Abstract
The African swine fever virus (ASFV) is currently causing a pandemic affecting wild and domestic swine from Western Europe to Asia. No commercial vaccines are available to prevent African swine fever (ASF), resulting in overwhelming economic losses to the swine industry. We recently developed a recombinant vaccine candidate, ASFVG-ΔI177L, by deleting the I177L gene from the genome of the highly virulent ASFV strain Georgia (ASFV-G). ASFV-G-ΔI177L has been proven safe and highly efficacious in challenge studies using parental ASFV-G. Here, we present data demonstrating that ASFV-G-ΔI177L can be administered by the oronasal (ON) route to achieve a similar efficacy to that of intramuscular (IM) administration. Animals receiving ON ASFV-G-ΔI177L were completely protected against virulent ASFV-G challenge. As previously described, similar results were obtained when ASFV-G-ΔI177L was given intramuscularly. Interestingly, viremias induced in animals inoculated oronasally were lower than those measured in IM-inoculated animals. ASFV-specific antibody responses, mediated by IgG1, IgG2 and IgM, do not differ in animals inoculated by the ON route from that had IM inoculations. Therefore, the ASFV-G-ΔI177L vaccine candidate can be administered oronasally, a critical attribute for potential vaccination of wild swine populations.
1. Introduction
African swine fever virus (ASFV) is the causative agent of African swine fever (ASF), a devastating disease affecting domestic and wild pigs. ASF outbreaks are currently affecting Central and Eastern Europe and Asia, causing significant economic losses on a global scale [1]. ASFV is a structurally complex enveloped virus containing a large (180–190 kilobase pairs) double-stranded DNA genome encoding for over 150 open reading frames (ORFs) [1]. Dissemination and maintenance of the virus in susceptible wild swine populations makes management of a disease outbreak difficult. Because no commercial vaccine is available, outbreak management relies on restricting animal movements and culling infected herds [1]. Live attenuated viruses, developed by genetic manipulation of virulent strains, have been shown experimentally to be effective vaccines [2,3,4,5,6,7,8,9,10]. Most of these experimental vaccines are administered by the parenteral route. Administering ASFV vaccines by the oral route would allow for their use in wild animals, facilitating the regional control or eradication of the disease.
Recently we reported the rational development of a live attenuated vaccine candidate, ASFV-G-ΔI177L, obtained by deletion of the I177L gene from the genome of ASFV-G [6]. ASFV-G-ΔI177L was shown to be safe when parenterally inoculated at high doses and highly efficacious in inducing protection against challenge with the parental, highly virulent ASFV-G when administered in a relatively low dose.
Here, we demonstrate that oronasal (ON) administration of ASFV-G-ΔI177L achieves similar efficacy as an intramuscular (IM) injection. ON-inoculated animals were protected against challenge with the virulent parental ASFV-G. Efficacy of protection as well as virus-specific antibody responses do not differ in animals inoculated with ASFV-G-ΔI177L by either the ON or IM route
2. Materials and Methods
2.1. Cell Culture and Viruses
Primary cultures of swine macrophages were prepared from swine blood, following procedures previously described. Preparation of macrophage cultures in 96-well plates for virus titration was also performed as previously described [11].
Development of the live attenuated vaccine candidate strain ASFV-G-ΔI177L has been previously described [6]. Briefly, partial deletion of the I177L gene in the genome of highly virulent isolate Georgia (ASFV-G) was replaced by mCherry under the ASFV p72 promoter.
Virus titration was performed on primary swine macrophage cell cultures in 96-well plates. Virus dilutions and cultures were performed using macrophage medium. The presence of virus was assessed by hemadsorption (HA) [6], and virus titers were calculated by the Reed and Muench method [12].
ASFV-G used in the animal challenge experiments is a field isolate kindly provided by Nino Vepkhvadze from the Laboratory of the Ministry of Agriculture (LMA) in Tbilisi, Republic of Georgia.
2.2. Animal Experiments
All animal experiments were done in a BSL-3ag facility at Plum Island Animal Disease Center (Greenport, NY, USA). Protective efficacy of ASFV-G-∆I177L was assessed using 80- to 90-pound commercial breed swine. Groups of crossbreed Yorkshire pigs (n = 5) were inoculated either intramuscularly with 102 HAD50 or oronasally with 2 × 106 HAD50 (106 HAD50 instilled in the rear of the nasal cavity and 106 HAD50 at the base of the tongue) of ASFV-G-∆I177L. A sixth animal (sentinel) was not inoculated and cohabitated for 28 days with the inoculated animals. Sentinel animals were removed at the time of challenge. A mock-inoculated group (n = 5) was included as a control. Clinical signs (anorexia, depression, fever, purple skin discoloration, staggering gait, diarrhea, and cough) and changes in body temperature were recorded daily throughout the experiment. Animals inoculated with ASFV-G-ΔI177L were given an IM challenge 28 days later with 102 HAD50 of the parental virulent ASFV-G strain. Clinical signs associated with the disease were recorded as described earlier [6].
2.3. Detection of Anti-ASFV Antibodies
The presence of virus-specific antibodies in the sera of the inoculated pigs was evaluated using an in-house indirect ELISA, which is described elsewhere [13]. The virus antigen was produced from Vero cells infected with a Vero-adapted ASFV strain. ELISA plates (MaxiSorp, Nunc, St. Louis, MO, USA) were coated with infected or uninfected cell extract (1 μg per well). The plates were blocked with 10% skim milk (Merck, Kenilworth, NJ, USA) in phosphate-buffered saline and 5% normal goat serum (Sigma, St. Louis, MO, USA). Each swine serum was tested at multiple dilutions against both infected and uninfected cell antigens. ASFV-specific antibodies in the swine sera were detected by an anti-swine IgM-, IgG-, IgG1- or IgG2-horseradish peroxidase conjugate (KPL, Gaithersburg, MD, USA) and SureBlue Reserve peroxidase substrate (KPL). Plates were read at an optical density of 630 nm (OD630) in an ELx808 plate reader (BioTek, Shoreline, WA, USA). Serum titers were expressed as the log10 of the highest dilution, where the OD630 reading of the tested sera at least duplicates the reading of the mock-infected sera.
3. Results
3.1. Replication of ASFV-G-ΔI177L in Animals Inoculated by Either IM or ON Route
We first evaluated the ability of ASFV-G-ΔI177L to replicate systemically in swine after ON administration and compared that with replication after IM inoculation. No ASF clinical signs were observed in any of the animals in either group, with animals remaining clinically normal until the day of the challenge, at 28 days post-infection (dpi) (Figure 1). Experimentally, ON inoculation of a high dose of ASFV-G-ΔI177L did not result in the appearance of clinical signs of ASF.
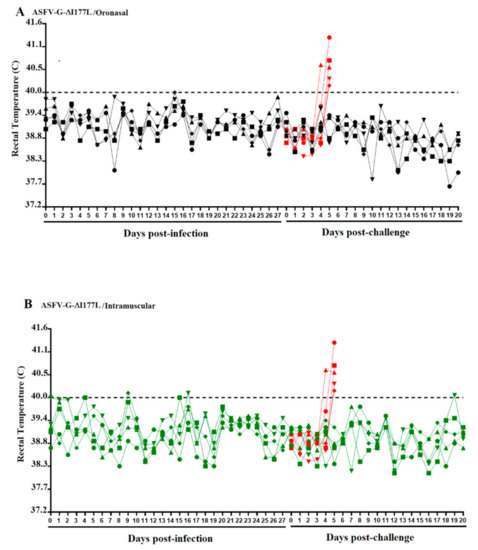
Figure 1.
Kinetics of body temperature values in pigs oronasally (A) or intramuscularly (B) inoculated with ASFV-G-ΔI177L before (Days post-infection) and after challenge (Days post-challenge) with ASFV-G. Each curve represents individual data from each of the animals under each of the treatments. Red curves represent a group of mock-inoculated animals used as a control during both challenge experiments.
Viremia, measured by assessing systemic virus replication, indicated that animals with IM ASFV-G-ΔI177L inoculation had high virus titers beginning at 7 dpi, with titers of 104–107 HAD50/mL peaking between 11 and 14 dpi (Figure 2). As previously observed [6], viremias in these animals evolved heterogeneously with two animals reaching challenge day (28 dpi) with relatively high titers (approximately 103 HAD50/mL) while the other three had undetectable viremias (<101.8 HAD50/mL) at the same time point. Conversely, viremias in animals that had an ON ASFV-G-ΔI177L inoculation remained low throughout the experimental period, with peak titers between 102 to 104 HAD50/mL at 4–7 dpi, then remaining at <103 HAD50/mL throughout the rest of the pre-challenge period with undetectable titers in 4 out 5 animals on challenge day. Based on this data, ON administration of ASFV-G-ΔI177L affected systemic virus replication, since viremias were lower than those measured after IM inoculation, despite the 10,000-fold higher ON inoculation dose. Sentinel animals cohabitating with IM- or ON-inoculated animals remained clinically normal during the 28-day observational period. In addition, no virus was detected in any of the samples from sentinels (all sampled blood time points as well as tonsil and spleen samples obtained at 28 dpi), indicating that transmission of ASFV-G-ΔI177L did not occur among animals even in those inoculated by the ON route. These results indicate that ASFV-G-ΔI177L-inoculated animals, regardless of the route of administration, may not shed enough virus to infect naive pigs during 28 days of cohabitation.
Figure 2.
Viremia titers in pigs either oronasally or intramuscularly inoculated with ASFV-G-ΔI177L before and after challenge with ASFV-G (A,B). Each curve represents individual data from each of the animals under each of the treatments. Red curves represent mock-inoculated animals used as controls during the challenge. The sensitivity of virus detection was ≥1.8 log10 HAD50/mL.
3.2. Assessment of Protective Efficacy of ASFV-G-ΔI177L Administered by the ON Route
In previous studies, protection induced by live attenuated viruses was dependent on the ability of the virus to replicate after inoculation [2,3,4,5,6,7,8,14,15,16]. The low viremia titers measured after ON administration of ASFV-G-ΔI177L may correlate with decreased protection after challenge with the parental virulent ASFV-G. Animals that had either an ON or IM inoculation with ASFV-G-ΔI177L were given an IM challenged at 28 days post-infection with 102 HAD50 of ASFV-G. An additional group of five naive animals were challenged as a mock-inoculated control group.
As expected, all control animals displayed ASF-related signs beginning at 5 days post-challenge (dpc), with an increased severity in clinical signs until euthanasia at 6 dpc (Table 1 and Figure 3). Animals inoculated with ASFV-G-ΔI177L, regardless of the route of administration, remained clinically normal and showed no signs of disease during the 21-day observational period, thus demonstrating that ASFV-G-ΔI177L offered protection against disease when challenged by the highly virulent parental virus.

Table 1.
Swine survival and fever response in animals inoculated either intramuscularly or oronasally with ASFV-G-ΔI177L and challenged at 28 dpi with 102 HAD50 of virulent ASFV-G.
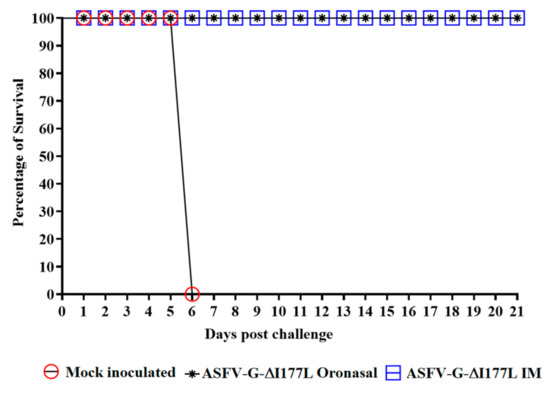
Figure 3.
Evolution of mortality in pigs either oronasally or intramuscularly inoculated with ASFV-G-ΔI177L or mock treated and challenged with 102 HAD50 of ASFV-G.
Viremia values from mock-inoculated animals infected with ASFV-G were as expected, with high titers (105 to 108.5 HAD50/mL) on day 4 pi, increasing (averaging HAD50/mL) until day 6 pi, when all animals were euthanized. Conversely, viremia values after challenge in animals intramuscularly inoculated with ASFV-G-ΔI177L progressively decreased in the two animals with high titers at the time of challenge until the end of the experimental period (21 days after challenge), at which time no virus could be detected in one animal, and very low titers (103.5 HAD50/mL) were measured in the other (Figure 2). Two of the three animals with undetectable viremia at challenge remained with undetectable titers until the end of the experiment (21 days post challenge - dpc) (Figure 2), while another animal experienced intermittent viremia peaks after challenge with undetectable titers by 21 dpc. After challenge, the group of animals oronasally inoculated with ASFV-G-ΔI177L presented low viremia titers (≤102.8 HAD50/mL) throughout the 21-day observational period or had undetectable viremias on the final day of the experiment (Figure 2).
These results indicate that both IM and ON administration of ASFV-G-ΔI177L effectively induces protection against challenge with the virulent parental ASFV-G.
3.3. Antibody Response in Animals ON Inoculated with ASFV-G-ΔI177L
Based on results obtained with different attenuated ASFV strains produced in our laboratory, the major immunological parameter consistently associated with protection against disease is the presence of virus-specific circulating antibodies in immunized animals [13]. We evaluated the ability of oronasally administered ASFV-G-ΔI177L to induce a circulating ASFV-specific antibody response and compared that with the response elicited in animals inoculated intramuscularly with ASFV-G-ΔI177L. Serum antibodies were detected using an in-house developed ELISA adapted to quantify antibody response mediated by different immunoglobulin isotypes [6]. The virus-specific IgM-mediated antibody response was detectable at 7 dpi in animals that had an ON inoculation and maintained relatively high titers (102–103) until 14 dpi. The IgM response in IM-inoculated animals appeared delayed and lower compared to that of the ON-inoculated animals (Figure 4A). An IgG-mediated ASFV-specific antibody response was detected by 11 dpi, increasing until 21 dpi. Maximum titers (104–105) were observed by the day of challenge (28 dpi). No differences were observed in the IgG titers of animals inoculated by either the ON or IM route (Figure 4B). Analysis of the virus-specific IgG1- and IgG2-mediated antibody response demonstrated that both isotypes equally contributed to the response, having similar kinetics as that described for IgG (Figure 4C,D). The ASFV-specific antibody response mediated by IgG1 or IgG2 observed in animals inoculated by the ON or the IM route did not differ. Therefore, no major differences were found in the antibody response to ASFV in animals inoculated by either route.
Figure 4.
Anti-ASFV antibody titers detected by ELISA in pigs oronasally (ON) or intramuscularly (IM) inoculated with ASFV-G-ΔI177L. Individual serum ASFV-specific antibody titers (indicated by individual symbols) mediated by IgM, total IgG, IgG1 and IgG2 are represented in panels (A), (B), (C) and (D), respectively. Titers are expressed as the log10 of the reciprocal of the highest dilution of sera at least duplicating OD readings of a pool of mock-infected sera.
4. Discussion
The only efficacious experimental vaccine candidates for the ASFV strain responsible for the current pandemic are live attenuated strains developed by deleting specific virulence-associated genes in the genome of the virulent strain [3,4,5,6,7,15]. Among the different attenuated strains developed in our laboratory [3,4,5,6], the safety and efficacy of ASFV-G-ΔI177L make this experimental vaccine a leading candidate over other live attenuated strains for its commercial potential [6].
An important factor when considering a vaccination program for ASFV is the involvement of wild swine, which has been a constant factor in epidemiological ASF scenarios in both endemic and disease-free areas affected by a disease outbreak [17]. The elimination of infected wild swine constitutes an important component in the control and eradication of ASFV from an affected region. The immunization of wild swine requires oral vaccine administration. To our knowledge, few reports have studied non-parenteral administration of ASF vaccines.
From the sparse reports detailing oral immunization studies, several observations indicate that an oral vaccination is a reasonable approach to protecting wild boar populations against ASF. It has been reported that intranasal (IN) administration of the naturally attenuated strain OURT88/3 provided complete protection against challenge with the virulent homologous isolate OURT88/1, while in this case IM inoculation resulted in less protection [18]. The oral administration of a non-hemadsorbing, naturally attenuated ASFV isolate, Lv17/WB/Rie1, induced protection in wild boars against challenge with the virulent homologous ASF virus isolate Arm07 [19]. A recombinant BeninΔDP148R virus, developed by deleting the DP148R gene from a virulent Benin 97/1 isolate, induced protection against virulent parental virus challenge when immunized by either the IM or the IN route [8]. These studies suggest it is possible to administer ASFV experimental vaccines orally or nasally to induce protection. This effect is likely due to the initial replication of the vaccine virus in the local mucosal entry site, which stimulates the induction of a systemic immune response that protects the animal against subsequent infection. Prior to this study we did not have any previous experience delivering ASFV-G-ΔI177L in the oral or nasal cavity. In this initial study, we decided to administer the vaccine candidate by combining both routes to increase the chances of successful vaccination.
In this study, we reported that the oronasal administration of ASFV-G-ΔI177L induced protection against challenge by the virulent parental virus ASFV-G and was comparable to that induced by parenteral inoculation of ASFV-G-ΔI177L. Although oronasally administered ASFV-G-ΔI177L replicated less efficiently when parenterally inoculated, it still induced a systemic antibody response that protected the animal against an ASFV-G challenge. These results suggest that the ON administration of ASFV-G-ΔI177L could be a viable delivery method for vaccines.
Author Contributions
Conceptualization, M.V.B., C.G.G. and D.P.G.; Data curation, E.R.-M., E.S., A.R. and S.P.; Formal analysis, M.V.B., E.R.-M., E.S., E.V., A.R., C.G.G., N.E., L.V.-S. and D.P.G.; Funding acquisition, M.V.B. and D.P.G.; Project administration, M.V.B., and D.P.G.; Writing—original draft, M.V.B., L.V.-S. and D.P.G.; Writing—review & editing, M.V.B., E.R.-M., E.S., E.V., S.P., C.G.G., N.E., L.V.-S. and D.P.G., All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Animal experiments to collect blood for swine macrophages were performed under biosafety level 3AG conditions in the animal facilities at the Plum Island Animal Disease Center (PIADC). All experimental procedures were carried out in compliance with the Animal Welfare Act (AWA), the 2011 Guide for Care and Use of Laboratory Animals, the 2002 PHS Policy for the Humane Care and Use of Laboratory Animals, U.S. Government Principles for Utilization and Care of Vertebrate Animals Used in Testing, Research and Training (IRAC 1985), as well as specific animal protocols reviewed and approved by the PIADC Institutional Animal Care and Use Committee of the U.S. Departments of Agriculture and Homeland Security (protocol numbers 205.03-17-R, 205.03-19-R and 225.02-19-R approved on 28 September 2017, 10 September 2019 and 10 September 2019, respectively).
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
We thank the Plum Island Animal Disease Center Animal Care Unit staff for their excellent technical assistance. We particularly thank Melanie V. Prarat for editing the manuscript. This research was supported in part by an appointment to the Plum Island Animal Disease Center (PIADC) Research Participation Program administered by the Oak Ridge Institute for Science and Education (ORISE) through an interagency agreement between the U.S. Department of Energy (DOE) and the U.S. Department of Agriculture (USDA). ORISE is managed by ORAU under DOE contract DE-SC0014664. All opinions expressed in this paper are those of the authors and do not necessarily reflect the policies and views of the USDA, ARS, APHIS, DHS, DOE, or ORAU/ORISE.
Conflicts of Interest
The authors Douglas Gladue and Manuel Borca have a patent filed for ASFV-G-ΔI177L as an effective vaccine against ASF. No other authors have a conflict of interest.
References
- Costard, S.; Wieland, B.; de Glanville, W.; Jori, F.; Rowlands, R.; Vosloo, W.; Roger, F.; Pfeiffer, D.U.; Dixon, L.K. African swine fever: How can global spread be prevented? Philos. Trans. R. Soc. Lond. Ser. B Biol. Sci. 2009, 364, 2683–2696. [Google Scholar] [CrossRef] [PubMed]
- Lewis, T.; Zsak, L.; Burrage, T.G.; Lu, Z.; Kutish, G.F.; Neilan, J.G.; Rock, D.L. An African swine fever virus ERV1-ALR homologue, 9GL, affects virion maturation and viral growth in macrophages and viral virulence in swine. J. Virol. 2000, 74, 1275–1285. [Google Scholar] [CrossRef] [PubMed]
- O’Donnell, V.; Holinka, L.G.; Gladue, D.P.; Sanford, B.; Krug, P.W.; Lu, X.; Arzt, J.; Reese, B.; Carrillo, C.; Risatti, G.R.; et al. African Swine Fever Virus Georgia Isolate Harboring Deletions of MGF360 and MGF505 Genes Is Attenuated in Swine and Confers Protection against Challenge with Virulent Parental Virus. J. Virol. 2015, 89, 6048–6056. [Google Scholar] [CrossRef] [PubMed]
- O’Donnell, V.; Holinka, L.G.; Krug, P.W.; Gladue, D.P.; Carlson, J.; Sanford, B.; Alfano, M.; Kramer, E.; Lu, Z.; Arzt, J.; et al. African swine fever virus Georgia 2007 with a deletion of virulence-associated gene 9GL (B119L), when administered at low doses, leads to virus attenuation in swine and induces an effective protection against homologous challenge. J. Virol. 2015, 89, 8556–8566. [Google Scholar] [CrossRef] [PubMed]
- O’Donnell, V.; Risatti, G.R.; Holinka, L.G.; Krug, P.W.; Carlson, J.; Velazquez-Salinas, L.; Azzinaro, P.A.; Gladue, D.P.; Borca, M.V. Simultaneous deletion of the 9GL and UK genes from the African swine fever virus Georgia 2007 isolate offers increased safety and protection against homologous challenge. J. Virol. 2017, 91. [Google Scholar] [CrossRef] [PubMed]
- Borca, M.V.; Ramirez-Medina, E.; Silva, E.; Vuono, E.; Rai, A.; Pruitt, S.; Holinka, L.G.; Velazquez-Salinas, L.; Zhu, J.; Gladue, D.P. Development of a Highly Effective African Swine Fever Virus Vaccine by Deletion of the I177L Gene Results in Sterile Immunity against the Current Epidemic Eurasia Strain. J. Virol. 2020, 94. [Google Scholar] [CrossRef] [PubMed]
- Monteagudo, P.L.; Lacasta, A.; Lopez, E.; Bosch, L.; Collado, J.; Pina-Pedrero, S.; Correa-Fiz, F.; Accensi, F.; Navas, M.J.; Vidal, E.; et al. BA71DeltaCD2: A New Recombinant Live Attenuated African Swine Fever Virus with Cross-Protective Capabilities. J. Virol. 2017, 91. [Google Scholar] [CrossRef]
- Reis, A.L.; Goatley, L.C.; Jabbar, T.; Sanchez-Cordon, P.J.; Netherton, C.L.; Chapman, D.A.G.; Dixon, L.K. Deletion of the African Swine Fever Virus Gene DP148R Does Not Reduce Virus Replication in Culture but Reduces Virus Virulence in Pigs and Induces High Levels of Protection against Challenge. J. Virol. 2017, 91. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Cordon, P.J.; Jabbar, T.; Berrezaie, M.; Chapman, D.; Reis, A.; Sastre, P.; Rueda, P.; Goatley, L.; Dixon, L.K. Evaluation of protection induced by immunisation of domestic pigs with deletion mutant African swine fever virus BeninDeltaMGF by different doses and routes. Vaccine 2018, 36, 707–715. [Google Scholar] [CrossRef]
- Moore, D.M.; Zsak, L.; Neilan, J.G.; Lu, Z.; Rock, D.L. The African swine fever virus thymidine kinase gene is required for efficient replication in swine macrophages and for virulence in swine. J. Virol. 1998, 72, 10310–10315. [Google Scholar] [CrossRef] [PubMed]
- Borca, M.V.; Berggren, K.A.; Ramirez-Medina, E.; Vuono, E.A.; Gladue, D.P. CRISPR/Cas Gene Editing of a Large DNA Virus: African Swine Fever Virus. BioProtocol 2018, 8. [Google Scholar] [CrossRef]
- Reed, L.J.; Muench, H. A simple method of estimating fifty percent endpoints. Am. J. Hyg. 1938, 27, 493–497. [Google Scholar]
- Carlson, J.; O’Donnell, V.; Alfano, M.; Velazquez Salinas, L.; Holinka, L.G.; Krug, P.W.; Gladue, D.P.; Higgs, S.; Borca, M.V. Association of the Host Immune Response with Protection Using a Live Attenuated African Swine Fever Virus Model. Viruses 2016, 8, 291. [Google Scholar] [CrossRef] [PubMed]
- O’Donnell, V.; Holinka, L.G.; Sanford, B.; Krug, P.W.; Carlson, J.; Pacheco, J.M.; Reese, B.; Risatti, G.R.; Gladue, D.P.; Borca, M.V. African swine fever virus Georgia isolate harboring deletions of 9GL and MGF360/505 genes is highly attenuated in swine but does not confer protection against parental virus challenge. Virus Res. 2016. [Google Scholar] [CrossRef]
- Chen, W.; Zhao, D.; He, X.; Liu, R.; Wang, Z.; Zhang, X.; Li, F.; Shan, D.; Chen, H.; Zhang, J.; et al. A seven-gene-deleted African swine fever virus is safe and effective as a live attenuated vaccine in pigs. Sci. China Life Sci. 2020, 63, 623–634. [Google Scholar] [CrossRef]
- Ramirez-Medina, E.; Vuono, E.; O’Donnell, V.; Holinka, L.G.; Silva, E.; Rai, A.; Pruitt, S.; Carrillo, C.; Gladue, D.P.; Borca, M.V. Differential Effect of the Deletion of African Swine Fever Virus Virulence-Associated Genes in the Induction of Attenuation of the Highly Virulent Georgia Strain. Viruses 2019, 11, 599. [Google Scholar] [CrossRef] [PubMed]
- European Food Safety Authority; Nielsen, S.S.; Alvarez, J.; Bicout, D.J.; Calistri, P.; Depner, K.; Drewe, J.A.; Garin-Bastuji, B.; Gonzales Rojas, J.L.; Gortazar Schmidt, C.; et al. ASF Exit Strategy: Providing cumulative evidence of the absence of African swine fever virus circulation in wild boar populations using standard surveillance measures. EFSA J. 2021, 19, e06419. [Google Scholar] [CrossRef]
- Sanchez-Cordon, P.J.; Chapman, D.; Jabbar, T.; Reis, A.L.; Goatley, L.; Netherton, C.L.; Taylor, G.; Montoya, M.; Dixon, L. Different routes and doses influence protection in pigs immunised with the naturally attenuated African swine fever virus isolate OURT88/3. Antivir. Res. 2017, 138, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Barasona, J.A.; Gallardo, C.; Cadenas-Fernandez, E.; Jurado, C.; Rivera, B.; Rodriguez-Bertos, A.; Arias, M.; Sanchez-Vizcaino, J.M. First Oral Vaccination of Eurasian Wild Boar Against African Swine Fever Virus Genotype II. Front. Vet. Sci. 2019, 6, 137. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).