Widespread Circulation of Flaviviruses in Horses and Birds in Northeastern Spain (Catalonia) between 2010 and 2019
Abstract
:1. Introduction
2. Materials and Methods
2.1. Laboratory Analyses
2.1.1. Birds
2.1.2. Horses
2.2. Spatial Analyses
3. Results
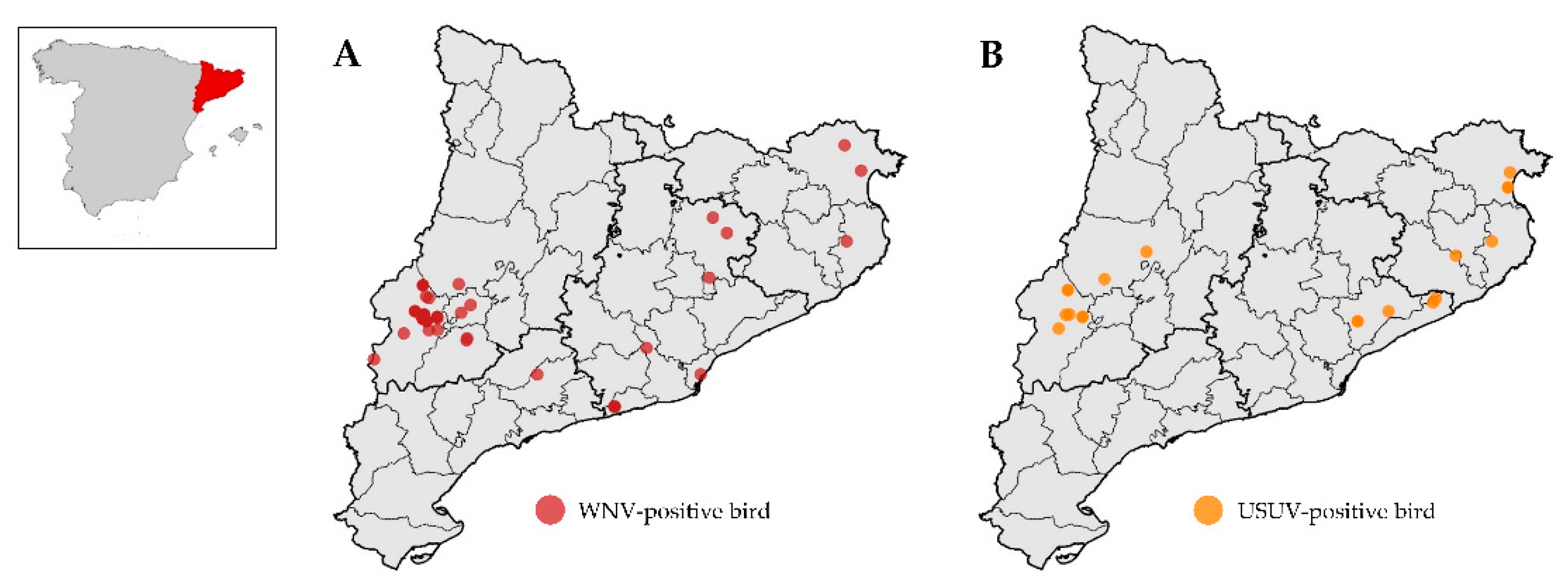
3.1. Flaviviruses in Birds
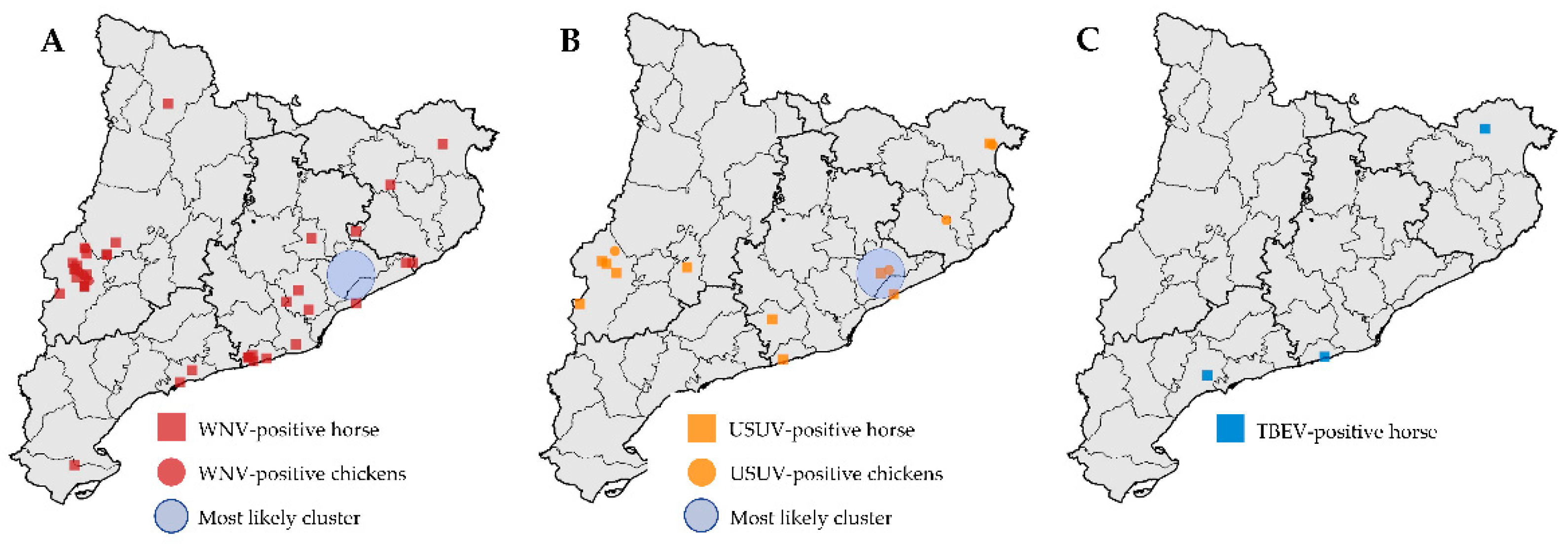
3.2. Flaviviruses in Horses
3.3. Spatial Analyses
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Barzon, L. Ongoing and emerging arbovirus threats in Europe. J. Clin. Virol. 2018, 107, 38–47. [Google Scholar] [CrossRef]
- Beck, C.; Jimenez-Clavero, M.A.; Leblond, A.; Durand, B.; Nowotny, N.; Leparc-Goffart, I.; Zientara, S.; Jourdain, E.; Lecollinet, S. Flaviviruses in Europe: Complex circulation patterns and their consequences for the diagnosis and control of West Nile disease. Int. J. Environ. Res. Public Health 2013, 10, 6049–6083. [Google Scholar] [CrossRef] [Green Version]
- Weaver, S.C.; Reisen, W.K. Present and future arboviral threats. Antiviral Res. 2010, 85, 328–345. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bakonyi, T.; Ivanics, E.; Erdélyi, K.; Ursu, K.; Ferenczi, E.; Weissenböck, H.; Nowotny, N. Lineage 1 and 2 strains of encephalitic West Nile virus; central Europe. Emerg. Infect. Dis. 2006, 12, 618–623. [Google Scholar] [CrossRef] [PubMed]
- Platonov, A.E.; Karan’, L.S.; Shopenskaia, T.A.; Fedorova, M.V.; Koliasnikova, N.M.; Rusakova, N.M.; Shishkina, L.V.; Arshba, T.E.; Zhuravlev, V.I.; Govorukhina, M.V.; et al. Genotyping of West Nile fever virus strains circulating in southern Russia as an epidemiological investigation method: Principles and results. Zhurnal Mikrobiologii Epidemiologii I Immunobiologii 2011, 2, 29–37. [Google Scholar]
- Hernández-Triana, L.M.; Jeffries, C.L.; Mansfield, K.L.; Carnell, G.; Fooks, A.R.; Johnson, N. Emergence of west nile virus lineage 2 in europe: A review on the introduction and spread of a mosquito-borne disease. Front. Public Health 2014, 2, 27. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- García-Bocanegra, I.; Jaén-Téllez, J.A.; Napp, S.; Arenas-Montes, A.; Fernández-Morente, M.; Fernández-Molera, V.; Arenas, A. West Nile fever outbreak in horses and humans; Spain; 2010. Emerg. Infect. Dis. 2011, 17, 2397–2399. [Google Scholar] [CrossRef]
- Busquets, N.; Laranjo-González, M.; Soler, M.; Nicolás, O.; Rivas, R.; Talavera, S.; Villalba, R.; San Miguel, E.; Torner, N.; Aranda, C.; et al. Detection of West Nile virus lineage 2 in North-Eastern Spain (Catalonia). Transbound. Emerg. Dis. 2019, 66, 617–621. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Camp, J.V.; Nowotny, N. The knowns and unknowns of West Nile virus in Europe: What did we learn from the 2018 outbreak? Expert Rev. Anti Infect. Ther. 2020, 18, 145–154. [Google Scholar] [CrossRef]
- Bakonyi, T.; Haussig, J.M. West Nile virus keeps on moving up in Europe. Euro Surveill. 2020, 25, 2001938. [Google Scholar] [CrossRef]
- Rodríguez-Alarcón, L.G.S.M.; Fernández-Martínez, B.; Sierra Moros, M.J.; Vázquez, A.; Julián Pachés, P.; García Villacieros, E.; Gómez Martín, M.B.; Figuerola Borras, J.; Lorusso, N.; Ramos Aceitero, J.M.; et al. Unprecedented increase of West Nile virus neuroinvasive disease; Spain; summer 2020. Euro Surveill. 2021, 26, 2002010. [Google Scholar]
- Weissenböck, H.; Kolodziejek, J.; Url, A.; Lussy, H.; Rebel-Bauder, B.; Nowotny, N. Emergence of Usutu virus; an African mosquito-borne flavivirus of the Japanese encephalitis virus group; central Europe. Emerg. Infect. Dis. 2002, 8, 652–656. [Google Scholar] [CrossRef] [PubMed]
- Weissenböck, H.; Bakonyi, T.; Rossi, G.; Mani, P.; Nowotny, N. Usutu virus; Italy; 1996. Emerg. Infect. Dis. 2013, 19, 274–277. [Google Scholar] [CrossRef]
- Busquets, N.; Alba, A.; Allepuz, A.; Aranda, C.; Ignacio Nuñez, J. Usutu virus sequences in Culex pipiens (Diptera: Culicidae); Spain. Emerg. Infect. Dis. 2008, 14, 861–863. [Google Scholar]
- Bakonyi, T.; Busquets, N.; Nowotny, N. Comparison of complete genome sequences of Usutu virus strains detected in Spain; Central Europe; and Africa. Vector Borne Zoonotic Dis. 2014, 14, 324–329. [Google Scholar] [CrossRef] [Green Version]
- Vázquez, A.; Ruiz, S.; Herrero, L.; Moreno, J.; Molero, F.; Magallanes, A.; Sánchez-Seco, M.P.; Figuerola, J.; Tenorio, A. West Nile and Usutu viruses in mosquitoes in Spain; 2008–2009. Am. J. Trop. Med. Hyg. 2011, 85, 178–181. [Google Scholar] [CrossRef]
- Clé, M.; Beck, C.; Salinas, S.; Lecollinet, S.; Gutierrez, S.; Van de Perre, P.; Baldet, T.; Foulongne, V.; Simonin, Y. Usutu virus: A new threat? Epidemiol. Infect. 2019, 147, e232. [Google Scholar] [CrossRef] [Green Version]
- Höfle, U.; Gamino, V.; de Mera, I.G.; Mangold, A.J.; Ortíz, J.A.; de la Fuente, J. Usutu virus in migratory song thrushes; Spain. Emerg. Infect. Dis. 2013, 19, 1173–1175. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Agüero, M.; Fernández-Pinero, J.; Buitrago, D.; Sánchez, A.; Elizalde, M.; San Miguel, E.; Villalba, R.; Llorente, F.; Jiménez-Clavero, M.A. Bagaza Virus in Partridges and Pheasants; Spain; 2010. Emerg. Infect. Dis. 2011, 17, 1498–1501. [Google Scholar] [CrossRef]
- Llorente, F.; Pérez-Ramírez, E.; Fernández-Pinero, J.; Soriguer, R.; Figuerola, J.; Jiménez-Clavero, M.A. Flaviviruses in game birds; southern Spain; 2011–2012. Emerg. Infect. Dis. 2013, 19, 1023–1025. [Google Scholar] [CrossRef]
- Napp, S.; Montalvo, T.; Piñol-Baena, C.; Gómez-Martín, M.B.; Nicolás-Francisco, O.; Soler, M.; Busquets, N. Usefulness of Eurasian Magpies (Pica pica) for West Nile virus Surveillance in Non-Endemic and Endemic Situations. Viruses 2019, 11, 716. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Estrada-Peña, A.; de la Fuente, J. The ecology of ticks and epidemiology of tick-borne viral diseases. Antiviral Res. 2014, 108, 104–128. [Google Scholar] [CrossRef] [PubMed]
- Beauté, J.; Spiteri, G.; Warns-Petit, E.; Zeller, H. Tick-borne encephalitis in Europe; 2012 to 2016. Euro Surveill. 2018, 23. [Google Scholar] [CrossRef] [Green Version]
- European Centre for Disease Prevention and Control (ECDC 2021). Tick-borne encephalitis. In ECDC. Annual Epidemiological Report for 2017; ECDC: Stockholm, Sweden, 2021; Available online: https://www.ecdc.europa.eu/sites/default/files/documents/AER-TBE-2019.pdf (accessed on 6 September 2021).
- Botelho-Nevers, E.; Gagneux-Brunon, A.; Velay, A.; Guerbois-Galla, M.; Grard, G.; Bretagne, C.; Mailles, A.; Verhoeven, P.O.; Pozzetto, B.; Gonzalo, S.; et al. Tick-Borne Encephalitis in Auvergne-Rhône-Alpes Region; France; 2017–2018. Emerg. Infect. Dis. 2019, 25, 1944–1948. [Google Scholar] [CrossRef]
- Waldenström, J.; Lundkvist, A.; Falk, K.I.; Garpmo, U.; Bergström, S.; Lindegren, G.; Sjöstedt, A.; Mejlon, H.; Fransson, T.; Haemig, P.D.; et al. Migrating birds and tickborne encephalitis virus. Emerg. Infect. Dis. 2007, 13, 1215–1218. [Google Scholar] [CrossRef] [PubMed]
- Beck, C.; Lowenski, S.; Durand, B.; Bahuon, C.; Zientara, S.; Lecollinet, S. Improved reliability of serological tools for the diagnosis of West Nile fever in horses within Europe. PLoS Negl. Trop. Dis. 2017, 11, e0005936. [Google Scholar] [CrossRef]
- Nikolay, B. A review of West Nile and Usutu virus co-circulation in Europe: How much do transmission cycles overlap? Trans. R. Soc. Trop. Med. Hyg. 2015, 109, 609–618. [Google Scholar] [CrossRef] [Green Version]
- Santos, P.D.; Michel, F.; Wylezich, C.; Höper, D.; Keller, M.; Holicki, C.M.; Szentiks, C.A.; Eiden, M.; Muluneh, A.; Neubauer-Juric, A.; et al. Co-infections: Simultaneous detections of West Nile virus and Usutu virus in birds from Germany. Transbound. Emerg. Dis. 2021, 2. [Google Scholar] [CrossRef]
- Alba, A.; Allepuz, A.; Napp, S.; Soler, M.; Selga, I.; Aranda, C.; Casal, J.; Pages, N.; Hayes, E.B.; Busquets, N. Ecological surveillance for West Nile in Catalonia (Spain); learning from a five-year period of follow-up. Zoonoses Public Health 2014, 61, 181–191. [Google Scholar] [CrossRef]
- Llorente, F.; García-Irazábal, A.; Pérez-Ramírez, E.; Cano-Gómez, C.; Sarasa, M.; Vázquez, A.; Jiménez-Clavero, M.A. Influence of flavivirus co-circulation in serological diagnostics and surveillance: A model of study using West Nile; Usutu and Bagaza viruses. Transbound. Emerg. Dis. 2019, 66, 2100–2106. [Google Scholar] [CrossRef]
- Beck, C.; Desprès, P.; Paulous, S.; Vanhomwegen, J.; Lowenski, S.; Nowotny, N.; Durand, B.; Garnier, A.; Blaise-Boisseau, S.; Guitton, E.; et al. A High-Performance Multiplex Immunoassay for Serodiagnosis of Flavivirus-Associated Neurological Diseases in Horses. Biomed Res. Int. 2015, 678084. [Google Scholar] [CrossRef] [Green Version]
- Kulldorff, M.; Nagarwalla, N. Spatial disease clusters: Detection and inference. Stat. Med. 1995, 14, 799–810. [Google Scholar] [CrossRef]
- Bravo-Barriga, D.; Aguilera-Sepúlveda, P.; Guerrero-Carvajal, F.; Llorente, F.; Reina, D.; Pérez-Martín, J.E.; Jiménez-Clavero, M.Á.; Frontera, E. West Nile and Usutu virus infections in wild birds admitted to rehabilitation centres in Extremadura; western Spain; 2017–2019. Vet. Microbiol. 2021, 255, 109020. [Google Scholar] [CrossRef]
- Engel, D.; Jöst, H.; Wink, M.; Börstler, J.; Bosch, S.; Garigliany, M.M.; Jöst, A.; Czajka, C.; Lühken, R.; Ziegler, U.; et al. Reconstruction of the Evolutionary History and Dispersal of Usutu Virus; a Neglected Emerging Arbovirus in Europe and Africa. mBio 2016, 7, e01938-15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jurado-Tarifa, E.; Napp, S.; Lecollinet, S.; Arenas, A.; Beck, C.; Cerdà-Cuéllar, M.; Fernández-Morente, M.; García-Bocanegra, I. Monitoring of West Nile virus; Usutu virus and Meaban virus in waterfowl used as decoys and wild raptors in southern Spain. Comp. Immunol. Microbiol. Infect. Dis. 2016, 49, 58–64. [Google Scholar] [CrossRef] [PubMed]
- Saiz, J.C.; Blazquez, A.B. Usutu virus: Current knowledge and future perspectives. Virus Adap. Treat. 2017, 9, 27–40. [Google Scholar] [CrossRef] [Green Version]
- Chvala, S.; Bakonyi, T.; Hackl, R.; Hess, M.; Nowotny, N.; Weissenböck, H. Limited pathogenicity of Usutu virus for the domestic chicken (Gallus domesticus). Avian Pathol. 2005, 34, 392–395. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chaintoutis, S.C.; Chaskopoulou, A.; Chassalevris, T.; Koehler, P.G.; Papanastassopoulou, M.; Dovas, C.I. West Nile virus lineage 2 strain in Greece; 2012. Emerg. Infect. Dis. 2013, 19, 827–829. [Google Scholar] [CrossRef]
- Roiz, D.; Vázquez, A.; Ruiz, S.; Tenorio, A.; Soriguer, R.; Figuerola, J. Evidence that Passerine Birds Act as Amplifying Hosts for Usutu Virus Circulation. Ecohealth 2019, 16, 734–742. [Google Scholar] [CrossRef] [PubMed]
- Vidaña, B.; Busquets, N.; Napp, S.; Pérez-Ramírez, E.; Jiménez-Clavero, M.Á.; Johnson, N. The Role of Birds of Prey in West Nile Virus Epidemiology. Vaccines 2020, 8, 550. [Google Scholar] [CrossRef]
- García-Bocanegra, I.; Arenas-Montes, A.; Napp, S.; Jaén-Téllez, J.A.; Fernández-Morente, M.; Fernández-Molera, V.; Arenas, A. Seroprevalence and risk factors associated to West Nile virus in horses from Andalusia; Southern Spain. Vet. Microbiol. 2012, 160, 341–346. [Google Scholar] [CrossRef]
- ICO. SIOC: Servidor d’informació Ornitològica de Catalunya; ICO: Barcelona, Spain, 2021; Available online: http://www.sioc.cat (accessed on 5 September 2021).
- Bournez, L.; Umhang, G.; Moinet, M.; Boucher, J.M.; Demerson, J.M.; Caillot, C.; Legras, L.; Devillers, E.; Hansmann, Y.; Velay, A.; et al. Disappearance of TBEV Circulation among Rodents in a Natural Focus in Alsace; Eastern France. Pathogens 2020, 9, 930. [Google Scholar] [CrossRef]
- European Centre for Disease Prevention and Control (ECDC 2020). Ixodes ricinus—Current Known Distribution. Available online: https://www.ecdc.europa.eu/en/publications-data/ixodes-ricinus-current-known-distribution-may-2020 (accessed on 6 September 2021).
- Espunyes, J.; Cabezón, O.; Pailler-García, L.; Dias-Alves, A.; Lobato-Bailón, L.; Marco, I.; Ribas, M.P.; Encinosa-Guzmán, P.E.; Valldeperes, M.; Napp, S. Hotspot of Crimean-Congo Hemorrhagic Fever Virus Seropositivity in Wildlife; Northeastern Spain. Emerg. Infect. Dis. 2021, 27, 2480–2484. [Google Scholar] [CrossRef]
- García-Bocanegra, I.; Jurado-Tarifa, E.; Cano-Terriza, D.; Martínez, R.; Pérez-Marín, J.E.; Lecollinet, S. Exposure to West Nile virus and tick-borne encephalitis virus in dogs in Spain. Transbound. Emerg. Dis. 2018, 65, 765–772. [Google Scholar] [CrossRef] [PubMed]
- Vanhomwegen, J.; Beck, C.; Desprès, P.; Figuerola, A.; García, R.; Lecollinet, S.; López-Roig, M.; Manuguerra, J.C.; Serra-Cobo, J. Circulation of Zoonotic Arboviruses in Equine Populations of Mallorca Island (Spain). Vector Borne Zoonotic Dis. 2017, 17, 340–346. [Google Scholar] [CrossRef] [PubMed]
- Camino, E.; Schmid, S.; Weber, F.; Pozo, P.; de Juan, L.; König, M.; Cruz-Lopez, F. Detection of antibodies against tick-borne encephalitis flaviviruses in breeding and sport horses from Spain. Ticks Tick-Borne Dis. 2020, 11, 101487. [Google Scholar] [CrossRef] [PubMed]
- Rushton, J.O.; Lecollinet, S.; Hubálek, Z.; Svobodová, P.; Lussy, H.; Nowotny, N. Tick-borne encephalitis virus in horses; Austria; 2011. Emerg. Infect. Dis. 2013, 19, 635–637. [Google Scholar] [CrossRef]
- Weingartl, H.M.; Drebot, M.A.; Hubálek, Z.; Halouzka, J.; Andonova, M.; Dibernardo, A.; Cottam-Birt, C.; Larence, J.; Marszal, P. Comparison of assays for the detection of West Nile virus antibodies in chicken serum. Can. J. Vet. Res. 2003, 67, 128–132. [Google Scholar]
- Arnal, A.; Gómez-Díaz, E.; Cerdà-Cuéllar, M.; Lecollinet, S.; Pearce-Duvet, J.; Busquets, N.; García-Bocanegra, I.; Pagès, N.; Vittecoq, M.; Hammouda, A.; et al. Circulation of a Meaban-like virus in yellow-legged gulls and seabird ticks in the western Mediterranean basin. PLoS ONE 2014, 9, e89601. [Google Scholar]
- Balseiro, A.; Royo, L.J.; Martínez, C.P.; Fernández de Mera, I.G.; Höfle, Ú.; Polledo, L.; Marreros, N.; Casais, R.; Marín, J.F. Louping ill in goats; Spain; 2011. Emerg. Infect. Dis. 2012, 18, 976–978. [Google Scholar] [CrossRef] [Green Version]
- Roesch, F.; Fajardo, A.; Moratorio, G.; Vignuzzi, M. Usutu Virus: An Arbovirus on the Rise. Viruses 2019, 11, 640. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fros, J.J.; Miesen, P.; Vogels, C.B.; Gaibani, P.; Sambri, V.; Martina, B.E.; Koenraadt, C.J.; van Rij, R.P.; Vlak, J.M.; Takken, W.; et al. Comparative Usutu and West Nile virus transmission potential by local Culex pipiens mosquitoes in north-western Europe. One Health 2015, 1, 31–36. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Scientific Name | Common Name | Family | Order | Undetermined Flavivirus | USUV | WNV | Total |
|---|---|---|---|---|---|---|---|
| Aquila fasciata | Bonelli’s eagle | Accipitridae | Accipitriformes | 1 (1/40) | 1 | ||
| Buteo buteo | Common buzzard | Accipitridae | Accipitriformes | 3 | 1 (1/10) | 1 (1/20) | 5 |
| Circaetus gallicus | Short-toed snake eagle | Accipitridae | Accipitriformes | 6 | 6 (1/20 to 1/160) | 12 | |
| Circus aeruginosus | Western marsh harrier | Accipitridae | Accipitriformes | 1 | 1 | ||
| Gypaetus barbatus | Bearded vulture | Accipitridae | Accipitriformes | 1 (1/10) | 5 (1/160 to 1/1280) | 6 | |
| Gyps fulvus | Griffon vulture | Accipitridae | Accipitriformes | 1 | 1 | ||
| Milvus migrans | Black kite | Accipitridae | Accipitriformes | 1 | 1 | ||
| Milvus milvus | Red kite | Accipitridae | Accipitriformes | 1 (1/20) | 1 | ||
| Pernis apivorus | European honey buzzard | Accipitridae | Accipitriformes | 1 | 3 (1/40 to 1/80) | 4 | |
| Anser anser | Greylag goose | Anatidae | Anseriformes | 1 (1/160) | 1 | ||
| Larus michahellis | Yellow-legged gull | Laridae | Charadriiformes | 4 | 4 | ||
| Ciconia ciconia | White stork | Ciconiidae | Ciconiiformes | 4 | 2 (1/20 to 1/640) | 4 (1/20 to 1/640) | 10 |
| Columba livia | Rock pigeon | Columbidae | Columbiformes | 2 | 2 (1/80) | 1 (1/40) | 5 |
| Columba palumbus | Common wood pigeon | Columbidae | Columbiformes | 1 (1/10) | 1 | ||
| Streptopelia decaocto | Eurasian collared dove | Columbidae | Columbiformes | 1 | 1 | ||
| Falco tinnunculus | Common kestrel | Falconidae | Falconiformes | 2 | 1 (1/320) | 4 (1/20 to 1/160) | 7 |
| Alectoris rufa | Red-legged partridge | Phasianidae | Galliformes | 1 (1/160) | 1 | ||
| Gallus gallus | Chicken | Phasianidae | Galliformes | 9 | 6 (1/10 to 1/640) | 34 (1/10 to 1/1280) | 49 |
| Corvus corax | Common raven | Corvidae | Passeriformes | 3 | 1 (1/10) | 4 | |
| Pica pica | Eurasian magpie | Corvidae | Passeriformes | 20 | 2 (1/10 to 1/160) | 55 (1/20 to 1/640) | 77 |
| Ardea cinerea | Grey heron | Ardeidae | Pelecaniformes | 3 | 2 (1/80 to 1/160) | 5 | |
| Asio otus | Long-eared owl | Strigidae | Strigiformes | 1 | 1 | ||
| Bubo bubo | Eurasian eagle-owl | Strigidae | Strigiformes | 1 (1/40) | 1 | ||
| Strix aluco | Brown owl | Strigidae | Strigiformes | 4 | 4 | ||
| Tyto alba | Western barn owl | Tytonidae | Strigiformes | 2 | 2 | ||
| Total | 68 | 19 | 118 | 205 |
| Titers | USUV | WNV | TBEV |
|---|---|---|---|
| 1/10 | 5 | 5 | |
| 1/20 | 1 | 15 | |
| 1/40 | 4 | 25 | 1 |
| 1/80 | 1 | 21 | 1 |
| 1/160 | 15 | ||
| 1/320 | 11 | 2 | |
| Total | 11 | 92 | 4 |
| Number of Cases | Expected Cases | Relative Risk | p-Value | |
|---|---|---|---|---|
| USUV | 3 | 0.72 | 5.0 | 0.12 |
| WNV | 0 | 2.28 | 0.0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Napp, S.; Llorente, F.; Beck, C.; Jose-Cunilleras, E.; Soler, M.; Pailler-García, L.; Amaral, R.; Aguilera-Sepúlveda, P.; Pifarré, M.; Molina-López, R.; et al. Widespread Circulation of Flaviviruses in Horses and Birds in Northeastern Spain (Catalonia) between 2010 and 2019. Viruses 2021, 13, 2404. https://doi.org/10.3390/v13122404
Napp S, Llorente F, Beck C, Jose-Cunilleras E, Soler M, Pailler-García L, Amaral R, Aguilera-Sepúlveda P, Pifarré M, Molina-López R, et al. Widespread Circulation of Flaviviruses in Horses and Birds in Northeastern Spain (Catalonia) between 2010 and 2019. Viruses. 2021; 13(12):2404. https://doi.org/10.3390/v13122404
Chicago/Turabian StyleNapp, Sebastian, Francisco Llorente, Cécile Beck, Eduard Jose-Cunilleras, Mercè Soler, Lola Pailler-García, Rayane Amaral, Pilar Aguilera-Sepúlveda, Maria Pifarré, Rafael Molina-López, and et al. 2021. "Widespread Circulation of Flaviviruses in Horses and Birds in Northeastern Spain (Catalonia) between 2010 and 2019" Viruses 13, no. 12: 2404. https://doi.org/10.3390/v13122404
APA StyleNapp, S., Llorente, F., Beck, C., Jose-Cunilleras, E., Soler, M., Pailler-García, L., Amaral, R., Aguilera-Sepúlveda, P., Pifarré, M., Molina-López, R., Obón, E., Nicolás, O., Lecollinet, S., Jiménez-Clavero, M. Á., & Busquets, N. (2021). Widespread Circulation of Flaviviruses in Horses and Birds in Northeastern Spain (Catalonia) between 2010 and 2019. Viruses, 13(12), 2404. https://doi.org/10.3390/v13122404










