Analysis of Synchronous and Asynchronous In Vitro Infections with Homologous Murine Norovirus Strains Reveals Time-Dependent Viral Interference Effects
Abstract
1. Introduction
2. Materials and Methods
2.1. Viruses and Cells
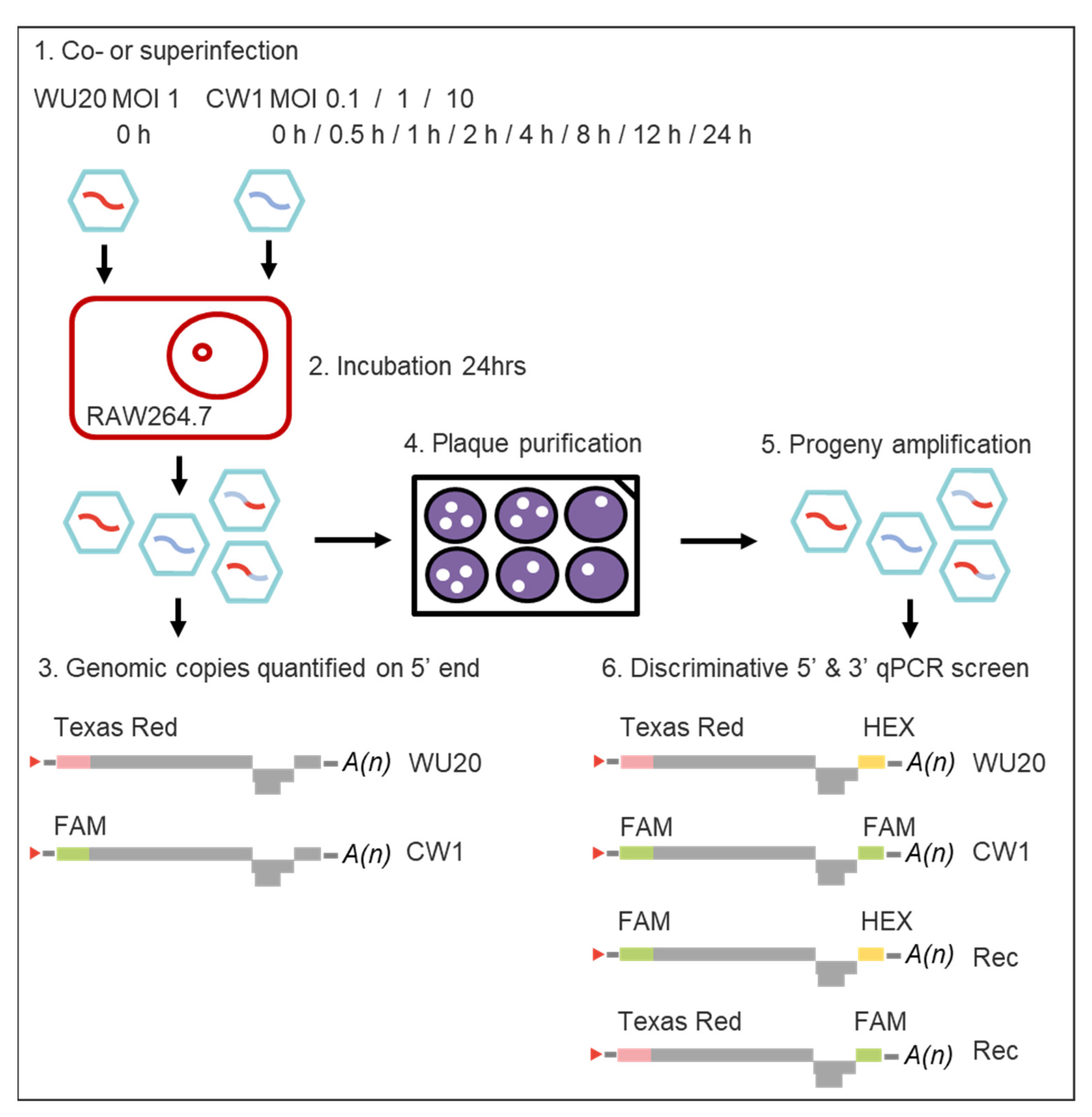
2.2. Coinfection and Superinfection of RAW264.7 Cells with Murine Noroviruses WU20 and CW1
2.3. Quantification of WU20 and CW1 Genomic Copies in Viral Progenies 24 h Post Co- or Superinfection
2.4. Isolation and Screening of Infectious Progeny Viruses
3. Results
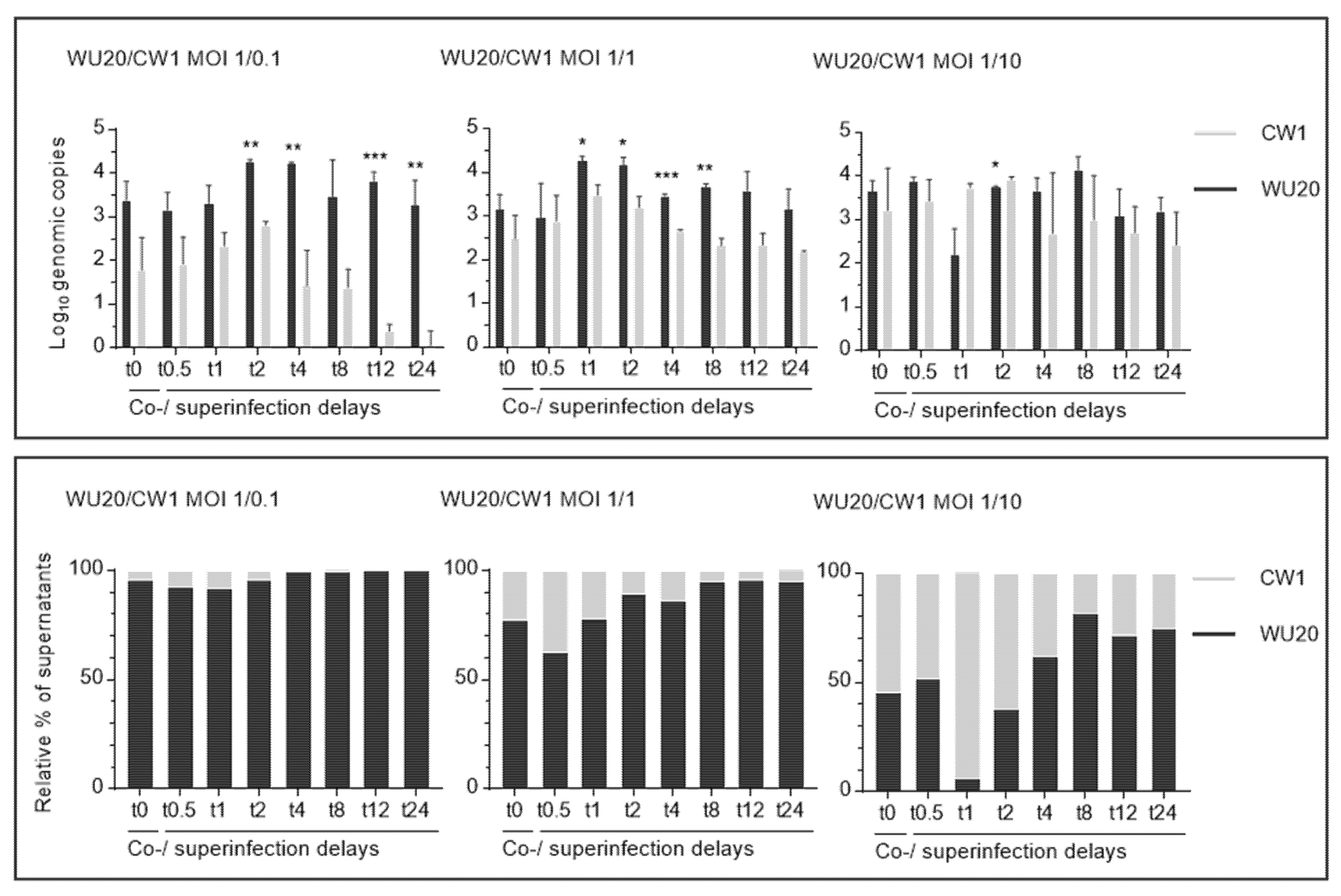
3.1. Absolute and Relative Quantification of Genomic Copies Reveals Skewed WU20 and CW1 Distributions and a WU20 Dominance in Most Viral Progenies 24 h Post Co- or Superinfection
3.2. Molecular Screening on Picked Lysis Plaques Demonstrates a WU20 Predominance in the Majority of Infectious Viral Progenies
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Robilotti, E.; Deresinski, S.; Pinsky, B.A. Norovirus. Clin. Microbiol. Rev. Internet 2015, 28, 134–164. [Google Scholar] [CrossRef]
- Bartsch, S.M.; Lopman, B.A.; Ozawa, S.; Hall, A.J.; Lee, B.Y. Global economic burden of norovirus gastroenteritis. PLoS ONE 2016, 11, e0151219. [Google Scholar] [CrossRef]
- Karst, S.M. Pathogenesis of noroviruses, emerging RNA viruses. Viruses 2010, 2, 748–781. [Google Scholar] [CrossRef] [PubMed]
- Harris, J.P.; Edmunds, W.J.; Pebody, R.; Brown, D.W.; Lopman, B.A. Deaths from norovirus among the elderly, England and Wales. Emerg. Infect. Dis. 2008, 14, 1546–1552. [Google Scholar] [CrossRef]
- Brown, J.R.; Gilmour, K.; Breuer, J. Norovirus Infections Occur in B-Cell-Deficient Patients. Clin. Infect. Dis. 2016, 62, 1136–1138. [Google Scholar] [CrossRef] [PubMed]
- Woodward, J.; Gkrania-Klotsas, E.; Kumararatne, D. Chronic norovirus infection and common variable immunodeficiency. Clin. Exp. Immunol. 2017, 188, 363–370. [Google Scholar] [CrossRef]
- Brown, J.R.; Roy, S.; Tutill, H.; Williams, R.; Breuer, J. Super-infections and relapses occur in chronic norovirus infections. J. Clin. Virol. 2017, 96, 44–48. [Google Scholar] [CrossRef]
- Vega, E.; Donaldson, E.; Huynh, J.; Barclay, L.; Lopman, B.; Baric, R.; Vinjé, J. RNA Populations in Immunocompromised Patients as Reservoirs for Novel Norovirus Variants. J. Virol. 2014, 88, 14184–14196. [Google Scholar] [CrossRef] [PubMed]
- Jones, M.K.; Grau, K.R.; Costantini, V.; Kolawole, A.O.; de Graaf, M.; Freiden, P.; Karst, S.M. Human norovirus culture in B cells. Nat. Protoc. 2015, 10, 1939–1947. [Google Scholar] [CrossRef]
- Ettayebi, K.; Crawford, S.E.; Murakami, K.; Broughman, J.R.; Karandikar, U.; Tenge, V.R.; Estes, M.K. Replication of human noroviruses in stem cell–derived human enteroids. Science 2016, 353, 1387–1393. [Google Scholar] [CrossRef]
- Van Dycke, J.; Ny, A.; Conceição-Neto, N.; Maes, J.; Hosmillo, M.; Cuvry, A.; Rocha-Pereira, J. A robust human norovirus replication model in zebrafish larvae. PLoS Pathog. 2019, 15, 1–21. [Google Scholar] [CrossRef]
- Todd, K.V.; Tripp, R.A. Human norovirus: Experimental models of infection. Viruses 2019, 11, 151. [Google Scholar] [CrossRef] [PubMed]
- Arias, A.; Bailey, D.; Chaudhry, Y.; Goodfellow, I. Development of a reverse-genetics system for murine norovirus 3: Long-term persistence occurs in the caecum and colon. J. Gen. Virol. 2012, 93, 1432–1441. [Google Scholar] [CrossRef]
- Yunus, M.A.; Chung, L.M.W.; Chaudhry, Y.; Bailey, D.; Goodfellow, I. Development of an optimized RNA-based murine norovirus reverse genetics system. J. Virol. Methods 2010, 169, 112–118. [Google Scholar] [CrossRef] [PubMed]
- Karst, S.M.; Wobus, C.E.; Lay, M.; Davidson, J.; Virgin, H.W. STAT1-dependent innate immunity to a Norwalk-like virus. Science 2003, 299, 1575–1578. [Google Scholar] [CrossRef] [PubMed]
- Wobus, C.E.; Thackray, L.B.; Virgin, H.W. Murine norovirus: A model system to study norovirus biology and pathogenesis. J. Virol. 2006, 80, 5104–5112. [Google Scholar] [CrossRef] [PubMed]
- Wobus, C.E.; Karst, S.M.; Thackray, L.B.; Chang, K.-O.; Sosnovtsev, S.V.; Belliot, G.; Virgin, H.W., 4th. Replication of Norovirus in Cell Culture Reveals a Tropism for Dendritic Cells and Macrophages. PLoS Biol. 2004, 30, e432. [Google Scholar] [CrossRef] [PubMed]
- Desselberger, U. Caliciviridae other than noroviruses. Viruses 2019, 11, 286. [Google Scholar] [CrossRef]
- Clarke, I.N.; Estes, M.K.; Green, K.Y.; Hansman, G.; Knowles, N.J.; Koopmans, M.K. Virus Taxonomy: Classification and Nomenclature of Viruses: Ninth Report of the International Committee on Taxonomy of Viruses; King, A.M.Q., Adams, M.J., Carstens, E.B., Lefkowitz, E.J., Eds.; Elsevier: San Diego, CA, USA, 2012; pp. 977–986. [Google Scholar]
- Karst, S.M.; Zhu, S.; Goodfellow, I.G. The molecular pathology of noroviruses. J. Pathol. 2015, 235, 206–216. [Google Scholar] [CrossRef]
- Karst, S.M.; Wobus, C.E.; Goodfellow, I.G.; Green, K.Y.; Virgin, H.W. Advances in Norovirus Biology. Cell Host Microbe 2014, 15, 668–680. [Google Scholar] [CrossRef]
- Thorne, L.G.; Goodfellow, I.G. Norovirus gene expression and replication. J. Gen. Virol. 2014, 95, 278–291. [Google Scholar] [CrossRef] [PubMed]
- McFadden, N.; Bailey, D.; Carrara, G.; Benson, A.; Chaudhry, Y.; Shortland, A.; Goodfellow, I. Norovirus regulation of the innate immune response and apoptosis occurs via the product of the alternative open reading frame 4. PLoS Pathog. 2011, 7, e1002413. [Google Scholar] [CrossRef] [PubMed]
- Parra, G.I. Emergence of norovirus strains: A tale of two genes. Virus. Evol. 2019, 5, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Bull, R.A.; Hansman, G.S.; Clancy, L.E.; Tanaka, M.M.; Rawlinson, W.D.; White, P. Norovirus Recombination in ORF1/ORF2 Overlap. Emerg. Infect. Dis. 2005, 11, 1079–1085. [Google Scholar] [CrossRef] [PubMed]
- Ludwig-Begall, L.F.; Mauroy, A.; Thiry, E. Norovirus recombinants: Recurrent in the field, recalcitrant in the lab—A scoping review of recombination and recombinant types of noroviruses. J. Gen. Virol. 2018, 99, 970–988. [Google Scholar] [CrossRef] [PubMed]
- Bull, R.A.; Tanaka, M.M.; White, P.A. Norovirus recombination. J. Gen. Virol. 2007, 88, 3347–3359. [Google Scholar] [CrossRef] [PubMed]
- Mathijs, E.; Muylkens, B.; Mauroy, A.; Ziant, D.; Delwiche, T.; Thiry, E. Experimental evidence of recombination in murine noroviruses. J. Gen. Virol. 2010, 91, 2723–2733. [Google Scholar] [CrossRef]
- Mathijs, E.; de Oliveira-Filho, E.F.; Dal Pozzo, F.; Mauroy, A.; Thiry, D.; Massart, F.; Thiry, E. Infectivity of a recombinant murine norovirus (RecMNV) in Balb/cByJ mice. Vet. Microbiol. 2016, 192, 118–122. [Google Scholar] [CrossRef]
- Ludwig-Begall, L.F.; Lu, J.; Hosmillo, M.; De Oliveira-Filho, E.F.; Mathijs, E.; Goodfellow, I.; Thiry, E. Replicative fitness recuperation of a recombinant murine norovirus—In vitro reciprocity of genetic shift and drift. J. Gen. Virol. 2020, 101, 510–522. [Google Scholar] [CrossRef]
- Worobey, M.; Holmes, E.C. Evolutionary aspects of recombination in RNA viruses. J. Gen. Virol. 1999, 80, 2535–2543. [Google Scholar] [CrossRef]
- Lowry, K.; Woodman, A.; Cook, J.; Evans, D.J. Recombination in enteroviruses is a biphasic replicative process involving the generation of greater-than genome length “imprecise” intermediates. PLoS Pathog. 2014, 10, e1004191. [Google Scholar] [CrossRef]
- Bagaya, B.S.; Tian, M.; Nickel, G.C.; Vega, J.F.; Li, Y.; He, P.; Gao, Y. An in vitro Model to Mimic Selection of Replication-Competent HIV-1 Intersubtype Recombination in Dual or Superinfected Patients. J. Mol. Biol. 2017, 429, 2246–2264. [Google Scholar] [CrossRef] [PubMed]
- Banner, L.R.; Mc Lai, M. Random nature of coronavirus RNA recombination in the absence of selection pressure. Virology 1991, 185, 441–445. [Google Scholar] [CrossRef]
- Sackman, A.M.; Reed, D.; Rokyta, D.R. Intergenic incompatibilities reduce fitness in hybrids of extremely closely related bacteriophages. PeerJ 2015, 3, 1320. [Google Scholar] [CrossRef] [PubMed]
- Erickson, A.K.; Jesudhasan, P.R.; Mayer, M.J.; Narbad, A.; Winter, S.E.; Pfeiffer, J.K. Bacteria Facilitate Enteric Virus Co-infection of Mammalian Cells and Promote Genetic Recombination. Cell Host Microbe 2018, 23, 77–88. [Google Scholar] [CrossRef] [PubMed]
- Folimonova, S.Y. Superinfection Exclusion Is an Active Virus-Controlled Function That Requires a Specific Viral Protein. J. Virol. 2012, 86, 5554–5561. [Google Scholar] [CrossRef]
- Bratt, M.A.; Rubin, H. Specific interference among strains of Newcastle disease virus: III. Mechanisms of interference. Virology 1968, 35, 395–407. [Google Scholar] [CrossRef]
- Huang, I.-C.; Li, W.; Sui, J.; Marasco, W.; Choe, H.; Farzan, M. Influenza A Virus Neuraminidase Limits Viral Superinfection. J. Virol. 2008, 82, 4834–4843. [Google Scholar] [CrossRef]
- Bergua, M.; Zwart, M.P.; El-Mohtar, C.; Shilts, T.; Elena, S.F.; Folimonova, S.Y. A viral protein mediates superinfection exclusion at the whole organism level while is not required for exclusion at the cellular level. J. Virol. 2014, 88, 11327–11338. [Google Scholar] [CrossRef]
- Adams, R.H.; Brown, D.T. BHK cells expressing Sindbis virus-induced homologous interference allow the translation of nonstructural genes of superinfecting virus. J. Virol. 1985, 54, 351–357. [Google Scholar] [CrossRef]
- Claus, C.; Tzeng, W.P.; Liebert, U.G.; Frey, T.K. Rubella virus-induced superinfection exclusion studied in cells with persisting replicons. J. Gen. Virol. 2007, 88, 2769–2773. [Google Scholar] [CrossRef] [PubMed]
- Tscherne, D.M.; Evans, M.J.; von Hahn, T.; Jones, C.T.; Stamataki, Z.; McKeating, J.; Rice, C.M. Superinfection exclusion in cells infected with hepatitis C virus. J. Virol. 2007, 81, 3693–3703. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.-M.; Tscherne, D.M.; Yun, S.-I.; Frolov, I.; Rice, C.M. Dual mechanisms of pestiviral superinfection exclusion at entry and RNA replication. J. Virol. 2005, 79, 3231–3242. [Google Scholar] [CrossRef]
- Zhou, X.; Sun, K.; Zhou, X.; Jackson, A.O.; Li, Z. The Matrix Protein of a Plant Rhabdovirus Mediates Superinfection Exclusion by Inhibiting Viral Transcription. J. Virol. 2019, 93, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Johnson, W.E. Origins and evolutionary consequences of ancient endogenous retroviruses. Nat. Rev. Microbiol. 2019, 17, 355–370. [Google Scholar] [CrossRef]
- Thackray, L.B.; Wobus, C.E.; Chachu, K.; Liu, B.; Alegre, E.R.; Henderson, K.S.; Virgin, H.W. Murine noroviruses comprising a single genogroup exhibit biological diversity despite limited sequence divergence. J. Virol. 2007, 81, 10460–10473. [Google Scholar] [CrossRef]
- Hyde, J.L.; Sosnovtsev, S.V.; Green, K.Y.; Wobus, C.; Virgin, H.W.; Mackenzie, J.M. Mouse norovirus replication is associated with virus-induced vesicle clusters originating from membranes derived from the secretory pathway. J. Virol. 2009, 83, 9709–9719. [Google Scholar] [CrossRef]
- Mauroy, A.; Poel, W.H.; Honing, R.H.-V.; Thys, C.; Thiry, E. Development and application of a SYBR green RT-PCR for first line screening and quantification of porcine sapovirus infection. BMC Vet. Res. 2012, 8, 193. [Google Scholar] [CrossRef]
- Zhang, H.; Cockrell, S.K.; Kolawole, A.O.; Rotem, A.; Serohijos, A.W.R.; Chang, C.B.; Pipas, J.M. Isolation and analysis of rare norovirus recombinants from co-infected mice using drop-based microfluidics. J. Virol. 2015, 89, 1137–1145. [Google Scholar] [CrossRef][Green Version]
- Mauroy, A.; Taminiau, B.; Nezer, C.; Ghurburrun, E.; Baurain, D.; Daube, G.; Thiry, E. High-throughput sequencing analysis reveals the genetic diversity of different regions of the murine norovirus genome during in vitro replication. Arch. Virol. 2017, 33, 1019–1023. [Google Scholar] [CrossRef]
- Stauffer Thompson, K.A.; Yin, J. Population dynamics of an RNA virus and its defective interfering particles in passage cultures. Virol. J. 2010, 7, 257. [Google Scholar] [CrossRef]
- Pathak, K.B.; Nagy, P.D. Defective interfering RNAs: Foes of viruses and friends of virologists. Viruses 2009, 1, 895–919. [Google Scholar] [CrossRef] [PubMed]
- Voinnet, O. Induction and suppression of RNA silencing: Insights from viral infections. Nat. Rev. Genet. 2005, 3, 206–220. [Google Scholar] [CrossRef] [PubMed]
- Webster, B.; Ott, M.; Greene, W.C. Evasion of superinfection exclusion and elimination of primary viral RNA by an adapted strain of hepatitis C virus. J. Virol. 2013, 87, 13354–13369. [Google Scholar] [CrossRef]
- Schaller, T.; Appel, N.; Koutsoudakis, G.; Kallis, S.; Lohmann, V.; Pietschmann, T.; Bartenschlager, R. Analysis of Hepatitis C Virus Superinfection Exclusion by Using Novel Fluorochrome Gene-Tagged Viral Genomes. J. Virol. 2007, 81, 4591–4603. [Google Scholar] [CrossRef] [PubMed]
- Zou, G.; Zhang, B.; Lim, P.-Y.; Yuan, Z.; Bernard, K.A.; Shi, P.-Y. Exclusion of West Nile Virus Superinfection through RNA Replication. J. Virol. 2009, 83, 11765–11776. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ludwig-Begall, L.F.; Di Felice, E.; Toffoli, B.; Ceci, C.; Di Martino, B.; Marsilio, F.; Mauroy, A.; Thiry, E. Analysis of Synchronous and Asynchronous In Vitro Infections with Homologous Murine Norovirus Strains Reveals Time-Dependent Viral Interference Effects. Viruses 2021, 13, 823. https://doi.org/10.3390/v13050823
Ludwig-Begall LF, Di Felice E, Toffoli B, Ceci C, Di Martino B, Marsilio F, Mauroy A, Thiry E. Analysis of Synchronous and Asynchronous In Vitro Infections with Homologous Murine Norovirus Strains Reveals Time-Dependent Viral Interference Effects. Viruses. 2021; 13(5):823. https://doi.org/10.3390/v13050823
Chicago/Turabian StyleLudwig-Begall, Louisa F., Elisabetta Di Felice, Barbara Toffoli, Chiara Ceci, Barbara Di Martino, Fulvio Marsilio, Axel Mauroy, and Etienne Thiry. 2021. "Analysis of Synchronous and Asynchronous In Vitro Infections with Homologous Murine Norovirus Strains Reveals Time-Dependent Viral Interference Effects" Viruses 13, no. 5: 823. https://doi.org/10.3390/v13050823
APA StyleLudwig-Begall, L. F., Di Felice, E., Toffoli, B., Ceci, C., Di Martino, B., Marsilio, F., Mauroy, A., & Thiry, E. (2021). Analysis of Synchronous and Asynchronous In Vitro Infections with Homologous Murine Norovirus Strains Reveals Time-Dependent Viral Interference Effects. Viruses, 13(5), 823. https://doi.org/10.3390/v13050823





