The Ecology of Nipah Virus in Bangladesh: A Nexus of Land-Use Change and Opportunistic Feeding Behavior in Bats
Abstract
1. Introduction
2. Materials and Methods
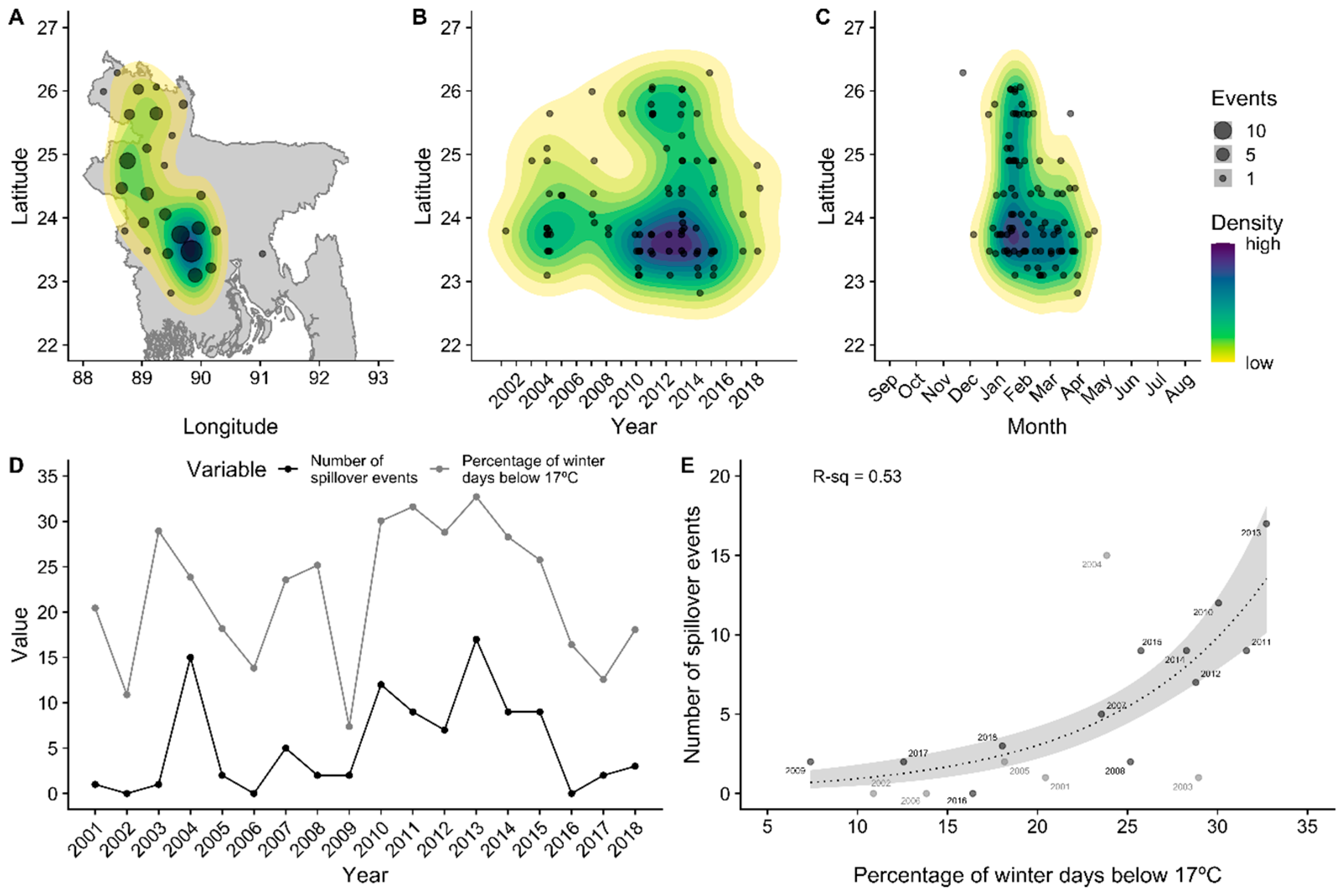
2.1. Nipah Virus Spillover Events
2.2. Climate Data
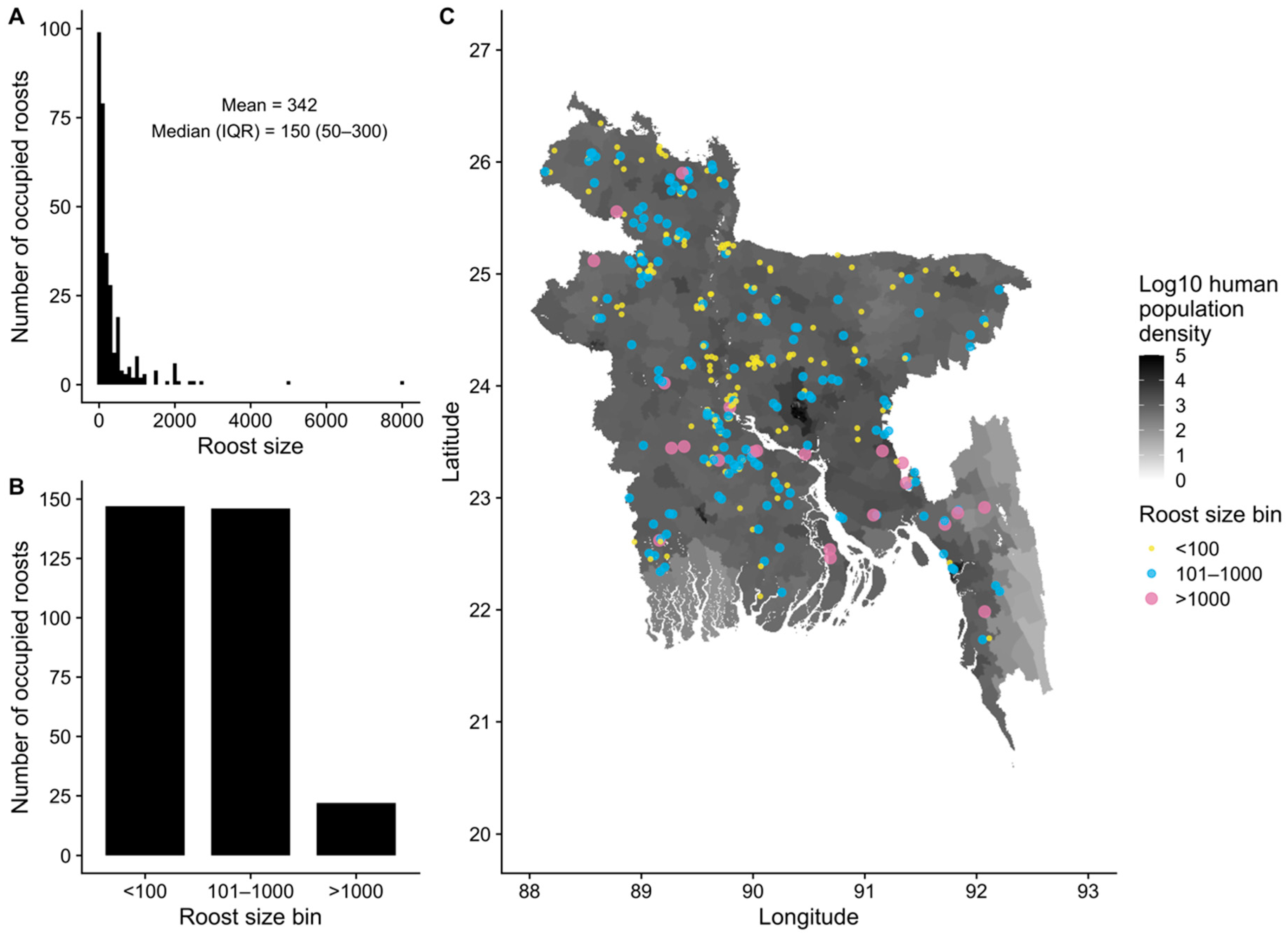
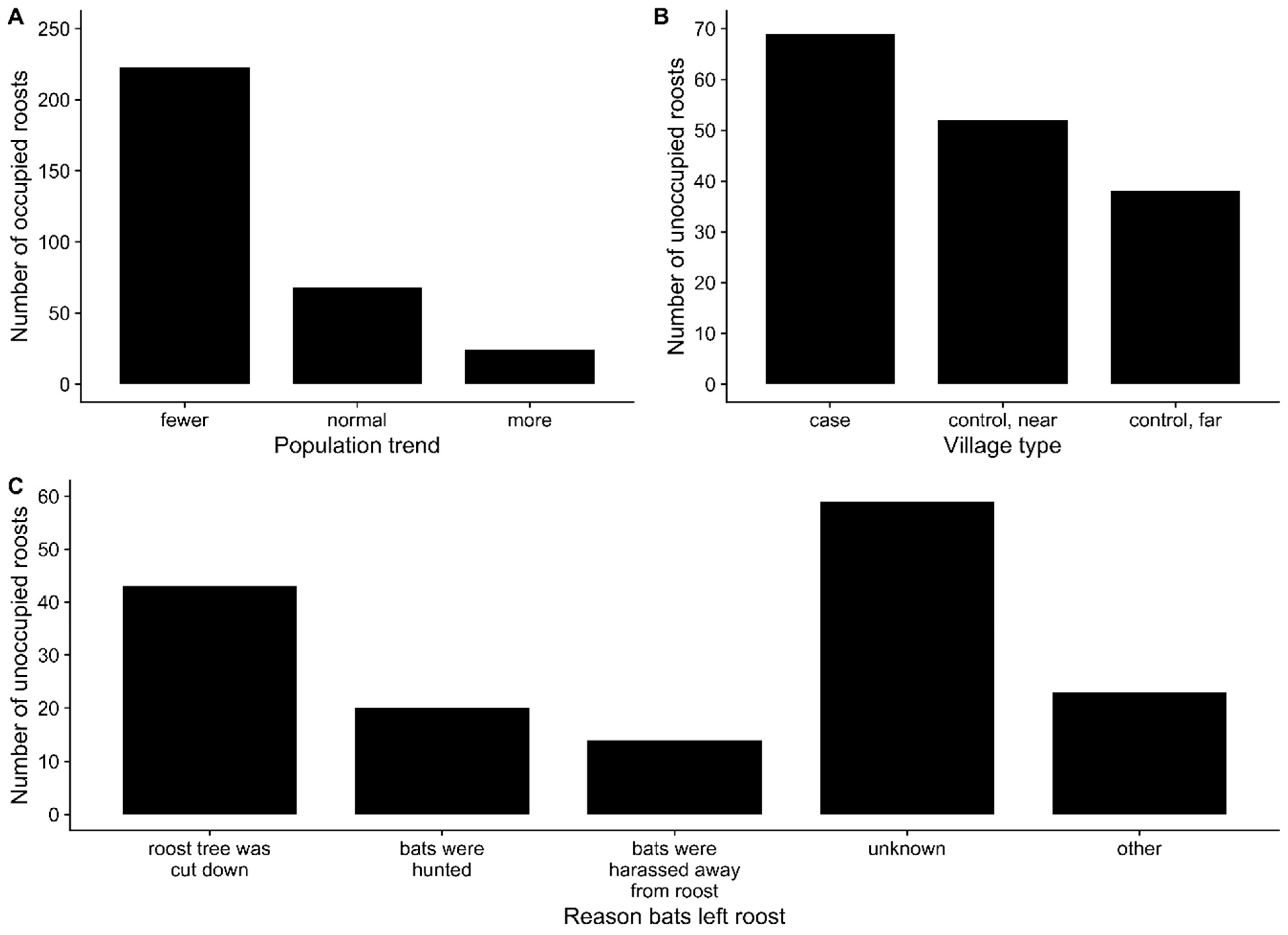
2.3. Survey of Bat Roost Sites and Food Resources
2.4. Spatial Covariates of Bat Roost Sites
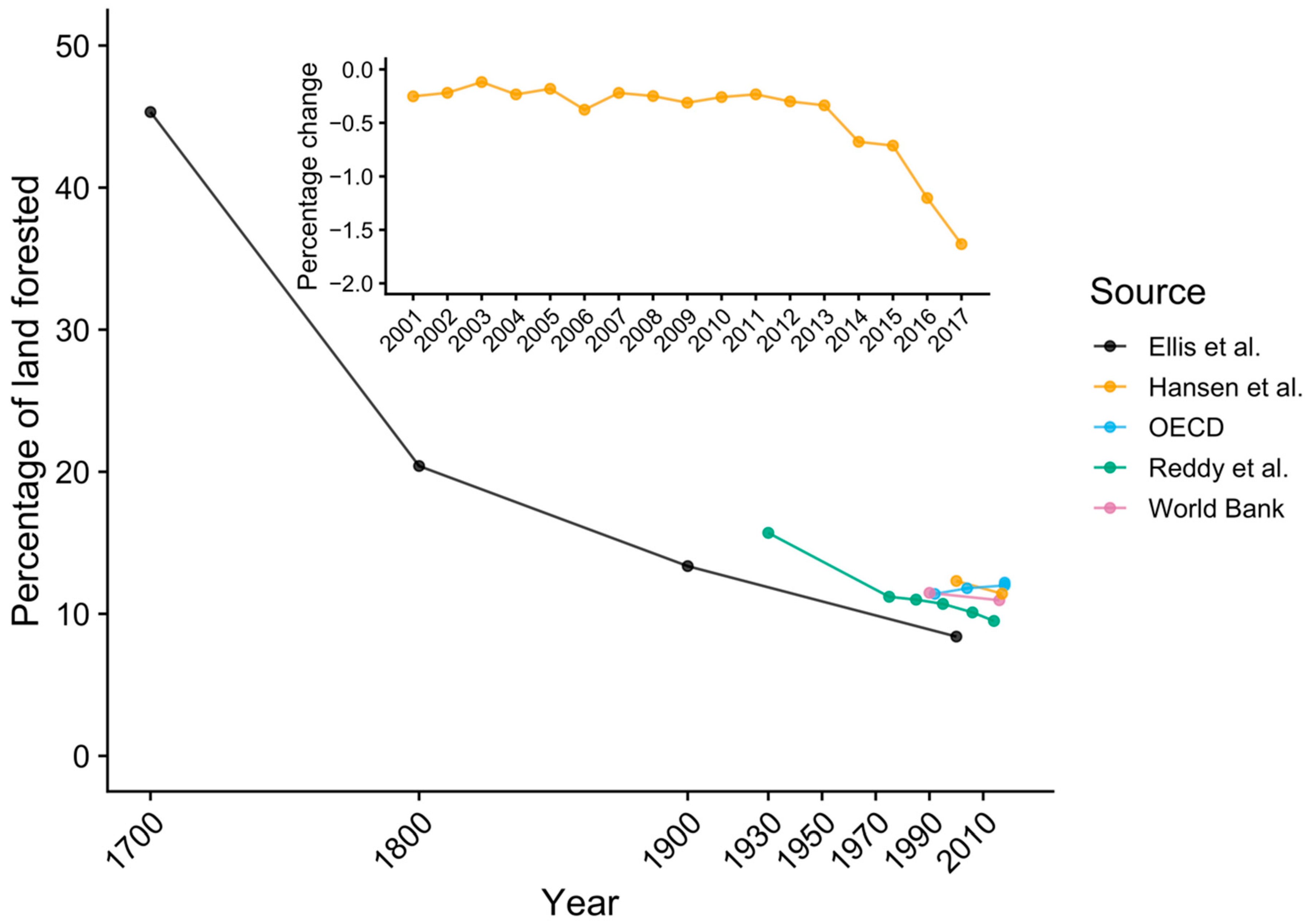
2.5. Historical Land-Use Data
2.6. Statistical Analysis
3. Results
3.1. Spatiotemporal Patterns of Nipah Virus Spillover
3.2. Spatial Distribution and Sizes of Pteropus medius Roosts
3.3. Historical Land-Use Change in Bangladesh
4. Discussion
4.1. Historical Land-Use Change, Bat Ecology, and Nipah Virus Spillover
4.2. Seasonality of Date Palm Sap Consumption and Spillovers
4.3. Roost Tree Loss and Pteropus Roosting Behavior
4.4. Possible Interventions to Prevent Nipah Virus Spillover
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Armstrong, G.L.; Conn, L.A.; Pinner, R.W. Trends in infectious disease mortality in the United States during the 20th century. JAMA 1999, 281, 61–66. [Google Scholar] [CrossRef] [PubMed]
- Cohen, M.L. Changing patterns of infectious disease. Nature 2000, 406, 762–767. [Google Scholar] [CrossRef]
- Murray, C.J.L.; Vos, T.; Lozano, R.; Naghavi, M.; Flaxman, A.D.; Michaud, C.; Ezzati, M.; Shibuya, K.; Salomon, J.A.; Abdalla, S.; et al. Disability-adjusted life years (DALYs) for 291 diseases and injuries in 21 regions, 1990–2010: A systematic analysis for the Global Burden of Disease Study 2010. Lancet 2012, 380, 2197–2223. [Google Scholar] [CrossRef]
- Hansen, V.; Oren, E.; Dennis, L.K.; Brown, H.E. Infectious disease mortality trends in the United States, 1980–2014. JAMA 2016, 316, 2149–2151. [Google Scholar] [CrossRef] [PubMed]
- Woolhouse, M.E.J.; Gowtage-Sequeria, S. Host range and emerging and reemerging pathogens. Emerg. Infect. Dis. 2005, 11, 1842–1847. [Google Scholar] [CrossRef]
- Jones, K.E.; Patel, N.G.; Levy, M.A.; Storeygard, A.; Balk, D.; Gittleman, J.L.; Daszak, P. Global trends in emerging infectious diseases. Nature 2008, 451, 990–993. [Google Scholar] [CrossRef]
- Plowright, R.K.; Parrish, C.R.; McCallum, H.; Hudson, P.J.; Ko, A.I.; Graham, A.L.; Lloyd-Smith, J.O. Pathways to zoonotic spillover. Nat. Rev. Microbiol. 2017, 15, 502–510. [Google Scholar] [CrossRef]
- Daszak, P.; Olival, K.J.; Li, H. A strategy to prevent future epidemics similar to the 2019-nCoV outbreak. Biosaf. Health 2020, 2, 6–8. [Google Scholar] [CrossRef]
- Hsu, V.P.; Hossain, M.J.; Parashar, U.D.; Ali, M.M.; Ksiazek, T.G.; Kuzmin, I.; Niezgoda, M.; Rupprecht, C.; Bresee, J.; Breiman, R.F. Nipah virus encephalitis reemergence, Bangladesh. Emerg. Infect. Dis. 2004, 10, 2082–2087. [Google Scholar] [CrossRef]
- Olival, K.J.; Latinne, A.; Islam, A.; Epstein, J.H.; Hersch, R.; Engstrand, R.C.; Gurley, E.S.; Amato, G.; Luby, S.P.; Daszak, P. Population genetics of fruit bat reservoir informs the dynamics, distribution and diversity of Nipah virus. Mol. Ecol. 2020, 29, 970–985. [Google Scholar] [CrossRef]
- Chua, K.B.; Koh, C.L.; Hooi, P.S.; Wee, K.F.; Khong, J.H.; Chua, B.H.; Chan, Y.P.; Lim, M.E.; Lam, S.K. Isolation of Nipah virus from Malaysian island flying-foxes. Microbes Infect. 2002, 4, 145–151. [Google Scholar] [CrossRef]
- Rahman, S.A.; Hassan, S.S.; Olival, K.J.; Mohamed, M.; Chang, L.-Y.; Hassan, L.; Saad, N.M.; Shohaimi, S.A.; Mamat, Z.C.; Naim, M.S.; et al. Characterization of Nipah virus from naturally infected Pteropus vampyrus bats, Malaysia. Emerg. Infect. Dis. 2010, 16, 1990–1993. [Google Scholar] [CrossRef] [PubMed]
- Sendow, I.; Field, H.E.; Adjid, A.; Ratnawati, A.; Breed, A.C.; Darminto; Morrissy, C.; Daniels, P. Screening for Nipah virus infection in West Kalimantan province, Indonesia. Zoonoses Public Health 2010, 57, 499–503. [Google Scholar] [CrossRef] [PubMed]
- Breed, A.C.; Meers, J.; Sendow, I.; Bossart, K.N.; Barr, J.A.; Smith, I.; Wacharapluesadee, S.; Wang, L.; Field, H.E. The distribution of henipaviruses in Southeast Asia and Australasia: Is Wallace’s line a barrier to Nipah virus? PLoS ONE 2013, 8, e61316. [Google Scholar] [CrossRef]
- Breed, A.C.; Yu, M.; Barr, J.A.; Crameri, G.; Thalmann, C.M.; Wang, L.-F. Prevalence of henipavirus and rubulavirus antibodies in pteropid bats, Papua New Guinea. Emerg. Infect. Dis. 2010, 16, 1997–1999. [Google Scholar] [CrossRef]
- Field, H.; de Jong, C.E.; Halpin, K.; Smith, C.S. Henipaviruses and fruit bats, Papua New Guinea. Emerg. Infect. Dis. 2013, 19, 670–671. [Google Scholar] [CrossRef]
- Wacharapluesadee, S.; Boongird, K.; Wanghongsa, S.; Ratanasetyuth, N.; Supavonwong, P.; Saengsen, D.; Gongal, G.N.; Hemachudha, T. A longitudinal study of the prevalence of Nipah virus in Pteropus lylei bats in Thailand: Evidence for seasonal preference in disease transmission. Vector Borne Zoonotic Dis. 2010, 10, 183–190. [Google Scholar] [CrossRef]
- Wacharapluesadee, S.; Samseeneam, P.; Phermpool, M.; Kaewpom, T.; Rodpan, A.; Maneeorn, P.; Srongmongkol, P.; Kanchanasaka, B.; Hemachudha, T. Molecular characterization of Nipah virus from Pteropus hypomelanus in Southern Thailand. Virol. J. 2016, 13, 53. [Google Scholar] [CrossRef]
- Reynes, J.-M.; Counor, D.; Ong, S.; Faure, C.; Seng, V.; Molia, S.; Walston, J.; Georges-Courbot, M.C.; Deubel, V.; Sarthou, J.-L. Nipah virus in Lyle’s flying foxes, Cambodia. Emerg. Infect. Dis. 2005, 11, 1042–1047. [Google Scholar] [CrossRef]
- Cappelle, J.; Hoem, T.; Hul, V.; Furey, N.; Nguon, K.; Prigent, S.; Dupon, L.; Ken, S.; Neung, C.; Hok, V.; et al. Nipah virus circulation at human–bat interfaces, Cambodia. Bull. World Health Organ. 2020, 98, 539–547. [Google Scholar] [CrossRef]
- Yob, J.M.; Field, H.; Rashdi, A.M.; Morrissy, C.; van der Heide, B.; Rota, P.; bin Adzhar, A.; White, J.; Daniels, P.; Jamaluddin, A.; et al. Nipah virus infection in bats (order Chiroptera) in peninsular Malaysia. Emerg. Infect. Dis. 2001, 7, 439–441. [Google Scholar] [CrossRef] [PubMed]
- Ching, P.K.G.; de los Reyes, V.C.; Sucaldito, M.N.; Tayag, E.; Columna-Vingno, A.B.; Malbas, F.F.; Bolo, G.C.; Sejvar, J.J.; Eagles, D.; Playford, G.; et al. Outbreak of henipavirus infection, Philippines, 2014. Emerg. Infect. Dis. 2015, 21, 328–331. [Google Scholar] [CrossRef] [PubMed]
- Halpin, K.; Young, P.L.; Mackenzie, J.S.; Field, H.E. Isolation of Hendra virus from pteropid bats: A natural reservoir of Hendra virus. J. Gen. Virol. 2000, 81, 1927–1932. [Google Scholar] [CrossRef] [PubMed]
- Marsh, G.A.; de Jong, C.; Barr, J.A.; Tachedjian, M.; Smith, C.; Middleton, D.; Yu, M.; Todd, S.; Foord, A.J.; Haring, V.; et al. Cedar virus: A novel henipavirus isolated from Australian bats. PLoS Pathog. 2012, 8, e1002836. [Google Scholar] [CrossRef]
- Brook, C.E.; Ranaivoson, H.C.; Broder, C.C.; Cunningham, A.A.; Héraud, J.; Peel, A.J.; Gibson, L.; Wood, J.L.N.; Metcalf, C.J.; Dobson, A.P. Disentangling serology to elucidate henipa- and filovirus transmission in Madagascar fruit bats. J. Anim. Ecol. 2019, 88, 1001–1016. [Google Scholar] [CrossRef]
- Drexler, J.F.; Corman, V.M.; Müller, M.A.; Maganga, G.D.; Vallo, P.; Binger, T.; Gloza-Rausch, F.; Rasche, A.; Yordanov, S.; Seebens, A.; et al. Bats host major mammalian paramyxoviruses. Nat. Commun. 2012, 3, 796. [Google Scholar] [CrossRef]
- Peel, A.J.; Sargan, D.R.; Baker, K.S.; Hayman, D.T.S.; Barr, J.A.; Crameri, G.; Suu-Ire, R.D.; Broder, C.C.; Lembo, T.; Wang, L.-F.; et al. Continent-wide panmixia of an African fruit bat facilitates transmission of potentially zoonotic viruses. Nat. Commun. 2013, 4, 2770. [Google Scholar] [CrossRef]
- Kessler, M.K.; Becker, D.J.; Peel, A.J.; Justice, N.V.; Lunn, T.; Crowley, D.E.; Jones, D.N.; Eby, P.; Sánchez, C.A.; Plowright, R.K. Changing resource landscapes and spillover of henipaviruses. Ann. N. Y. Acad. Sci. 2018, 1429, 78–99. [Google Scholar] [CrossRef]
- Williamson, M.M.; Hooper, P.T.; Selleck, P.W.; Westbury, H.A.; Slocombe, R.F. Experimental Hendra virus infection in pregnant guinea-pigs and fruit bats (Pteropus poliocephalus). J. Comp. Pathol. 2000, 122, 201–207. [Google Scholar] [CrossRef]
- Halpin, K.; Hyatt, A.D.; Fogarty, R.; Middleton, D.; Bingham, J.; Epstein, J.H.; Rahman, S.A.; Hughes, T.; Smith, C.; Field, H.E.; et al. Pteropid bats are confirmed as the reservoir hosts of henipaviruses: A comprehensive experimental study of virus transmission. Am. J. Trop. Med. Hyg. 2011, 85, 946–951. [Google Scholar] [CrossRef]
- Almeida, F.C.; Giannini, N.P.; Simmons, N.B.; Helgen, K.M. Each flying fox on its own branch: A phylogenetic tree for Pteropus and related genera (Chiroptera: Pteropodidae). Mol. Phylogenet. Evol. 2014, 77, 83–95. [Google Scholar] [CrossRef] [PubMed]
- Almeida, F.C.; Giannini, N.P.; Simmons, N.B. The evolutionary history of the African fruit bats (Chiroptera: Pteropodidae). Acta Chiropterol. 2016, 18, 73–90. [Google Scholar] [CrossRef]
- Almeida, F.C.; Simmons, N.B.; Giannini, N.P. A species-level phylogeny of Old World fruit bats with a new higher-level classification of the family Pteropodidae. Am. Mus. Novit. 2020, 2020, 1–24. [Google Scholar] [CrossRef]
- Tsang, S.M.; Wiantoro, S.; Veluz, M.J.; Sugita, N.; Nguyen, Y.; Simmons, N.B.; Lohman, D.J. Dispersal out of Wallacea spurs diversification of Pteropus flying foxes, the world’s largest bats (Mammalia: Chiroptera). J. Biogeogr. 2019, 47, 527–537. [Google Scholar] [CrossRef]
- Stribna, T.; Romportl, D.; Demjanovič, J.; Vogeler, A.; Tschapka, M.; Benda, P.; Horáček, I.; Juste, J.; Goodman, S.M.; Hulva, P. Pan African phylogeography and palaeodistribution of rousettine fruit bats: Ecogeographic correlation with Pleistocene climate vegetation cycles. J. Biogeogr. 2019, 46, 2336–2349. [Google Scholar] [CrossRef]
- Chua, K.B.; Goh, K.J.; Wong, K.T.; Kamarulzaman, A.; Tan, P.S.K.; Ksiazek, T.G.; Zaki, S.R.; Paul, G.; Lam, S.K.; Tan, C.T. Fatal encephalitis due to Nipah virus among pig-farmers in Malaysia. Lancet 1999, 354, 1257–1259. [Google Scholar] [CrossRef]
- Paton, N.I.; Leo, Y.S.; Zaki, S.R.; Auchus, A.P.; Lee, K.E.; Ling, A.E.; Chew, S.K.; Ang, B.; Rollin, P.E.; Umapathi, T.; et al. Outbreak of Nipah-virus infection among abattoir workers in Singapore. Lancet 1999, 354, 1253–1256. [Google Scholar] [CrossRef]
- Goh, K.J.; Tan, C.T.; Chew, N.K.; Tan, P.S.K.; Kamarulzaman, A.; Sarji, S.A.; Wong, K.T.; Abdullah, B.J.J.; Chua, K.B.; Lam, S.K. Clinical features of Nipah virus encephalitis among pig farmers in Malaysia. N. Engl. J. Med. 2000, 342, 1229–1235. [Google Scholar] [CrossRef]
- Parashar, U.D.; Sunn, L.M.; Ong, F.; Mounts, A.W.; Arif, M.T.; Ksiazek, T.G.; Kamaluddin, M.A.; Mustafa, A.N.; Kaur, H.; Ding, L.M.; et al. Case-control study of risk factors for human infection with a new zoonotic paramyxovirus, Nipah virus, during a 1998–1999 outbreak of severe encephalitis in Malaysia. J. Infect. Dis. 2000, 181, 1755–1759. [Google Scholar] [CrossRef]
- Chua, K.B. Nipah virus outbreak in Malaysia. J. Clin. Virol. 2003, 26, 265–275. [Google Scholar] [CrossRef]
- Chadha, M.S.; Comer, J.A.; Lowe, L.; Rota, P.A.; Rollin, P.E.; Bellini, W.J.; Ksiazek, T.G.; Mishra, A.C. Nipah virus-associated encephalitis outbreak, Siliguri, India. Emerg. Infect. Dis. 2006, 12, 235–240. [Google Scholar] [CrossRef] [PubMed]
- Nikolay, B.; Salje, H.; Khan, A.K.M.D.; Sazzad, H.M.S.; Satter, S.M.; Rahman, M.; Doan, S.; Knust, B.; Flora, M.S.; Luby, S.P.; et al. A framework to monitor changes in transmission and epidemiology of emerging pathogens: Lessons from Nipah virus. J. Infect. Dis. 2020, 221, S363–S369. [Google Scholar] [CrossRef] [PubMed]
- Arunkumar, G.; Chandni, R.; Mourya, D.T.; Singh, S.K.; Sadanandan, R.; Sudan, P.; Bhargava, B.; Gangakhedkar, R.R.; Gupta, N.; Bhargava, B.; et al. Outbreak investigation of Nipah virus disease in Kerala, India, 2018. J. Infect. Dis. 2019, 219, 1867–1878. [Google Scholar] [CrossRef] [PubMed]
- Cortes, M.C.; Cauchemez, S.; Lefrancq, N.; Luby, S.P.; Jahangir Hossain, M.; Sazzad, H.M.S.; Rahman, M.; Daszak, P.; Salje, H.; Gurley, E.S. Characterization of the spatial and temporal distribution of Nipah virus spillover events in Bangladesh, 2007–2013. J. Infect. Dis. 2018, 217, 1390–1394. [Google Scholar] [CrossRef] [PubMed]
- Luby, S.P.; Rahman, M.; Hossain, M.J.; Blum, L.; Husain, M.M.; Gurley, E.; Khan, R.; Ahmed, B.-N.; Rahman, S.; Nahar, N.; et al. Foodborne transmission of Nipah virus, Bangladesh. Emerg. Infect. Dis. 2006, 12, 1888–1894. [Google Scholar] [CrossRef]
- Rahman, M.A.; Hossain, M.J.; Sultana, S.; Homaira, N.; Khan, S.U.; Rahman, M.; Gurley, E.S.; Rollin, P.E.; Lo, M.K.; Comer, J.A.; et al. Date palm sap linked to Nipah virus outbreak in Bangladesh, 2008. Vector Borne Zoonotic Dis. 2012, 12, 65–72. [Google Scholar] [CrossRef]
- Islam, M.S.; Sazzad, H.M.S.; Satter, S.M.; Sultana, S.; Hossain, M.J.; Hasan, M.; Rahman, M.; Campbell, S.; Cannon, D.L.; Ströher, U.; et al. Nipah virus transmission from bats to humans associated with drinking traditional liquor made from date palm sap, Bangladesh, 2011–2014. Emerg. Infect. Dis. 2016, 22, 664–670. [Google Scholar] [CrossRef]
- Gurley, E.S.; Hegde, S.T.; Hossain, K.; Sazzad, H.M.S.; Hossain, M.J.; Rahman, M.; Sharker, M.A.Y.; Salje, H.; Islam, M.S.; Epstein, J.H.; et al. Convergence of humans, bats, trees, and culture in Nipah virus transmission, Bangladesh. Emerg. Infect. Dis. 2017, 23, 1446–1453. [Google Scholar] [CrossRef]
- Harcourt, B.H.; Lowe, L.; Tamin, A.; Liu, X.; Bankamp, B.; Bowden, N.; Rollin, P.E.; Comer, J.A.; Ksiazek, T.G.; Hossain, M.J.; et al. Genetic characterization of Nipah virus, Bangladesh, 2004. Emerg. Infect. Dis. 2005, 11, 1594–1597. [Google Scholar] [CrossRef]
- Gurley, E.S.; Montgomery, J.M.; Hossain, M.J.; Bell, M.; Azad, A.K.; Islam, M.R.; Molla, M.A.R.; Carroll, D.S.; Ksiazek, T.G.; Rota, P.A.; et al. Person-to-person transmission of Nipah virus in a Bangladeshi community. Emerg. Infect. Dis. 2007, 13, 1031–1037. [Google Scholar] [CrossRef]
- Nikolay, B.; Salje, H.; Hossain, M.J.; Khan, A.K.M.D.; Sazzad, H.M.S.; Rahman, M.; Daszak, P.; Ströher, U.; Pulliam, J.R.C.; Kilpatrick, A.M.; et al. Transmission of Nipah virus—14 years of investigations in Bangladesh. N. Engl. J. Med. 2019, 380, 1804–1814. [Google Scholar] [CrossRef] [PubMed]
- Arankalle, V.A.; Bandyopadhyay, B.T.; Ramdasi, A.Y.; Jadi, R.; Patil, D.R.; Rahman, M.; Majumdar, M.; Banerjee, P.S.; Hati, A.K.; Goswami, R.P.; et al. Genomic characterization of Nipah virus, West Bengal, India. Emerg. Infect. Dis. 2011, 17, 907–909. [Google Scholar] [CrossRef] [PubMed]
- Kumar, C.P.G.; Sugunan, A.P.; Yadav, P.; Kurup, K.K.; Aarathee, R.; Manickam, P.; Bhatnagar, T.; Radhakrishnan, C.; Thomas, B.; Kumar, A.; et al. Infections among contacts of patients with Nipah Virus, India. Emerg. Infect. Dis. 2019, 25, 1007–1010. [Google Scholar] [CrossRef] [PubMed]
- Tan, C.T.; Tan, K.S. Nosocomial transmissibility of Nipah virus. J. Infect. Dis. 2001, 184, 1367. [Google Scholar] [CrossRef][Green Version]
- Mounts, A.W.; Kaur, H.; Parashar, U.D.; Ksiazek, T.G.; Cannon, D.; Arokiasamy, J.T.; Anderson, L.J.; Lye, M.S. A cohort study of health care workers to assess nosocomial transmissibility of Nipah virus, Malaysia, 1999. J. Infect. Dis. 2001, 183, 810–813. [Google Scholar] [CrossRef]
- Altizer, S.; Becker, D.J.; Epstein, J.H.; Forbes, K.M.; Gillespie, T.R.; Hall, R.J.; Hawley, D.M.; Hernandez, S.M.; Martin, L.B.; Plowright, R.K.; et al. Food for contagion: Synthesis and future directions for studying host–parasite responses to resource shifts in anthropogenic environments. Philos. Trans. R. Soc. B Biol. Sci. 2018, 373, 20170102. [Google Scholar] [CrossRef]
- Chua, K.B.; Chua, B.H.; Wang, C.W. Anthropogenic deforestation, El Niño and the emergence of Nipah virus in Malaysia. Malays. J. Pathol. 2002, 24, 15–21. [Google Scholar]
- Pulliam, J.R.C.; Epstein, J.H.; Dushoff, J.; Rahman, S.A.; Bunning, M.; Jamaluddin, A.A.; Hyatt, A.D.; Field, H.E.; Dobson, A.P.; Daszak, P. Agricultural intensification, priming for persistence and the emergence of Nipah virus: A lethal bat-borne zoonosis. J. R. Soc. Interface 2012, 9, 89–101. [Google Scholar] [CrossRef]
- Nahar, N.; Sultana, R.; Gurley, E.S.; Hossain, M.J.; Luby, S.P. Date palm sap collection: Exploring opportunities to prevent Nipah transmission. Ecohealth 2010, 7, 196–203. [Google Scholar] [CrossRef]
- Khan, M.S.U.; Hossain, J.; Gurley, E.S.; Nahar, N.; Sultana, R.; Luby, S.P.; Khan, M.S.U.; Hossain, J.; Gurley, E.S.; Nahar, N.; et al. Use of infrared camera to understand bats’ access to date palm sap: Implications for preventing Nipah virus transmission. Ecohealth 2010, 7, 517–525. [Google Scholar] [CrossRef]
- Luby, S.P.; Hossain, M.J.; Gurley, E.S.; Ahmed, B.-N.; Banu, S.; Khan, S.U.; Homaira, N.; Rota, P.A.; Rollin, P.E.; Comer, J.A.; et al. Recurrent zoonotic transmission of Nipah virus into humans, Bangladesh, 2001–2007. Emerg. Infect. Dis. 2009, 15, 1229–1235. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.; Rahman, J. Medicinal value and nutrient status of indigenous fruits in Bangladesh. Nov. J. Med. Biol. Sci. 2014, 3, 1–10. [Google Scholar] [CrossRef]
- KewScience. Phoenix sylvestris. Available online: http://wcsp.science.kew.org/namedetail.do?name_id=152708 (accessed on 14 October 2020).
- Chaluvadi, S.R.; Young, P.; Thompson, K.; Bahri, B.A.; Gajera, B.; Narayanan, S.; Krueger, R.; Bennetzen, J.L. Phoenix phylogeny, and analysis of genetic variation in a diverse collection of date palm (Phoenix dactylifera) and related species. Plant Divers. 2019, 41, 330–339. [Google Scholar] [CrossRef] [PubMed]
- Annett, H.E.; Lele, G.K.; Amin, B.M. The date sugar industry in Bengal: An investigation into its chemistry and agriculture. Mem. Dep. Agric. India 1913, 2, 281–389. [Google Scholar]
- Blatter, E. The Palms of British India and Ceylon; Oxford University Press: Oxford, UK, 1926. [Google Scholar]
- Epstein, J.H.; Anthony, S.J.; Islam, A.; Kilpatrick, A.M.; Ali Khan, S.; Balkey, M.D.; Ross, N.; Smith, I.; Zambrana-Torrelio, C.; Tao, Y.; et al. Nipah virus dynamics in bats and implications for spillover to humans. Proc. Natl. Acad. Sci. USA 2020, 117, 29190–29201. [Google Scholar] [CrossRef] [PubMed]
- Fogarty, R.; Halpin, K.; Hyatt, A.D.; Daszak, P.; Mungall, B.A. Henipavirus susceptibility to environmental variables. Virus Res. 2008, 132, 140–144. [Google Scholar] [CrossRef]
- De Wit, E.; Prescott, J.; Falzarano, D.; Bushmaker, T.; Scott, D.; Feldmann, H.; Munster, V.J. Foodborne transmission of Nipah virus in Syrian hamsters. PLoS Pathog. 2014, 10, e1004001. [Google Scholar] [CrossRef]
- Nahar, N.; Asaduzzaman, M.; Mandal, U.K.; Rimi, N.A.; Gurley, E.S.; Rahman, M.; Garcia, F.; Zimicki, S.; Sultana, R.; Luby, S.P. Hunting bats for human consumption in Bangladesh. Ecohealth 2020, 17, 139–151. [Google Scholar] [CrossRef]
- Khan, M.A.R. Status and distribution of bats in Bangladesh with notes on their ecology. Zoos Print J. 2001, 16, 479–483. [Google Scholar] [CrossRef]
- Mickleburgh, S.P.; Hutson, A.M.; Racey, P.A. A review of the global conservation status of bats. Oryx 2002, 36, 18–34. [Google Scholar] [CrossRef]
- Epstein, J.H.; Olival, K.J.; Pulliam, J.R.C.; Smith, C.; Westrum, J.; Hughes, T.; Dobson, A.P.; Zubaid, A.; Rahman, S.A.; Basir, M.M.; et al. Pteropus vampyrus, a hunted migratory species with a multinational home-range and a need for regional management. J. Appl. Ecol. 2009, 46, 991–1002. [Google Scholar] [CrossRef]
- Oleksy, R.; Racey, P.A.; Jones, G. High-resolution GPS tracking reveals habitat selection and the potential for long-distance seed dispersal by Madagascan flying foxes Pteropus rufus. Glob. Ecol. Conserv. 2015, 3, 678–692. [Google Scholar] [CrossRef]
- Roberts, B.J.; Catterall, C.P.; Eby, P.; Kanowski, J. Long-distance and frequent movements of the flying-fox Pteropus poliocephalus: Implications for management. PLoS ONE 2012, 7, e42532. [Google Scholar] [CrossRef]
- United Nations. World Population Prospects 2019, Online Edition. Available online: https://population.un.org/wpp/ (accessed on 17 May 2020).
- Hahn, M.B.; Epstein, J.H.; Gurley, E.S.; Islam, M.S.; Luby, S.P.; Daszak, P.; Patz, J.A. Roosting behaviour and habitat selection of Pteropus giganteus reveal potential links to Nipah virus epidemiology. J. Appl. Ecol. 2014, 51, 376–387. [Google Scholar] [CrossRef] [PubMed]
- Hahn, M.B.; Gurley, E.S.; Epstein, J.H.; Islam, M.S.; Patz, J.A.; Daszak, P.; Luby, S.P. The role of landscape composition and configuration on Pteropus giganteus roosting ecology and Nipah virus spillover risk in Bangladesh. Am. J. Trop. Med. Hyg. 2014, 90, 247–255. [Google Scholar] [CrossRef] [PubMed]
- National Climatic Data Center (NCDC). NCDC Climate Data Online. Available online: https://www.ncdc.noaa.gov/cdo-web/search (accessed on 15 January 2020).
- Japan Agency for Marine-Earth Science and Technology Application Laboratory. APL VirtualEarth. Available online: http://www.jamstec.go.jp/virtualearth/general/en/index.html (accessed on 12 November 2020).
- National Oceanic and Atmospheric Administration Physical Sciences Laboratory. Multivariate ENSO Index Version 2 (MEI.v2). Available online: https://psl.noaa.gov/enso/mei/ (accessed on 12 November 2020).
- Lloyd, C.T.; Chamberlain, H.; Kerr, D.; Yetman, G.; Pistolesi, L.; Stevens, F.R.; Gaughan, A.E.; Nieves, J.J.; Hornby, G.; MacManus, K.; et al. Global spatio-temporally harmonised datasets for producing high-resolution gridded population distribution datasets. Big Earth Data 2019, 3, 108–139. [Google Scholar] [CrossRef] [PubMed]
- Lloyd, C.T.; Sorichetta, A.; Tatem, A.J. High resolution global gridded data for use in population studies. Sci. Data 2017, 4, 170001. [Google Scholar] [CrossRef]
- Doxsey-Whitfield, E.; MacManus, K.; Adamo, S.B.; Pistolesi, L.; Squires, J.; Borkovska, O.; Baptista, S.R. Taking advantage of the improved availability of census data: A first look at the gridded population of the world, version 4. Pap. Appl. Geogr. 2015, 1, 226–234. [Google Scholar] [CrossRef]
- Fick, S.E.; Hijmans, R.J. WorldClim 2: New 1-km spatial resolution climate surfaces for global land areas. Int. J. Climatol. 2017, 37, 4302–4315. [Google Scholar] [CrossRef]
- Hansen, M.C.; Potapov, P.V.; Moore, R.; Hancher, M.; Turubanova, S.A.; Tyukavina, A.; Thau, D.; Stehman, S.V.; Goetz, S.J.; Loveland, T.R.; et al. High-resolution global maps of 21st-century forest cover change. Science 2013, 342, 850–853. [Google Scholar] [CrossRef]
- Ellis, E.C.; Klein Goldewijk, K.; Siebert, S.; Lightman, D.; Ramankutty, N. Anthropogenic transformation of the biomes, 1700 to 2000. Glob. Ecol. Biogeogr. 2010, 19, 589–606. [Google Scholar] [CrossRef]
- Klein Goldewijk, K.; Beusen, A.; Van Drecht, G.; de Vos, M. The HYDE 3.1 spatially explicit database of human-induced global land-use change over the past 12,000 years. Glob. Ecol. Biogeogr. 2011, 20, 73–86. [Google Scholar] [CrossRef]
- OECD. Land Cover in Countries and Regions. Available online: https://stats.oecd.org/Index.aspx?DataSetCode=LAND_COVER (accessed on 6 August 2020).
- ESA-CCI. Land Cover. Available online: http://maps.elie.ucl.ac.be/CCI/viewer/ (accessed on 6 August 2020).
- World Bank. Forest Area (% of Land Area)—Bangladesh. Available online: https://data.worldbank.org/indicator/AG.LND.FRST.ZS?end=2016&locations=BD&start=1990 (accessed on 6 August 2020).
- Reddy, C.S.; Pasha, S.V.; Jha, C.S.; Diwakar, P.G.; Dadhwal, V.K. Development of national database on long-term deforestation (1930–2014) in Bangladesh. Glob. Planet. Chang. 2016, 139, 173–182. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing. Available online: http://www.r-project.org (accessed on 17 May 2020).
- Cooper, M.; Zvoleff, A. Gfcanalysis: Tools for Working with Hansen et al. Global Forest Change Dataset. Available online: https://cran.r-project.org/package=gfcanalysis (accessed on 17 May 2020).
- Ripley, B.; Venables, B.; Bates, D.M.; Hornik, K.; Gebhardt, A.; Firth, D. MASS: Support Functions and Datasets for Venables and Ripley’s MASS. Available online: https://cran.r-project.org/package=MASS (accessed on 17 May 2020).
- Burnham, K.P.; Anderson, D.R. Multimodel inference: Understanding AIC and BIC in model selection. Sociol. Methods Res. 2004, 33, 261–304. [Google Scholar] [CrossRef]
- Bartoń, K. MuMIn: Multi-Model Inference. Available online: http://cran.r-project.org/package=MuMIn (accessed on 17 May 2020).
- Guyon, I. A scaling law for the validation-set training-set size ratio. AT&T Bell Lab. 1997, 1, 1–11. [Google Scholar]
- Friedman, J.; Hastie, T.; Tibshirani, R. Regularization paths for generalized linear models via coordinate descent. J. Stat. Softw. 2010, 33, 1–22. [Google Scholar] [CrossRef]
- Breiman, L. Random forests. Mach. Learn. 2001, 45, 5–32. [Google Scholar] [CrossRef]
- Kuhn, M.; Wing, J.; Weston, S.; Williams, A.; Keefer, C.; Engelhardt, A.; Cooper, T.; Mayer, Z.; Kenkel, B.; R Core Team; et al. Caret: Classification and Regression Training. Available online: https://cran.r-project.org/package=caret (accessed on 17 May 2020).
- Friedman, J.; Hastie, T.; Tibshirani, R.; Narasimhan, B.; Tay, K.; Simon, N.; Qian, J. Glmnet: Lasso and Elastic-Net Regularized Generalized Linear Models. Available online: https://cran.r-project.org/package=glmnet (accessed on 17 May 2020).
- Wright, M.N.; Wager, S.; Probst, P. Ranger: A Fast Implementation of Random Forests. Available online: https://cran.r-project.org/package=ranger (accessed on 17 May 2020).
- Krystufek, B. Indian flying fox Pteropus giganteus colony in Peradeniya Botanical Gardens, Sri Lanka. Hystrix Ital. J. Mammal. 2009, 20, 29–35. [Google Scholar]
- Kumar, R.; Elangovan, V. Effect of tree characteristics on roost selection of the Indian flying fox, Pteropus giganteus. J. Bat Res. Conserv. 2019, 12, 100–106. [Google Scholar] [CrossRef]
- Kumar, J.; Kanaujia, A. Distribution and population status of fruit-bat (Pteropus giganteus) in district Lakhimpur-Kheri, Uttar Pradesh, India. G J. Environ. Sci. Technol. 2015, 3, 23–28. [Google Scholar]
- Neupane, K.R.; Basnet, K.; Katuwal, H.B. Plight of Indian flying fox (Pteropus giganteus) in lowlands of eastern Nepal. World J. Zool. 2016, 11, 81–85. [Google Scholar]
- Sharma, B.; Subedi, A.; Gyawali, K.; Ghimire, P.; Bist, B.S.; Baniya, S. Can Pteropus giganteus Brünnich, 1782 co-exist in a human dominated landscape? A case study in Pokhara valley, western Nepal. J. Bat Res. Conserv. 2018, 11, 1–7. [Google Scholar]
- Gulraiz, T.L.; Javid, A.; Mahmood-Ul-Hassan, M.; Maqbool, A.; Ashraf, S.; Hussain, M.; Daud, S. Roost characteristics and habitat preferences of Indian flying fox (Pteropus giganteus) in urban areas of Lahore, Pakistan. Turkish J. Zool. 2015, 39, 388–394. [Google Scholar] [CrossRef]
- Khan, W.; Nisa, N.N.; Khan, A.R.; Rahbar, B.; Mehmood, S.A.; Ahmed, S.; Kamal, M.; Shah, M.; Rasool, A.; Pahanwar, W.A.; et al. Roosting ecology and morphometric analysis of Pteropus medius (Indian flying fox) in Lower Dir, district, Pakistan. Braz. J. Biol. 2020. [Google Scholar] [CrossRef] [PubMed]
- Chaiyes, A.; Duengkae, P.; Wacharapluesadee, S.; Pongpattananurak, N.; Olival, K.J.; Hemachudha, T. Assessing the distribution, roosting site characteristics, and population of Pteropus lylei in Thailand. Raffles Bull. Zool. 2017, 65, 670–680. [Google Scholar]
- Giles, J.R.; Plowright, R.K.; Eby, P.; Peel, A.J.; McCallum, H. Models of eucalypt phenology predict bat population flux. Ecol. Evol. 2016, 6, 7230–7245. [Google Scholar] [CrossRef]
- Welbergen, J.A.; Klose, S.M.; Markus, N.; Eby, P. Climate change and the effects of temperature extremes on Australian flying-foxes. Proc. R. Soc. B Biol. Sci. 2008, 275, 419–425. [Google Scholar] [CrossRef]
- Van der Ree, R.; McDonnell, M.J.; Temby, I.; Nelson, J.; Whittingham, E. The establishment and dynamics of a recently established urban camp of flying foxes (Pteropus poliocephalus) outside their geographic range. J. Zool. 2006, 268, 177–185. [Google Scholar] [CrossRef]
- Hosmer, D.W.; Lemeshow, S. Applied Logistic Regression, 2nd ed.; Wiley and Sons, Inc.: New York, NY, USA, 2000; ISBN 9780471654025. [Google Scholar]
- Van Schendel, W. A History of Bangladesh; Cambridge University Press: Cambridge, UK, 2009; ISBN 9780511997419. [Google Scholar]
- Bangladesh Bureau of Statistics. Bangladesh Agricultural Statistics Yearbook 2017; Bangladesh Bureau of Statistics: Dhaka, Bangladesh, 2018.
- Alam, G.M.M.; Khatun, M.N. Sweetening food security in Bangladesh: Present situation and future strategy. Pak. Sugar J. 2012, 27, 10–18. [Google Scholar]
- Aung, M.H.; Steinberg, D.I.; Aung-Thwin, M.A. Myanmar. Available online: https://www.britannica.com/place/Myanmar (accessed on 16 November 2020).
- Ahmed, F.U. Traditional agroforestry systems in Bangladesh. Agrofor. Newsl. 1995, 3, 6–9. [Google Scholar]
- Sun, B.; Jia, L.; Liang, B.; Chen, Q.; Liu, D. Phylogeography, transmission, and viral proteins of Nipah virus. Virol. Sin. 2018, 33, 385–393. [Google Scholar] [CrossRef] [PubMed]
- Whitmer, S.L.M.; Lo, M.K.; Sazzad, H.M.S.; Zufan, S.; Gurley, E.S.; Sultana, S.; Amman, B.; Ladner, J.T.; Rahman, M.Z.; Doan, S.; et al. Inference of Nipah virus evolution, 1999–2015. Virus Evol. 2020. [Google Scholar] [CrossRef]
- Hegde, S.T.; Salje, H.; Sazzad, H.M.S.; Hossain, M.J.; Rahman, M.; Daszak, P.; Klena, J.D.; Nichol, S.T.; Luby, S.P.; Gurley, E.S. Using healthcare-seeking behaviour to estimate the number of Nipah outbreaks missed by hospital-based surveillance in Bangladesh. Int. J. Epidemiol. 2019, 48, 1219–1227. [Google Scholar] [CrossRef] [PubMed]
- Páez, D.J.; Giles, J.; McCallum, H.; Field, H.; Jordan, D.; Peel, A.J.; Plowright, R.K. Conditions affecting the timing and magnitude of Hendra virus shedding across pteropodid bat populations in Australia. Epidemiol. Infect. 2017, 145, 3143–3153. [Google Scholar] [CrossRef] [PubMed]
- Plowright, R.K.; Eby, P.; Hudson, P.J.; Smith, I.L.; Westcott, D.; Bryden, W.L.; Middleton, D.; Reid, P.A.; McFarlane, R.A.; Martin, G.; et al. Ecological dynamics of emerging bat virus spillover. Proc. R. Soc. B Biol. Sci. 2015, 282, 20142124. [Google Scholar] [CrossRef]
- Hayman, D.T.S. Biannual birth pulses allow filoviruses to persist in bat populations. Proc. R. Soc. B Biol. Sci. 2015, 282, 20142591. [Google Scholar] [CrossRef]
- Mahmood-Ul-Hassan, M.; Gulraiz, T.L.; Rana, S.A.; Javid, A. The diet of Indian flying-foxes (Pteropus giganteus) in urban habitats of Pakistan. Acta Chiropterol. 2010, 12, 341–347. [Google Scholar] [CrossRef]
- Walter, M.H.; Verdong, A.; Olmos, V.; Weiss, C.C.; Vial, L.-R.; Putra, A.; Müller, J.; Tschapka, M.; Schnitzler, H.-U. Discrimination of small sugar concentration differences helps the nectar-feeding bat Leptonycteris yerbabuenae cover energetic demands. J. Exp. Biol. 2020, 223, jeb.215053. [Google Scholar] [CrossRef]
- Plowright, R.K.; Peel, A.J.; Streicker, D.G.; Gilbert, A.T.; McCallum, H.; Wood, J.; Baker, M.L.; Restif, O. Transmission or within-host dynamics driving pulses of zoonotic viruses in reservoir–host populations. PLoS Negl. Trop. Dis. 2016, 10, e0004796. [Google Scholar] [CrossRef]
- Edson, D.W.; Field, H.E.; McMichael, L.; Jordan, D.; Kung, N.Y.; Mayer, D.; Smith, C.S. Flying-fox roost disturbance and Hendra virus spillover risk. PLoS ONE 2015, 10, e0125881. [Google Scholar] [CrossRef]
- McMichael, L.; Edson, D.; Mayer, D.; Broos, A.; Kopp, S.; Meers, J.; Field, H. Physiologic biomarkers and Hendra virus infection in Australian black flying foxes (Pteropus alecto). J. Wildl. Dis. 2017, 53, 111. [Google Scholar] [CrossRef] [PubMed]
- McMichael, L.; Edson, D.; Smith, C.; Mayer, D.; Smith, I.; Kopp, S.; Meers, J.; Field, H. Physiological stress and Hendra virus in flying-foxes (Pteropus spp.), Australia. PLoS ONE 2017, 12, e0182171. [Google Scholar] [CrossRef] [PubMed]
- Dey, S.; Roy, U.S.; Chattopadhyay, S. Distribution and abundance of three populations of Indian flying fox (Pteropus giganteus) from Purulia district of West Bengal, India. TAPROBANICA J. Asian Biodivers. 2013, 5, 60. [Google Scholar] [CrossRef]
- Manandhar, S.; Thapa, S.; Shrestha, T.K.; Jyakhwo, R.; Wright, W.; Aryal, A. Population status and diurnal behaviour of the Indian flying fox Pteropus giganteus (Brünnich, 1782) in Kathmandu Valley, Nepal. Proc. Zool. Soc. 2018, 71, 363–375. [Google Scholar] [CrossRef]
- Khan, S.U.; Gurley, E.S.; Hossain, M.J.; Nahar, N.; Sharker, M.A.Y.; Luby, S.P. A randomized controlled trial of interventions to impede date palm sap contamination by bats to prevent Nipah virus transmission in Bangladesh. PLoS ONE 2012, 7, e42689. [Google Scholar] [CrossRef]
- Nahar, N.; Mondal, U.K.; Sultana, R.; Hossain, M.J.; Khan, M.S.U.; Gurley, E.S.; Oliveras, E.; Luby, S.P. Piloting the use of indigenous methods to prevent Nipah virus infection by interrupting bats’ access to date palm sap in Bangladesh. Health Promot. Int. 2013, 28, 378–386. [Google Scholar] [CrossRef]
- Nahar, N.; Asaduzzaman, M.; Sultana, R.; Garcia, F.; Paul, R.C.; Abedin, J.; Sazzad, H.M.S.; Rahman, M.; Gurley, E.S.; Luby, S.P. A large-scale behavior change intervention to prevent Nipah transmission in Bangladesh: Components and costs. BMC Res. Notes 2017, 10, 225. [Google Scholar] [CrossRef]
- Pernet, O.; Schneider, B.S.; Beaty, S.M.; Lebreton, M.; Yun, T.E.; Park, A.; Zachariah, T.T.; Bowden, T.A.; Hitchens, P.; Ramirez, C.M.; et al. Evidence for henipavirus spillover into human populations in Africa. Nat. Commun. 2014, 5, 1–10. [Google Scholar] [CrossRef]
- Das, P.; Sazzad, H.M.S.; Aleem, M.A.; Rahman, M.Z.; Rahman, M.; Anthony, S.J.; Lipkin, W.I.; Gurley, E.S.; Luby, S.P.; Openshaw, J.J. Hospital-based zoonotic disease surveillance in Bangladesh: Design, field data and difficulties. Philos. Trans. R. Soc. B Biol. Sci. 2019, 374, 20190019. [Google Scholar] [CrossRef]
- Hegde, S.T.; Sazzad, H.M.S.; Hossain, M.J.; Alam, M.-U.; Kenah, E.; Daszak, P.; Rollin, P.; Rahman, M.; Luby, S.P.; Gurley, E.S. Investigating rare risk factors for Nipah virus in Bangladesh: 2001–2012. Ecohealth 2016, 13, 720–728. [Google Scholar] [CrossRef]
- Chowdhury, S.; Khan, S.U.; Crameri, G.; Epstein, J.H.; Broder, C.C.; Islam, A.; Peel, A.J.; Barr, J.A.; Daszak, P.; Wang, L.-F.; et al. Serological evidence of henipavirus exposure in cattle, goats and pigs in Bangladesh. PLoS Negl. Trop. Dis. 2014, 8, e3302. [Google Scholar] [CrossRef] [PubMed]
- Yadav, P.D.; Shete, A.M.; Kumar, G.A.; Sarkale, P.; Sahay, R.R.; Radhakrishnan, C.; Lakra, R.; Pardeshi, P.; Gupta, N.; Gangakhedkar, R.R.; et al. Nipah virus sequences from humans and bats during Nipah outbreak, Kerala, India, 2018. Emerg. Infect. Dis. 2019, 25, 1003–1006. [Google Scholar] [CrossRef] [PubMed]


| Response Variable | Set | Model | Response Error | RMSE | MAE | R2 | AUC |
|---|---|---|---|---|---|---|---|
| Occupancy (presence/absence of bats) | Training (n = 380) | GLM | 0.48 | 0.45 | 0.42 | 0.12 | 0.7 |
| Random forest | 0.48 | 0.41 | 0.04 | 0.61 | |||
| Test (n = 94) | GLM | 0.46 | 0.46 | 0.43 | 0.02 | 0.59 | |
| Random forest | 0.51 | 0.43 | 0 | 0.49 | |||
| Abundance (roost size) | Training (n = 255) | GLM | 670 | 631 | 314 | 0.14 | |
| Random forest | 643 | 312 | 0.09 | ||||
| Test (n = 60) | GLM | 744 | 711 | 320 | 0.1 | ||
| Random forest | 709 | 327 | 0.08 |
| Covariate | Median (IQR) |
|---|---|
| Human population density (persons/km2) | 996 (858–1260) |
| Distance to nearest inland water (km) | 0.6 (0.3–1) |
| Distance to nearest feeding site (km) | 2 (0.9–3.6) |
| Distance to nearest date palm tree (km) | 1.2 (0.2–2.7) |
| Number of feeding sites within 15 km of roost site | 11 (3–20) |
| Number of date palm trees within 15 km of roost site | 80 (29–307) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
McKee, C.D.; Islam, A.; Luby, S.P.; Salje, H.; Hudson, P.J.; Plowright, R.K.; Gurley, E.S. The Ecology of Nipah Virus in Bangladesh: A Nexus of Land-Use Change and Opportunistic Feeding Behavior in Bats. Viruses 2021, 13, 169. https://doi.org/10.3390/v13020169
McKee CD, Islam A, Luby SP, Salje H, Hudson PJ, Plowright RK, Gurley ES. The Ecology of Nipah Virus in Bangladesh: A Nexus of Land-Use Change and Opportunistic Feeding Behavior in Bats. Viruses. 2021; 13(2):169. https://doi.org/10.3390/v13020169
Chicago/Turabian StyleMcKee, Clifton D., Ausraful Islam, Stephen P. Luby, Henrik Salje, Peter J. Hudson, Raina K. Plowright, and Emily S. Gurley. 2021. "The Ecology of Nipah Virus in Bangladesh: A Nexus of Land-Use Change and Opportunistic Feeding Behavior in Bats" Viruses 13, no. 2: 169. https://doi.org/10.3390/v13020169
APA StyleMcKee, C. D., Islam, A., Luby, S. P., Salje, H., Hudson, P. J., Plowright, R. K., & Gurley, E. S. (2021). The Ecology of Nipah Virus in Bangladesh: A Nexus of Land-Use Change and Opportunistic Feeding Behavior in Bats. Viruses, 13(2), 169. https://doi.org/10.3390/v13020169






