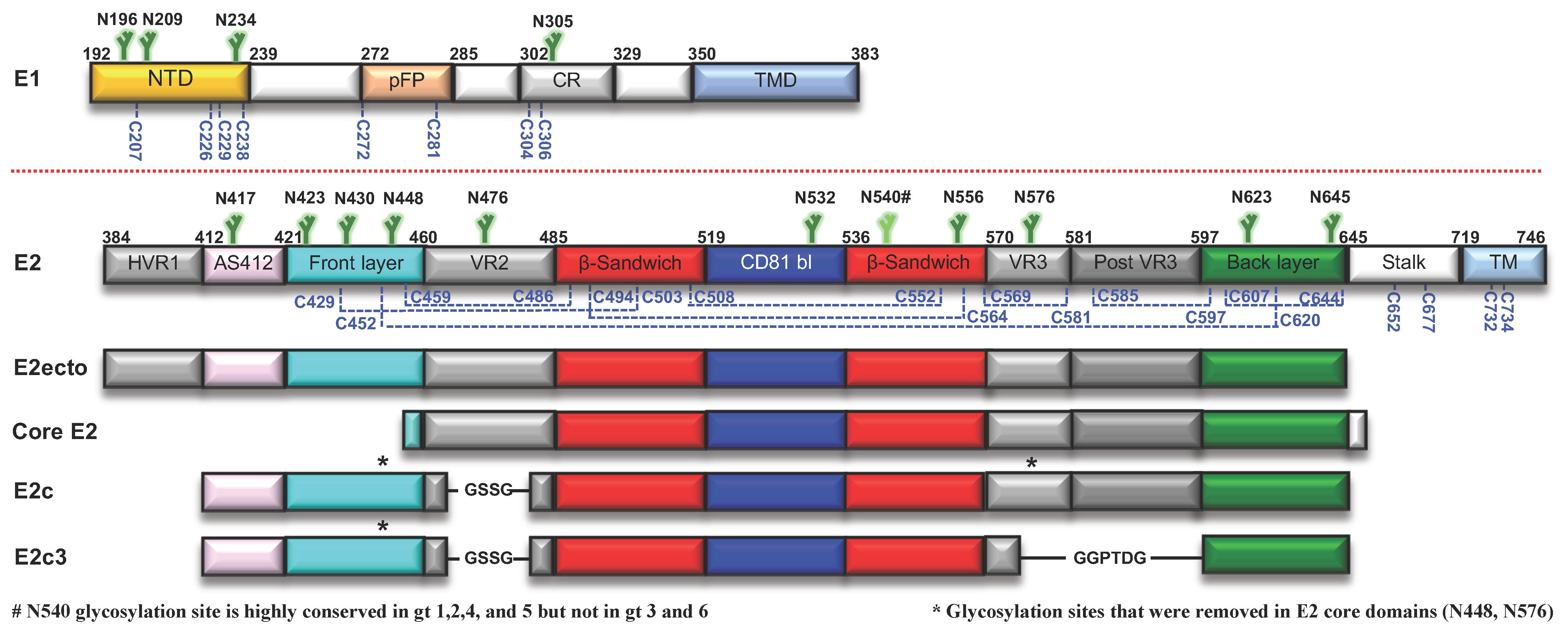
2.2. E1 Env
Structural Studies of E1 Env
2.3. E2 Env
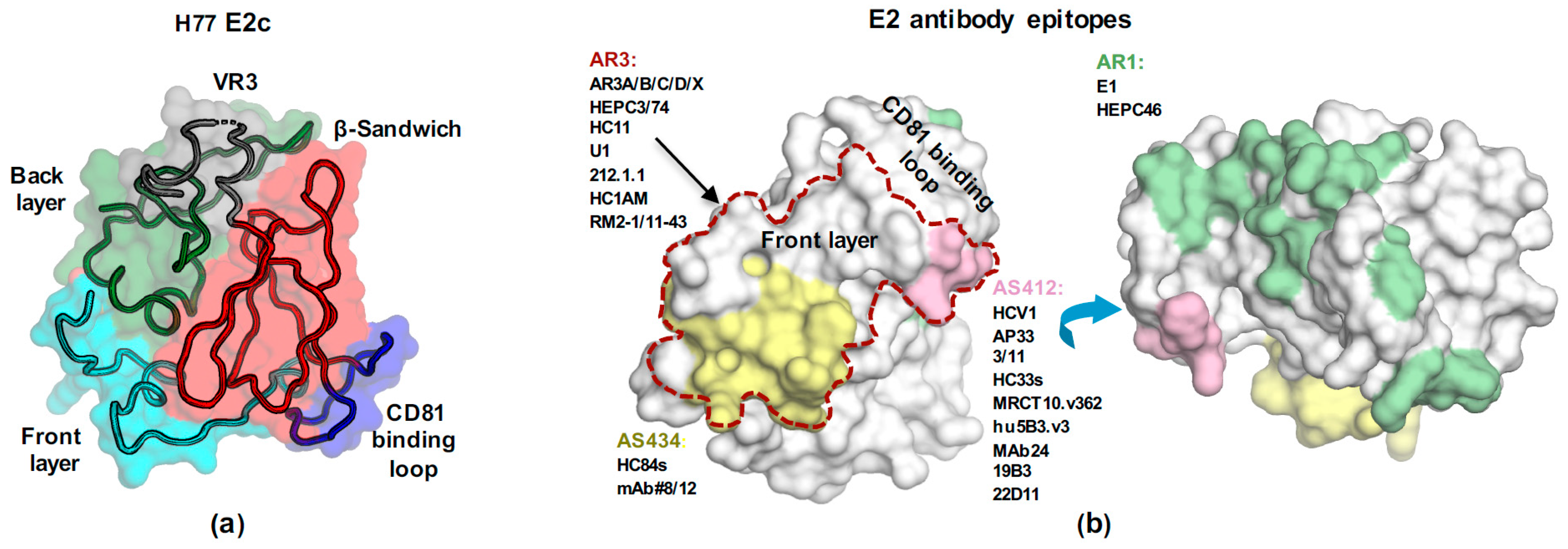
2.3.1. Structural Studies of E2 Env
AS412
AS434
Amino Acids 529–540
E2 Core Structures
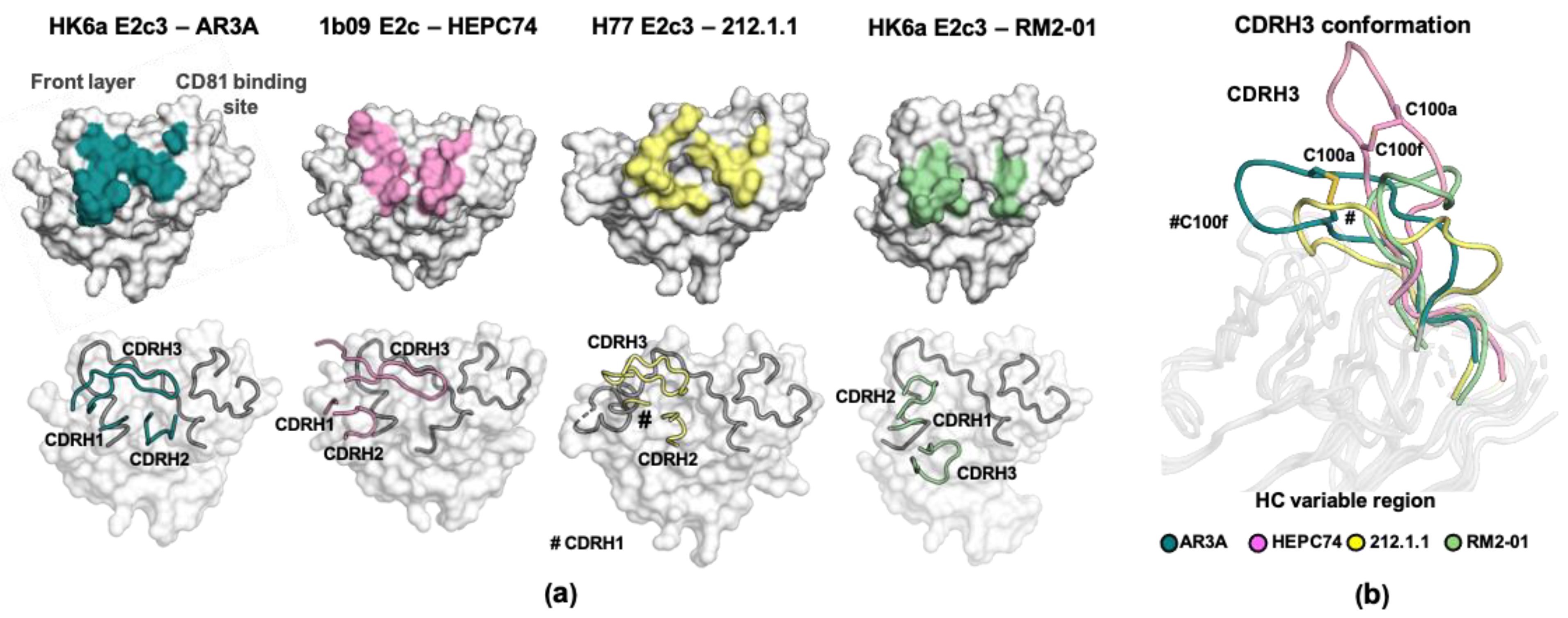
2.3.2. The Binding Mode of AR3-Targeting bnAbs
2.3.3. E2 Immunodominant Decoy Epitopes
2.3.4. Structural Plasticity of E2
3. Structure-Based and Germline-Targeting Vaccine Design
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- New Hepatitis Data Highlight Need for Urgent Global Response. 2017. Available online: https://www.who.int/hepatitis/publications/global-hepatitis-report2017/en/ (accessed on 27 April 2021).
- Messina, J.P.; Humphreys, I.; Flaxman, A.; Brown, A.; Cooke, G.S.; Pybus, O.G.; Barnes, E. Global distribution and prevalence of hepatitis C virus genotypes. Hepatology 2015, 61, 77–87. [Google Scholar] [CrossRef] [PubMed]
- Petruzziello, A.; Marigliano, S.; Loquercio, G.; Cozzolino, A.; Cacciapuoti, C. Global epidemiology of hepatitis C virus infection: An up-date of the distribution and circulation of hepatitis C virus genotypes. World J. Gastroenterol. 2016, 22, 7824–7840. [Google Scholar] [CrossRef]
- Surveillance for Viral Hepatitis—United States 2017. 2019. Available online: https://www.cdc.gov/hepatitis/statistics/2017surveillance/index.htm (accessed on 27 April 2021).
- Buhler, S.; Bartenschlager, R. Promotion of hepatocellular carcinoma by hepatitis C virus. Dig. Dis. 2012, 30, 445–452. [Google Scholar] [CrossRef]
- Cox, A.L. MEDICINE. Global control of hepatitis C virus. Science 2015, 349, 790–791. [Google Scholar] [CrossRef]
- Midgard, H.; Bjoro, B.; Maeland, A.; Konopski, Z.; Kileng, H.; Damas, J.K.; Paulsen, J.; Heggelund, L.; Sandvei, P.K.; Ringstad, J.O.; et al. Hepatitis C reinfection after sustained virological response. J. Hepatol. 2016, 64, 1020–1026. [Google Scholar] [CrossRef]
- Al-Khazraji, A.; Patel, I.; Saleh, M.; Ashraf, A.; Lieber, J.; Malik, R. Identifying barriers to the treatment of chronic hepatitis C infection. Dig. Dis. 2020, 38, 46–52. [Google Scholar] [CrossRef] [PubMed]
- Smith, D.B.; Bukh, J.; Kuiken, C.; Muerhoff, A.S.; Rice, C.M.; Stapleton, J.T.; Simmonds, P. A Web Resource to Manage the Classification and Genotype and Subtype Assignments of Hepatitis C Virus. 2017. Available online: https://talk.ictvonline.org/ictv_wikis/flaviviridae/w/sg_flavi/56/hcv-classification (accessed on 27 April 2021).
- Farci, P. New insights into the HCV quasispecies and compartmentalization. Semin. Liver Dis. 2011, 31, 356–374. [Google Scholar] [CrossRef] [PubMed]
- Baumert, T.F.; Fauvelle, C.; Chen, D.Y.; Lauer, G.M. A prophylactic hepatitis C virus vaccine: A distant peak still worth climbing. J. Hepatol. 2014, 61, S34–S44. [Google Scholar] [CrossRef] [PubMed]
- Shoukry, N.H. Hepatitis C vaccines, antibodies, and T cells. Front. Immunol. 2018, 9, 1480. [Google Scholar] [CrossRef]
- Osburn, W.O.; Snider, A.E.; Wells, B.L.; Latanich, R.; Bailey, J.R.; Thomas, D.L.; Cox, A.L.; Ray, S.C. Clearance of hepatitis C infection is associated with the early appearance of broad neutralizing antibody responses. Hepatology 2014, 59, 2140–2151. [Google Scholar] [CrossRef]
- Walker, M.R.; Leung, P.; Eltahla, A.A.; Underwood, A.; Abayasingam, A.; Brasher, N.A.; Li, H.; Wu, B.R.; Maher, L.; Luciani, F.; et al. Clearance of hepatitis C virus is associated with early and potent but narrowly-directed, Envelope-specific antibodies. Sci. Rep. 2019, 9, 13300. [Google Scholar] [CrossRef] [PubMed]
- Smith, S.; Honegger, J.R.; Walker, C. T-Cell Immunity against the hepatitis C virus: A persistent research priority in an era of highly effective therapy. Cold Spring Harb. Perspect. Med. 2021, 11, a036954. [Google Scholar] [CrossRef] [PubMed]
- Sepulveda-Crespo, D.; Resino, S.; Martinez, I. Hepatitis C virus vaccine design: Focus on the humoral immune response. J. Biomed. Sci. 2020, 27, 78. [Google Scholar] [CrossRef] [PubMed]
- Fuerst, T.R.; Pierce, B.G.; Keck, Z.Y.; Foung, S.K.H. Designing a B Cell-based vaccine against a highly variable hepatitis C virus. Front. Microbiol. 2017, 8, 2692. [Google Scholar] [CrossRef] [PubMed]
- Duncan, J.D.; Urbanowicz, R.A.; Tarr, A.W.; Ball, J.K. Hepatitis C virus vaccine: Challenges and prospects. Vaccines (Basel) 2020, 8, 90. [Google Scholar] [CrossRef]
- Bailey, J.R.; Barnes, E.; Cox, A.L. Approaches, progress, and challenges to hepatitis C vaccine development. Gastroenterology 2019, 156, 418–430. [Google Scholar] [CrossRef]
- Kelly, C.; Swadling, L.; Brown, A.; Capone, S.; Folgori, A.; Salio, M.; Klenerman, P.; Barnes, E. Cross-reactivity of hepatitis C virus specific vaccine-induced T cells at immunodominant epitopes. Eur. J. Immunol. 2015, 45, 309–316. [Google Scholar] [CrossRef]
- Barnes, E.; Folgori, A.; Capone, S.; Swadling, L.; Aston, S.; Kurioka, A.; Meyer, J.; Huddart, R.; Smith, K.; Townsend, R.; et al. Novel adenovirus-based vaccines induce broad and sustained T cell responses to HCV in man. Sci. Transl. Med. 2012, 4, 115ra111. [Google Scholar] [CrossRef] [PubMed]
- Folgori, A.; Capone, S.; Ruggeri, L.; Meola, A.; Sporeno, E.; Ercole, B.B.; Pezzanera, M.; Tafi, R.; Arcuri, M.; Fattori, E.; et al. A T-cell HCV vaccine eliciting effective immunity against heterologous virus challenge in chimpanzees. Nat. Med. 2006, 12, 190–197. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Wan, Q.; Feng, Y.; Liu, M.; Wu, J.; Chen, X.; Zhang, X.L. Engineering of N-glycosylation of hepatitis C virus envelope protein E2 enhances T cell responses for DNA immunization. Vaccine 2007, 25, 1544–1551. [Google Scholar] [CrossRef] [PubMed]
- Frey, S.E.; Houghton, M.; Coates, S.; Abrignani, S.; Chien, D.; Rosa, D.; Pileri, P.; Ray, R.; Di Bisceglie, A.M.; Rinella, P.; et al. Safety and immunogenicity of HCV E1E2 vaccine adjuvanted with MF59 administered to healthy adults. Vaccine 2010, 28, 6367–6373. [Google Scholar] [CrossRef]
- Ray, R.; Meyer, K.; Banerjee, A.; Basu, A.; Coates, S.; Abrignani, S.; Houghton, M.; Frey, S.E.; Belshe, R.B. Characterization of antibodies induced by vaccination with hepatitis C virus envelope glycoproteins. J. Infect. Dis. 2010, 202, 862–866. [Google Scholar] [CrossRef] [PubMed]
- Logan, M.; Law, J.; Wong, J.A.J.; Hockman, D.; Landi, A.; Chen, C.; Crawford, K.; Kundu, J.; Baldwin, L.; Johnson, J.; et al. Native folding of a recombinant gpE1/gpE2 heterodimer vaccine antigen from a precursor protein fused with Fc IgG. J. Virol. 2017, 91. [Google Scholar] [CrossRef] [PubMed]
- Wong, J.A.; Bhat, R.; Hockman, D.; Logan, M.; Chen, C.; Levin, A.; Frey, S.E.; Belshe, R.B.; Tyrrell, D.L.; Law, J.L.; et al. Recombinant hepatitis C virus envelope glycoprotein vaccine elicits antibodies targeting multiple epitopes on the envelope glycoproteins associated with broad cross-neutralization. J. Virol. 2014, 88, 14278–14288. [Google Scholar] [CrossRef] [PubMed]
- Masavuli, M.G.; Wijesundara, D.K.; Torresi, J.; Gowans, E.J.; Grubor-Bauk, B. Preclinical development and production of virus-like particles as vaccine candidates for hepatitis C. Front. Microbiol. 2017, 8, 2413. [Google Scholar] [CrossRef] [PubMed]
- Collett, S.; Torresi, J.; Earnest-Silveira, L.; Christiansen, D.; Elbourne, A.; Ramsland, P.A. Probing and pressing surfaces of hepatitis C virus-like particles. J. Colloid Interface Sci. 2019, 545, 259–268. [Google Scholar] [CrossRef]
- Bazzill, J.D.; Ochyl, L.J.; Giang, E.; Castillo, S.; Law, M.; Moon, J.J. Interrogation of antigen display on individual vaccine nanoparticles for achieving neutralizing antibody responses against hepatitis C virus. Nano Lett. 2018, 18, 7832–7838. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.; Wang, X.; Lou, P.; Hu, Z.; Qu, P.; Li, D.; Li, Q.; Xu, Y.; Niu, J.; He, Y.; et al. A nanoparticle-based HCV vaccine with enhanced potency. J. Infect. Dis. 2020, 221, 1304–1314. [Google Scholar] [CrossRef] [PubMed]
- Cox, A.L. Challenges and promise of a hepatitis C virus vaccine. Cold Spring Harb. Perspect. Med. 2020, 10, a036947. [Google Scholar] [CrossRef] [PubMed]
- Drane, D.; Maraskovsky, E.; Gibson, R.; Mitchell, S.; Barnden, M.; Moskwa, A.; Shaw, D.; Gervase, B.; Coates, S.; Houghton, M.; et al. Priming of CD4+ and CD8+ T cell responses using a HCV core ISCOMATRIX vaccine: A phase I study in healthy volunteers. Hum. Vaccin 2009, 5, 151–157. [Google Scholar] [CrossRef] [PubMed]
- Meunier, J.C.; Gottwein, J.M.; Houghton, M.; Russell, R.S.; Emerson, S.U.; Bukh, J.; Purcell, R.H. Vaccine-induced cross-genotype reactive neutralizing antibodies against hepatitis C virus. J. Infect. Dis. 2011, 204, 1186–1190. [Google Scholar] [CrossRef]
- Cox, A.L.; Page, K.; Melia, M.; Veenhuis, R.; Massaccesi, G.; Osburn, W.; Wagner, K.; Giudice, L.; Stein, E.; Asher, A.K.; et al. LB10. A randomized, double-blind, placebo-controlled efficacy trial of a vaccine to prevent chronic hepatitis C virus infection in an at-risk population. Open Forum Infect. Dis. 2019, 6, S997. [Google Scholar] [CrossRef]
- Page, K.; Melia, M.T.; Veenhuis, R.T.; Winter, M.; Rousseau, K.E.; Massaccesi, G.; Osburn, W.O.; Forman, M.; Thomas, E.; Thornton, K.; et al. Randomized trial of a vaccine regimen to prevent chronic HCV infection. N. Engl. J. Med. 2021, 384, 541–549. [Google Scholar] [CrossRef] [PubMed]
- Choo, Q.L.; Kuo, G.; Ralston, R.; Weiner, A.; Chien, D.; Van Nest, G.; Han, J.; Berger, K.; Thudium, K.; Kuo, C.; et al. Vaccination of chimpanzees against infection by the hepatitis C virus. Proc. Natl. Acad. Sci. USA 1994, 91, 1294–1298. [Google Scholar] [CrossRef] [PubMed]
- Stamataki, Z.; Coates, S.; Evans, M.J.; Wininger, M.; Crawford, K.; Dong, C.; Fong, Y.L.; Chien, D.; Abrignani, S.; Balfe, P.; et al. Hepatitis C virus envelope glycoprotein immunization of rodents elicits cross-reactive neutralizing antibodies. Vaccine 2007, 25, 7773–7784. [Google Scholar] [CrossRef]
- Meunier, J.C.; Russell, R.S.; Goossens, V.; Priem, S.; Walter, H.; Depla, E.; Union, A.; Faulk, K.N.; Bukh, J.; Emerson, S.U.; et al. Isolation and characterization of broadly neutralizing human monoclonal antibodies to the e1 glycoprotein of hepatitis C virus. J. Virol. 2008, 82, 966–973. [Google Scholar] [CrossRef] [PubMed]
- Law, J.L.; Chen, C.; Wong, J.; Hockman, D.; Santer, D.M.; Frey, S.E.; Belshe, R.B.; Wakita, T.; Bukh, J.; Jones, C.T.; et al. A hepatitis C virus (HCV) vaccine comprising envelope glycoproteins gpE1/gpE2 derived from a single isolate elicits broad cross-genotype neutralizing antibodies in humans. PLoS ONE 2013, 8, e59776. [Google Scholar] [CrossRef] [PubMed]
- Cowton, V.M.; Singer, J.B.; Gifford, R.J.; Patel, A.H. Predicting the effectiveness of hepatitis C virus neutralizing antibodies by bioinformatic analysis of conserved epitope residues using public sequence data. Front. Immunol. 2018, 9, 1470. [Google Scholar] [CrossRef] [PubMed]
- Keck, M.L.; Wrensch, F.; Pierce, B.G.; Baumert, T.F.; Foung, S.K.H. Mapping determinants of virus neutralization and viral escape for rational design of a hepatitis C virus vaccine. Front. Immunol. 2018, 9, 1194. [Google Scholar] [CrossRef] [PubMed]
- Giang, E.; Dorner, M.; Prentoe, J.C.; Dreux, M.; Evans, M.J.; Bukh, J.; Rice, C.M.; Ploss, A.; Burton, D.R.; Law, M. Human broadly neutralizing antibodies to the envelope glycoprotein complex of hepatitis C virus. Proc. Natl. Acad. Sci. USA 2012, 109, 6205–6210. [Google Scholar] [CrossRef] [PubMed]
- Kong, L.; Giang, E.; Nieusma, T.; Kadam, R.U.; Cogburn, K.E.; Hua, Y.; Dai, X.; Stanfield, R.L.; Burton, D.R.; Ward, A.B.; et al. Hepatitis C virus E2 envelope glycoprotein core structure. Science 2013, 342, 1090–1094. [Google Scholar] [CrossRef]
- Owsianka, A.M.; Timms, J.M.; Tarr, A.W.; Brown, R.J.; Hickling, T.P.; Szwejk, A.; Bienkowska-Szewczyk, K.; Thomson, B.J.; Patel, A.H.; Ball, J.K. Identification of conserved residues in the E2 envelope glycoprotein of the hepatitis C virus that are critical for CD81 binding. J. Virol. 2006, 80, 8695–8704. [Google Scholar] [CrossRef] [PubMed]
- Moradpour, D.; Penin, F.; Rice, C.M. Replication of hepatitis C virus. Nat. Rev. Microbiol. 2007, 5, 453–463. [Google Scholar] [CrossRef] [PubMed]
- Catanese, M.T.; Uryu, K.; Kopp, M.; Edwards, T.J.; Andrus, L.; Rice, W.J.; Silvestry, M.; Kuhn, R.J.; Rice, C.M. Ultrastructural analysis of hepatitis C virus particles. Proc. Natl. Acad. Sci. USA 2013, 110, 9505–9510. [Google Scholar] [CrossRef]
- Wrensch, F.; Crouchet, E.; Ligat, G.; Zeisel, M.B.; Keck, Z.Y.; Foung, S.K.H.; Schuster, C.; Baumert, T.F. Hepatitis C virus (HCV)-apolipoprotein interactions and immune evasion and their impact on HCV vaccine design. Front. Immunol. 2018, 9, 1436. [Google Scholar] [CrossRef]
- Fauvelle, C.; Felmlee, D.J.; Crouchet, E.; Lee, J.; Heydmann, L.; Lefevre, M.; Magri, A.; Hiet, M.S.; Fofana, I.; Habersetzer, F.; et al. Apolipoprotein E mediates evasion from Hepatitis C virus neutralizing antibodies. Gastroenterology 2016, 150, 206–217 e204. [Google Scholar] [CrossRef] [PubMed]
- Dreux, M.; Pietschmann, T.; Granier, C.; Voisset, C.; Ricard-Blum, S.; Mangeot, P.E.; Keck, Z.; Foung, S.; Vu-Dac, N.; Dubuisson, J.; et al. High density lipoprotein inhibits hepatitis C virus-neutralizing antibodies by stimulating cell entry via activation of the scavenger receptor BI. J. Biol. Chem. 2006, 281, 18285–18295. [Google Scholar] [CrossRef] [PubMed]
- Deng, L.; Jiang, W.; Wang, X.; Merz, A.; Hiet, M.S.; Chen, Y.; Pan, X.; Jiu, Y.; Yang, Y.; Yu, B.; et al. Syntenin regulates hepatitis C virus sensitivity to neutralizing antibody by promoting E2 secretion through exosomes. J. Hepatol. 2019, 71, 52–61. [Google Scholar] [CrossRef]
- Bankwitz, D.; Doepke, M.; Hueging, K.; Weller, R.; Bruening, J.; Behrendt, P.; Lee, J.Y.; Vondran, F.W.R.; Manns, M.P.; Bartenschlager, R.; et al. Maturation of secreted HCV particles by incorporation of secreted ApoE protects from antibodies by enhancing infectivity. J. Hepatol. 2017, 67, 480–489. [Google Scholar] [CrossRef] [PubMed]
- Moradpour, D.; Penin, F. Hepatitis C virus proteins: From structure to function. Curr. Top. Microbiol. Immunol. 2013, 369, 113–142. [Google Scholar] [PubMed]
- Douam, F.; Lavillette, D.; Cosset, F.L. The mechanism of HCV entry into host cells. Prog. Mol. Biol. Transl. Sci. 2015, 129, 63–107. [Google Scholar]
- Cocquerel, L.; Wychowski, C.; Minner, F.; Penin, F.; Dubuisson, J. Charged residues in the transmembrane domains of hepatitis C virus glycoproteins play a major role in the processing, subcellular localization, and assembly of these envelope proteins. J. Virol. 2000, 74, 3623–3633. [Google Scholar] [CrossRef]
- Op De Beeck, A.; Montserret, R.; Duvet, S.; Cocquerel, L.; Cacan, R.; Barberot, B.; Le Maire, M.; Penin, F.; Dubuisson, J. The transmembrane domains of hepatitis C virus envelope glycoproteins E1 and E2 play a major role in heterodimerization. J. Biol. Chem. 2000, 275, 31428–31437. [Google Scholar] [CrossRef]
- Michalak, J.P.; Wychowski, C.; Choukhi, A.; Meunier, J.C.; Ung, S.; Rice, C.M.; Dubuisson, J. Characterization of truncated forms of hepatitis C virus glycoproteins. J. Gen. Virol. 1997, 78 Pt 9, 2299–2306. [Google Scholar] [CrossRef] [PubMed]
- Flint, M.; Dubuisson, J.; Maidens, C.; Harrop, R.; Guile, G.R.; Borrow, P.; McKeating, J.A. Functional characterization of intracellular and secreted forms of a truncated hepatitis C virus E2 glycoprotein. J. Virol. 2000, 74, 702–709. [Google Scholar] [CrossRef]
- Spaete, R.R.; Alexander, D.; Rugroden, M.E.; Choo, Q.L.; Berger, K.; Crawford, K.; Kuo, C.; Leng, S.; Lee, C.; Ralston, R.; et al. Characterization of the hepatitis C virus E2/NS1 gene product expressed in mammalian cells. Virology 1992, 188, 819–830. [Google Scholar] [CrossRef]
- Whidby, J.; Mateu, G.; Scarborough, H.; Demeler, B.; Grakoui, A.; Marcotrigiano, J. Blocking hepatitis C virus infection with recombinant form of envelope protein 2 ectodomain. J. Virol. 2009, 83, 11078–11089. [Google Scholar] [CrossRef] [PubMed]
- Ruwona, T.B.; Giang, E.; Nieusma, T.; Law, M. Fine mapping of murine antibody responses to immunization with a novel soluble form of hepatitis C virus envelope glycoprotein complex. J. Virol. 2014, 88, 10459–10471. [Google Scholar] [CrossRef]
- Tello, D.; Rodriguez-Rodriguez, M.; Yelamos, B.; Gomez-Gutierrez, J.; Ortega, S.; Pacheco, B.; Peterson, D.L.; Gavilanes, F. Expression and structural properties of a chimeric protein based on the ectodomains of E1 and E2 hepatitis C virus envelope glycoproteins. Protein Expr. Purif. 2010, 71, 123–131. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Rey, F.A.; Lok, S.M. Common features of enveloped viruses and implications for immunogen design for next-generation vaccines. Cell 2018, 172, 1319–1334. [Google Scholar] [CrossRef] [PubMed]
- Cao, L.; Yu, B.; Kong, D.; Cong, Q.; Yu, T.; Chen, Z.; Hu, Z.; Chang, H.; Zhong, J.; Baker, D.; et al. Functional expression and characterization of the envelope glycoprotein E1E2 heterodimer of hepatitis C virus. PLoS Pathog. 2019, 15, e1007759. [Google Scholar] [CrossRef]
- Castelli, M.; Clementi, N.; Pfaff, J.; Sautto, G.A.; Diotti, R.A.; Burioni, R.; Doranz, B.J.; Dal Peraro, M.; Clementi, M.; Mancini, N. A biologically-validated HCV E1E2 heterodimer structural model. Sci. Rep. 2017, 7, 214. [Google Scholar] [CrossRef] [PubMed]
- Tong, Y.; Lavillette, D.; Li, Q.; Zhong, J. Role of hepatitis C virus envelope glycoprotein E1 in virus entry and assembly. Front. Immunol. 2018, 9, 1411. [Google Scholar] [CrossRef]
- El Omari, K.; Iourin, O.; Kadlec, J.; Sutton, G.; Harlos, K.; Grimes, J.M.; Stuart, D.I. Unexpected structure for the N-terminal domain of hepatitis C virus envelope glycoprotein E1. Nat. Commun. 2014, 5, 4874. [Google Scholar] [CrossRef] [PubMed]
- Wahid, A.; Helle, F.; Descamps, V.; Duverlie, G.; Penin, F.; Dubuisson, J. Disulfide bonds in hepatitis C virus glycoprotein E1 control the assembly and entry functions of E2 glycoprotein. J. Virol. 2013, 87, 1605–1617. [Google Scholar] [CrossRef] [PubMed]
- Banda, D.H.; Perin, P.M.; Brown, R.J.P.; Todt, D.; Solodenko, W.; Hoffmeyer, P.; Kumar Sahu, K.; Houghton, M.; Meuleman, P.; Muller, R.; et al. A central hydrophobic E1 region controls the pH range of hepatitis C virus membrane fusion and susceptibility to fusion inhibitors. J. Hepatol. 2019, 70, 1082–1092. [Google Scholar] [CrossRef] [PubMed]
- Cheng, J.J.; Li, J.R.; Huang, M.H.; Ma, L.L.; Wu, Z.Y.; Jiang, C.C.; Li, W.J.; Li, Y.H.; Han, Y.X.; Li, H.; et al. CD36 is a co-receptor for hepatitis C virus E1 protein attachment. Sci. Rep. 2016, 6, 21808. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.Y.; Acosta, E.G.; Stoeck, I.K.; Long, G.; Hiet, M.S.; Mueller, B.; Fackler, O.T.; Kallis, S.; Bartenschlager, R. Apolipoprotein E likely contributes to a maturation step of infectious hepatitis C virus particles and interacts with viral envelope glycoproteins. J. Virol. 2014, 88, 12422–12437. [Google Scholar] [CrossRef] [PubMed]
- Mazumdar, B.; Banerjee, A.; Meyer, K.; Ray, R. Hepatitis C virus E1 envelope glycoprotein interacts with apolipoproteins in facilitating entry into hepatocytes. Hepatology 2011, 54, 1149–1156. [Google Scholar] [CrossRef]
- Keck, Z.Y.; Sung, V.M.; Perkins, S.; Rowe, J.; Paul, S.; Liang, T.J.; Lai, M.M.; Foung, S.K. Human monoclonal antibody to hepatitis C virus E1 glycoprotein that blocks virus attachment and viral infectivity. J. Virol. 2004, 78, 7257–7263. [Google Scholar] [CrossRef]
- Kong, L.; Kadam, R.U.; Giang, E.; Ruwona, T.B.; Nieusma, T.; Culhane, J.C.; Stanfield, R.L.; Dawson, P.E.; Wilson, I.A.; Law, M. Structure of hepatitis C virus envelope glycoprotein E1 antigenic site 314-324 in complex with antibody IGH526. J. Mol. Biol. 2015, 427, 2617–2628. [Google Scholar] [CrossRef]
- Spadaccini, R.; D’Errico, G.; D’Alessio, V.; Notomista, E.; Bianchi, A.; Merola, M.; Picone, D. Structural characterization of the transmembrane proximal region of the hepatitis C virus E1 glycoprotein. Biochim. Biophys. Acta 2010, 1798, 344–353. [Google Scholar] [CrossRef] [PubMed]
- Kong, L.; Lee, D.E.; Kadam, R.U.; Liu, T.; Giang, E.; Nieusma, T.; Garces, F.; Tzarum, N.; Woods, V.L., Jr.; Ward, A.B.; et al. Structural flexibility at a major conserved antibody target on hepatitis C virus E2 antigen. Proc. Natl. Acad. Sci. USA 2016, 113, 12768–12773. [Google Scholar] [CrossRef] [PubMed]
- Goffard, A.; Dubuisson, J. Glycosylation of hepatitis C virus envelope proteins. Biochimie 2003, 85, 295–301. [Google Scholar] [CrossRef]
- Lavie, M.; Hanoulle, X.; Dubuisson, J. Glycan shielding and modulation of hepatitis C virus neutralizing antibodies. Front. Immunol. 2018, 9, 910. [Google Scholar] [CrossRef]
- Prentoe, J.; Velazquez-Moctezuma, R.; Augestad, E.H.; Galli, A.; Wang, R.; Law, M.; Alter, H.; Bukh, J. Hypervariable region 1 and N-linked glycans of hepatitis C regulate virion neutralization by modulating envelope conformations. Proc. Natl. Acad. Sci. USA 2019, 116, 10039–10047. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.G.; Whidby, J.; Miller, M.T.; Scarborough, H.; Zatorski, A.V.; Cygan, A.; Price, A.A.; Yost, S.A.; Bohannon, C.D.; Jacob, J.; et al. Structure of the core ectodomain of the hepatitis C virus envelope glycoprotein 2. Nature 2014, 509, 381–384. [Google Scholar] [CrossRef] [PubMed]
- Stejskal, L.; Lees, W.D.; Moss, D.S.; Palor, M.; Bingham, R.J.; Shepherd, A.J.; Grove, J. Flexibility and intrinsic disorder are conserved features of hepatitis C virus E2 glycoprotein. PLoS Comput. Biol. 2020, 16, e1007710. [Google Scholar] [CrossRef] [PubMed]
- Guest, J.D.; Pierce, B.G. Computational modeling of hepatitis C virus envelope glycoprotein structure and recognition. Front. Immunol. 2018, 9, 1117. [Google Scholar] [CrossRef]
- Stroh, L.J.; Nagarathinam, K.; Krey, T. Conformational flexibility in the CD81-bindingsite of the hepatitis C virus glycoprotein E2. Front. Immunol. 2018, 9, 1396. [Google Scholar] [CrossRef] [PubMed]
- Tzarum, N.; Wilson, I.A.; Law, M. The neutralizing face of hepatitis C virus E2 envelope glycoprotein. Front. Immunol. 2018, 9, 1315. [Google Scholar] [CrossRef]
- Flint, M.; Maidens, C.; Loomis-Price, L.D.; Shotton, C.; Dubuisson, J.; Monk, P.; Higginbottom, A.; Levy, S.; McKeating, J.A. Characterization of hepatitis C virus E2 glycoprotein interaction with a putative cellular receptor, CD81. J. Virol. 1999, 73, 6235–6244. [Google Scholar] [CrossRef]
- Owsianka, A.; Tarr, A.W.; Juttla, V.S.; Lavillette, D.; Bartosch, B.; Cosset, F.L.; Ball, J.K.; Patel, A.H. Monoclonal antibody AP33 defines a broadly neutralizing epitope on the hepatitis C virus E2 envelope glycoprotein. J. Virol. 2005, 79, 11095–11104. [Google Scholar] [CrossRef] [PubMed]
- Broering, T.J.; Garrity, K.A.; Boatright, N.K.; Sloan, S.E.; Sandor, F.; Thomas, W.D., Jr.; Szabo, G.; Finberg, R.W.; Ambrosino, D.M.; Babcock, G.J. Identification and characterization of broadly neutralizing human monoclonal antibodies directed against the E2 envelope glycoprotein of hepatitis C virus. J. Virol. 2009, 83, 12473–12482. [Google Scholar] [CrossRef] [PubMed]
- Sabo, M.C.; Luca, V.C.; Prentoe, J.; Hopcraft, S.E.; Blight, K.J.; Yi, M.; Lemon, S.M.; Ball, J.K.; Bukh, J.; Evans, M.J.; et al. Neutralizing monoclonal antibodies against hepatitis C virus E2 protein bind discontinuous epitopes and inhibit infection at a postattachment step. J. Virol. 2011, 85, 7005–7019. [Google Scholar] [CrossRef]
- Keck, Z.; Wang, W.; Wang, Y.; Lau, P.; Carlsen, T.H.; Prentoe, J.; Xia, J.; Patel, A.H.; Bukh, J.; Foung, S.K. Cooperativity in virus neutralization by human monoclonal antibodies to two adjacent regions located at the amino terminus of hepatitis C virus E2 glycoprotein. J. Virol. 2013, 87, 37–51. [Google Scholar] [CrossRef] [PubMed]
- Pantua, H.; Diao, J.; Ultsch, M.; Hazen, M.; Mathieu, M.; McCutcheon, K.; Takeda, K.; Date, S.; Cheung, T.K.; Phung, Q.; et al. Glycan shifting on hepatitis C virus (HCV) E2 glycoprotein is a mechanism for escape from broadly neutralizing antibodies. J. Mol. Biol. 2013, 425, 1899–1914. [Google Scholar] [CrossRef]
- Alhammad, Y.; Gu, J.; Boo, I.; Harrison, D.; McCaffrey, K.; Vietheer, P.T.; Edwards, S.; Quinn, C.; Coulibaly, F.; Poumbourios, P.; et al. Monoclonal antibodies directed toward the Hepatitis C virus glycoprotein E2 detect antigenic differences modulated by the N-terminal hypervariable region 1 (HVR1), HVR2, and intergenotypic variable region. J. Virol. 2015, 89, 12245–12261. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.P.; Gottwein, J.M.; Scheel, T.K.; Jensen, T.B.; Bukh, J. MicroRNA-122 antagonism against hepatitis C virus genotypes 1-6 and reduced efficacy by host RNA insertion or mutations in the HCV 5’ UTR. Proc. Natl. Acad. Sci. USA 2011, 108, 4991–4996. [Google Scholar] [CrossRef]
- Kong, L.; Giang, E.; Nieusma, T.; Robbins, J.B.; Deller, M.C.; Stanfield, R.L.; Wilson, I.A.; Law, M. Structure of hepatitis C virus envelope glycoprotein E2 antigenic site 412 to 423 in complex with antibody AP33. J. Virol. 2012, 86, 13085–13088. [Google Scholar] [CrossRef]
- Kong, L.; Giang, E.; Robbins, J.B.; Stanfield, R.L.; Burton, D.R.; Wilson, I.A.; Law, M. Structural basis of hepatitis C virus neutralization by broadly neutralizing antibody HCV1. Proc. Natl. Acad. Sci. USA 2012, 109, 9499–9504. [Google Scholar] [CrossRef]
- Aleman, F.; Tzarum, N.; Kong, L.; Nagy, K.; Zhu, J.; Wilson, I.A.; Law, M. Immunogenetic and structural analysis of a class of HCV broadly neutralizing antibodies and their precursors. Proc. Natl. Acad. Sci. USA 2018, 115, 7569–7574. [Google Scholar] [CrossRef] [PubMed]
- Gu, J.; Hardy, J.; Boo, I.; Vietheer, P.; McCaffrey, K.; Alhammad, Y.; Chopra, A.; Gaudieri, S.; Poumbourios, P.; Coulibaly, F.; et al. Escape of hepatitis c virus from epitope I neutralization increases sensitivity of other neutralization epitopes. J. Virol. 2018, 92. [Google Scholar] [CrossRef] [PubMed]
- Potter, J.A.; Owsianka, A.M.; Jeffery, N.; Matthews, D.J.; Keck, Z.Y.; Lau, P.; Foung, S.K.; Taylor, G.L.; Patel, A.H. Toward a hepatitis C virus vaccine: The structural basis of hepatitis C virus neutralization by AP33, a broadly neutralizing antibody. J. Virol. 2012, 86, 12923–12932. [Google Scholar] [CrossRef]
- Li, Y.; Pierce, B.G.; Wang, Q.; Keck, Z.Y.; Fuerst, T.R.; Foung, S.K.; Mariuzza, R.A. Structural basis for penetration of the glycan shield of hepatitis C virus E2 glycoprotein by a broadly neutralizing human antibody. J. Biol. Chem. 2015, 290, 10117–10125. [Google Scholar] [CrossRef] [PubMed]
- Keck, Z.Y.; Girard-Blanc, C.; Wang, W.; Lau, P.; Zuiani, A.; Rey, F.A.; Krey, T.; Diamond, M.S.; Foung, S.K. Antibody response to hypervariable region 1 interferes with broadly neutralizing antibodies to Hepatitis C virus. J. Virol. 2016, 90, 3112–3122. [Google Scholar] [CrossRef]
- Meola, A.; Tarr, A.W.; England, P.; Meredith, L.W.; McClure, C.P.; Foung, S.K.; McKeating, J.A.; Ball, J.K.; Rey, F.A.; Krey, T. Structural flexibility of a conserved antigenic region in hepatitis C virus glycoprotein E2 recognized by broadly neutralizing antibodies. J. Virol. 2015, 89, 2170–2181. [Google Scholar] [CrossRef] [PubMed]
- Tarr, A.W.; Owsianka, A.M.; Timms, J.M.; McClure, C.P.; Brown, R.J.; Hickling, T.P.; Pietschmann, T.; Bartenschlager, R.; Patel, A.H.; Ball, J.K. Characterization of the hepatitis C virus E2 epitope defined by the broadly neutralizing monoclonal antibody AP33. Hepatology 2006, 43, 592–601. [Google Scholar] [CrossRef]
- Krey, T.; Meola, A.; Keck, Z.Y.; Damier-Piolle, L.; Foung, S.K.; Rey, F.A. Structural basis of HCV neutralization by human monoclonal antibodies resistant to viral neutralization escape. PLoS Pathog. 2013, 9, e1003364. [Google Scholar] [CrossRef] [PubMed]
- Keck, Z.Y.; Wang, Y.; Lau, P.; Lund, G.; Rangarajan, S.; Fauvelle, C.; Liao, G.C.; Holtsberg, F.W.; Warfield, K.L.; Aman, M.J.; et al. Affinity maturation of a broadly neutralizing human monoclonal antibody that prevents acute hepatitis C virus infection in mice. Hepatology 2016, 64, 1922–1933. [Google Scholar] [CrossRef] [PubMed]
- Deng, L.; Zhong, L.; Struble, E.; Duan, H.; Ma, L.; Harman, C.; Yan, H.; Virata-Theimer, M.L.; Zhao, Z.; Feinstone, S.; et al. Structural evidence for a bifurcated mode of action in the antibody-mediated neutralization of hepatitis C virus. Proc. Natl. Acad. Sci. USA 2013, 110, 7418–7422. [Google Scholar] [CrossRef]
- Deng, L.; Ma, L.; Virata-Theimer, M.L.; Zhong, L.; Yan, H.; Zhao, Z.; Struble, E.; Feinstone, S.; Alter, H.; Zhang, P. Discrete conformations of epitope II on the hepatitis C virus E2 protein for antibody-mediated neutralization and nonneutralization. Proc. Natl. Acad. Sci. USA 2014, 111, 10690–10695. [Google Scholar] [CrossRef] [PubMed]
- Vasiliauskaite, I.; Owsianka, A.; England, P.; Khan, A.G.; Cole, S.; Bankwitz, D.; Foung, S.K.H.; Pietschmann, T.; Marcotrigiano, J.; Rey, F.A.; et al. Conformational flexibility in the immunoglobulin-like domain of the Hepatitis C virus glycoprotein E2. mBio 2017, 8, e00382-00317. [Google Scholar] [CrossRef] [PubMed]
- Balasco, N.; Barone, D.; Iaccarino, E.; Sandomenico, A.; De Simone, A.; Ruvo, M.; Vitagliano, L. Intrinsic structural versatility of the highly conserved 412-423 epitope of the Hepatitis C Virus E2 protein. Int. J. Biol. Macromol. 2018, 116, 620–632. [Google Scholar] [CrossRef] [PubMed]
- El-Diwany, R.; Cohen, V.J.; Mankowski, M.C.; Wasilewski, L.N.; Brady, J.K.; Snider, A.E.; Osburn, W.O.; Murrell, B.; Ray, S.C.; Bailey, J.R. Extra-epitopic hepatitis C virus polymorphisms confer resistance to broadly neutralizing antibodies by modulating binding to scavenger receptor B1. PLoS Pathog. 2017, 13, e1006235. [Google Scholar] [CrossRef]
- Keck, Z.Y.; Li, S.H.; Xia, J.; von Hahn, T.; Balfe, P.; McKeating, J.A.; Witteveldt, J.; Patel, A.H.; Alter, H.; Rice, C.M.; et al. Mutations in hepatitis C virus E2 located outside the CD81 binding sites lead to escape from broadly neutralizing antibodies but compromise virus infectivity. J. Virol. 2009, 83, 6149–6160. [Google Scholar] [CrossRef]
- Keck, Z.Y.; Xia, J.; Wang, Y.; Wang, W.; Krey, T.; Prentoe, J.; Carlsen, T.; Li, A.Y.; Patel, A.H.; Lemon, S.M.; et al. Human monoclonal antibodies to a novel cluster of conformational epitopes on HCV E2 with resistance to neutralization escape in a genotype 2a isolate. PLoS Pathog. 2012, 8, e1002653. [Google Scholar] [CrossRef] [PubMed]
- Chung, R.T.; Gordon, F.D.; Curry, M.P.; Schiano, T.D.; Emre, S.; Corey, K.; Markmann, J.F.; Hertl, M.; Pomposelli, J.J.; Pomfret, E.A.; et al. Human monoclonal antibody MBL-HCV1 delays HCV viral rebound following liver transplantation: A randomized controlled study. Am. J. Transplant. 2013, 13, 1047–1054. [Google Scholar] [CrossRef]
- Dhillon, S.; Witteveldt, J.; Gatherer, D.; Owsianka, A.M.; Zeisel, M.B.; Zahid, M.N.; Rychlowska, M.; Foung, S.K.; Baumert, T.F.; Angus, A.G.; et al. Mutations within a conserved region of the hepatitis C virus E2 glycoprotein that influence virus-receptor interactions and sensitivity to neutralizing antibodies. J. Virol. 2010, 84, 5494–5507. [Google Scholar] [CrossRef] [PubMed]
- Gal-Tanamy, M.; Keck, Z.Y.; Yi, M.; McKeating, J.A.; Patel, A.H.; Foung, S.K.; Lemon, S.M. In vitro selection of a neutralization-resistant hepatitis C virus escape mutant. Proc. Natl. Acad. Sci. USA 2008, 105, 19450–19455. [Google Scholar] [CrossRef]
- Morin, T.J.; Broering, T.J.; Leav, B.A.; Blair, B.M.; Rowley, K.J.; Boucher, E.N.; Wang, Y.; Cheslock, P.S.; Knauber, M.; Olsen, D.B.; et al. Human monoclonal antibody HCV1 effectively prevents and treats HCV infection in chimpanzees. PLoS Pathog. 2012, 8, e1002895. [Google Scholar] [CrossRef] [PubMed]
- Drummer, H.E.; Boo, I.; Maerz, A.L.; Poumbourios, P. A conserved Gly436-Trp-Leu-Ala-Gly-Leu-Phe-Tyr motif in hepatitis C virus glycoprotein E2 is a determinant of CD81 binding and viral entry. J. Virol. 2006, 80, 7844–7853. [Google Scholar] [CrossRef] [PubMed]
- Duan, H.; Kachko, A.; Zhong, L.; Struble, E.; Pandey, S.; Yan, H.; Harman, C.; Virata-Theimer, M.L.; Deng, L.; Zhao, Z.; et al. Amino acid residue-specific neutralization and nonneutralization of hepatitis C virus by monoclonal antibodies to the E2 protein. J. Virol. 2012, 86, 12686–12694. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Gopal, R.; Jackson, K.; Tzarum, N.; Kong, L.; Ettenger, A.; Guest, J.; Pfaff, J.M.; Barnes, T.; Honda, A.; Giang, E.; et al. Probing the antigenicity of hepatitis C virus envelope glycoprotein complex by high-throughput mutagenesis. PLoS Pathog. 2017, 13, e1006735. [Google Scholar] [CrossRef] [PubMed]
- Flyak, A.I.; Ruiz, S.; Colbert, M.D.; Luong, T.; Crowe, J.E., Jr.; Bailey, J.R.; Bjorkman, P.J. HCV broadly neutralizing antibodies use a CDRH3 disulfide motif to recognize an E2 glycoprotein site that can be targeted for vaccine design. Cell Host Microbe 2018, 24, 703–716.e3. [Google Scholar] [CrossRef] [PubMed]
- Tzarum, N.; Giang, E.; Kong, L.; He, L.; Prentoe, J.; Augestad, E.; Hua, Y.; Castillo, S.; Lauer, G.M.; Bukh, J.; et al. Genetic and structural insights into broad neutralization of hepatitis C virus by human VH1-69 antibodies. Sci. Adv. 2019, 5, eaav1882. [Google Scholar] [CrossRef] [PubMed]
- Flyak, A.I.; Ruiz, S.E.; Salas, J.; Rho, S.; Bailey, J.R.; Bjorkman, P.J. An ultralong CDRH2 in HCV neutralizing antibody demonstrates structural plasticity of antibodies against E2 glycoprotein. Elife 2020, 9, e53169. [Google Scholar] [CrossRef] [PubMed]
- Tzarum, N.; Giang, E.; Kadam, R.U.; Chen, F.; Nagy, K.; Augestad, E.H.; Velazquez-Moctezuma, R.; Keck, Z.Y.; Hua, Y.; Stanfield, R.L.; et al. An alternate conformation of HCV E2 neutralizing face as an additional vaccine target. Sci. Adv. 2020, 6, eabb5642. [Google Scholar] [CrossRef]
- Stroh, L.J.; Krey, T. HCV glycoprotein sructure and implications for B-cell vaccine development. Int. J. Mol. Sci. 2020, 21, 6781. [Google Scholar] [CrossRef]
- Merat, S.J.; Molenkamp, R.; Wagner, K.; Koekkoek, S.M.; van de Berg, D.; Yasuda, E.; Bohne, M.; Claassen, Y.B.; Grady, B.P.; Prins, M.; et al. Hepatitis C virus broadly neutralizing monoclonal antibodies isolated 25 years after spontaneous clearance. PLoS ONE 2016, 11, e0165047. [Google Scholar] [CrossRef]
- Law, M.; Maruyama, T.; Lewis, J.; Giang, E.; Tarr, A.W.; Stamataki, Z.; Gastaminza, P.; Chisari, F.V.; Jones, I.M.; Fox, R.I.; et al. Broadly neutralizing antibodies protect against hepatitis C virus quasispecies challenge. Nat. Med. 2008, 14, 25–27. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; Nagy, K.; Chavez, D.; Willis, S.; McBride, R.; Giang, E.; Honda, A.; Bukh, J.; Ordoukhanian, P.; Zhu, J.; et al. Antibody responses to immunization with HCV envelope glycoproteins as a baseline for B-cell-based vaccine development. Gastroenterology 2020, 158, 1058–1071.e6. [Google Scholar] [CrossRef] [PubMed]
- Bailey, J.R.; Flyak, A.I.; Cohen, V.J.; Li, H.; Wasilewski, L.N.; Snider, A.E.; Wang, S.; Learn, G.H.; Kose, N.; Loerinc, L.; et al. Broadly neutralizing antibodies with few somatic mutations and hepatitis C virus clearance. JCI Insight 2017, 2, 92872. [Google Scholar] [CrossRef] [PubMed]
- Keck, Z.Y.; Pierce, B.G.; Lau, P.; Lu, J.; Wang, Y.; Underwood, A.; Bull, R.A.; Prentoe, J.; Velázquez-Moctezuma, R.; Walker, M.R.; et al. Broadly neutralizing antibodies from an individual that naturally cleared multiple hepatitis C virus infections uncover molecular determinants for E2 targeting and vaccine design. PLoS Pathog. 2019, 15, e1007772. [Google Scholar] [CrossRef] [PubMed]
- Keck, Z.Y.; Saha, A.; Xia, J.; Wang, Y.; Lau, P.; Krey, T.; Rey, F.A.; Foung, S.K. Mapping a region of hepatitis C virus E2 that is responsible for escape from neutralizing antibodies and a core CD81-binding region that does not tolerate neutralization escape mutations. J. Virol. 2011, 85, 10451–10463. [Google Scholar] [CrossRef] [PubMed]
- Merat, S.J.; Bru, C.; van de Berg, D.; Molenkamp, R.; Tarr, A.W.; Koekkoek, S.; Kootstra, N.A.; Prins, M.; Ball, J.K.; Bakker, A.Q.; et al. Cross-genotype AR3-specific neutralizing antibodies confer long-term protection in injecting drug users after HCV clearance. J. Hepatol. 2019, 71, 14–24. [Google Scholar] [CrossRef] [PubMed]
- Yi, C.; Xia, J.; He, L.; Ling, Z.; Wang, X.; Yan, Y.; Wang, J.; Zhao, X.; Fan, W.; Sun, X.; et al. Junctional and somatic hypermutation-induced CX4C motif is critical for the recognition of a highly conserved epitope on HCV E2 by a human broadly neutralizing antibody. Cell Mol. Immunol. 2021, 18, 675–685. [Google Scholar] [CrossRef] [PubMed]
- Velazquez-Moctezuma, R.; Galli, A.; Law, M.; Bukh, J.; Prentoe, J. Hepatitis C virus escape studies of human antibody AR3A reveal a high barrier to resistance and novel insights on viral antibody evasion mechanisms. J. Virol. 2019, 93. [Google Scholar] [CrossRef] [PubMed]
- McLellan, J.S.; Chen, M.; Leung, S.; Graepel, K.W.; Du, X.; Yang, Y.; Zhou, T.; Baxa, U.; Yasuda, E.; Beaumont, T.; et al. Structure of RSV fusion glycoprotein trimer bound to a prefusion-specific neutralizing antibody. Science 2013, 340, 1113–1117. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; Tzarum, N.; Lin, X.; Giang, E.; Velazquez-Moctezuma, R.; Augestad, E.H.; Nagy, K.; He, L.; Hernandez, M.; Fouch, M.E.; et al. Functional convergence of a germline-encoded neutralizing antibody response in rhesus macaques immunized with HCV envelope glycoproteins. Immunity 2021, 54, 781–796.e4. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; Tzarum, N.; Wilson, I.A.; Law, M. VH1-69 antiviral broadly neutralizing antibodies: Genetics, structures, and relevance to rational vaccine design. Curr. Opin. Virol. 2019, 34, 149–159. [Google Scholar] [CrossRef]
- Briney, B.; Inderbitzin, A.; Joyce, C.; Burton, D.R. Commonality despite exceptional diversity in the baseline human antibody repertoire. Nature 2019, 566, 393–397. [Google Scholar] [CrossRef] [PubMed]
- He, L.; Tzarum, N.; Lin, X.; Shapero, B.; Sou, C.; Mann, C.J.; Stano, A.; Zhang, L.; Nagy, K.; Giang, E.; et al. Proof of concept for rational design of hepatitis C virus E2 core nanoparticle vaccines. Sci. Adv. 2020, 6, eaaz6225. [Google Scholar] [CrossRef] [PubMed]
- Prentoe, J.; Bukh, J. Hypervariable region 1 in envelope protein 2 of hepatitis C virus: A linchpin in neutralizingantibody evasion and viral entry. Front. Immunol. 2018, 9, 2146. [Google Scholar] [CrossRef]
- Law, J.L.M.; Logan, M.; Wong, J.; Kundu, J.; Hockman, D.; Landi, A.; Chen, C.; Crawford, K.; Wininger, M.; Johnson, J.; et al. Role of the E2 Hypervariable Region (HVR1) in the Immunogenicity of a Recombinant Hepatitis C Virus Vaccine. J. Virol. 2018, 92. [Google Scholar] [CrossRef] [PubMed]
- Vietheer, P.T.; Boo, I.; Gu, J.; McCaffrey, K.; Edwards, S.; Owczarek, C.; Hardy, M.P.; Fabri, L.; Center, R.J.; Poumbourios, P.; et al. The core domain of hepatitis C virus glycoprotein E2 generates potent cross-neutralizing antibodies in guinea pigs. Hepatology 2017, 65, 1117–1131. [Google Scholar] [CrossRef] [PubMed]
- Khera, T.; Behrendt, P.; Bankwitz, D.; Brown, R.J.P.; Todt, D.; Doepke, M.; Khan, A.G.; Schulze, K.; Law, J.; Logan, M.; et al. Functional and immunogenic characterization of diverse HCV glycoprotein E2 variants. J. Hepatol. 2019, 70, 593–602. [Google Scholar] [CrossRef]
- He, L.; Zhu, J. Computational tools for epitope vaccine design and evaluation. Curr. Opin. Virol. 2015, 11, 103–112. [Google Scholar] [CrossRef] [PubMed]
- Plemper, R.K. Cell entry of enveloped viruses. Curr. Opin. Virol. 2011, 1, 92–100. [Google Scholar] [CrossRef] [PubMed]
- Bhattarai, N.; McLinden, J.H.; Xiang, J.; Kaufman, T.M.; Stapleton, J.T. Conserved motifs within hepatitis C virus envelope (E2) RNA and protein independently inhibit T cell activation. PLoS Pathog. 2015, 11, e1005183. [Google Scholar] [CrossRef]
- Cowton, V.M.; Owsianka, A.M.; Fadda, V.; Ortega-Prieto, A.M.; Cole, S.J.; Potter, J.A.; Skelton, J.K.; Jeffrey, N.; Di Lorenzo, C.; Dorner, M.; et al. Development of a structural epitope mimic: An idiotypic approach to HCV vaccine design. NPJ Vaccines 2021, 6, 7. [Google Scholar] [CrossRef] [PubMed]
- He, L.; Cheng, Y.; Kong, L.; Azadnia, P.; Giang, E.; Kim, J.; Wood, M.R.; Wilson, I.A.; Law, M.; Zhu, J. Approaching rational epitope vaccine design for hepatitis C virus with meta-server and multivalent scaffolding. Sci. Rep. 2015, 5, 12501. [Google Scholar] [CrossRef]
- Meuleman, T.J.; Cowton, V.M.; Patel, A.H.; Liskamp, R.M.J. Design and synthesis of HCV-E2 glycoprotein epitope mimics in molecular construction of potential synthetic vaccines. Viruses 2021, 13, 326. [Google Scholar] [CrossRef]
- Pierce, B.G.; Boucher, E.N.; Piepenbrink, K.H.; Ejemel, M.; Rapp, C.A.; Thomas, W.D., Jr.; Sundberg, E.J.; Weng, Z.; Wang, Y. Structure-based design of Hepatitis C virus vaccines that elicit neutralizing antibody responses to a conserved epitope. J. Virol. 2017, 91, e01032-01017. [Google Scholar] [CrossRef] [PubMed]
- Wheatley, A.K.; Whittle, J.R.; Lingwood, D.; Kanekiyo, M.; Yassine, H.M.; Ma, S.S.; Narpala, S.R.; Prabhakaran, M.S.; Matus-Nicodemos, R.A.; Bailer, R.T.; et al. H5N1 vaccine-elicited memory B cells are genetically constrained by the IGHV locus in the recognition of a neutralizing epitope in the hemagglutinin stem. J. Immunol. 2015, 195, 602–610. [Google Scholar] [CrossRef] [PubMed]
- Zhou, T.; Lynch, R.M.; Chen, L.; Acharya, P.; Wu, X.; Doria-Rose, N.A.; Joyce, M.G.; Lingwood, D.; Soto, C.; Bailer, R.T.; et al. Structural repertoire of HIV-1-neutralizing antibodies targeting the CD4 supersite in 14 donors. Cell 2015, 161, 1280–1292. [Google Scholar] [CrossRef] [PubMed]
- Setliff, I.; McDonnell, W.J.; Raju, N.; Bombardi, R.G.; Murji, A.A.; Scheepers, C.; Ziki, R.; Mynhardt, C.; Shepherd, B.E.; Mamchak, A.A.; et al. Multi-donor longitudinal antibody repertoire sequencing reveals the existence of public antibody clonotypes in HIV-1 infection. Cell Host Microbe 2018, 23, 845–854.e6. [Google Scholar] [CrossRef] [PubMed]
- Parameswaran, P.; Liu, Y.; Roskin, K.M.; Jackson, K.K.; Dixit, V.P.; Lee, J.Y.; Artiles, K.L.; Zompi, S.; Vargas, M.J.; Simen, B.B.; et al. Convergent antibody signatures in human dengue. Cell Host Microbe 2013, 13, 691–700. [Google Scholar] [CrossRef] [PubMed]
- Sandomenico, A.; Leonardi, A.; Berisio, R.; Sanguigno, L.; Foca, G.; Foca, A.; Ruggiero, A.; Doti, N.; Muscariello, L.; Barone, D.; et al. Generation and characterization of monoclonal antibodies against a cyclic variant of hepatitis C virus E2 epitope 412-422. J. Virol. 2016, 90, 3745–3759. [Google Scholar] [CrossRef] [PubMed]
- Burton, D.R. Advancing an HIV vaccine; advancing vaccinology. Nat. Rev. Immunol. 2019, 19, 77–78. [Google Scholar] [CrossRef]
- Jardine, J.G.; Ota, T.; Sok, D.; Pauthner, M.; Kulp, D.W.; Kalyuzhniy, O.; Skog, P.D.; Thinnes, T.C.; Bhullar, D.; Briney, B.; et al. Priming a broadly neutralizing antibody response to HIV-1 using a germline-targeting immunogen. Science 2015, 349, 156–161. [Google Scholar] [CrossRef] [PubMed]
- Andrews, S.F.; McDermott, A.B. Shaping a universally broad antibody response to influenza amidst a variable immunoglobulin landscape. Curr. Opin. Immunol. 2018, 53, 96–101. [Google Scholar] [CrossRef] [PubMed]
- Joyce, M.G.; Wheatley, A.K.; Thomas, P.V.; Chuang, G.Y.; Soto, C.; Bailer, R.T.; Druz, A.; Georgiev, I.S.; Gillespie, R.A.; Kanekiyo, M.; et al. Vaccine-induced antibodies that neutralize group 1 and group 2 influenza A viruses. Cell 2016, 166, 609–623. [Google Scholar] [CrossRef] [PubMed]
- Yuan, M.; Liu, H.; Wu, N.C.; Lee, C.D.; Zhu, X.; Zhao, F.; Huang, D.; Yu, W.; Hua, Y.; Tien, H.; et al. Structural basis of a shared antibody response to SARS-CoV-2. Science 2020, 369, 1119–1123. [Google Scholar] [CrossRef] [PubMed]
- Pieper, K.; Tan, J.; Piccoli, L.; Foglierini, M.; Barbieri, S.; Chen, Y.; Silacci-Fregni, C.; Wolf, T.; Jarrossay, D.; Anderle, M.; et al. Public antibodies to malaria antigens generated by two LAIR1 insertion modalities. Nature 2017, 548, 597–601. [Google Scholar] [CrossRef] [PubMed]
- Ramesh, A.; Darko, S.; Hua, A.; Overman, G.; Ransier, A.; Francica, J.R.; Trama, A.; Tomaras, G.D.; Haynes, B.F.; Douek, D.C.; et al. Structure and diversity of the rhesus macaque immunoglobulin loci through multiple de novo genome assemblies. Front. Immunol. 2017, 8, 1407. [Google Scholar] [CrossRef] [PubMed]
- Sundling, C.; Li, Y.; Huynh, N.; Poulsen, C.; Wilson, R.; O’Dell, S.; Feng, Y.; Mascola, J.R.; Wyatt, R.T.; Karlsson Hedestam, G.B. High-resolution definition of vaccine-elicited B cell responses against the HIV primary receptor binding site. Sci. Transl. Med. 2012, 4, 142ra196. [Google Scholar] [CrossRef]

| Epitope | mAbs | Antigen Conformation | PDB ID | References | |
|---|---|---|---|---|---|
| AS412 | HCV1 | Human bnAb | β-hairpin | 4DGV/Y | [94] |
| AP33 | Mouse bnAb | β-hairpin | 4G6A | [93,97] | |
| 4GAG/J | |||||
| MRCT10.v362 | Humanized and affinity-matured bnAb | β-hairpin | 4HS6 | [90] | |
| Hu5B3.V3 | Humanized and affinity-matured bnAb | -hairpin | 4HS8 | [90] | |
| 3/11 | Rat bnAb | open | 4WHY/T | [100] | |
| HC33.1 | Human bnAb | semiopen | 4XVJ | [98] | |
| HC33.4 | Human bnAb | semiopen | 5FGB | [99] | |
| HC33.8 | Human bnAb | semiopen | 5FGC | [99] | |
| MAb24 | Mouse bnAb | β-hairpin | 5VXR | [96] | |
| 19B3 | Mouse bnAb | β-hairpin | 6BZU | [95] | |
| 22D11 | Mouse bnAb | β-hairpin | 6BZY | [95] | |
| 19B3 GL | Mouse bnAb | β-hairpin | 6BZW | [95] | |
| 22D11 GL | Mouse bnAb | β-hairpin | 6BZV | [95] | |
| AS434 | HC84.1 | Human bnAb | 1.5-turn α-helix | 4JZN | [102] |
| HC84.27 | Human bnAb | 1.5-turn α-helix | 4JZO | [102] | |
| HC84.26 | Human bnAb | 1.5-turn α-helix | 5ERW | [103] | |
| HC84.26 AM | Affinity-matured human bnAb | 1.5-turn α-helix | 4Z0X | [103] | |
| 8 | Murine-neutralizing mAb (genotype 1a only) | 1.5-turn α-helix | 4HZL | [104] | |
| 12 | Murine-non-neutralizing mAb | 1.5-turn α-helix | 4Q0X | [105] | |
| a.a. 529–540 | DAO5 | Non-neutralization mAb | one-turn α-helix | 5NPH/I/J | [106] |
| E2 Domain | HCV Isolate 1 | mAbs | Front Layer 2 | CDRH2 Tip Sequence 3 | CDRH3 Length 4 | CxGGxC Motif | HC Dominancy | PDB ID | REF | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| E2c3 | HK6a (6a) | AR3A | Human bnAb from chronically infected | A | FIPMF | 18 | √ | HC only | 6BKB, 6UYG | [119] | ||
| E2c3, E2mc3 | HK6a (6a) | AR3B | Human bnAb from chronically infected | A | IIPAF | 19 | HC only | 6BKC, 6UYF | [119,136] | |||
| E2c, E2mc3 | H77 (1a) | AR3C | Human bnAb from chronically infected | A | VVPLF | 18 | √ | HC only | 4MWF, 6UYD/M | [44,136] | ||
| E2c3 | HK6a (6a) | AR3D | Human bnAb from chronically infected | A | IIPFF | 22 | HC only | 6BKD | [119] | |||
| E2c3 | HK6a (6a) | U1 | Human bnAb from chronically infected | A | ITPIF | 17 | HC dominant | 6WO3 | [121] | |||
| E2c3 | HK6a (6a) | HC11 | Human bnAb from chronically infected | A | IIPMF | 17 | √ | HC dominant | 6WO4 | [121] | ||
| E2ecto 5 | 1b09 (1b), 1a53 (1a) | HEPC3 | Human bnAb from spontaneously cleared patient | A | ITPIF | 17 | √ | HC only | 6MEI/J/K | [118] | ||
| E2ecto, E2c | 1b09 (1b) | HEPC74 | Human bnAb from spontaneously cleared patient | A | MSPIS | 18 | √ | HC only | 6MEH | [118] | ||
| E2c3 5 | H77 (1a) | HC1AM | Human bnAb from chronically infected | B | FIPMF | 15 | HC dominant | 6WOQ | [121] | |||
| E2c3 5 | H77 (1a) | 212.1.1 | Human nAb from spontaneously cleared patient | B | SIPIL | 16 | HC dominant | 6WO5 | [121] | |||
| E2c3 | HK6a (6a) | RM2-01 | bnAb from immunized rhesus macaques | A | IVPLG | 15 | HC only | 7JTF | ||||
| E2c3 | HK6a (6a) | RM11-43 | bnAb from immunized rhesus macaques | A | IIPLG | 19 | HC only | 7JTG | ||||
| E2ecto | 1b09 (1b) | AR3X | Human bnAb from chronically infected | A | INPIS | 19 | √ | 6URH | [120] | |||
| Core E2 6 | J6 | 2A12 | Non-neutralization mAb | 4WEB | [106] | |||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yechezkel, I.; Law, M.; Tzarum, N. From Structural Studies to HCV Vaccine Design. Viruses 2021, 13, 833. https://doi.org/10.3390/v13050833
Yechezkel I, Law M, Tzarum N. From Structural Studies to HCV Vaccine Design. Viruses. 2021; 13(5):833. https://doi.org/10.3390/v13050833
Chicago/Turabian StyleYechezkel, Itai, Mansun Law, and Netanel Tzarum. 2021. "From Structural Studies to HCV Vaccine Design" Viruses 13, no. 5: 833. https://doi.org/10.3390/v13050833
APA StyleYechezkel, I., Law, M., & Tzarum, N. (2021). From Structural Studies to HCV Vaccine Design. Viruses, 13(5), 833. https://doi.org/10.3390/v13050833






