Superiority of MALDI-TOF Mass Spectrometry over Real-Time PCR for SARS-CoV-2 RNA Detection
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Collecton, Storage and Workflow
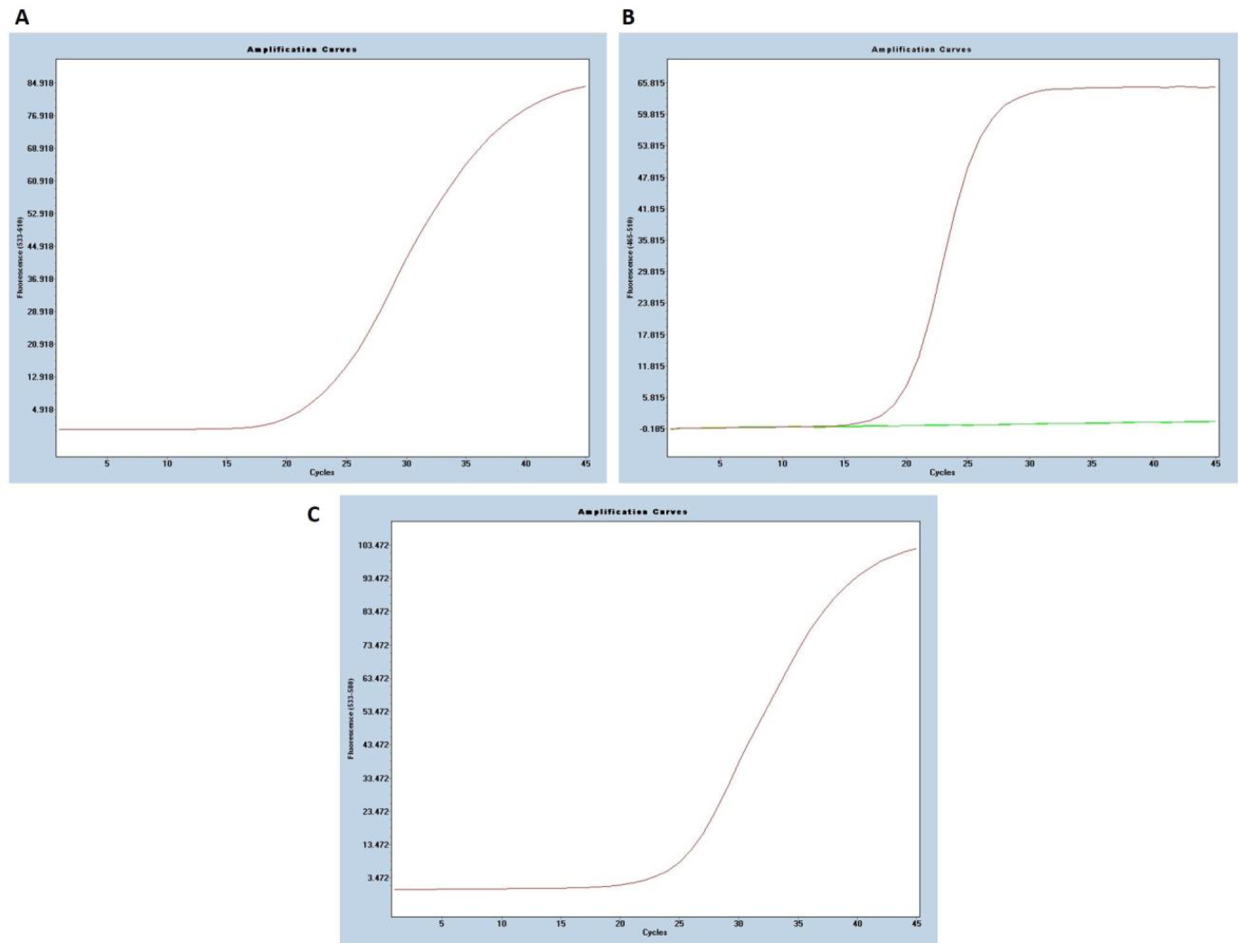
2.2. Real–Time PCR
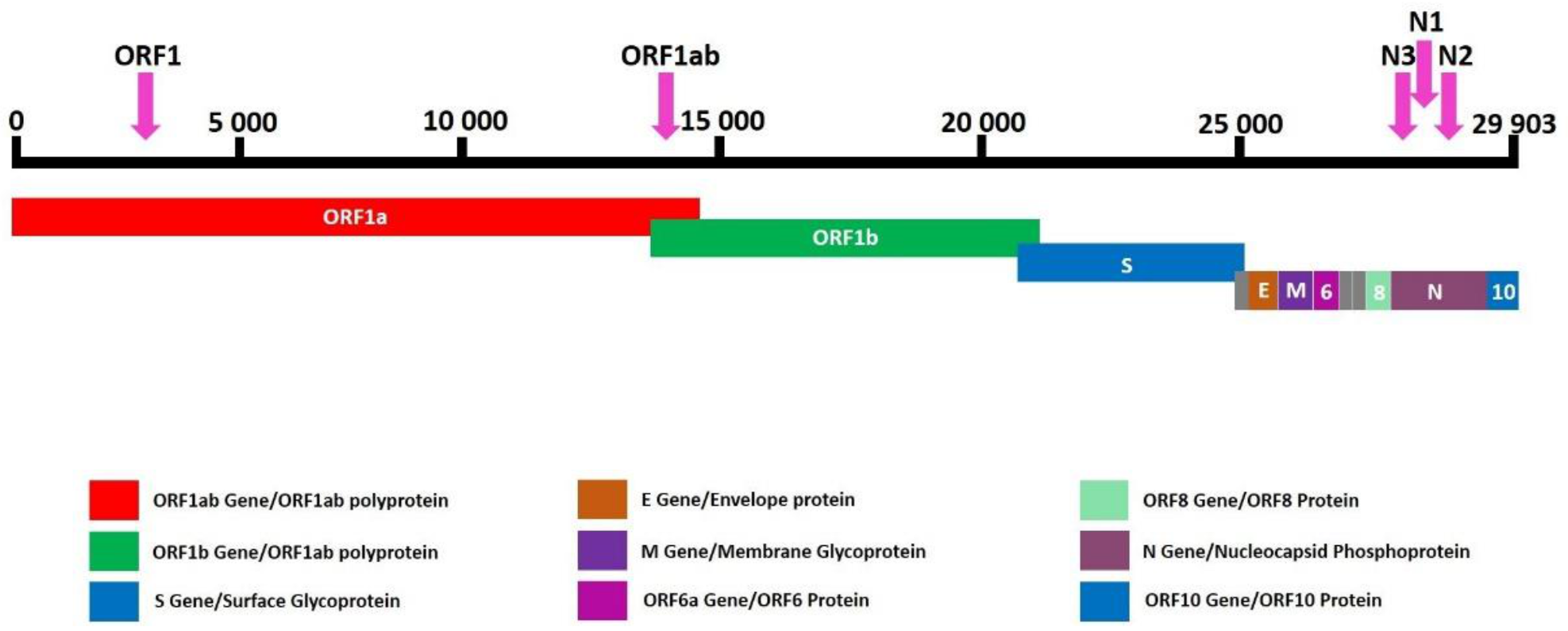
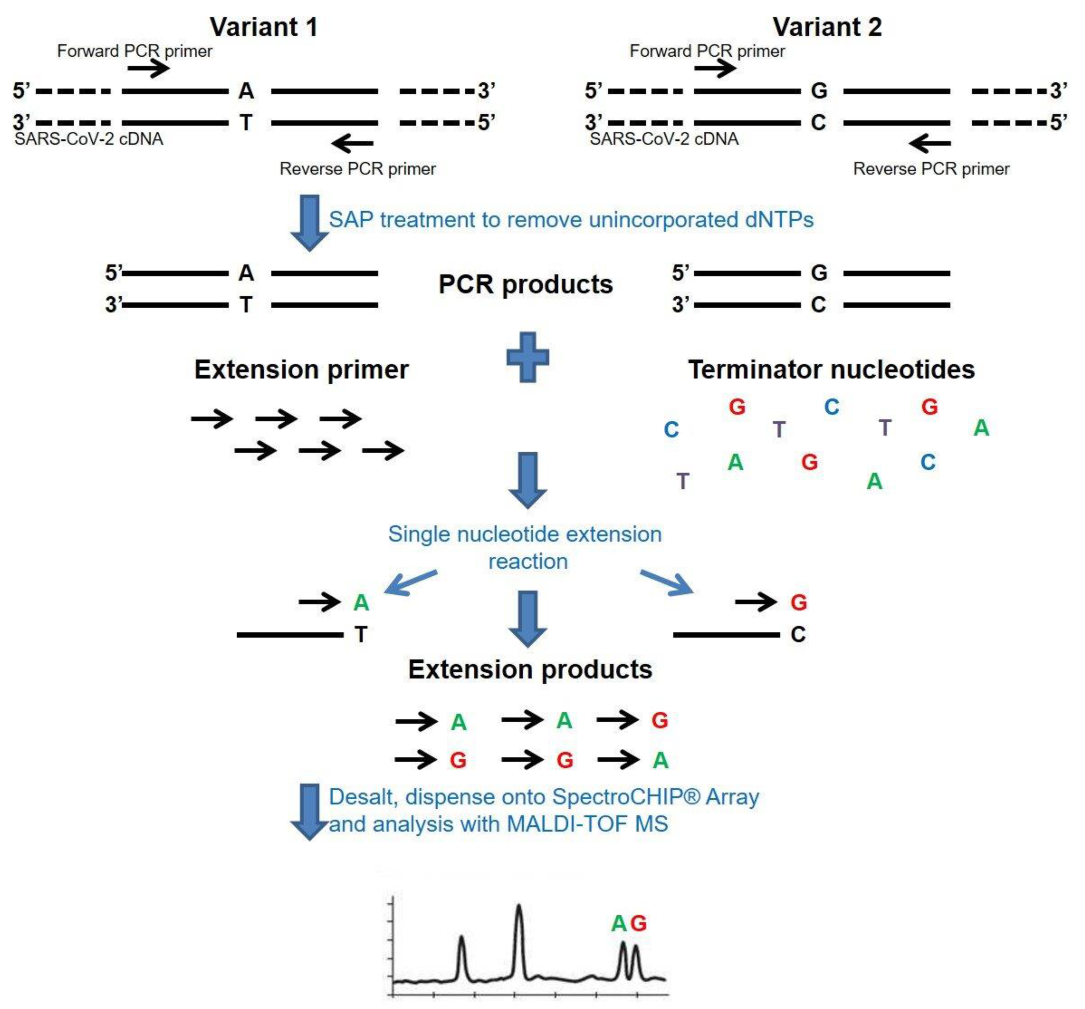
2.3. Mass Spectrometry-Based Assay
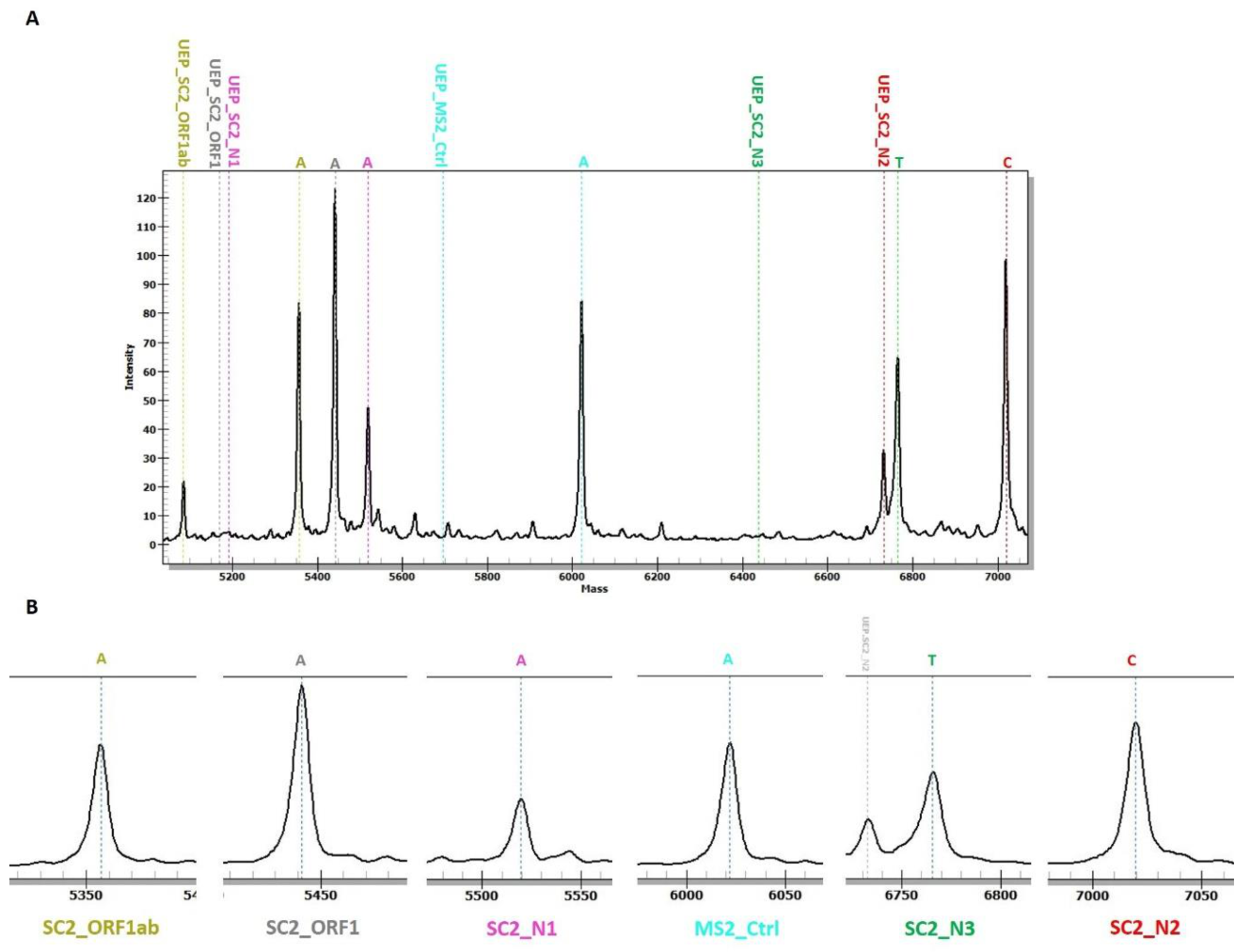
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Coronavirus Update (Live): 92,895,303 Cases and 1,989,457 Deaths from COVID-19 Virus Pandemic—Worldometer. Available online: https://www.worldometers.info/coronavirus (accessed on 14 January 2021).
- Bartoli, A.; Gabrielli, F.; Alicandro, T.; Nascimbeni, F.; Andreone, P. COVID-19 treatment options: A difficult journey between failed attempts and experimental drugs. Intern. Emerg. Med. 2021, 16, 281–308. [Google Scholar] [CrossRef] [PubMed]
- Mukhopadhyay, C.D.; Sharma, P.; Sinha, K.; Rajarshi, K. Recent trends in analytical and digital techniques for the detection of the SARS-Cov-2. Biophys. Chem. 2021, 270, 106538. [Google Scholar] [CrossRef] [PubMed]
- Kanji, J.N.; Zelyas, N.; MacDonald, C.; Pabbaraju, K.; Khan, M.N.; Prasad, A.; Hu, J.; Diggle, M.; Berenger, B.M.; Tipples, G. False negative rate of COVID-19 PCR testing: A discordant testing analysis. Virol. J. 2021, 18, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Arevalo-Rodriguez, I.; Buitrago-Garcia, D.; Simancas-Racines, D.; Zambrano-Achig, P.; Del Campo, R.; Ciapponi, A.; Sued, O.; Martinez-Garcia, L.; Rutjes, A.W.; Low, N.; et al. False-negative results of initial RT-PCR assays for COVID-19: A systematic review. PLoS ONE 2020, 15, e0242958. [Google Scholar] [CrossRef] [PubMed]
- Carter, L.J.; Garner, L.V.; Smoot, J.W.; Li, Y.; Zhou, Q.; Saveson, C.J.; Sasso, J.M.; Gregg, A.C.; Soares, D.J.; Beskid, T.R.; et al. Assay Techniques and Test Development for COVID-19 Diagnosis. ACS Cent. Sci. 2020, 6, 591–605. [Google Scholar] [CrossRef] [PubMed]
- Basu, P.; Chandna, P.; Bamezai, R.N.K.; Siddiqi, M.; Saranath, D.; Lear, A.; Ratnam, S. MassARRAY spectrometry is more sensitive than PreTect HPV-Proofer and consensus PCR for type-specific detection of high-risk oncogenic human papillomavirus genotypes in cervical cancer. J. Clin. Microbiol. 2011, 49, 3537–3544. [Google Scholar] [CrossRef] [PubMed]
- Rybicka, M.; Stalke, P.; Dreczewski, M.; Smiatacz, T.; Bielawski, K.P. High-Throughput Matrix-Assisted Laser Desorption Ionization-Time of Flight Mass Spectrometry as an Alternative Approach to Monitoring Drug Resistance of Hepatitis B Virus. J. Clin. Microbiol. 2014, 52, 9–14. [Google Scholar] [CrossRef] [PubMed]
- Xiu, L.; Zhang, C.; Wu, Z.; Peng, J. Establishment and application of a universal coronavirus screening method using MALDI-TOF mass spectrometry. Front. Microbiol. 2017, 8, 1510. [Google Scholar] [CrossRef] [PubMed]
- Zeng, L.; Li, D.; Tong, W.; Shi, T.; Ning, B. Biochemical features and mutations of key proteins in SARS-CoV-2 and their impacts on RNA therapeutics. Biochem. Pharmacol. 2021, 114424. [Google Scholar] [CrossRef] [PubMed]
- Greaney, A.J.; Starr, T.N.; Gilchuk, P.; Zost, S.J.; Binshtein, E.; Loes, A.N.; Hilton, S.K.; Huddleston, J.; Eguia, R.; Crawford, K.H.; et al. Complete Mapping of Mutations to the SARS-CoV-2 Spike Receptor-Binding Domain that Escape Antibody Recognition. Cell Host Microbe 2021, 29, 44–57. [Google Scholar] [CrossRef] [PubMed]
- van Kasteren, P.B.; van Der Veer, B.; van den Brink, S.; Wijsman, L.; de Jonge, J.; van den Brandt, A.; Molenkamp, R.; Reusken, C.B.; Meijer, A. Comparison of seven commercial RT-PCR diagnostic kits for COVID-19. J. Clin. Virol. 2020, 128, 104412. [Google Scholar] [CrossRef]
- Lu, Y.; Li, L.; Ren, S.; Liu, X.; Zhang, L.; Li, W.; Yu, H. Comparison of the diagnostic efficacy between two PCR test kits for SARS-CoV-2 nucleic acid detection. J. Clin. Lab. Anal. 2020, 34, e23554. [Google Scholar] [CrossRef]
- Rahbari, R.; Moradi, N.; Abdi, M. rRT-PCR for SARS-CoV-2: Analytical considerations. Clin. Chim. Acta 2021, 516, 1–7. [Google Scholar] [CrossRef]
- Zhou, R.; Li, F.; Chen, F.; Liu, H.; Zheng, J.; Lei, C.; Wu, X. Viral dynamics in asymptomatic patients with COVID-19. Int. J. Infect. Dis. 2020, 96, 288–290. [Google Scholar] [CrossRef] [PubMed]

| Reagent | Per Reaction (µL) |
|---|---|
| RT-PCR | |
| 10×PCR Buffer | 0.500 |
| MgCl2, 25mM | 0.400 |
| dNTP Mix, 25mM | 0.100 |
| UNG | 0.050 |
| RNase Inhibitor | 0.125 |
| PCR Enzyme | 0.200 |
| MMLV Enzyme | 0.125 |
| SARS-CoV-2 PCR Primers | 0.500 |
| Sample RNA | 3 |
| SAP Cocktail | |
| HPLC-grade water | 1.53 |
| SAP Buffer | 0.17 |
| Shrimp Alkaline Phosphatase (SAP) | 0.3 |
| Extension Cocktail | |
| HPLC-grade water | 0.62 |
| iPLEX Buffer Plus, GPR | 0.20 |
| iPLEX Termination Mix | 0.20 |
| iPLEX Pro Enzyme | 0.04 |
| SARS-CoV-2 Extend Primers | 0.94 |
| SARS-CoV-2 Targets | MS2 | QC Status | Judgement |
|---|---|---|---|
| ≥2 SARS-CoV-2 target detected | Detected | Passed | Positive |
| <2 SARS-CoV-2 target detected | Detected | Passed | Suspected |
| 0–5 SARS-CoV-2 target detected | Not detected | Failed | Invalid |
| Sample No | Official Report by RT-PCR a | Results of RT-PCR b | Results of MassARRAY®,c | MassARRAY® Judgment | |||||
|---|---|---|---|---|---|---|---|---|---|
| ORF1ab | N | ORF1ab | ORF1 | N1 | N2 | N3 | |||
| 1 | S | 35.92 | ND | ND | P | P | P | P | P |
| 2 | S | ND | 38.37 | ND | P | P | ND | P | P |
| 3 | S | ND | ND | ND | P | ND | ND | ND | S |
| 4 | S | ND | ND | P | ND | ND | P | ND | P |
| 5 | S | ND | 36.24 | ND | P | P | ND | P | P |
| 6 | S | ND | 36.85 | ND | P | P | P | P | P |
| 7 | S | ND | 34.26 | P | ND | ND | P | ND | P |
| 8 | S | ND | 37.20 | ND | P | P | ND | P | P |
| 9 | S | ND | ND | ND | P | ND | ND | ND | S |
| 10 | S | ND | 35.96 | ND | ND | P | P | ND | P |
| 11 | S | 34.72 | ND | P | P | P | ND | P | P |
| 12 | S | 36.20 | ND | P | P | P | P | P | P |
| 13 | S | 35.44 | ND | ND | ND | P | P | P | P |
| 14 | S | ND | 35.35 | P | P | P | P | P | P |
| 15 | S | ND | 35.48 | P | P | P | P | P | P |
| 16 | N | ND | ND | ND | ND | ND | ND | ND | N |
| 17 | N | ND | ND | ND | P | ND | ND | ND | S |
| 18 | N | ND | ND | ND | ND | ND | ND | ND | N |
| 19 | N | ND | ND | ND | ND | ND | ND | ND | N |
| 20 | N | ND | ND | ND | ND | ND | ND | ND | N |
| 21 | N | ND | ND | ND | ND | ND | ND | ND | N |
| 22 | N | ND | ND | ND | ND | P | ND | P | P |
| 23 | N | ND | ND | ND | ND | ND | ND | ND | N |
| 24 | N | ND | ND | ND | P | ND | ND | ND | S |
| 25 | N | ND | ND | ND | ND | ND | ND | ND | N |
| 26 | N | ND | ND | ND | ND | ND | ND | ND | N |
| 27 | N | ND | ND | ND | ND | ND | ND | ND | N |
| 28 | N | ND | ND | ND | ND | ND | ND | ND | N |
| 29 | N | ND | ND | ND | P | P | ND | P | P |
| 30 | N | ND | ND | ND | ND | ND | ND | ND | N |
| 31 | N | ND | ND | ND | ND | ND | ND | ND | N |
| 32 | N | ND | ND | ND | ND | ND | ND | ND | N |
| 33 | N | ND | ND | ND | ND | ND | ND | ND | N |
| 34 | N | ND | ND | ND | ND | ND | ND | ND | N |
| 35 | N | ND | ND | ND | ND | ND | ND | ND | N |
| Technique | RT-PCR | MassARRAY® System |
|---|---|---|
| Targets | ORF1ab, N | ORF1ab, ORF1, N1, N2, N3 |
| RNA volume | 5 µL | 3 µL |
| Time needed * | 4–6 h | 8 h |
| Cost | ~10 EUR/sample | ~10 EUR/sample |
| Report method | Qualitative | Qualitative |
| Advantages | Short time of analyses; specific, confirms active cases; useful in clinical decision-making | Highly sensitive, accurate, high-throughput, easy data analysis, cost-effective, simple partially automated workflow, runs up to eight 96-well plates per day, may process two more plates overnight. |
| Disadvantages | Unable to simultaneously detect virus and its mutations, less accurate in low viral load samples | Multiple preparation steps, requires specialized equipment |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rybicka, M.; Miłosz, E.; Bielawski, K.P. Superiority of MALDI-TOF Mass Spectrometry over Real-Time PCR for SARS-CoV-2 RNA Detection. Viruses 2021, 13, 730. https://doi.org/10.3390/v13050730
Rybicka M, Miłosz E, Bielawski KP. Superiority of MALDI-TOF Mass Spectrometry over Real-Time PCR for SARS-CoV-2 RNA Detection. Viruses. 2021; 13(5):730. https://doi.org/10.3390/v13050730
Chicago/Turabian StyleRybicka, Magda, Ewa Miłosz, and Krzysztof Piotr Bielawski. 2021. "Superiority of MALDI-TOF Mass Spectrometry over Real-Time PCR for SARS-CoV-2 RNA Detection" Viruses 13, no. 5: 730. https://doi.org/10.3390/v13050730
APA StyleRybicka, M., Miłosz, E., & Bielawski, K. P. (2021). Superiority of MALDI-TOF Mass Spectrometry over Real-Time PCR for SARS-CoV-2 RNA Detection. Viruses, 13(5), 730. https://doi.org/10.3390/v13050730






