Heterologous Immunity of Virus-Specific T Cells Leading to Alloreactivity: Possible Implications for Solid Organ Transplantation
Abstract
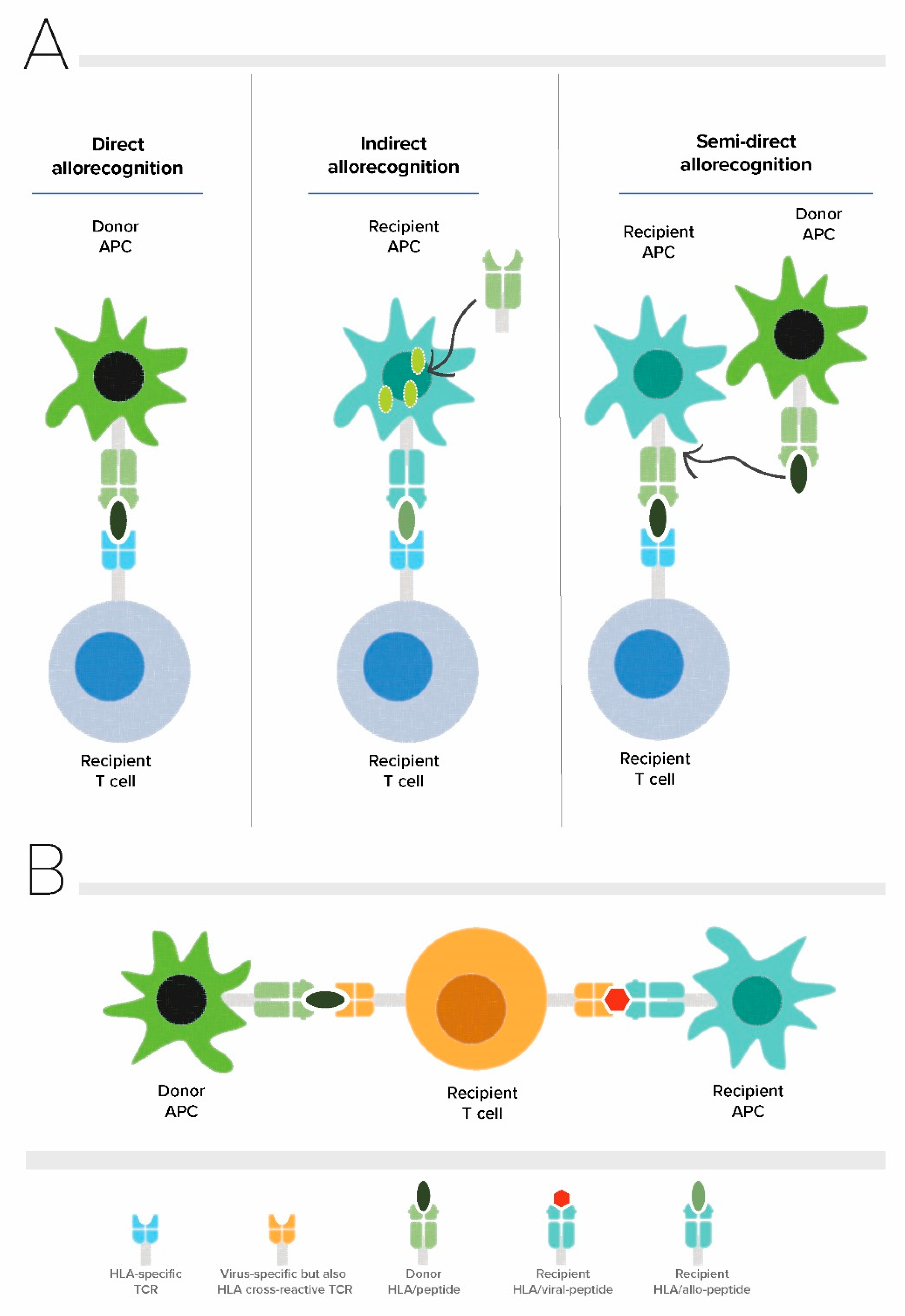
1. Introduction
2. Relevance of Heterologous Immunity in Transplantation: Evidence from Murine Studies
3. Cross-Reactive Virus-Specific Memory T Cells: How Predictable Are They in Humans?
4. Heterologous Immunity: Shaping the Alloreactive T Cell Repertoire in Humans
5. Impact of Donor-HLA Cross-Reactive Virus-Specific T Cells on Allograft Rejection in Humans
6. Future Directions and Concluding Remarks
Author Contributions
Funding
Conflicts of Interest
References
- Opelz, G.; Wujciak, T.; Dohler, B.; Scherer, S.; Mytilineos, J. HLA compatibility and organ transplant survival. Collaborative Transplant Study. Rev. Immunogenet. 1999, 1, 334–342. [Google Scholar]
- Echterdiek, F.; Latus, J.; Dohler, B.; Schwenger, V.; Susal, C. Impact of HLA compatibility in recipients of kidneys from expanded criteria donors: A Collaborative Transplant Study Report. Int. J. Immunogenet. 2021, 48, 201–210. [Google Scholar] [CrossRef]
- Sellares, J.; de Freitas, D.G.; Mengel, M.; Reeve, J.; Einecke, G.; Sis, B.; Hidalgo, L.G.; Famulski, K.; Matas, A.; Halloran, P.F. Understanding the causes of kidney transplant failure: The dominant role of antibody-mediated rejection and nonadherence. Am. J. Transplant. 2012, 12, 388–399. [Google Scholar] [CrossRef]
- Halloran, P.F.; Chang, J.; Famulski, K.; Hidalgo, L.G.; Salazar, I.D.; Merino Lopez, M.; Matas, A.; Picton, M.; de Freitas, D.; Bromberg, J.; et al. Disappearance of T Cell-Mediated Rejection Despite Continued Antibody-Mediated Rejection in Late Kidney Transplant Recipients. J. Am. Soc. Nephrol. 2015, 26, 1711–1720. [Google Scholar] [CrossRef]
- Podesta, M.A.; Binder, C.; Sellberg, F.; DeWolf, S.; Shonts, B.; Ho, S.H.; Obradovic, A.; Waffarn, E.; Danzl, N.; Berglund, D.; et al. Siplizumab selectively depletes effector memory T cells and promotes a relative expansion of alloreactive regulatory T cells in vitro. Am. J. Transplant. 2020, 20, 88–100. [Google Scholar] [CrossRef]
- Morris, H.; DeWolf, S.; Robins, H.; Sprangers, B.; LoCascio, S.A.; Shonts, B.A.; Kawai, T.; Wong, W.; Yang, S.; Zuber, J.; et al. Tracking donor-reactive T cells: Evidence for clonal deletion in tolerant kidney transplant patients. Sci. Transl. Med. 2015, 7, 272ra10. [Google Scholar] [CrossRef]
- Ali, J.M.; Bolton, E.M.; Bradley, J.A.; Pettigrew, G.J. Allorecognition pathways in transplant rejection and tolerance. Transplantation 2013, 96, 681–688. [Google Scholar] [CrossRef]
- Baker, R.J.; Hernandez-Fuentes, M.P.; Brookes, P.A.; Chaudhry, A.N.; Lechler, R.I. The role of the allograft in the induction of donor-specific T cell hyporesponsiveness. Transplantation 2001, 72, 480–485. [Google Scholar] [CrossRef]
- Yin, Y.; Mariuzza, R.A. The multiple mechanisms of T cell receptor cross-reactivity. Immunity 2009, 31, 849–851. [Google Scholar] [CrossRef]
- Zinkernagel, R.M.; Doherty, P.C. MHC-restricted cytotoxic T cells: Studies on the biological role of polymorphic major transplantation antigens determining T-cell restriction-specificity, function, and responsiveness. Adv. Immunol. 1979, 27, 51–177. [Google Scholar]
- Colf, L.A.; Bankovich, A.J.; Hanick, N.A.; Bowerman, N.A.; Jones, L.L.; Kranz, D.M.; Garcia, K.C. How a single T cell receptor recognizes both self and foreign MHC. Cell 2007, 129, 135–146. [Google Scholar] [CrossRef] [PubMed]
- Macdonald, W.A.; Chen, Z.; Gras, S.; Archbold, J.K.; Tynan, F.E.; Clements, C.S.; Bharadwaj, M.; Kjer-Nielsen, L.; Saunders, P.M.; Wilce, M.C.; et al. T cell allorecognition via molecular mimicry. Immunity 2009, 31, 897–908. [Google Scholar] [CrossRef]
- Burrows, S.R.; Khanna, R.; Silins, S.L.; Moss, D.J. The influence of antiviral T-cell responses on the alloreactive repertoire. Immunol. Today 1999, 20, 203–207. [Google Scholar] [CrossRef]
- Ford, W.L.; Atkins, R.C. The proportion of lymphocytes capable of recognizing strong transplantation antigens in vivo. Adv. Exp. Med. Biol. 1973, 29, 255–262. [Google Scholar] [CrossRef] [PubMed]
- Suchin, E.J.; Langmuir, P.B.; Palmer, E.; Sayegh, M.H.; Wells, A.D.; Turka, L.A. Quantifying the frequency of alloreactive T cells in vivo: New answers to an old question. J. Immunol. 2001, 166, 973–981. [Google Scholar] [CrossRef]
- Macedo, C.; Orkis, E.A.; Popescu, I.; Elinoff, B.D.; Zeevi, A.; Shapiro, R.; Lakkis, F.G.; Metes, D. Contribution of naive and memory T-cell populations to the human alloimmune response. Am. J. Transplant. 2009, 9, 2057–2066. [Google Scholar] [CrossRef]
- Felix, N.J.; Allen, P.M. Specificity of T-cell alloreactivity. Nat. Rev. Immunol. 2007, 7, 942–953. [Google Scholar] [CrossRef]
- Golshayan, D.; Wyss, J.C.; Buckland, M.; Hernandez-Fuentes, M.; Lechler, R.I. Differential role of naive and memory CD4 T-cell subsets in primary alloresponses. Am. J. Transplant. 2010, 10, 1749–1759. [Google Scholar] [CrossRef]
- Lakkis, F.G.; Sayegh, M.H. Memory T cells: A hurdle to immunologic tolerance. J. Am. Soc. Nephrol. 2003, 14, 2402–2410. [Google Scholar] [CrossRef]
- Valujskikh, A.; Pantenburg, B.; Heeger, P.S. Primed allospecific T cells prevent the effects of costimulatory blockade on prolonged cardiac allograft survival in mice. Am. J. Transplant. 2002, 2, 501–509. [Google Scholar] [CrossRef]
- Emerson, R.O.; Mathew, J.M.; Konieczna, I.M.; Robins, H.S.; Leventhal, J.R. Defining the alloreactive T cell repertoire using high-throughput sequencing of mixed lymphocyte reaction culture. PLoS ONE 2014, 9, e111943. [Google Scholar] [CrossRef][Green Version]
- Heeger, P.S.; Greenspan, N.S.; Kuhlenschmidt, S.; Dejelo, C.; Hricik, D.E.; Schulak, J.A.; Tary-Lehmann, M. Pretransplant frequency of donor-specific, IFN-gamma-producing lymphocytes is a manifestation of immunologic memory and correlates with the risk of posttransplant rejection episodes. J. Immunol. 1999, 163, 2267–2275. [Google Scholar]
- Gebauer, B.S.; Hricik, D.E.; Atallah, A.; Bryan, K.; Riley, J.; Tary-Lehmann, M.; Greenspan, N.S.; Dejelo, C.; Boehm, B.O.; Hering, B.J.; et al. Evolution of the enzyme-linked immunosorbent spot assay for post-transplant alloreactivity as a potentially useful immune monitoring tool. Am. J. Transplant. 2002, 2, 857–866. [Google Scholar] [CrossRef]
- Nickel, P.; Presber, F.; Bold, G.; Biti, D.; Schonemann, C.; Tullius, S.G.; Volk, H.D.; Reinke, P. Enzyme-linked immunosorbent spot assay for donor-reactive interferon-gamma-producing cells identifies T-cell presensitization and correlates with graft function at 6 and 12 months in renal-transplant recipients. Transplantation 2004, 78, 1640–1646. [Google Scholar] [CrossRef]
- Burrows, S.R.; Khanna, R.; Burrows, J.M.; Moss, D.J. An alloresponse in humans is dominated by cytotoxic T lymphocytes (CTL) cross-reactive with a single Epstein-Barr virus CTL epitope: Implications for graft-versus-host disease. J. Exp. Med. 1994, 179, 1155–1161. [Google Scholar] [CrossRef]
- Welsh, R.M.; Selin, L.K. No one is naive: The significance of heterologous T-cell immunity. Nat. Rev. Immunol. 2002, 2, 417–426. [Google Scholar] [CrossRef] [PubMed]
- Amir, A.L.; D’Orsogna, L.J.; Roelen, D.L.; van Loenen, M.M.; Hagedoorn, R.S.; de Boer, R.; van der Hoorn, M.A.; Kester, M.G.; Doxiadis, I.I.N.; Falkenburg, J.H.; et al. Allo-HLA reactivity of virus-specific memory T cells is common. Blood 2010, 115, 3146–3157. [Google Scholar] [CrossRef]
- Van den Heuvel, H.; Heutinck, K.M.; van der Meer-Prins, E.M.W.; Yong, S.L.; van Miert, P.; Anholts, J.D.H.; Franke-van Dijk, M.E.I.; Zhang, X.Q.; Roelen, D.L.; Ten Berge, R.J.M.; et al. Allo-HLA Cross-Reactivities of Cytomegalovirus-, Influenza-, and Varicella Zoster Virus-Specific Memory T Cells Are Shared by Different Healthy Individuals. Am. J. Transplant. 2017, 17, 2033–2044. [Google Scholar] [CrossRef] [PubMed]
- Huisman, W.; Leboux, D.A.T.; van der Maarel, L.E.; Hageman, L.; Amsen, D.; Falkenburg, J.H.F.; Jedema, I. Magnitude of Off-Target Allo-HLA Reactivity by Third-Party Donor-Derived Virus-Specific T Cells Is Dictated by HLA-Restriction. Front. Immunol. 2021, 12, 630440. [Google Scholar] [CrossRef] [PubMed]
- Adams, A.B.; Williams, M.A.; Jones, T.R.; Shirasugi, N.; Durham, M.M.; Kaech, S.M.; Wherry, E.J.; Onami, T.; Lanier, J.G.; Kokko, K.E.; et al. Heterologous immunity provides a potent barrier to transplantation tolerance. J. Clin. Investig. 2003, 111, 1887–1895. [Google Scholar] [CrossRef]
- Sachs, D.H. Tolerance: Of mice and men. J. Clin. Investig. 2003, 111, 1819–1821. [Google Scholar] [CrossRef]
- Brehm, M.A.; Daniels, K.A.; Priyadharshini, B.; Thornley, T.B.; Greiner, D.L.; Rossini, A.A.; Welsh, R.M. Allografts stimulate cross-reactive virus-specific memory CD8 T cells with private specificity. Am. J. Transplant. 2010, 10, 1738–1748. [Google Scholar] [CrossRef]
- Davis, M.M.; Bjorkman, P.J. T-cell antigen receptor genes and T-cell recognition. Nature 1988, 334, 395–402. [Google Scholar] [CrossRef]
- Krogsgaard, M.; Davis, M.M. How T cells ‘see’ antigen. Nat. Immunol. 2005, 6, 239–245. [Google Scholar] [CrossRef]
- Emerson, R.O.; DeWitt, W.S.; Vignali, M.; Gravley, J.; Hu, J.K.; Osborne, E.J.; Desmarais, C.; Klinger, M.; Carlson, C.S.; Hansen, J.A.; et al. Immunosequencing identifies signatures of cytomegalovirus exposure history and HLA-mediated effects on the T cell repertoire. Nat. Genet. 2017, 49, 659–665. [Google Scholar] [CrossRef] [PubMed]
- Carter, J.A.; Preall, J.B.; Grigaityte, K.; Goldfless, S.J.; Jeffery, E.; Briggs, A.W.; Vigneault, F.; Atwal, G.S. Single T Cell Sequencing Demonstrates the Functional Role of alphabeta TCR Pairing in Cell Lineage and Antigen Specificity. Front. Immunol. 2019, 10, 1516. [Google Scholar] [CrossRef] [PubMed]
- Mason, D. A very high level of crossreactivity is an essential feature of the T-cell receptor. Immunol. Today 1998, 19, 395–404. [Google Scholar] [CrossRef]
- Mazza, C.; Auphan-Anezin, N.; Gregoire, C.; Guimezanes, A.; Kellenberger, C.; Roussel, A.; Kearney, A.; van der Merwe, P.A.; Schmitt-Verhulst, A.M.; Malissen, B. How much can a T-cell antigen receptor adapt to structurally distinct antigenic peptides? EMBO J. 2007, 26, 1972–1983. [Google Scholar] [CrossRef]
- Lombardi, G.; Sidhu, S.; Daly, M.; Batchelor, J.R.; Makgoba, W.; Lechler, R.I. Are primary alloresponses truly primary? Int. Immunol. 1990, 2, 9–13. [Google Scholar] [CrossRef]
- Gill, R.G.; Burrack, A.L. Diverse Routes of Allograft Tolerance Disruption by Memory T Cells. Front. Immunol. 2020, 11, 580483. [Google Scholar] [CrossRef]
- Rowntree, L.C.; Nguyen, T.H.; Gras, S.; Kotsimbos, T.C.; Mifsud, N.A. Deciphering the clinical relevance of allo-human leukocyte antigen cross-reactivity in mediating alloimmunity following transplantation. Curr. Opin. Organ Transplant. 2016, 21, 29–39. [Google Scholar] [CrossRef]
- Rowntree, L.C.; van den Heuvel, H.; Sun, J.; D’Orsogna, L.J.; Nguyen, T.H.O.; Claas, F.H.J.; Rossjohn, J.; Kotsimbos, T.C.; Purcell, A.W.; Mifsud, N.A. Preferential HLA-B27 Allorecognition Displayed by Multiple Cross-Reactive Antiviral CD8(+) T Cell Receptors. Front. Immunol. 2020, 11, 248. [Google Scholar] [CrossRef]
- Almeida, C.A.; van Miert, P.; O’Driscoll, K.; Zoet, Y.M.; Chopra, A.; Watson, M.; de Santis, D.; Witt, C.; John, M.; Claas, F.H.J.; et al. Stimulation of HIV-specific T cell clonotypes using allogeneic HLA. Cell. Immunol. 2017, 316, 32–40. [Google Scholar] [CrossRef] [PubMed]
- Van der Zwan, A.; van der Meer-Prins, E.M.W.; van Miert, P.; van den Heuvel, H.; Anholts, J.D.H.; Roelen, D.L.; Claas, F.H.J.; Heidt, S. Cross-Reactivity of Virus-Specific CD8+ T Cells Against Allogeneic HLA-C: Possible Implications for Pregnancy Outcome. Front. Immunol. 2018, 9, 2880. [Google Scholar] [CrossRef] [PubMed]
- Rist, M.; Smith, C.; Bell, M.J.; Burrows, S.R.; Khanna, R. Cross-recognition of HLA DR4 alloantigen by virus-specific CD8+ T cells: A new paradigm for self-/nonself-recognition. Blood 2009, 114, 2244–2253. [Google Scholar] [CrossRef] [PubMed]
- Umetsu, D.T.; Yunis, E.J.; Matsui, Y.; Jabara, H.H.; Geha, R.S. HLA-DR-4-associated alloreactivity of an HLA-DR-3-restricted human tetanus toxoid-specific T cell clone: Inhibition of both reactivities by an alloantiserum. Eur. J. Immunol. 1985, 15, 356–361. [Google Scholar] [CrossRef]
- Elkington, R.; Khanna, R. Cross-recognition of human alloantigen by cytomegalovirus glycoprotein-specific CD4+ cytotoxic T lymphocytes: Implications for graft-versus-host disease. Blood 2005, 105, 1362–1364. [Google Scholar] [CrossRef]
- D’Orsogna, L.J.; Roelen, D.L.; van der Meer-Prins, E.M.; van der Pol, P.; Franke-van Dijk, M.E.; Eikmans, M.; Anholts, J.; Rossjohn, J.; McCluskey, J.; Mulder, A.; et al. Tissue specificity of cross-reactive allogeneic responses by EBV EBNA3A-specific memory T cells. Transplantation 2011, 91, 494–500. [Google Scholar] [CrossRef]
- D’Orsogna, L.J.; van den Heuvel, H.; van der Meer-Prins, E.M.; Roelen, D.L.; Doxiadis, I.I.N.; Claas, F.H. Stimulation of human EBV- and CMV-specific cytolytic effector function using allogeneic HLA molecules. J. Immunol. 2012, 189, 4825–4831. [Google Scholar] [CrossRef]
- Argaet, V.P.; Schmidt, C.W.; Burrows, S.R.; Silins, S.L.; Kurilla, M.G.; Doolan, D.L.; Suhrbier, A.; Moss, D.J.; Kieff, E.; Sculley, T.B.; et al. Dominant selection of an invariant T cell antigen receptor in response to persistent infection by Epstein-Barr virus. J. Exp. Med. 1994, 180, 2335–2340. [Google Scholar] [CrossRef]
- D’Orsogna, L.J.; Amir, A.L.; Zoet, Y.M.; van der Meer-Prins, P.M.; van der Slik, A.R.; Kester, M.G.; Heemskerk, M.H.; Doxiadis, I.I.N.; Roelen, D.L.; Claas, F.H. New tools to monitor the impact of viral infection on the alloreactive T-cell repertoire. Tissue Antigens 2009, 74, 290–297. [Google Scholar] [CrossRef] [PubMed]
- Burrows, S.R.; Silins, S.L.; Moss, D.J.; Khanna, R.; Misko, I.S.; Argaet, V.P. T cell receptor repertoire for a viral epitope in humans is diversified by tolerance to a background major histocompatibility complex antigen. J. Exp. Med. 1995, 182, 1703–1715. [Google Scholar] [CrossRef]
- Gras, S.; Burrows, S.R.; Kjer-Nielsen, L.; Clements, C.S.; Liu, Y.C.; Sullivan, L.C.; Bell, M.J.; Brooks, A.G.; Purcell, A.W.; McCluskey, J.; et al. The shaping of T cell receptor recognition by self-tolerance. Immunity 2009, 30, 193–203. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.H.; Rowntree, L.C.; Pellicci, D.G.; Bird, N.L.; Handel, A.; Kjer-Nielsen, L.; Kedzierska, K.; Kotsimbos, T.C.; Mifsud, N.A. Recognition of distinct cross-reactive virus-specific CD8+ T cells reveals a unique TCR signature in a clinical setting. J. Immunol. 2014, 192, 5039–5049. [Google Scholar] [CrossRef]
- Van Besouw, N.M.; van der Mast, B.J.; de Kuiper, P.; Smak Gregoor, P.J.; Vaessen, L.M.; JN, I.J.; van Gelder, T.; Weimar, W. Donor-specific T-cell reactivity identifies kidney transplant patients in whom immunosuppressive therapy can be safely reduced. Transplantation 2000, 70, 136–143. [Google Scholar] [PubMed]
- Van den Heuvel, H.; van der Meer-Prins, E.M.W.; van Miert, P.; Zhang, X.; Anholts, J.D.H.; Claas, F.H.J. Infection with a virus generates a polyclonal immune response with broad alloreactive potential. Hum. Immunol. 2019, 80, 97–102. [Google Scholar] [CrossRef] [PubMed]
- Danziger-Isakov, L.; Cherkassky, L.; Siegel, H.; McManamon, M.; Kramer, K.; Budev, M.; Sawinski, D.; Augustine, J.J.; Hricik, D.E.; Fairchild, R.; et al. Effects of influenza immunization on humoral and cellular alloreactivity in humans. Transplantation 2010, 89, 838–844. [Google Scholar] [CrossRef]
- Danziger-Isakov, L.; Kumar, D.; Practice, A.I.C.o. Vaccination of solid organ transplant candidates and recipients: Guidelines from the American society of transplantation infectious diseases community of practice. Clin. Transplant. 2019, 33, e13563. [Google Scholar] [CrossRef]
- Caillard, S.; Thaunat, O. COVID-19 vaccination in kidney transplant recipients. Nat. Rev. Nephrol. 2021, 17, 785–787. [Google Scholar] [CrossRef] [PubMed]
- D’Orsogna, L.; van den Heuvel, H.; van Kooten, C.; Heidt, S.; Claas, F.H.J. Infectious pathogens may trigger specific allo-HLA reactivity via multiple mechanisms. Immunogenetics 2017, 69, 631–641. [Google Scholar] [CrossRef]
- D’Orsogna, L.J.; van Besouw, N.M.; van der Meer-Prins, E.M.; van der Pol, P.; Franke-van Dijk, M.; Zoet, Y.M.; van der Slik, A.; Weimar, W.; van Kooten, C.; Mulder, A.; et al. Vaccine-Induced Allo-HLA-Reactive Memory T Cells in a Kidney Transplantation Candidate. Transplantation 2011, 91, 645–651. [Google Scholar] [CrossRef]
- Heldman, M.R.; Limaye, A.P. SARS-CoV-2 Vaccines in Kidney Transplant Recipients: Will They Be Safe and Effective and How Will We Know? J. Am. Soc. Nephrol. 2021, 32, 1021–1024. [Google Scholar] [CrossRef]
- Prendecki, M.; Thomson, T.; Clarke, C.L.; Martin, P.; Gleeson, S.; De Aguiar, R.C.; Edwards, H.; Mortimer, P.; McIntyre, S.; Mokreri, D.; et al. Immunological responses to SARS-CoV-2 vaccines in kidney transplant recipients. Lancet 2021, 398, 1482–1484. [Google Scholar] [CrossRef]
- Burrows, S.R.; Silins, S.L.; Khanna, R.; Burrows, J.M.; Rischmueller, M.; McCluskey, J.; Moss, D.J. Cross-reactive memory T cells for Epstein-Barr virus augment the alloresponse to common human leukocyte antigens: Degenerate recognition of major histocompatibility complex-bound peptide by T cells and its role in alloreactivity. Eur. J. Immunol. 1997, 27, 1726–1736. [Google Scholar] [CrossRef]
- D’Orsogna, L.J.; van der Meer-Prins, E.M.; Zoet, Y.M.; Roelen, D.L.; Doxiadis, I.I.N.; Claas, F.H. Detection of allo-HLA cross-reactivity by virus-specific memory T-cell clones using single HLA-transfected K562 cells. Methods Mol. Biol. 2012, 882, 339–349. [Google Scholar] [CrossRef]
- Heidt, S.; Feltkamp, M.C.; Karahan, G.E.; de Brouwer, C.S.; Langerak-Langerak, J.; Mulder, A.; Claas, F.H.J. No Evidence for Cross-reactivity of Virus-specific Antibodies With HLA Alloantigens. Transplantation 2018, 102, 1844–1849. [Google Scholar] [CrossRef] [PubMed]
- Russell, M.R.; Halnon, N.J.; Alejos, J.C.; Salem, M.M.; Reardon, L.C. COVID-19 in a pediatric heart transplant recipient: Emergence of donor-specific antibodies. J. Heart Lung Transplant. 2020, 39, 732–733. [Google Scholar] [CrossRef] [PubMed]
- Roll, G.R.; Lunow-Luke, T.; Braun, H.J.; Buenaventura, O.; Mallari, M.; Stock, P.G.; Rajalingam, R. COVID-19 does not impact HLA antibody profile in a series of waitlisted renal transplant candidates. Hum. Immunol. 2021, 82, 568–573. [Google Scholar] [CrossRef]
- Adams, A.B.; Pearson, T.C.; Larsen, C.P. Heterologous immunity: An overlooked barrier to tolerance. Immunol. Rev. 2003, 196, 147–160. [Google Scholar] [CrossRef] [PubMed]
- Mifsud, N.A.; Nguyen, T.H.; Tait, B.D.; Kotsimbos, T.C. Quantitative and functional diversity of cross-reactive EBV-specific CD8+ T cells in a longitudinal study cohort of lung transplant recipients. Transplantation 2010, 90, 1439–1449. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.H.; Westall, G.P.; Bull, T.E.; Meehan, A.C.; Mifsud, N.A.; Kotsimbos, T.C. Cross-reactive anti-viral T cells increase prior to an episode of viral reactivation post human lung transplantation. PLoS ONE 2013, 8, e56042. [Google Scholar] [CrossRef]
- Heutinck, K.M.; Yong, S.L.; Tonneijck, L.; van den Heuvel, H.; van der Weerd, N.C.; van der Pant, K.A.; Bemelman, F.J.; Claas, F.H.; Ten Berge, I.J. Virus-Specific CD8(+) T Cells Cross-Reactive to Donor-Alloantigen Are Transiently Present in the Circulation of Kidney Transplant Recipients Infected With CMV and/or EBV. Am. J. Transplant. 2016, 16, 1480–1491. [Google Scholar] [CrossRef]
- Stranavova, L.; Pelak, O.; Svaton, M.; Hruba, P.; Fronkova, E.; Slavcev, A.; Osickova, K.; Maluskova, J.; Hubacek, P.; Fronek, J.; et al. Heterologous Cytomegalovirus and Allo-Reactivity by Shared T Cell Receptor Repertoire in Kidney Transplantation. Front. Immunol. 2019, 10, 2549. [Google Scholar] [CrossRef]
- D’Orsogna, L.J.; Nguyen, T.H.; Claas, F.H.; Witt, C.; Mifsud, N.A. Endogenous-peptide-dependent alloreactivity: New scientific insights and clinical implications. Tissue Antigens 2013, 81, 399–407. [Google Scholar] [CrossRef] [PubMed]
- Van den Heuvel, H.; Heutinck, K.M.; van der Meer-Prins, E.M.W.; Franke-van Dijk, M.E.I.; van Miert, P.; Zhang, X.; Ten Berge, I.J.M.; Claas, F.H.J. The avidity of cross-reactive virus-specific T cells for their viral and allogeneic epitopes is variable and depends on epitope expression. Hum. Immunol. 2018, 79, 39–50. [Google Scholar] [CrossRef]
- Naesens, M.; Kuypers, D.R.; Sarwal, M. Calcineurin inhibitor nephrotoxicity. Clin. J. Am. Soc. Nephrol. 2009, 4, 481–508. [Google Scholar] [CrossRef] [PubMed]
- Kumar, J.; Reccia, I.; Virdis, F.; Podda, M.; Sharma, A.K.; Halawa, A. Belatacept in renal transplantation in comparison to tacrolimus and molecular understanding of resistance pattern: Meta-analysis and systematic review. World J. Transplant. 2021, 11, 70–86. [Google Scholar] [CrossRef]
- Gaston, R.S. Chronic calcineurin inhibitor nephrotoxicity: Reflections on an evolving paradigm. Clin. J. Am. Soc. Nephrol. 2009, 4, 2029–2034. [Google Scholar] [CrossRef]
- Kaufman, D.B.; Woodle, E.S.; Shields, A.R.; Leone, J.; Matas, A.; Wiseman, A.; West-Thielke, P.; Sa, T.; King, E.C.; Alloway, R.R.; et al. Belatacept for Simultaneous Calcineurin Inhibitor and Chronic Corticosteroid Immunosuppression Avoidance: Two-Year Results of a Prospective, Randomized Multicenter Trial. Clin. J. Am. Soc. Nephrol. 2021, 16, 1387–1397. [Google Scholar] [CrossRef]
- De Graav, G.N.; Baan, C.C.; Clahsen-van Groningen, M.C.; Kraaijeveld, R.; Dieterich, M.; Verschoor, W.; von der Thusen, J.H.; Roelen, D.L.; Cadogan, M.; van de Wetering, J.; et al. A Randomized Controlled Clinical Trial Comparing Belatacept With Tacrolimus After De Novo Kidney Transplantation. Transplantation 2017, 101, 2571–2581. [Google Scholar] [CrossRef]
- Mathews, D.V.; Wakwe, W.C.; Kim, S.C.; Lowe, M.C.; Breeden, C.; Roberts, M.E.; Farris, A.B.; Strobert, E.A.; Jenkins, J.B.; Larsen, C.P.; et al. Belatacept-Resistant Rejection Is Associated With CD28(+) Memory CD8 T Cells. Am. J. Transplant. 2017, 17, 2285–2299. [Google Scholar] [CrossRef]
- Karadkhele, G.; Hogan, J.; Magua, W.; Zhang, W.; Badell, I.R.; Mehta, A.; Lyon, M.; Pastan, S.; Pearson, T.C.; Larsen, C.P. CMV high-risk status and posttransplant outcomes in kidney transplant recipients treated with belatacept. Am. J. Transplant. 2021, 21, 208–221. [Google Scholar] [CrossRef] [PubMed]
- Chavarot, N.; Divard, G.; Scemla, A.; Amrouche, L.; Aubert, O.; Leruez-Ville, M.; Timsit, M.O.; Tinel, C.; Zuber, J.; Legendre, C.; et al. Increased incidence and unusual presentations of CMV disease in kidney transplant recipients after conversion to belatacept. Am. J. Transplant. 2021, 21, 2448–2458. [Google Scholar] [CrossRef] [PubMed]
- Pantenburg, B.; Heinzel, F.; Das, L.; Heeger, P.S.; Valujskikh, A. T cells primed by Leishmania major infection cross-react with alloantigens and alter the course of allograft rejection. J. Immunol. 2002, 169, 3686–3693. [Google Scholar] [CrossRef] [PubMed]
- Melenhorst, J.J.; Leen, A.M.; Bollard, C.M.; Quigley, M.F.; Price, D.A.; Rooney, C.M.; Brenner, M.K.; Barrett, A.J.; Heslop, H.E. Allogeneic virus-specific T cells with HLA alloreactivity do not produce GVHD in human subjects. Blood 2010, 116, 4700–4702. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Karahan, G.E.; Claas, F.H.J.; Heidt, S. Heterologous Immunity of Virus-Specific T Cells Leading to Alloreactivity: Possible Implications for Solid Organ Transplantation. Viruses 2021, 13, 2359. https://doi.org/10.3390/v13122359
Karahan GE, Claas FHJ, Heidt S. Heterologous Immunity of Virus-Specific T Cells Leading to Alloreactivity: Possible Implications for Solid Organ Transplantation. Viruses. 2021; 13(12):2359. https://doi.org/10.3390/v13122359
Chicago/Turabian StyleKarahan, Gonca E., Frans H. J. Claas, and Sebastiaan Heidt. 2021. "Heterologous Immunity of Virus-Specific T Cells Leading to Alloreactivity: Possible Implications for Solid Organ Transplantation" Viruses 13, no. 12: 2359. https://doi.org/10.3390/v13122359
APA StyleKarahan, G. E., Claas, F. H. J., & Heidt, S. (2021). Heterologous Immunity of Virus-Specific T Cells Leading to Alloreactivity: Possible Implications for Solid Organ Transplantation. Viruses, 13(12), 2359. https://doi.org/10.3390/v13122359





