Persistent Airway Hyperresponsiveness Following Recovery from Infection with Pneumonia Virus of Mice
Abstract
1. Introduction
2. Materials and Methods
2.1. Mice
2.2. Virus Preparation and Inoculation Strategies
2.3. Profiling and ELISAs
2.4. Histology
2.5. Characterization of Leukocytes in BAL by Flow Cytometry
2.6. Assessment of Bronchoconstriction with Precision Cut Lung Slices (PCLS)
2.7. Measurements of Airway Resistance (FlexiVent)
2.8. Statistical Analyses
3. Results
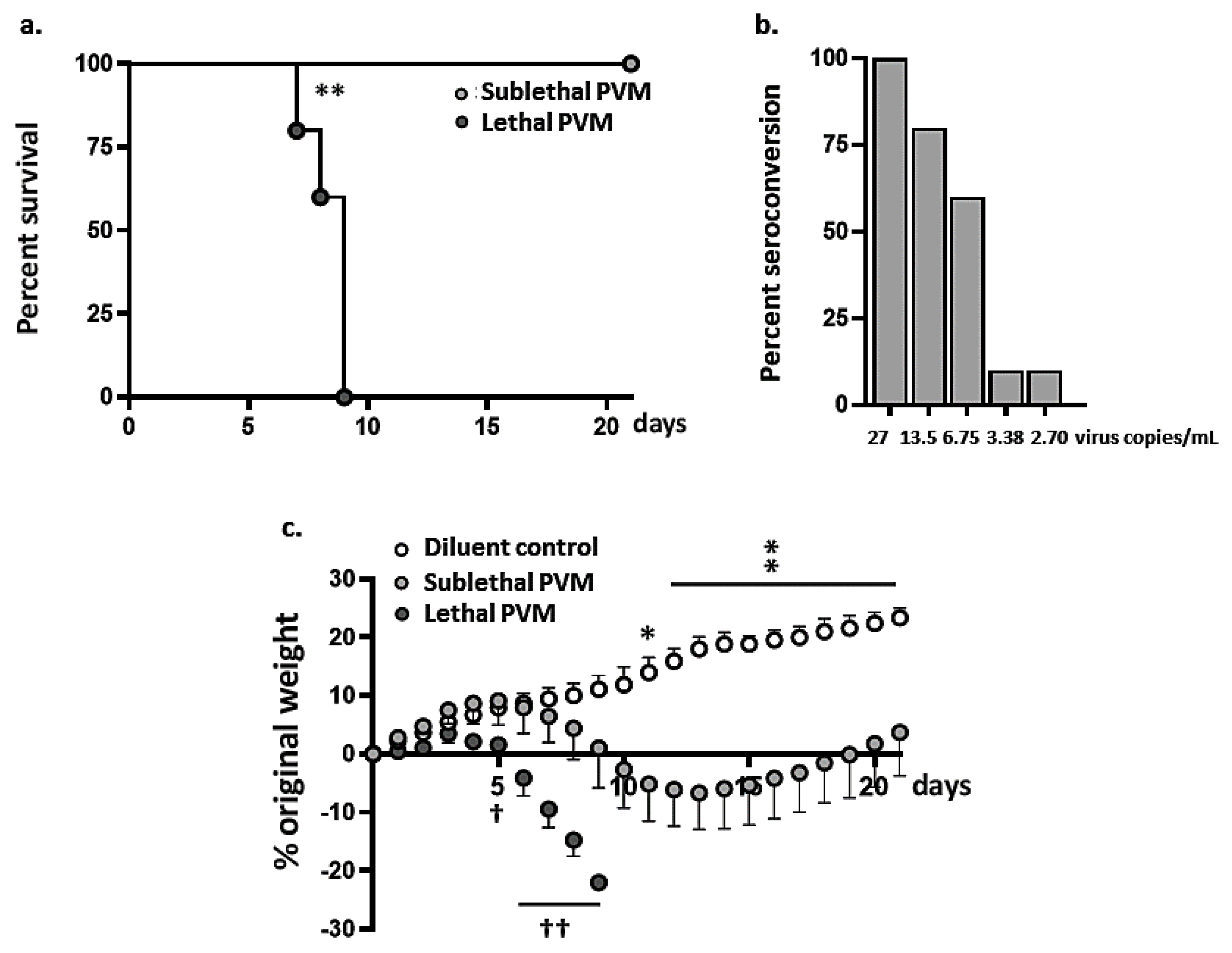
3.1. Symptomatic Sublethal PVM Infection in Wild-Type BALB/c Mice
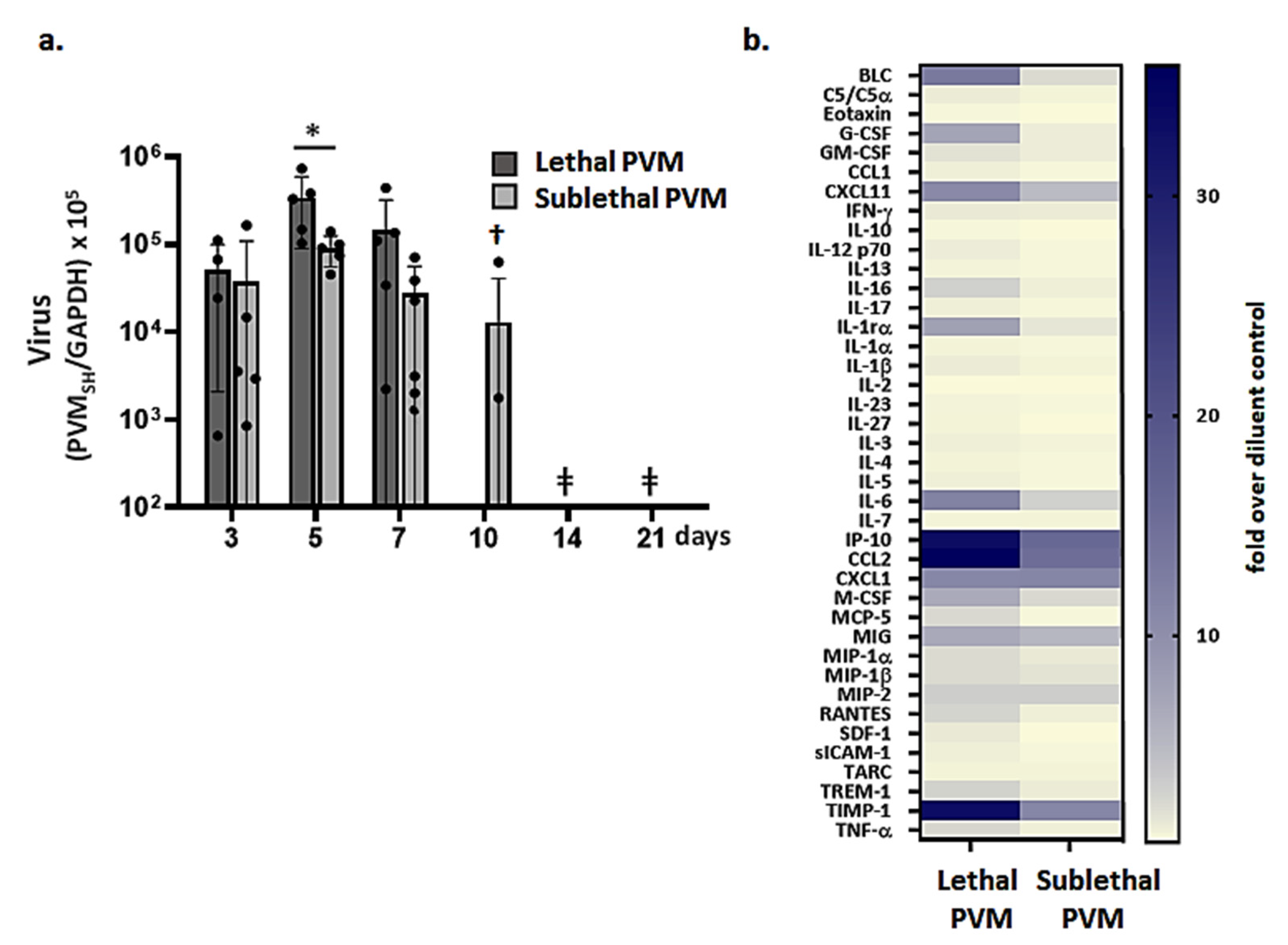
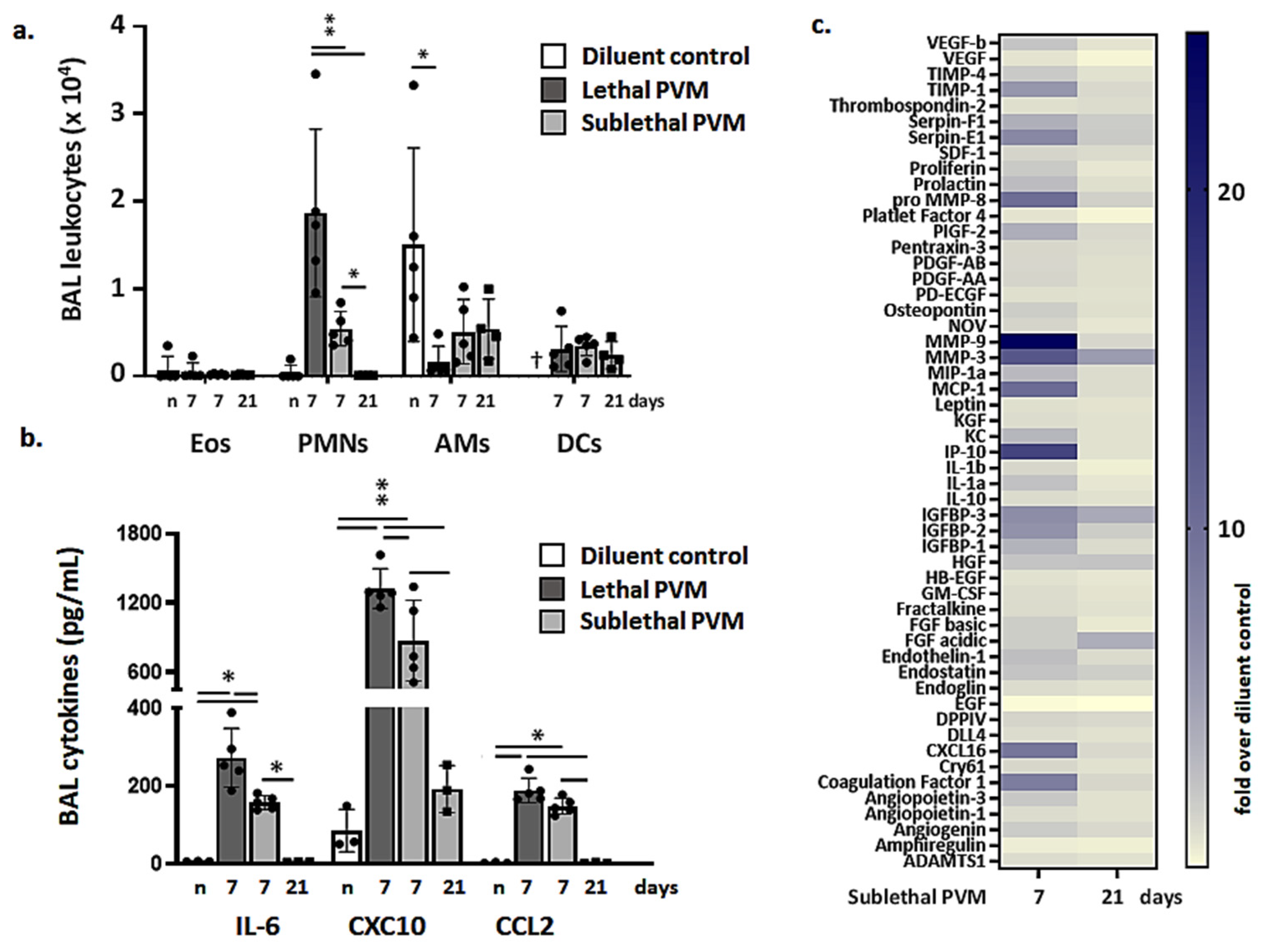
3.2. Resolution of Inflammation in Response to Sublethal PVM Infection
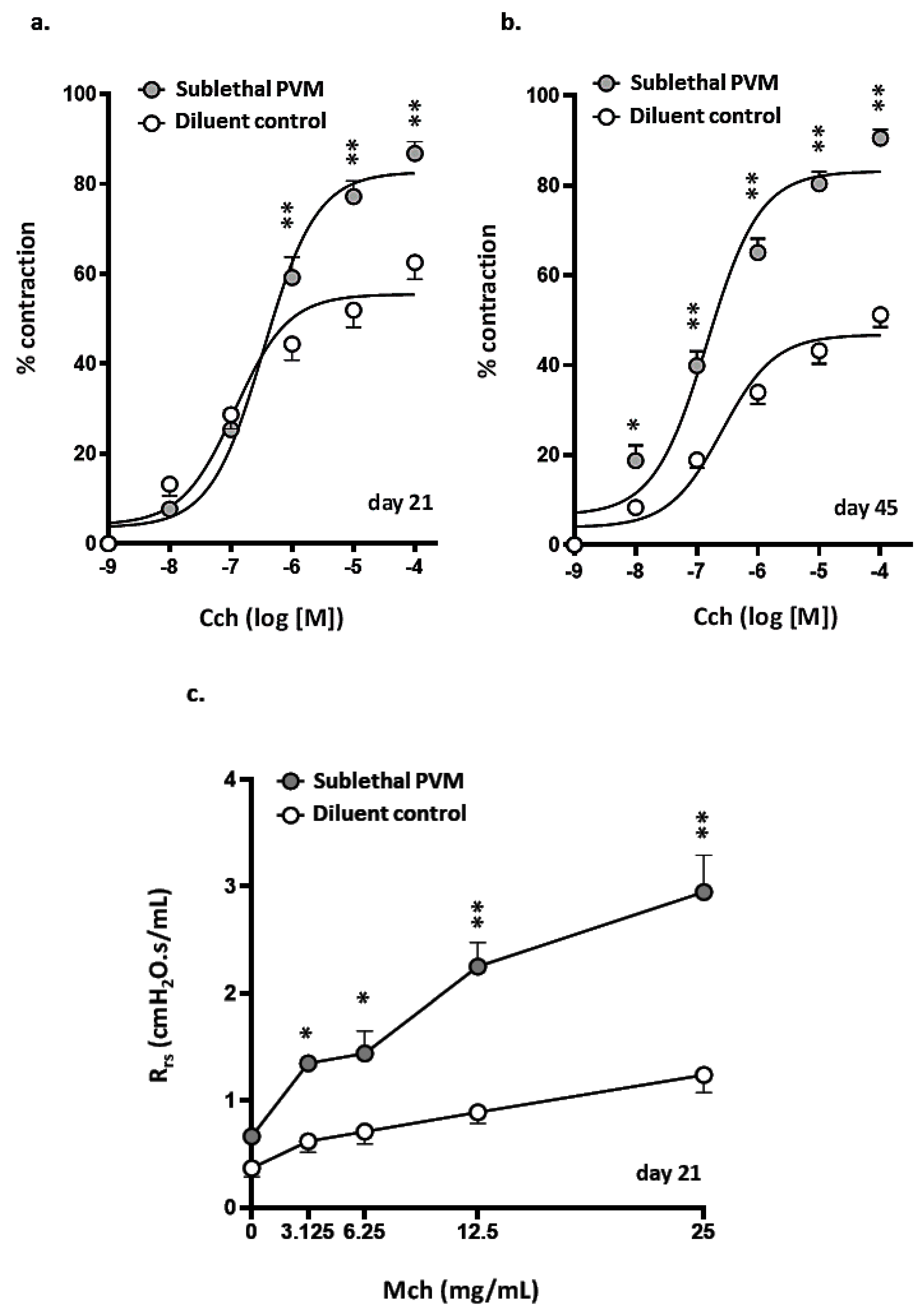
3.3. Airway Hyperresponsiveness after Recovery from Acute Sublethal PVM Infection
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ong, K.C.; Ng, A.W.; Lee, L.S.; Kaw, G.; Kwek, S.K.; Leow, M.K.; Earnest, A. One-year pulmonary function and health status in survivors of severe acute respiratory syndrome. Chest 2005, 128, 1393–1400. [Google Scholar] [CrossRef]
- Bellan, M.; Soddu, D.; Balbo, P.E.; Baricich, A.; Zeppegno, P.; Avanzi, G.C.; Baldon, G.; Bartolomei, G.; Battaglia, M.; Battistini, S.; et al. Respiratory and psychosocial sequelae among patients with COVID-19 four months after hospital discharge. JAMA Netw. Open 2021, 4, e2036142. [Google Scholar] [CrossRef] [PubMed]
- Munoz, F. The impact of influenza in children. Semin. Pediatr. Infect Dis. 2002, 13, 72–78. [Google Scholar] [CrossRef]
- Abraha, H.Y.; Lanctôt, K.L.; Paes, B. Risk of respiratory syncytial virus infection in preterm infants: Reviewing the need for prevention. Expert Rev. Respir. Med. 2015, 9, 779–799. [Google Scholar] [CrossRef] [PubMed]
- Greenough, A.; Broughton, S. Chronic manifestations of respiratory syncytial virus infection in premature infants. Pediatr. Infect. Dis. J. 2005, 24, S184–S187. [Google Scholar] [CrossRef] [PubMed]
- Jartti, T.; Gern, J.E. Role of viral infections in the development and exacerbation of asthma in children. Clin. Rev. Allergy Immunol. 2017, 140, 895–906. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.M.; Moore, P.E.; Gern, J.E.; Lemanske, R.F., Jr.; Hartert, T.V. Bronchiolitis to asthma: A review and call for studies of gene-virus interactions in asthma causation. Am. J. Respir. Crit. Care Med. 2007, 175, 108–119. [Google Scholar] [CrossRef]
- Jackson, D.J.; Lemanske, R.F., Jr. The role of respiratory virus infections in childhood asthma inception. Immunol. Allergy Clin. North Am. 2010, 30, 513–522. [Google Scholar] [CrossRef]
- Ruotsalainen, M.; Hyvärinen, M.K.; Piippo-Savolainen, E.; Korppi, M. Adolescent asthma after rhinovirus and respiratory syncytial virus bronchiolitis. Pediatr. Pulmonol. 2013, 48, 633–639. [Google Scholar] [CrossRef]
- Stein, R.T.; Sherrill, D.; Morgan, W.J.; Holberg, C.J.; Halonen, M.; Taussig, L.M.; Wright, A.L.; Martinez, F.D. Respiratory syncytial virus in early life and risk of wheeze and allergy by age 13 years. Lancet 1999, 354, 541–545. [Google Scholar] [CrossRef]
- Yoshihara, S.; Kusuda, S.; Mochizuki, H.; Okada, K.; Nishima, S.; Simões, E.A.; C-CREW Investigators. Effect of palivizumab prophylaxis on subsequent recurrent wheezing in preterm infants. Pediatrics 2013, 132, 811–818. [Google Scholar] [CrossRef] [PubMed]
- Mochizuki, H.; Kusuda, S.; Okada, K.; Yoshihara, S.; Furuya, H.; Simões, E.A.F.; The Scientific Committee for Elucidation of Infantile Asthma. Palivizumab prophylaxis in preterm infants and subsequent recurrent wheezing. Six-year follow-up study. Am. J. Respir. Crit. Care Med. 2017, 196, 29–38. [Google Scholar] [CrossRef] [PubMed]
- Brunwasser, S.M.; Snyder, B.M.; Driscoll, A.J.; Fell, D.B.; Savitz, D.A.; Feikin, D.R.; Skidmore, B.; Bhat, N.; Bont, L.J.; Dupont, W.D.; et al. Assessing the strength of evidence for a causal effect of respiratory syncytial virus lower respiratory tract infections on subsequent wheezing illness: A systematic review and meta-analysis. Lancet Respir. Med. 2020, 8, 795–806. [Google Scholar] [CrossRef]
- Driscoll, A.J.; Arshad, S.H.; Bont, L.; Brunwasser, S.M.; Cherian, T.; Englund, J.A.; Fell, D.B.; Hammitt, L.L.; Hartert, T.V.; Innis, B.L.; et al. Does respiratory syncytial virus lower respiratory illness in early life cause recurrent wheeze of early childhood and asthma? Critical review of the evidence and guidance for future studies from a World Health Organization-sponsored meeting. Vaccine 2020, 38, 2435–2448. [Google Scholar] [CrossRef]
- Dyer, K.D.; Garcia-Crespo, K.E.; Glineur, S.; Domachowske, J.B.; Rosenberg, H.F. The pneumonia virus of mice (PVM) model of acute respiratory infection. Viruses 2012, 4, 3494–3510. [Google Scholar] [CrossRef]
- Rosenberg, H.F.; Domachowske, J.B. Pneumonia virus of mice: Severe respiratory infection in a natural host. Immunol. Lett. 2008, 118, 6–12. [Google Scholar] [CrossRef] [PubMed]
- Bem, R.A.; Domachowske, J.B.; Rosenberg, H.F. Animal models of human respiratory syncytial virus disease. Am. J. Physiol. Lung Cell Mol. Physiol. 2011, 301, L148–L156. [Google Scholar] [CrossRef]
- Taylor, G. Animal models of respiratory syncytial virus infection. Vaccine 2017, 35, 469–480. [Google Scholar] [CrossRef]
- Rosenberg, H.F.; Bonville, C.A.; Easton, A.J.; Domachowske, J.B. The pneumonia virus of mice infection model for severe respiratory syncytial virus infection: Identifying novel targets for therapeutic intervention. Pharmacol. Ther. 2005, 105, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Bonville, C.A.; Bennett, N.J.; Koehnlein, M.; Haines, D.M.; Ellis, J.A.; DelVecchio, A.M.; Rosenberg, H.F.; Domachowske, J.B. Respiratory dysfunction and proinflammatory chemokines in the pneumonia virus of mice (PVM) model of viral bronchiolitis. Virology 2006, 349, 87–95. [Google Scholar] [CrossRef] [PubMed]
- Walsh, K.B.; Teijaro, J.R.; Brock, L.G.; Fremgen, D.M.; Collins, P.L.; Rosen, H.; Oldstone, M.B. Animal model of respiratory syncytial virus: CD8+ T cells cause a cytokine storm that is chemically tractable by sphingosine-1-phosphate 1 receptor agonist therapy. J. Virol. 2014, 88, 6281–6293. [Google Scholar] [CrossRef]
- Bem, R.A.; van Woensel, J.B.; Bos, A.P.; Koski, A.; Farnand, A.W.; Domachowske, J.B.; Rosenberg, H.F.; Martin, T.R.; Matute-Bello, G. Mechanical ventilation enhances lung inflammation and caspase activity in a model of mouse pneumovirus infection. Am. J. Physiol. Lung Cell Mol. Physiol. 2009, 296, L46–L56. [Google Scholar] [CrossRef] [PubMed]
- Bonville, C.A.; Lau, V.K.; DeLeon, J.M.; Gao, J.L.; Easton, A.J.; Rosenberg, H.F.; Domachowske, J.B. Functional antagonism of chemokine receptor CCR1 reduced mortality in acute pneumovirus infection in vivo. J. Virol. 2004, 78, 7984–7989. [Google Scholar] [CrossRef]
- Bonville, C.A.; Rosenberg, H.F.; Domachowske, J.B. Ribavirin and cysteinyl leukotriene-1 receptor blockade as treatment for severe bronchiolitis. Antiviral Res. 2006, 69, 53–59. [Google Scholar] [CrossRef] [PubMed]
- Bondue, B.; Vosters, O.; de Nadai, P.; Glineur, S.; De Henau, O.; Luangsay, S.; van Gool, F.; Communi, D.; De Vuyst, P.; Desmecht, V.; et al. ChemR23 dampens lung inflammation and enhances anti-viral immunity in a mouse model of acute viral pneumonia. PLoS Pathog. 2011, 7, e1002358. [Google Scholar] [CrossRef] [PubMed]
- Vanderstocken, G.; Van de Paar, E.; Robaye, B.; di Pietrantonio, L.; Bondue, B.; Boeynaems, J.M.; Desmecht, D.; Communi, D. Protective role of P2Y2 receptor against lung infection induced by pneumonia virus of mice. PLoS ONE 2012, 7, e50385. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Percopo, C.M.; Ma, M.; Brenner, T.A.; Krumholz, J.O.; Break, T.J.; Laky, K.; Rosenberg, H.F. Critical adverse impact of IL-6 in acute pneumovirus infection. J. Immunol. 2019, 202, 871–882. [Google Scholar] [CrossRef] [PubMed]
- Rice, T.A.; Brenner, T.A.; Percopo, C.M.; Ma, M.; Keicher, J.D.; Domachowske, J.B.; Rosenberg, H.F. Signaling via pattern recognition receptors NOD2 and TLR2 contributes to immunomodulatory control of lethal pneumovirus infection. Antiviral Res. 2016, 132, 131–140. [Google Scholar] [CrossRef][Green Version]
- Percopo, C.M.; Rice, T.A.; Brenner, T.A.; Dyer, K.D.; Luo, J.L.; Kanakabandi, K.; Sturdevant, D.E.; Porcella, S.F.; Domachowske, J.B.; Keicher, J.D.; et al. Immunobiotic Lactobacillus administered post-exposure averts the lethal sequelae of respiratory virus infection. Antiviral Res. 2015, 121, 109–119. [Google Scholar] [CrossRef] [PubMed]
- Gabryszewski, S.J.; Bachar, O.; Dyer, K.D.; Percopo, C.M.; Killoran, K.E.; Domachowske, J.B.; Rosenberg, H.F. Lactobacillus-mediated priming of the respiratory mucosa protects against lethal pneumovirus infection. J. Immunol. 2011, 186, 1151–1161. [Google Scholar] [CrossRef]
- Percopo, C.M.; Dyer, K.D.; Karpe, K.A.; Domachowske, J.B.; Rosenberg, H.F. Eosinophils and respiratory virus infection: A dual-standard curve qRT-PCR-based method for determining virus recovery from mouse lung tissue. Methods Mol. Biol. 2014, 1178, 257–266. [Google Scholar]
- Ellis, J.A.; Martin, B.V.; Waldner, C.; Dyer, K.D.; Domachowske, J.B.; Rosenberg, H.F. Mucosal inoculation with an attenuated mouse pneumovirus strain protects against virulent challenge in wild type and interferon-gamma receptor-deficient mice. Vaccine 2007, 25, 1085–1095. [Google Scholar] [CrossRef] [PubMed]
- Mai, E.; Percopo, C.M.; Limkar, A.R.; Sek, A.C.; Ma, M.; Rosenberg, H.F. Respiratory epithelial cells respond to Lactobacillus plantarum but provide no cross-protection against virus-induced inflammation. Viruses 2020, 13, 2. [Google Scholar] [CrossRef]
- Liu, G.; Betts, C.; Cunoosamy, D.M.; Åberg, P.M.; Hornberg, J.J.; Sivars, K.B.; Cohen, T.S. Use of precision-cut lung slices as a translational model for the study of lung biology. Respir. Res. 2019, 20, 162. [Google Scholar] [CrossRef] [PubMed]
- Jude, J.; Botelho, D.; Karmacharya, N.; Cao, G.Y.; Jester, W.; Panettieri, R., Jr. A. Salicylic acid amplifies carbachol-induced bronchoconstriction in human precision-cut lung slides. Respir. Res. 2019, 20, 72. [Google Scholar] [CrossRef]
- O’Byrne, P.M.; Inman, M.D. Airway hyperresponsiveness. Chest 2003, 123, 411S–416S. [Google Scholar] [CrossRef] [PubMed]
- Fang, L.; Sun, Q.; Roth, M. Immunologic and non-immunologic mechanisms leading to airway remodeling in asthma. Int. J. Mol. Sci. 2020, 21, 757. [Google Scholar] [CrossRef] [PubMed]
- D’Urzo, K.A.; Mok, F.; D’Urzo, A.D. Variation among spirometry interpretation algorithms. Respir. Care 2020, 65, 1585–1590. [Google Scholar] [CrossRef] [PubMed]
- Verheijden, K.A.T.; Henricks, P.A.J.; Redegeld, R.A.; Garssen, J.; Folkerts, G. Measurement of airway function using invasive and non-invasive methods in mild and severe models for allergy airway inflammation in mice. Front. Pharmacol. 2014, 5, 190. [Google Scholar] [CrossRef]
- Hoymann, H.G. Invasive and noninvasive lung function measurements in rodents. J. Pharmacol. Toxicol. Methods 2007, 55, 16–26. [Google Scholar] [CrossRef]
- Bozanich, E.M.; Gualano, R.C.; Zosky, G.R.; Larcombe, A.N.; Turner, D.J.; Hantos, Z.; Sly, P.D. Acute influenza A infection-induced bronchial hyper-responsiveness in mice. Respir. Physiol. Neurobiol. 2008, 162, 190–196. [Google Scholar] [CrossRef] [PubMed]
- Dakhama, A.; Lee, Y.M.; Gelfand, E.W. Virus-induced airway dysfunction: Pathogenesis and biomechanisms. Pediatr. Infect. Dis. J. 2005, 24, S159–S169. [Google Scholar] [CrossRef]
- Mejías, A.; Chávez-Bueno, S.; Jafri, H.S.; Ramilo, O. Respiratory syncytial virus infections: Old challenges and new opportunities. Pediatr. Infect. Dis. J. 2005, 24, S189–S196. [Google Scholar] [CrossRef]
- Van Schaik, S.M.; Enhorning, G.; Vargas, I.; Welliver, R.C. Respiratory syncytial virus affects pulmonary function in BALB/c mice. J. Infect. Dis. 1998, 177, 269–276. [Google Scholar] [CrossRef]
- You, D.; Siefker, D.T.; Shrestha, B.; Saravia, J.; Cormier, S.A. Building a better neonatal mouse model to understand infant respiratory syncytial virus disease. Respir. Res. 2015, 16, 91. [Google Scholar] [CrossRef] [PubMed]
- Estripeaut, D.; Torres, J.P.; Somers, C.S.; Tagliabue, C.; Khokhar, S.; Bhoj, V.G.; Grube, S.M.; Wozniakowski, A.; Gomez, A.M.; Ramilo, O.; et al. Respiratory syncytial virus persistence in the lungs correlates with airway hyperreactivity in the mouse model. J. Infect. Dis. 2008, 198, 1435–1443. [Google Scholar] [CrossRef] [PubMed]
- Rosas-Salazar, C.; Shilts, M.H.; Tovchigrechko, A.; Schobel, S.; Chappell, J.D.; Larkin, E.K.; Gebretsadik, T.; Halpin, R.A.; Nelson, K.E.; Moore, M.L.; et al. Nasopharyngeal Lactobacillus is associated with a reduced risk of childhood wheezing illnesses following acute respiratory syncytial virus infection in infancy. J. Allergy Clin. Immunol. 2018, 142, 1447–1456. [Google Scholar] [CrossRef] [PubMed]
- Tomosada, Y.; Chiba, E.; Zelaya, H.; Takahashi, T.; Tsukida, K.; Kitazawa, H.; Alvarez, S.; Villena, J. Nasally administered Lactobacillus rhamnosus strains differentially modulate respiratory antiviral immune responses and induced protection against respiratory syncytial virus infection. BMC Immunol. 2013, 14, 40. [Google Scholar] [CrossRef]
- Park, M.K.; Ngo, V.; Kwon, Y.M.; Lee, Y.T.; Yoo, S.; Cho, Y.H.; Hong, S.M.; Hwang, H.S.; Ko, E.J.; Jung, Y.J.; et al. Lactobacillus plantarum DK119 as a probiotic confers protection against influenza virus by modulating innate immunity. PLoS ONE 2013, 8, e75368. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Limkar, A.R.; Percopo, C.M.; Redes, J.L.; Druey, K.M.; Rosenberg, H.F. Persistent Airway Hyperresponsiveness Following Recovery from Infection with Pneumonia Virus of Mice. Viruses 2021, 13, 728. https://doi.org/10.3390/v13050728
Limkar AR, Percopo CM, Redes JL, Druey KM, Rosenberg HF. Persistent Airway Hyperresponsiveness Following Recovery from Infection with Pneumonia Virus of Mice. Viruses. 2021; 13(5):728. https://doi.org/10.3390/v13050728
Chicago/Turabian StyleLimkar, Ajinkya R., Caroline M. Percopo, Jamie L. Redes, Kirk M. Druey, and Helene F. Rosenberg. 2021. "Persistent Airway Hyperresponsiveness Following Recovery from Infection with Pneumonia Virus of Mice" Viruses 13, no. 5: 728. https://doi.org/10.3390/v13050728
APA StyleLimkar, A. R., Percopo, C. M., Redes, J. L., Druey, K. M., & Rosenberg, H. F. (2021). Persistent Airway Hyperresponsiveness Following Recovery from Infection with Pneumonia Virus of Mice. Viruses, 13(5), 728. https://doi.org/10.3390/v13050728





