The Antiviral Activities of Poly-ADP-Ribose Polymerases
Abstract
1. Introduction
2. Inhibition of Viral Cycle Steps by the PARPs
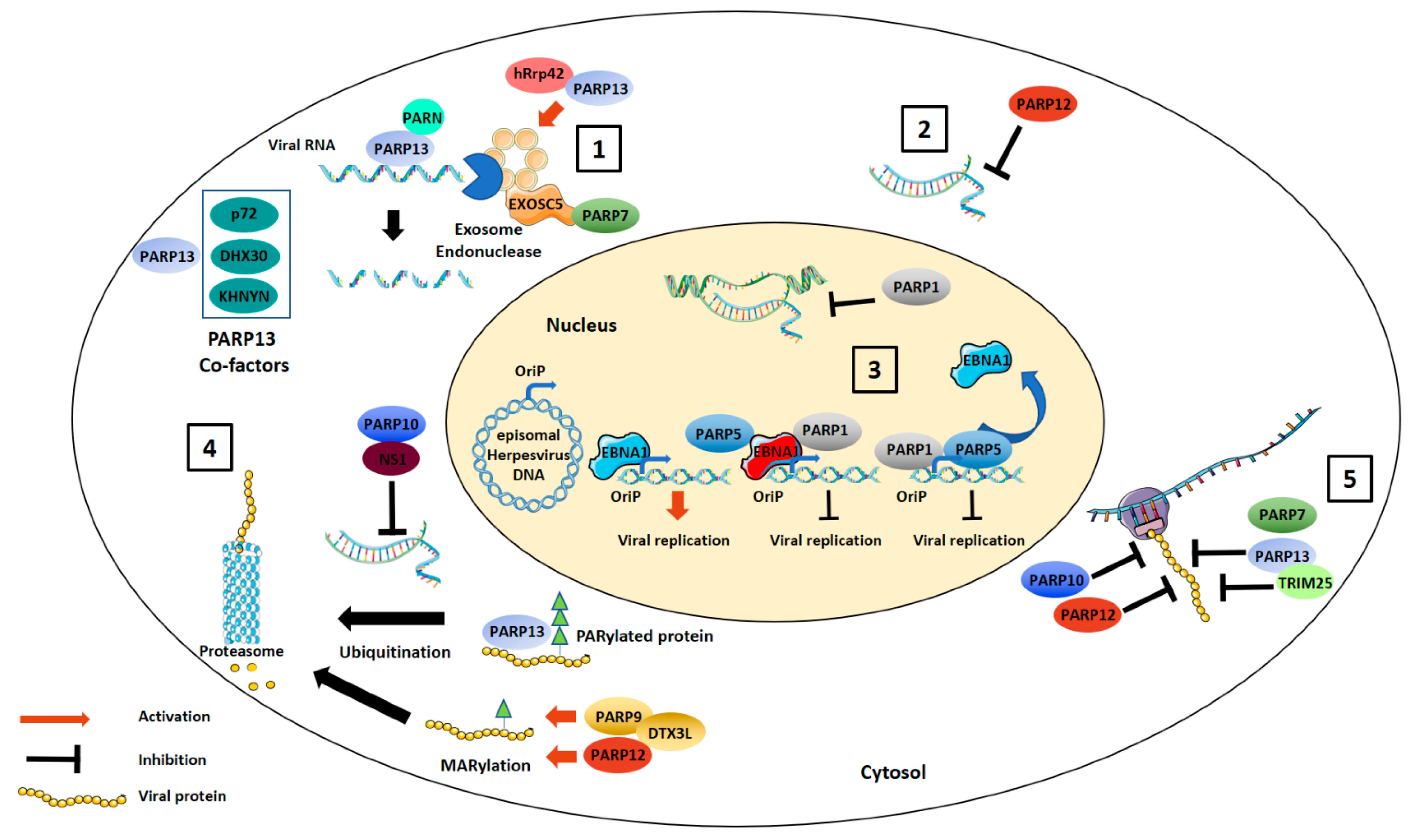
2.1. Degradation of Viral Nucleic Acids
2.2. Inhibition of Viral Replication
2.3. Translation Inhibition
2.4. Targeting Viral Proteins
3. Immunomodulation
4. Strategies to Escape the Antiviral Activities of PARPs
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Schreiber, V.; Dantzer, F.; Ame, J.-C.; de Murcia, G. Poly(ADP-Ribose): Novel Functions for an Old Molecule. Nat. Rev. Mol. Cell Biol. 2006, 7, 517–528. [Google Scholar] [CrossRef]
- Gibson, B.A.; Kraus, W.L. New Insights into the Molecular and Cellular Functions of Poly(ADP-Ribose) and PARPs. Nat. Rev. Mol. Cell Biol. 2012, 13, 411–424. [Google Scholar] [CrossRef]
- Holbourn, K.P.; Shone, C.C.; Acharya, K.R. A Family of Killer Toxins: Exploring the Mechanism of ADP-Ribosylating Toxins. FEBS J. 2006, 273, 4579–4593. [Google Scholar] [CrossRef]
- Citarelli, M.; Teotia, S.; Lamb, R.S. Evolutionary History of the Poly(ADP-Ribose) Polymerase Gene Family in Eukaryotes. BMC Evol. Biol. 2010, 10, 308. [Google Scholar] [CrossRef]
- Leidecker, O.; Bonfiglio, J.J.; Colby, T.; Zhang, Q.; Atanassov, I.; Zaja, R.; Palazzo, L.; Stockum, A.; Ahel, I.; Matic, I. Serine Is a New Target Residue for Endogenous ADP-Ribosylation on Histones. Nat. Chem. Biol. 2016, 12, 998–1000. [Google Scholar] [CrossRef] [PubMed]
- Gagné, J.-P.; Ethier, C.; Defoy, D.; Bourassa, S.; Langelier, M.-F.; Riccio, A.A.; Pascal, J.M.; Moon, K.-M.; Foster, L.J.; Ning, Z.; et al. Quantitative Site-Specific ADP-Ribosylation Profiling of DNA-Dependent PARPs. DNA Repair. 2015, 30, 68–79. [Google Scholar] [CrossRef] [PubMed]
- Feijs, K.L.H.; Verheugd, P.; Lüscher, B. Expanding Functions of Intracellular Resident Mono-ADP-Ribosylation in Cell Physiology. FEBS J. 2013, 280, 3519–3529. [Google Scholar] [CrossRef] [PubMed]
- Ke, Y.; Wang, C.; Zhang, J.; Zhong, X.; Wang, R.; Zeng, X.; Ba, X. The Role of PARPs in Inflammation—And Metabolic—Related Diseases: Molecular Mechanisms and Beyond. Cells 2019, 8, 1047. [Google Scholar] [CrossRef] [PubMed]
- Krishnakumar, R.; Gamble, M.J.; Frizzell, K.M.; Berrocal, J.G.; Kininis, M.; Kraus, W.L. Reciprocal Binding of PARP-1 and Histone H1 at Promoters Specifies Transcriptional Outcomes. Science 2008, 319, 819–821. [Google Scholar] [CrossRef]
- Posavec Marjanović, M.; Crawford, K.; Ahel, I. PARP, Transcription and Chromatin Modeling. Semin. Cell Dev. Biol. 2017, 63, 102–113. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Luo, W.; Wang, Y. PARP-1 and Its Associated Nucleases in DNA Damage Response. DNA Repair 2019, 81, 102651. [Google Scholar] [CrossRef] [PubMed]
- Smith, S.; Giriat, I.; Schmitt, A.; de Lange, T. Tankyrase, a Poly(ADP-Ribose) Polymerase at Human Telomeres. Science 1998, 282, 1484–1487. [Google Scholar] [CrossRef]
- Grunewald, M.E.; Shaban, M.G.; Mackin, S.R.; Fehr, A.R.; Perlman, S. Murine Coronavirus Infection Activates the Aryl Hydrocarbon Receptor in an Indoleamine 2,3-Dioxygenase-Independent Manner, Contributing to Cytokine Modulation and Proviral TCDD-Inducible-PARP Expression. J. Virol. 2020, 94. [Google Scholar] [CrossRef]
- Heer, C.D.; Sanderson, D.J.; Voth, L.S.; Alhammad, Y.M.O.; Schmidt, M.S.; Trammell, S.A.J.; Perlman, S.; Cohen, M.S.; Fehr, A.R.; Brenner, C. Coronavirus Infection and PARP Expression Dysregulate the NAD Metabolome: An Actionable Component of Innate Immunity. J. Biol. Chem. 2020, 295, 17986–17996. [Google Scholar] [CrossRef] [PubMed]
- Gao, G.; Guo, X.; Goff, S.P. Inhibition of Retroviral RNA Production by ZAP, a CCCH-Type Zinc Finger Protein. Sci. New Ser. 2002, 297, 1703–1706. [Google Scholar] [CrossRef] [PubMed]
- Kerns, J.A.; Emerman, M.; Malik, H.S. Positive Selection and Increased Antiviral Activity Associated with the PARP-Containing Isoform of Human Zinc-Finger Antiviral Protein. PLoS Genet. 2008, 4, e21. [Google Scholar] [CrossRef]
- Li, M.M.H.; Aguilar, E.G.; Michailidis, E.; Pabon, J.; Park, P.; Wu, X.; de Jong, Y.P.; Schneider, W.M.; Molina, H.; Rice, C.M.; et al. Characterization of Novel Splice Variants of Zinc Finger Antiviral Protein (ZAP). J. Virol. 2019, 93. [Google Scholar] [CrossRef]
- Bick, M.J.; Carroll, J.-W.N.; Gao, G.; Goff, S.P.; Rice, C.M.; MacDonald, M.R. Expression of the Zinc-Finger Antiviral Protein Inhibits Alphavirus Replication. JVI 2003, 77, 11555–11562. [Google Scholar] [CrossRef]
- Müller, S.; Möller, P.; Bick, M.J.; Wurr, S.; Becker, S.; Günther, S.; Kümmerer, B.M. Inhibition of Filovirus Replication by the Zinc Finger Antiviral Protein. JVI 2007, 81, 2391–2400. [Google Scholar] [CrossRef]
- Wang, X.; Tu, F.; Zhu, Y.; Gao, G. Zinc-Finger Antiviral Protein Inhibits XMRV Infection. PLoS ONE 2012, 7, e39159. [Google Scholar] [CrossRef][Green Version]
- Zhu, Y.; Chen, G.; Lv, F.; Wang, X.; Ji, X.; Xu, Y.; Sun, J.; Wu, L.; Zheng, Y.-T.; Gao, G. Zinc-Finger Antiviral Protein Inhibits HIV-1 Infection by Selectively Targeting Multiply Spliced Viral MRNAs for Degradation. Proc. Natl. Acad. Sci. USA 2011, 108, 15834–15839. [Google Scholar] [CrossRef] [PubMed]
- Mao, R.; Nie, H.; Cai, D.; Zhang, J.; Liu, H.; Yan, R.; Cuconati, A.; Block, T.M.; Guo, J.-T.; Guo, H. Inhibition of Hepatitis B Virus Replication by the Host Zinc Finger Antiviral Protein. PLoS Pathog. 2013, 9, e1003494. [Google Scholar] [CrossRef]
- Tang, Q.; Wang, X.; Gao, G. The Short Form of the Zinc Finger Antiviral Protein Inhibits Influenza A Virus Protein Expression and Is Antagonized by the Virus-Encoded NS1. J. Virol. 2017, 91, e01909-16. [Google Scholar] [CrossRef] [PubMed]
- Chiu, H.-P.; Chiu, H.; Yang, C.-F.; Lee, Y.-L.; Chiu, F.-L.; Kuo, H.-C.; Lin, R.-J.; Lin, Y.-L. Inhibition of Japanese Encephalitis Virus Infection by the Host Zinc-Finger Antiviral Protein. PLOS Pathog. 2018, 14, e1007166. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Yan, K.; Wei, L.; Yang, J.; Lu, C.; Xiong, F.; Zheng, C.; Xu, W. Zinc Finger Antiviral Protein Inhibits Coxsackievirus B3 Virus Replication and Protects against Viral Myocarditis. Antivir. Res. 2015, 123, 50–61. [Google Scholar] [CrossRef]
- Kozaki, T.; Takahama, M.; Misawa, T.; Matsuura, Y.; Akira, S.; Saitoh, T. Role of Zinc-Finger Anti-Viral Protein in Host Defense against Sindbis Virus. Int. Immunol. 2015, 27, 357–364. [Google Scholar] [CrossRef]
- Guo, X.; Carroll, J.-W.N.; MacDonald, M.R.; Goff, S.P.; Gao, G. The Zinc Finger Antiviral Protein Directly Binds to Specific Viral MRNAs through the CCCH Zinc Finger Motifs. JVI 2004, 78, 12781–12787. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Xu, Y.; Zhang, K.; Wang, X.; Gao, G.; Liu, Y. Structure of N-Terminal Domain of ZAP Indicates How a Zinc-Finger Protein Recognizes Complex RNA. Nat. Struct. Mol. Biol. 2012, 19, 430–435. [Google Scholar] [CrossRef]
- Luo, X.; Wang, X.; Gao, Y.; Zhu, J.; Liu, S.; Gao, G.; Gao, P. Molecular Mechanism of RNA Recognition by Zinc-Finger Antiviral Protein. Cell Rep. 2020, 30, 46–52.e4. [Google Scholar] [CrossRef]
- Guo, X.; Ma, J.; Sun, J.; Gao, G. The Zinc-Finger Antiviral Protein Recruits the RNA Processing Exosome to Degrade the Target MRNA. Proc. Natl. Acad. Sci. USA 2007, 104, 151–156. [Google Scholar] [CrossRef] [PubMed]
- Nchioua, R.; Kmiec, D.; Müller, J.A.; Conzelmann, C.; Groß, R.; Swanson, C.M.; Neil, S.J.D.; Stenger, S.; Sauter, D.; Münch, J.; et al. SARS-CoV-2 Is Restricted by Zinc Finger Antiviral Protein despite Preadaptation to the Low-CpG Environment in Humans. mBio 2020, 11, e01930-20. [Google Scholar] [CrossRef]
- Kmiec, D.; Nchioua, R.; Sherrill-Mix, S.; Stürzel, C.M.; Heusinger, E.; Braun, E.; Gondim, M.V.P.; Hotter, D.; Sparrer, K.M.J.; Hahn, B.H.; et al. CpG Frequency in the 5′ Third of the Env Gene Determines Sensitivity of Primary HIV-1 Strains to the Zinc-Finger Antiviral Protein. mBio 2020, 11. [Google Scholar] [CrossRef]
- MacDonald, M.R.; Machlin, E.S.; Albin, O.R.; Levy, D.E. The Zinc Finger Antiviral Protein Acts Synergistically with an Interferon-Induced Factor for Maximal Activity against Alphaviruses. JVI 2007, 81, 13509–13518. [Google Scholar] [CrossRef]
- Chen, G.; Guo, X.; Lv, F.; Xu, Y.; Gao, G. P72 DEAD Box RNA Helicase Is Required for Optimal Function of the Zinc-Finger Antiviral Protein. Proc. Natl. Acad. Sci. USA 2008, 105, 4352–4357. [Google Scholar] [CrossRef] [PubMed]
- Ye, P.; Liu, S.; Zhu, Y.; Chen, G.; Gao, G. DEXH-Box Protein DHX30 Is Required for Optimal Function of the Zinc-Finger Antiviral Protein. Protein Cell 2010, 1, 956–964. [Google Scholar] [CrossRef] [PubMed]
- Ficarelli, M.; Wilson, H.; Pedro Galão, R.; Mazzon, M.; Antzin-Anduetza, I.; Marsh, M.; Neil, S.J.; Swanson, C.M. KHNYN Is Essential for the Zinc Finger Antiviral Protein (ZAP) to Restrict HIV-1 Containing Clustered CpG Dinucleotides. Elife 2019, 8. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.-H.; Zhou, L.; Chen, G.; Krug, R.M. Battle between Influenza A Virus and a Newly Identified Antiviral Activity of the PARP-Containing ZAPL Protein. Proc. Natl. Acad. Sci. USA 2015, 112, 14048–14053. [Google Scholar] [CrossRef] [PubMed]
- Schwerk, J.; Soveg, F.W.; Ryan, A.P.; Thomas, K.R.; Hatfield, L.D.; Ozarkar, S.; Forero, A.; Kell, A.M.; Roby, J.A.; So, L.; et al. RNA-Binding Protein Isoforms ZAP-S and ZAP-L Have Distinct Antiviral and Immune Resolution Functions. Nat. Immunol. 2019, 20, 1610–1620. [Google Scholar] [CrossRef] [PubMed]
- Gläsker, S.; Töller, M.; Kümmerer, B.M. The Alternate Triad Motif of the Poly(ADP-Ribose) Polymerase-like Domain of the Human Zinc Finger Antiviral Protein Is Essential for Its Antiviral Activity. J. Gen. Virol. 2014, 95, 816–822. [Google Scholar] [CrossRef]
- Kozaki, T.; Komano, J.; Kanbayashi, D.; Takahama, M.; Misawa, T.; Satoh, T.; Takeuchi, O.; Kawai, T.; Shimizu, S.; Matsuura, Y.; et al. Mitochondrial Damage Elicits a TCDD-Inducible Poly(ADP-Ribose) Polymerase-Mediated Antiviral Response. Proc. Natl. Acad. Sci. USA 2017, 114, 2681–2686. [Google Scholar] [CrossRef]
- Ji, Y.; Tulin, A.V. The Roles of PARP1 in Gene Control and Cell Differentiation. Curr. Opin. Genet. Dev. 2010, 20, 512–518. [Google Scholar] [CrossRef]
- Deng, Z.; Lezina, L.; Chen, C.-J.; Shtivelband, S.; So, W.; Lieberman, P.M. Telomeric Proteins Regulate Episomal Maintenance of Epstein-Barr Virus Origin of Plasmid Replication. Mol. Cell 2002, 9, 493–503. [Google Scholar] [CrossRef]
- Tempera, I.; Deng, Z.; Atanasiu, C.; Chen, C.-J.; D’Erme, M.; Lieberman, P.M. Regulation of Epstein-Barr Virus OriP Replication by Poly(ADP-Ribose) Polymerase 1. JVI 2010, 84, 4988–4997. [Google Scholar] [CrossRef] [PubMed]
- Deng, Z.; Atanasiu, C.; Zhao, K.; Marmorstein, R.; Sbodio, J.I.; Chi, N.-W.; Lieberman, P.M. Inhibition of Epstein-Barr Virus OriP Function by Tankyrase, a Telomere-Associated Poly-ADP Ribose Polymerase That Binds and Modifies EBNA1. JVI 2005, 79, 4640–4650. [Google Scholar] [CrossRef] [PubMed]
- Ohsaki, E.; Ueda, K.; Sakakibara, S.; Do, E.; Yada, K.; Yamanishi, K. Poly(ADP-Ribose) Polymerase 1 Binds to Kaposi’s Sarcoma-Associated Herpesvirus (KSHV) Terminal Repeat Sequence and Modulates KSHV Replication in Latency. J. Virol. 2004, 78, 9936–9946. [Google Scholar] [CrossRef]
- Lupey-Green, L.N.; Moquin, S.A.; Martin, K.A.; McDevitt, S.M.; Hulse, M.; Caruso, L.B.; Pomerantz, R.T.; Miranda, J.L.; Tempera, I. PARP1 Restricts Epstein Barr Virus Lytic Reactivation by Binding the BZLF1 Promoter. Virology 2017, 507, 220–230. [Google Scholar] [CrossRef] [PubMed]
- Gwack, Y.; Nakamura, H.; Lee, S.H.; Souvlis, J.; Yustein, J.T.; Gygi, S.; Kung, H.-J.; Jung, J.U. Poly(ADP-Ribose) Polymerase 1 and Ste20-Like Kinase HKFC Act as Transcriptional Repressors for Gamma-2 Herpesvirus Lytic Replication. MCB 2003, 23, 8282–8294. [Google Scholar] [CrossRef] [PubMed]
- Parent, M.; Yung, T.M.C.; Rancourt, A.; Ho, E.L.Y.; Vispé, S.; Suzuki-Matsuda, F.; Uehara, A.; Wada, T.; Handa, H.; Satoh, M.S. Poly(ADP-Ribose) Polymerase-1 Is a Negative Regulator of HIV-1 Transcription through Competitive Binding to TAR RNA with Tat·Positive Transcription Elongation Factor b (p-TEFb) Complex. J. Biol. Chem. 2005, 280, 448–457. [Google Scholar] [CrossRef]
- Bueno, M.T.D.; Reyes, D.; Valdes, L.; Saheba, A.; Urias, E.; Mendoza, C.; Fregoso, O.I.; Llano, M. Poly(ADP-Ribose) Polymerase 1 Promotes Transcriptional Repression of Integrated Retroviruses. J. Virol. 2013, 87, 2496–2507. [Google Scholar] [CrossRef]
- Gutierrez, D.A.; Valdes, L.; Serguera, C.; Llano, M. Poly(ADP-Ribose) Polymerase-1 Silences Retroviruses Independently of Viral DNA Integration or Heterochromatin Formation. J. Gen. Virol. 2016, 97, 1686–1692. [Google Scholar] [CrossRef][Green Version]
- Kameoka, M.; Nukuzuma, S.; Itaya, A.; Tanaka, Y.; Ota, K.; Inada, Y.; Ikuta, K.; Yoshihara, K. Poly(ADP-Ribose)Polymerase-1 Is Required for Integration of the Human Immunodeficiency Virus Type 1 Genome near Centromeric Alphoid DNA in Human and Murine Cells. Biochem. Biophys. Res. Commun. 2005, 334, 412–417. [Google Scholar] [CrossRef]
- Ha, H.C.; Juluri, K.; Zhou, Y.; Leung, S.; Hermankova, M.; Snyder, S.H. Poly(ADP-Ribose) Polymerase-1 Is Required for Efficient HIV-1 Integration. Proc. Natl. Acad. Sci. USA 2001, 98, 3364–3368. [Google Scholar] [CrossRef] [PubMed]
- Ariumi, Y.; Turelli, P.; Masutani, M.; Trono, D. DNA Damage Sensors ATM, ATR, DNA-PKcs, and PARP-1 Are Dispensable for Human Immunodeficiency Virus Type 1 Integration. J. Virol. 2005, 79, 2973–2978. [Google Scholar] [CrossRef] [PubMed]
- Siva, A.C.; Bushman, F. Poly(ADP-Ribose) Polymerase 1 Is Not Strictly Required for Infection of Murine Cells by Retroviruses. J. Virol. 2002, 76, 11904–11910. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.-Y.; Sanchez, D.J.; Aliyari, R.; Lu, S.; Cheng, G. Systematic Identification of Type I and Type II Interferon-Induced Antiviral Factors. Proc. Natl. Acad. Sci. USA 2012, 109, 4239–4244. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Wang, X.; Goff, S.P.; Gao, G. Translational Repression Precedes and Is Required for ZAP-Mediated MRNA Decay: ZAP-Mediated Translational Repression versus MRNA Decay. EMBO J. 2012, 31, 4236–4246. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.; Wang, X.; Tu, F.; Wang, Q.; Fan, Z.; Gao, G. TRIM25 Is Required for the Antiviral Activity of Zinc Finger Antiviral Protein. J. Virol. 2017, 91. [Google Scholar] [CrossRef] [PubMed]
- Li, M.M.H.; Lau, Z.; Cheung, P.; Aguilar, E.G.; Schneider, W.M.; Bozzacco, L.; Molina, H.; Buehler, E.; Takaoka, A.; Rice, C.M.; et al. TRIM25 Enhances the Antiviral Action of Zinc-Finger Antiviral Protein (ZAP). PLoS Pathog. 2017, 13. [Google Scholar] [CrossRef]
- Ohlmann, T.; Mengardi, C.; López-Lastra, M. Translation Initiation of the HIV-1 MRNA. Translation 2014, 2. [Google Scholar] [CrossRef] [PubMed]
- Atasheva, S.; Frolova, E.I.; Frolov, I. Interferon-Stimulated Poly(ADP-Ribose) Polymerases Are Potent Inhibitors of Cellular Translation and Virus Replication. J. Virol. 2014, 88, 2116–2130. [Google Scholar] [CrossRef]
- Atasheva, S.; Akhrymuk, M.; Frolova, E.I.; Frolov, I. New PARP Gene with an Anti-Alphavirus Function. J. Virol. 2012, 86, 8147–8160. [Google Scholar] [CrossRef] [PubMed]
- Welsby, I.; Hutin, D.; Gueydan, C.; Kruys, V.; Rongvaux, A.; Leo, O. PARP12, an Interferon-Stimulated Gene Involved in the Control of Protein Translation and Inflammation. J. Biol. Chem. 2014, 289, 26642–26657. [Google Scholar] [CrossRef] [PubMed]
- Aguiar, R.C.T.; Takeyama, K.; He, C.; Kreinbrink, K.; Shipp, M.A. B-Aggressive Lymphoma Family Proteins Have Unique Domains That Modulate Transcription and Exhibit Poly(ADP-Ribose) Polymerase Activity. J. Biol. Chem. 2005, 280, 33756–33765. [Google Scholar] [CrossRef]
- Takeyama, K.; Aguiar, R.C.T.; Gu, L.; He, C.; Freeman, G.J.; Kutok, J.L.; Aster, J.C.; Shipp, M.A. The BAL-Binding Protein BBAP and Related Deltex Family Members Exhibit Ubiquitin-Protein Isopeptide Ligase Activity. J. Biol. Chem. 2003, 278, 21930–21937. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.-S.; Jividen, K.; Spencer, A.; Dworak, N.; Ni, L.; Oostdyk, L.T.; Chatterjee, M.; Kuśmider, B.; Reon, B.; Parlak, M.; et al. Ubiquitin Modification by the E3 Ligase/ADP-Ribosyltransferase Dtx3L/Parp9. Mol. Cell 2017, 66, 503–516.e5. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Mao, D.; Roswit, W.T.; Jin, X.; Patel, A.C.; Patel, D.A.; Agapov, E.; Wang, Z.; Tidwell, R.M.; Atkinson, J.J.; et al. PARP9-DTX3L Ubiquitin Ligase Targets Host Histone H2BJ and Viral 3C Protease to Enhance Interferon Signaling and Control Viral Infection. Nat. Immunol. 2015, 16, 1215–1227. [Google Scholar] [CrossRef] [PubMed]
- Yu, M.; Zhang, C.; Yang, Y.; Yang, Z.; Zhao, L.; Xu, L.; Wang, R.; Zhou, X.; Huang, P. The Interaction between the PARP10 Protein and the NS1 Protein of H5N1 AIV and Its Effect on Virus Replication. Virol. J. 2011, 8, 546. [Google Scholar] [CrossRef]
- Li, L.; Zhao, H.; Liu, P.; Li, C.; Quanquin, N.; Ji, X.; Sun, N.; Du, P.; Qin, C.-F.; Lu, N.; et al. PARP12 Suppresses Zika Virus Infection through PARP-Dependent Degradation of NS1 and NS3 Viral Proteins. Sci. Signal. 2018, 11, eaas9332. [Google Scholar] [CrossRef]
- Rosado, M.M.; Bennici, E.; Novelli, F.; Pioli, C. Beyond DNA Repair, the Immunological Role of PARP-1 and Its Siblings. Immunology 2013, 139, 428–437. [Google Scholar] [CrossRef]
- Bai, P.; Virág, L. Role of Poly(ADP-Ribose) Polymerases in the Regulation of Inflammatory Processes. FEBS Lett. 2012, 586, 3771–3777. [Google Scholar] [CrossRef]
- Laudisi, F.; Sambucci, M.; Pioli, C. Poly (ADP-Ribose) Polymerase-1 (PARP-1) as Immune Regulator. Endocr. Metab. Immune Disord. Drug Targets 2011, 11, 326–333. [Google Scholar] [CrossRef] [PubMed]
- Yélamos, J.; Moreno-Lama, L.; Jimeno, J.; Ali, S.O. Immunomodulatory Roles of PARP-1 and PARP-2: Impact on PARP-Centered Cancer Therapies. Cancers 2020, 12, 392. [Google Scholar] [CrossRef]
- Xia, C.; Wolf, J.J.; Sun, C.; Xu, M.; Studstill, C.J.; Chen, J.; Ngo, H.; Zhu, H.; Hahm, B. PARP1 Enhances Influenza A Virus Propagation by Facilitating Degradation of Host Type I Interferon Receptor. J. Virol. 2020, 94. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Li, Q.; Li, W.; Xie, L.; Zhou, M.; Xie, J. The Role of PARP-1 in Host−Pathogen Interaction and Cellular Stress Responses. CRE 2015, 25. [Google Scholar] [CrossRef]
- Guo, T.; Zuo, Y.; Qian, L.; Liu, J.; Yuan, Y.; Xu, K.; Miao, Y.; Feng, Q.; Chen, X.; Jin, L.; et al. ADP-Ribosyltransferase PARP11 Modulates the Interferon Antiviral Response by Mono-ADP-Ribosylating the Ubiquitin E3 Ligase β-TrCP. Nat. Microbiol. 2019, 4, 1872–1884. [Google Scholar] [CrossRef] [PubMed]
- Hayakawa, S.; Shiratori, S.; Yamato, H.; Kameyama, T.; Kitatsuji, C.; Kashigi, F.; Goto, S.; Kameoka, S.; Fujikura, D.; Yamada, T.; et al. ZAPS Is a Potent Stimulator of Signaling Mediated by the RNA Helicase RIG-I during Antiviral Responses. Nat. Immunol. 2011, 12, 37–44. [Google Scholar] [CrossRef]
- Yamada, T.; Horimoto, H.; Kameyama, T.; Hayakawa, S.; Yamato, H.; Dazai, M.; Takada, A.; Kida, H.; Bott, D.; Zhou, A.C.; et al. Constitutive Aryl Hydrocarbon Receptor Signaling Constrains Type I Interferon–Mediated Antiviral Innate Defense. Nat. Immunol. 2016, 17, 687–694. [Google Scholar] [CrossRef]
- Verheugd, P.; Forst, A.H.; Milke, L.; Herzog, N.; Feijs, K.L.H.; Kremmer, E.; Kleine, H.; Lüscher, B. Regulation of NF-ΚB Signalling by the Mono-ADP-Ribosyltransferase ARTD10. Nat. Commun. 2013, 4, 1683. [Google Scholar] [CrossRef]
- Juszczynski, P.; Kutok, J.L.; Li, C.; Mitra, J.; Aguiar, R.C.T.; Shipp, M.A. BAL1 and BBAP Are Regulated by a Gamma Interferon-Responsive Bidirectional Promoter and Are Overexpressed in Diffuse Large B-Cell Lymphomas with a Prominent Inflammatory Infiltrate. MCB 2006, 26, 5348–5359. [Google Scholar] [CrossRef]
- Mehrotra, P.; Riley, J.P.; Patel, R.; Li, F.; Voss, L.; Goenka, S. PARP-14 Functions as a Transcriptional Switch for Stat6-Dependent Gene Activation. J. Biol. Chem. 2011, 286, 1767–1776. [Google Scholar] [CrossRef]
- Iwata, H.; Goettsch, C.; Sharma, A.; Ricchiuto, P.; Goh, W.W.B.; Halu, A.; Yamada, I.; Yoshida, H.; Hara, T.; Wei, M.; et al. PARP9 and PARP14 Cross-Regulate Macrophage Activation via STAT1 ADP-Ribosylation. Nat. Commun. 2016, 7, 12849. [Google Scholar] [CrossRef]
- Kaplan, M.H.; Schindler, U.; Smiley, S.T.; Grusby, M.J. Stat6 Is Required for Mediating Responses to IL-4 and for the Development of Th2 Cells. Immunity 1996, 4, 313–319. [Google Scholar] [CrossRef]
- Mehrotra, P.; Hollenbeck, A.; Riley, J.P.; Li, F.; Patel, R.J.; Akhtar, N.; Goenka, S. Poly (ADP-Ribose) Polymerase 14 and Its Enzyme Activity Regulates TH2 Differentiation and Allergic Airway Disease. J. Allergy Clin. Immunol. 2013, 131, 521–531.e12. [Google Scholar] [CrossRef]
- Pehrson, J.R.; Fuji, R.N. Evolutionary Conservation of Histone MacroH2A Subtypes and Domains. Nucleic Acids Res. 1998, 26, 2837–2842. [Google Scholar] [CrossRef]
- Neuvonen, M.; Ahola, T. Differential Activities of Cellular and Viral Macro Domain Proteins in Binding of ADP-Ribose Metabolites. J. Mol. Biol. 2009, 385, 212–225. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Debing, Y.; Jankevicius, G.; Neyts, J.; Ahel, I.; Coutard, B.; Canard, B. Viral Macro Domains Reverse Protein ADP-Ribosylation. J. Virol. 2016, 90, 8478–8486. [Google Scholar] [CrossRef]
- Alhammad, Y.M.O.; Fehr, A.R. The Viral Macrodomain Counters Host Antiviral ADP-Ribosylation. Viruses 2020, 12, 384. [Google Scholar] [CrossRef] [PubMed]
- Abraham, R.; Hauer, D.; McPherson, R.L.; Utt, A.; Kirby, I.T.; Cohen, M.S.; Merits, A.; Leung, A.K.L.; Griffin, D.E. ADP-Ribosyl–Binding and Hydrolase Activities of the Alphavirus NsP3 Macrodomain Are Critical for Initiation of Virus Replication. Proc. Natl. Acad. Sci. USA 2018, 115, E10457–E10466. [Google Scholar] [CrossRef] [PubMed]
- Fehr, A.R.; Athmer, J.; Channappanavar, R.; Phillips, J.M.; Meyerholz, D.K.; Perlman, S. The Nsp3 Macrodomain Promotes Virulence in Mice with Coronavirus-Induced Encephalitis. J. Virol. 2014, 89, 1523–1536. [Google Scholar] [CrossRef] [PubMed]
- Grunewald, M.E.; Chen, Y.; Kuny, C.; Maejima, T.; Lease, R.; Ferraris, D.; Aikawa, M.; Sullivan, C.S.; Perlman, S.; Fehr, A.R. The Coronavirus Macrodomain Is Required to Prevent PARP-Mediated Inhibition of Virus Replication and Enhancement of IFN Expression. PLoS Pathog. 2019, 15, e1007756. [Google Scholar] [CrossRef] [PubMed]
- Lei, X.; Sun, Z.; Liu, X.; Jin, Q.; He, B.; Wang, J. Cleavage of the Adaptor Protein TRIF by Enterovirus 71 3C Inhibits Antiviral Responses Mediated by Toll-like Receptor 3. J. Virol. 2011, 85, 8811–8818. [Google Scholar] [CrossRef]
- Lei, X.; Han, N.; Xiao, X.; Jin, Q.; He, B.; Wang, J. Enterovirus 71 3C Inhibits Cytokine Expression through Cleavage of the TAK1/TAB1/TAB2/TAB3 Complex. J. Virol. 2014, 88, 9830–9841. [Google Scholar] [CrossRef]
- Xie, L.; Lu, B.; Zheng, Z.; Miao, Y.; Liu, Y.; Zhang, Y.; Zheng, C.; Ke, X.; Hu, Q.; Wang, H. The 3C Protease of Enterovirus A71 Counteracts the Activity of Host Zinc-Finger Antiviral Protein (ZAP). J. Gen. Virol. 2018, 99, 73–85. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.-T.; Usherwood, E.J.; Stewart, J.P.; Nash, A.A.; Sun, R. Rta of Murine Gammaherpesvirus 68 Reactivates the Complete Lytic Cycle from Latency. J. Virol. 2000, 74, 3659–3667. [Google Scholar] [CrossRef]
- Cheong, W.-C.; Park, J.-H.; Kang, H.-R.; Song, M.J. Downregulation of Poly(ADP-Ribose) Polymerase 1 by a Viral Processivity Factor Facilitates Lytic Replication of Gammaherpesvirus. J. Virol. 2015, 89, 9676–9682. [Google Scholar] [CrossRef][Green Version]
- Li, Z.; Yamauchi, Y.; Kamakura, M.; Murayama, T.; Goshima, F.; Kimura, H.; Nishiyama, Y. Herpes Simplex Virus Requires Poly(ADP-Ribose) Polymerase Activity for Efficient Replication and Induces Extracellular Signal-Related Kinase-Dependent Phosphorylation and ICP0-Dependent Nuclear Localization of Tankyrase 1. J. Virol. 2012, 86, 492–503. [Google Scholar] [CrossRef] [PubMed]
- Su, C.; Zhang, J.; Zheng, C. Herpes Simplex Virus 1 UL41 Protein Abrogates the Antiviral Activity of HZAP by Degrading Its MRNA. Virol. J. 2015, 12. [Google Scholar] [CrossRef]
- Laukoter, S.; Rauschka, H.; Tröscher, A.R.; Köck, U.; Saji, E.; Jellinger, K.; Lassmann, H.; Bauer, J. Differences in T Cell Cytotoxicity and Cell Death Mechanisms between Progressive Multifocal Leukoencephalopathy, Herpes Simplex Virus Encephalitis and Cytomegalovirus Encephalitis. Acta Neuropathol. 2017, 133, 613–627. [Google Scholar] [CrossRef]
- Xu, G.; Li, S.; Liu, X.; Gao, P.; Chen, X.; Wang, H.; Zhang, M.; Yang, Y.; Gao, G.F.; Zhang, F. PARP-1 Mediated Cell Death Is Directly Activated by ZIKV Infection. Virology 2019, 537, 254–262. [Google Scholar] [CrossRef]
- Nebenzahl-Sharon, K.; Sharf, R.; Amer, J.; Shalata, H.; Khoury-Haddad, H.; Sohn, S.-Y.; Ayoub, N.; Hearing, P.; Kleinberger, T. An Interaction with PARP-1 and Inhibition of Parylation Contribute to Attenuation of DNA Damage Signaling by the Adenovirus E4orf4 Protein. J. Virol. 2019, 93. [Google Scholar] [CrossRef]

| Name | Other Names | PARP Activity | PARP Subfamilies | Characteristic Domains | Antiviral Activity | Viruses Targeted |
|---|---|---|---|---|---|---|
| PARP1 | ARTD1 | PARylation | DNA-dependent PARPS | BRCT, WGR | Transcription and replication inhibition | EBV, HIV, KSHV, MLV |
| PARP2 | ARTD2 | PARylation | DNA-dependent PARPS | WGR | ND | ND |
| PARP3 | ARTD3 | PARylation | DNA-dependent PARPS | WGR | ND | ND |
| PARP4 | ARTD4 KIAA0177 | MARylation | Unclassified | BRCT | ND | ND |
| PARP5a | ARTD5 TANK1 TIN1 | PARylation | Tankyrases | ANK | Replication inhibition | EBV |
| PARP5b | ARTD6 TANK2 TNKL | PARylation | Tankyrases | ANK | Replication inhibition | EBV |
| PARP6 | ARTD17 | MARylation | Unclassified | HPS | ND | ND |
| PARP7 | ARTD14 TIPARP | MARylation | CCCH PARPs | Zinc-fingers, WWE | Replication and translation inhibition | SINV, Rubella virus, VEEV |
| PARP8 | ARTD16 | MARylation | Unclassified | HPS | ND | ND |
| PARP9 +DTX3L | ARTD9 BAL1 | MARylation | MacroPARPs | Macrodomains | Viral protein degradation | EMCV |
| PARP10 | ARTD10 | MARylation | Unclassified | UIM | Transcription and replication inhibition Viral protein degradation | AIV, VEEV |
| PARP11 | ARTD11 | MARylation | Unclassified | WWE | ND | ND |
| PARP12 | ARTD12 ZC3HDC1 | MARylation | CCCH PARPs | Zinc-fingers, WWE | Transcription and replication inhibition Viral protein degradation | CHIKV, EMCV, RFVF, SINV, VEEV, VSV |
| PARP13 | ZAP ARTD13 ZC3HDC2 | Inactive | CCCH PARPs | Zinc-fingers, WWE | Replication and translation inhibition Viral RNA and protein degradation | HIV, IAV, HBV, SINV, XMRV, Ebola virus, Marburg virus, MHV68 |
| PARP14 | ARTD8 BAL2 | MARylation | MacroPARPs | Macrodomains, WWE | ND | ND |
| PARP15 | ARTD7 BAL3 | MARylation | MacroPARPs | Macrodomains | ND | ND |
| PARP16 | ARTD15 | MARylation | Unclassified | TMD | ND | ND |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Malgras, M.; Garcia, M.; Jousselin, C.; Bodet, C.; Lévêque, N. The Antiviral Activities of Poly-ADP-Ribose Polymerases. Viruses 2021, 13, 582. https://doi.org/10.3390/v13040582
Malgras M, Garcia M, Jousselin C, Bodet C, Lévêque N. The Antiviral Activities of Poly-ADP-Ribose Polymerases. Viruses. 2021; 13(4):582. https://doi.org/10.3390/v13040582
Chicago/Turabian StyleMalgras, Mathilde, Magali Garcia, Clément Jousselin, Charles Bodet, and Nicolas Lévêque. 2021. "The Antiviral Activities of Poly-ADP-Ribose Polymerases" Viruses 13, no. 4: 582. https://doi.org/10.3390/v13040582
APA StyleMalgras, M., Garcia, M., Jousselin, C., Bodet, C., & Lévêque, N. (2021). The Antiviral Activities of Poly-ADP-Ribose Polymerases. Viruses, 13(4), 582. https://doi.org/10.3390/v13040582






