Israeli Rousettus aegyptiacus Pox Virus (IsrRAPXV) Infection in Juvenile Egyptian Fruit Bat (Rousettus aegyptiacus): Clinical Findings and Molecular Detection
Abstract
1. Introduction
2. Materials and Methods
2.1. Bats: Sampling and Processing
2.2. Virus Isolation
2.3. DNA Extraction and PCR Amplification
2.4. Quantitative Real Time PCR (qPCR)
2.5. Histology
3. Results
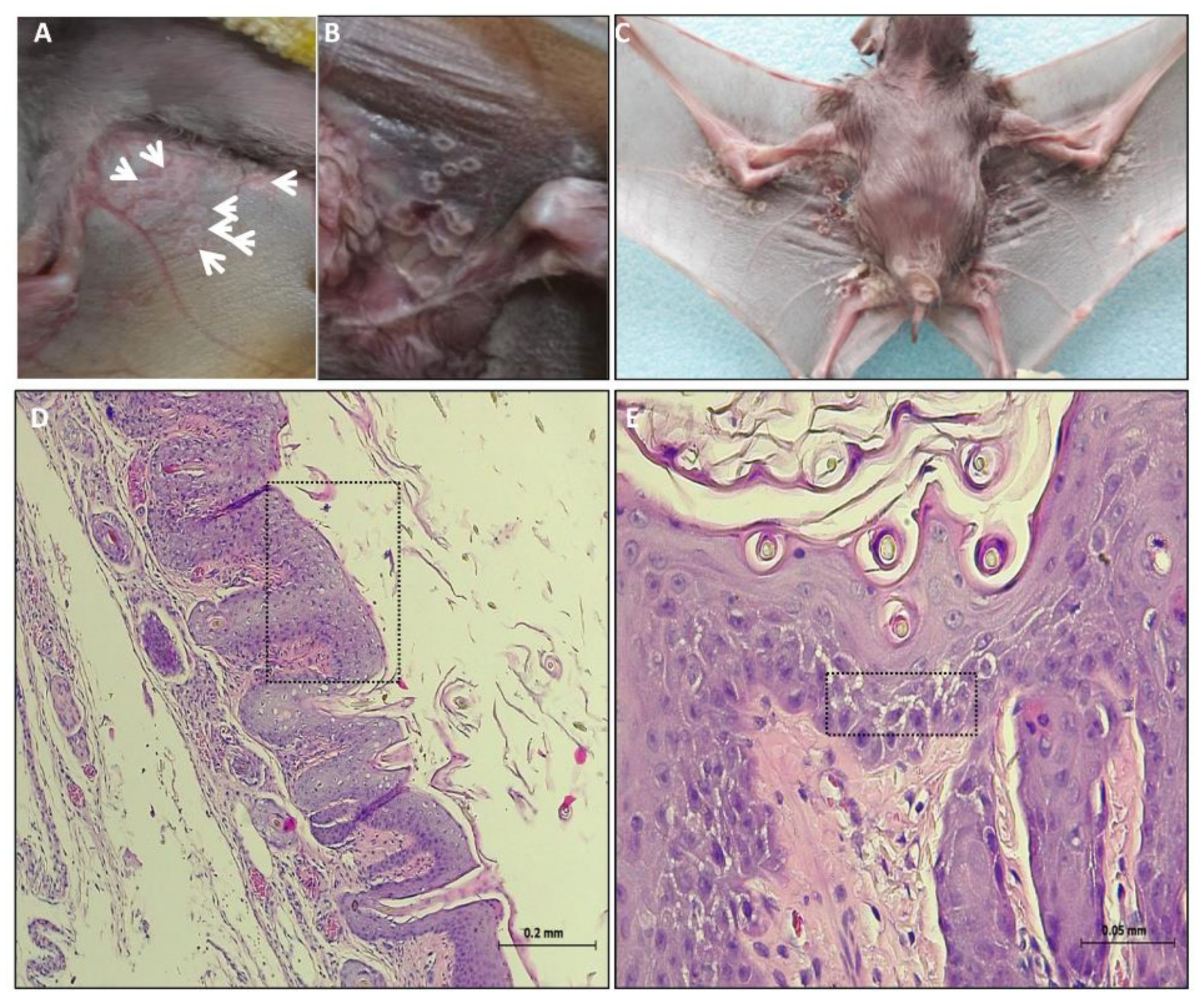
3.1. Clinical Signs and Post Mortem (PM) Examination
3.2. Histopathology
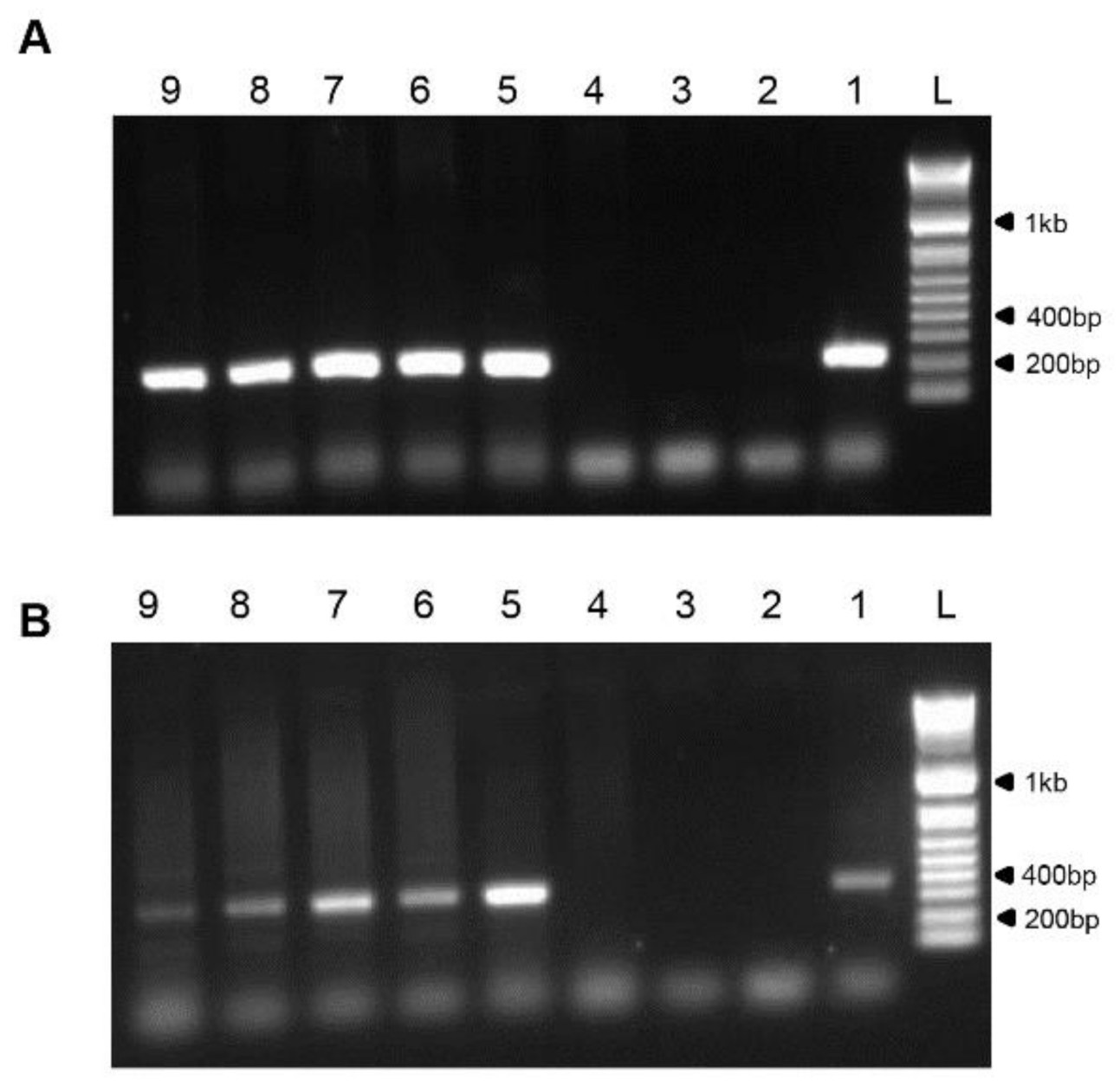
3.3. Molecular Diagnosis of Isolated Viruses by PCR
3.4. Virus Isolation
3.5. Molecular Detection of IsrRAPXV by Novel qPCR
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Drexler, J.F.; Corman, V.M.; Wegner, T.; Tateno, A.F.; Zerbinati, R.M.; Gloza-Rausch, F.; Seebens, A.; Müller, M.A.; Drosten, C. Amplification of emerging viruses in a bat colony. Emerg. Infect. Dis. 2011, 17, 449–456. [Google Scholar] [CrossRef] [PubMed]
- Calisher, C.H.; Childs, J.E.; Field, H.E.; Holmes, K.V.; Schountz, T. Bats: Important reservoir hosts of emerging viruses. Clin. Microbiol. Rev. 2006, 19, 531–545. [Google Scholar] [CrossRef] [PubMed]
- Messenger, S.L.; Rupprecht, C.; Smith, J.S. Bat Ecology; Kunz, T.H., Fenton, M.B., Eds.; Illinois University: Chicago, IL, USA, 2003; pp. 622–679. [Google Scholar]
- Halpin, K.; Hyatt, A.D.; Fogarty, R.; Middleton, D.; Bingham, J.; Epstein, J.H.; Rahman, S.A.; Hughes, T.; Smith, C.; Field, H.E.; et al. Pteropid bats are confirmed as the reservoir hosts of henipaviruses: A comprehensive experimental study of virus transmission. Am. J. Trop. Med. Hyg. 2011, 85, 946–951. [Google Scholar] [CrossRef] [PubMed]
- Amman, B.R.; Bird, B.H.; Bakarr, I.A.; Bangura, J.; Schuh, A.J.; Johnny, J.; Sealy, T.K.; Conteh, I.; Koroma, A.H.; Foday, I.; et al. Isolation of Angola-like Marburg virus from Egyptian rousette bats from West Africa. Nat. Commun. 2020, 11. [Google Scholar] [CrossRef] [PubMed]
- Kuzmin, I.V.; Orciari, L.A.; Arai, Y.T.; Smith, J.S.; Hanlon, C.A.; Kameoka, Y.; Rupprecht, C.E. Bat lyssaviruses (Aravan and Khujand) from Central Asia: Phylogenetic relationships according to N, P and G gene sequences. Virus Res. 2003, 97, 65–79. [Google Scholar] [CrossRef]
- Lau, S.K.P.; Woo, P.C.Y.; Li, K.S.M.; Huang, Y.; Tsoi, H.W.; Wong, B.H.L.; Wong, S.S.Y.; Leung, S.Y.; Chan, K.H.; Yuen, K.Y. Severe acute respiratory syndrome coronavirus-like virus in Chinese horseshoe bats. Proc. Natl. Acad. Sci. USA 2005, 102, 14040–14045. [Google Scholar] [CrossRef] [PubMed]
- Van Boheemen, S.; de Graaf, M.; Lauber, C.; Bestebroer, T.M.; Raj, V.S.; Zaki, A.M.; Osterhaus, A.D.M.E.; Haagmans, B.L.; Gorbalenya, A.E.; Snijder, E.J.; et al. Genomic characterization of a newly discovered coronavirus associated with acute respiratory distress syndrome in humans. MBio 2012, 3. [Google Scholar] [CrossRef] [PubMed]
- Boni, M.F.; Lemey, P.; Jiang, X.; Lam, T.T.Y.; Perry, B.W.; Castoe, T.A.; Rambaut, A.; Robertson, D.L. Evolutionary origins of the SARS-CoV-2 sarbecovirus lineage responsible for the COVID-19 pandemic. Nat. Microbiol. 2020, 5, 1408–1417. [Google Scholar] [CrossRef] [PubMed]
- Yom-Tov, Y. Seeing Sound. Bats-Between Myth and Reality (in Hebrew); Dan Perry Publishing: Jerusalem, Israel, 2018. [Google Scholar]
- Amman, B.R.; Schuh, A.J.; Sealy, T.K.; Spengler, J.R.; Welch, S.R.; Kirejczyk, S.G.M.; Albariño, C.G.; Nichol, S.T.; Towner, J.S. Experimental infection of Egyptian rousette bats (Rousettus aegyptiacus) with Sosuga virus demonstrates potential transmission routes for a bat-borne human pathogenic paramyxovirus. PLoS Negl. Trop. Dis. 2020, 14, e0008092. [Google Scholar] [CrossRef] [PubMed]
- Essbauer, S.; Pfeffer, M.; Meyer, H. Zoonotic poxviruses. Vet. Microbiol. 2010, 140, 229–236. [Google Scholar] [CrossRef] [PubMed]
- Lelli, D.; Lavazza, A.; Prosperi, A.; Sozzi, E.; Faccin, F.; Baioni, L.; Trogu, T.; Cavallari, G.L.; Mauri, M.; Gibellini, A.M.; et al. Hypsugopoxvirus: A novel poxvirus isolated from hypsugo savii in italy. Viruses 2019, 11, 568. [Google Scholar] [CrossRef] [PubMed]
- Emerson, G.L.; Nordhausen, R.; Garner, M.M.; Huckabee, J.R.; Johnson, S.; Wohrle, R.D.; Davidson, W.B.; Wilkins, K.; Li, Y.; Doty, J.B.; et al. Novel poxvirus in big brown bats, northwestern United States. Emerg. Infect. Dis. 2013, 19, 1002–1004. [Google Scholar] [CrossRef] [PubMed]
- David, D.; Davidson, I.; Berkowitz, A.; Karniely, S.; Edery, N.; Bumbarov, V.; Laskar, O.; Elazari-Volcani, R. A novel poxvirus isolated from an Egyptian fruit bat in Israel. Vet. Med. Sci. 2020, 6, 587–590. [Google Scholar] [CrossRef] [PubMed]
- O’Dea, M.A.; Tu, S.L.; Pang, S.; de Ridder, T.; Jackson, B.; Upton, C. Genomic characterization of a novel poxvirus from a flying fox: Evidence for a new genus? J. Gen. Virol. 2016, 97, 2363–2375. [Google Scholar] [CrossRef] [PubMed]
- Tuomi, P.A.; Murray, M.J.; Garner, M.M.; Goertz, C.E.C.; Nordhausen, R.W.; Burek-Huntington, K.A.; Getzy, D.M.; Nielsen, O.; Archer, L.L.; Maness, H.T.D.; et al. Novel poxvirus infection in northern and southern sea otters (enhydra lutris kenyoni and enhydra lutris neiris), Alaska and California, USA. J. Wildland Dis. 2014, 50, 607–615. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Meyer, H.; Zhao, H.; Damon, I.K. GC content-based pan-pox universal PCR assays for poxvirus detection. J. Clin. Microbiol. 2010, 48, 268–276. [Google Scholar] [CrossRef] [PubMed]



| Bat (#) | Gender | Sample Location | Clinical Signs Detection (Date) | Clinical Signs Duration (Days) | Death | Sampling Date (Skin Swab) |
|---|---|---|---|---|---|---|
| 1334 | F | Qiryat Yam | 18/3/19 | 6 | 23/3/19 | 18/3/19 |
| 1374 | F | Qiryat Yam | 23/3/19 | 8 | 30/3/19 | 23/3/19 |
| 1375 | F | Qiryat Yam | 23/3/19 | 8 | 31/3/19 | 2/4/19 |
| 1827 | M | Ramat Gan | 2/8/19 | 6 | 7/8/19 | 15/8/19 |
| 1840 | M | Tel Aviv | NT | NT | 20/8/19 | 25/8/19 |
| Name | Sequence of Primers (5′–3′) | Size (bp) | Reference |
|---|---|---|---|
| Poxpol_556F | GAYTAYAAYWSNYTNTAYCCNAAYGTITG | 338 | [17] |
| Poxpol_673R | RAANCCCATNARNCCRTAIAC | ||
| Insulin metalloproteinase-like | ACACCAAAAACTCATATAACTTCT | 220 | [18] |
| IMV | CCTATTTTACTCCTTAGTAAATGAT | ||
| IsrRAPXV-F | GAGGTGAAGTGTTTAAATTGGTTCG | 102 | This study |
| IsrRAPXV-Probe | FAM-CAAAAGGTAACAATAGACTATTGTT-BHQ1 | ||
| IsrRAPXV-R | TGTAAATCTTATAAATATTGTTTCG | ||
| IsrRAPXV-037F | ATGGAGGTGAAGTGTTTAAATT | 432 | This study |
| IsrRAPXV-037411R | TCAGTGCAATGATAACACCCT |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
David, D.; Davidson, I.; Karniely, S.; Edery, N.; Rosenzweig, A.; Sol, A. Israeli Rousettus aegyptiacus Pox Virus (IsrRAPXV) Infection in Juvenile Egyptian Fruit Bat (Rousettus aegyptiacus): Clinical Findings and Molecular Detection. Viruses 2021, 13, 407. https://doi.org/10.3390/v13030407
David D, Davidson I, Karniely S, Edery N, Rosenzweig A, Sol A. Israeli Rousettus aegyptiacus Pox Virus (IsrRAPXV) Infection in Juvenile Egyptian Fruit Bat (Rousettus aegyptiacus): Clinical Findings and Molecular Detection. Viruses. 2021; 13(3):407. https://doi.org/10.3390/v13030407
Chicago/Turabian StyleDavid, Dan, Irit Davidson, Sharon Karniely, Nir Edery, Ariela Rosenzweig, and Asaf Sol. 2021. "Israeli Rousettus aegyptiacus Pox Virus (IsrRAPXV) Infection in Juvenile Egyptian Fruit Bat (Rousettus aegyptiacus): Clinical Findings and Molecular Detection" Viruses 13, no. 3: 407. https://doi.org/10.3390/v13030407
APA StyleDavid, D., Davidson, I., Karniely, S., Edery, N., Rosenzweig, A., & Sol, A. (2021). Israeli Rousettus aegyptiacus Pox Virus (IsrRAPXV) Infection in Juvenile Egyptian Fruit Bat (Rousettus aegyptiacus): Clinical Findings and Molecular Detection. Viruses, 13(3), 407. https://doi.org/10.3390/v13030407





