Role of Serum Vitamin D, Interleukin 13, and microRNA-135a in Hepatocellular Carcinoma and Treatment Failure in Egyptian HCV-Infected Patients Receiving Direct Antiviral Agents
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Study Subjects
2.3. Sample Processing
2.4. Quantitative Real-Time Polymerase Chain Reaction (RT-qPCR)
2.5. Determination of Vit D by ELISA
2.6. Determination of Human IL-13 by ELISA
2.7. Quantification of HCV Viral Load
2.8. Statistical Analysis
3. Results
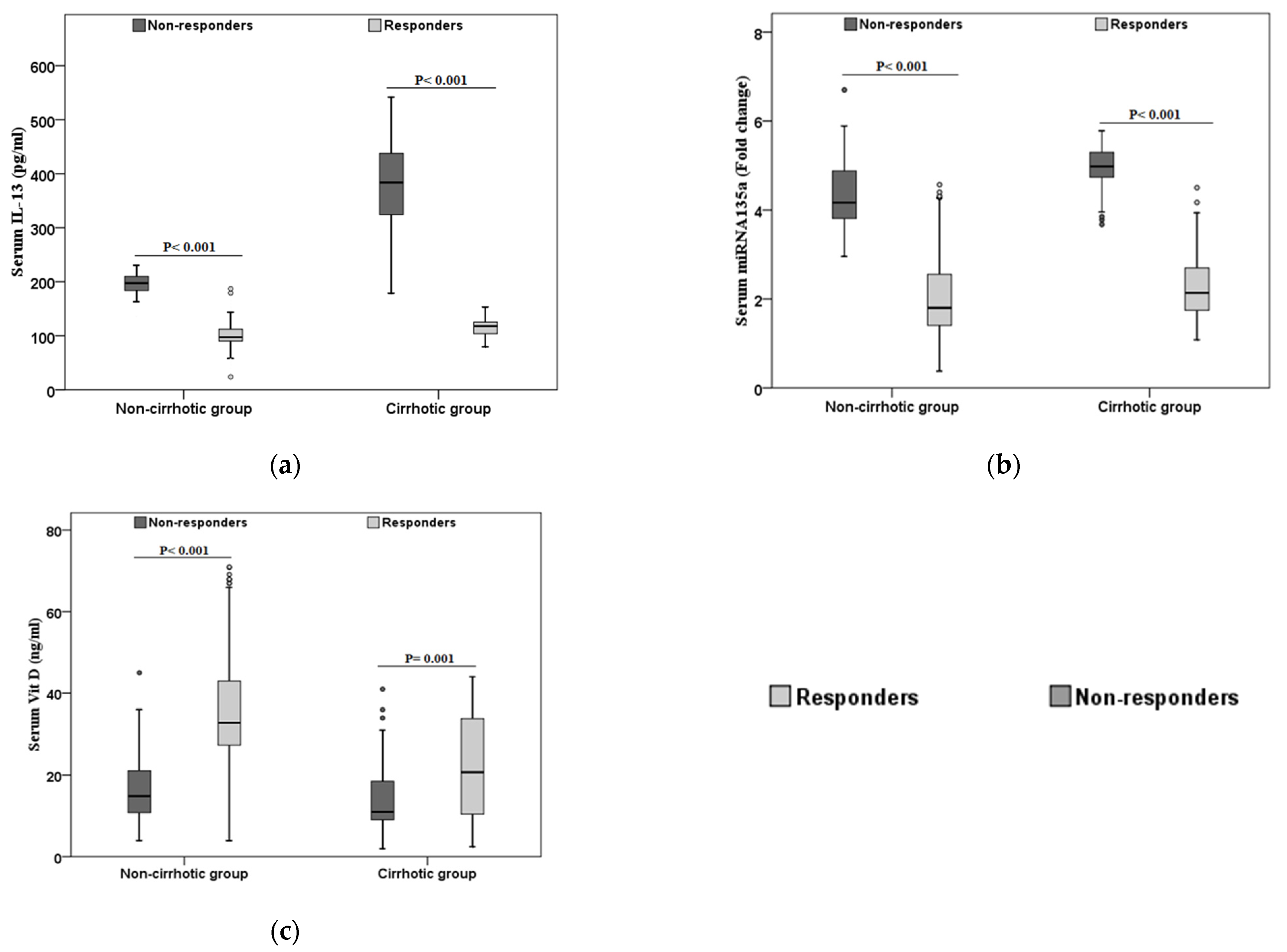
3.1. Characteristics of the Study Population According to the Treatment Response
3.2. Characteristics of the Non-Responders According to the HCC Development
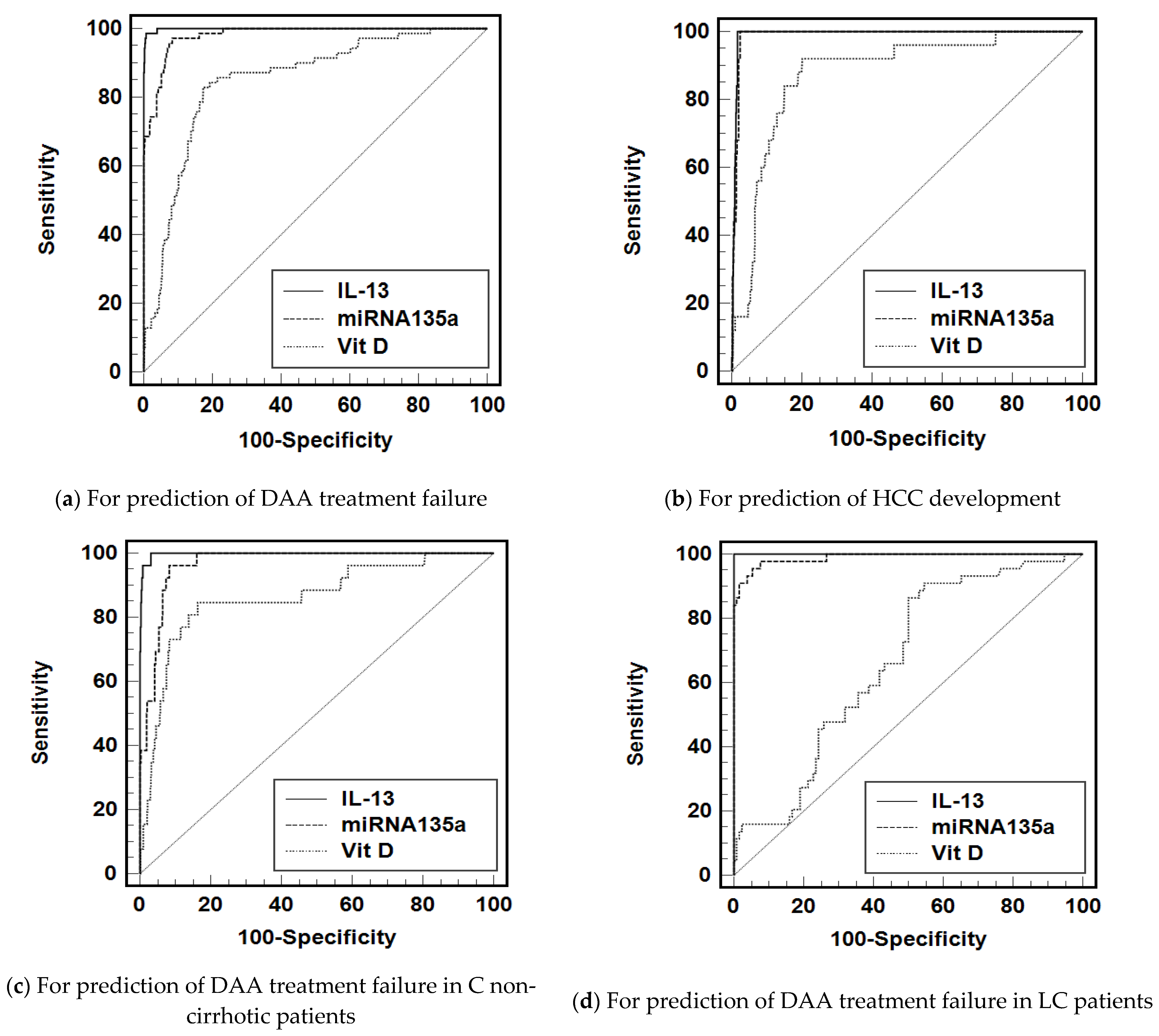
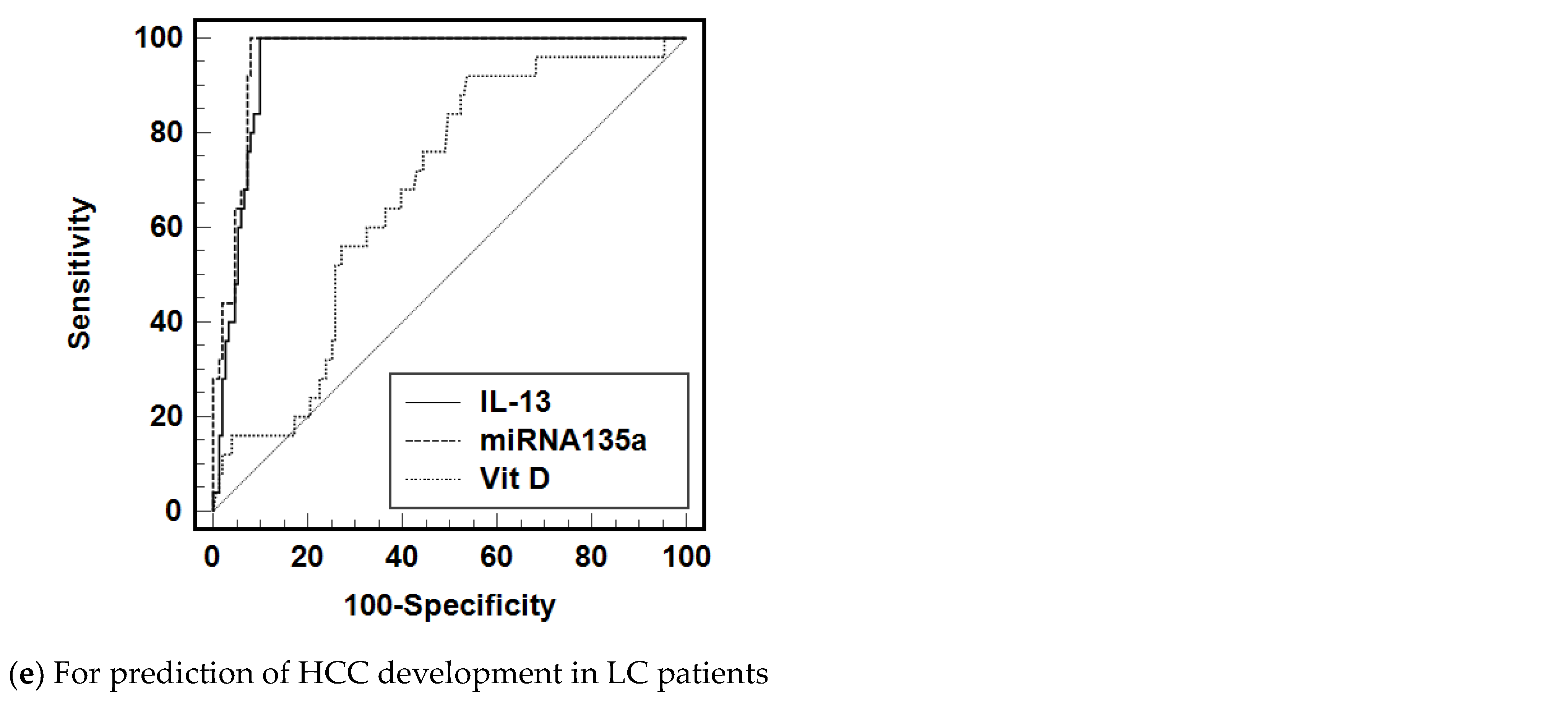
3.3. Diagnostic Accuracy of IL-13, miRNA-135a, and Vit D to Predict the Treatment Failure and HCC Development
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Deterding, K.; zu Siederdissen, C.H.; Port, K.; Solbach, P.; Sollik, L.; Kirschner, J.; Wedemeyer, H. Improvement of liver function parameters in advanced HCV-associated liver cirrhosis by IFN-free antiviral therapies. Aliment. Pharmacol. Ther. 2015, 42, 889–901. [Google Scholar] [CrossRef] [PubMed]
- Lok, A.S.; Seeff, L.B.; Morgan, T.R.; di Bisceglie, A.M.; Sterling, R.K.; Curto, T.M.; HALT-C Trial Group. Incidence of Hepatocellular Carcinoma and Associated Risk Factors in Hepatitis C-Related Advanced Liver Disease. Gastroenterology 2009, 136, 138–148. [Google Scholar] [CrossRef]
- Lombardi, A.; Mondelli, M.U.; ESCMID Study Group for Viral Hepatitis (ESGVH). Hepatitis C: Is eradication possible? Liver Int. 2019, 39, 416–426. [Google Scholar] [CrossRef] [PubMed]
- Ghany, M.G.; Strader, D.B.; Thomas, D.L.; Seeff, L.B. Diagnosis, management, and treatment of hepatitis C: An update. Hepatology 2008, 49, 1335–1374. [Google Scholar] [CrossRef] [PubMed]
- Vezali, E.; Aghemo, A.; Colombo, M. A review of the treatment of chronic hepatitis C virus infection in cirrhosis. Clin. Ther. 2010, 32, 2117–2138. [Google Scholar] [CrossRef]
- Zhuo, Y.; Hayashi, T.; Chen, Q.; Aggarwal, R.; Hutin, Y.; Chhatwal, J. Estimating the price at which hepatitis C treatment with direct-acting antivirals would be cost-saving in Japan. Sci. Rep. 2020, 10, 1–9. [Google Scholar] [CrossRef]
- Das, D.; Pandya, M. Recent advancement of direct-acting antiviral agents (DAAs) in hepatitis C therapy. Mini Rev. Med. Chem. 2018, 18, 584–596. [Google Scholar] [CrossRef]
- Wyles, D.L. Resistance to DAAs: When to Look and When It Matters. Curr. HIV/AIDS Rep. 2017, 14, 229–237. [Google Scholar] [CrossRef]
- Chen, Z.-W.; Li, H.; Ren, H.; Hu, P. Global prevalence of pre-existing HCV variants resistant to direct-acting antiviral agents (DAAs): Mining the GenBank HCV genome data. Sci. Rep. 2016, 6, 20310. [Google Scholar] [CrossRef] [PubMed]
- Bourlière, M.; Gordon, S.C.; Schiff, E.R.; Tran, T.T.; Ravendhran, N.; Landis, C.S.; Hyland, R.H.; Stamm, L.M.; Zhang, J.; Dvory-Sobol, H.; et al. Deferred treatment with sofosbuvir–velpatasvir–voxilaprevir for patients with chronic hepatitis C virus who were previously treated with an NS5A inhibitor: An open-label substudy of POLARIS-1. Lancet Gastroenterol. Hepatol. 2018, 3, 559–565. [Google Scholar] [CrossRef]
- Hézode, C.; Chevaliez, S.; Scoazec, G.; Soulier, A.; Varaut, A.; Bouvier-Alias, M.; Ruiz, I.; Roudot-Thoraval, F.; Mallat, A.; Féray, C.; et al. Retreatment with sofosbuvir and simeprevir of patients with hepatitis C virus genotype 1 or 4 who previously failed a daclatasvir-containing regimen. Hepatology 2016, 63, 1809–1816. [Google Scholar] [CrossRef] [PubMed]
- Echeverría, N. Hepatitis C virus genetic variability and evolution. World J. Hepatol. 2015, 7, 831–845. [Google Scholar] [CrossRef]
- Cuypers, L.; Ceccherini-Silberstein, F.; Van Laethem, K.; Li, G.; Vandamme, A.-M.; Rockstroh, J.K. Impact of HCV genotype on treatment regimens and drug resistance: A snapshot in time. Rev. Med. Virol. 2016, 26, 408–434. [Google Scholar] [CrossRef] [PubMed]
- Forns, X.; Lawitz, E.; Zeuzem, S.; Gane, E.; Bronowicki, J.P.; Andreone, P.; Horban, A.; Brown, A.; Peeters, M.; Lenz, O.; et al. Simeprevir with Peginterferon and Ribavirin Leads to High Rates of SVR in Patients with HCV Genotype 1 Who Relapsed after Previous Therapy: A Phase 3 Trial. Gastroenterology 2014, 146, 1669–1679. [Google Scholar] [CrossRef]
- Fourati, S.; Pawlotsky, J.-M. Virologic Tools for HCV Drug Resistance Testing. Viruses 2015, 7, 6346–6359. [Google Scholar] [CrossRef]
- Rahman, A.H.; Branch, A.D. Vitamin D for your patients with chronic hepatitis C? J. Hepatol. 2013, 58, 184–189. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.; Read, S.A.; Shackel, N.A.; Hebbard, L.; George, J.; Ahlenstiel, G. The Role of Micronutrients in the Infection and Subsequent Response to Hepatitis C Virus. Cells 2019, 8, 603. [Google Scholar] [CrossRef]
- Gal-Tanamy, M.; Bachmetov, L.; Ravid, A.; Koren, R.; Erman, A.; Tur-Kaspa, R.; Zemel, R. Vitamin D: An innate antiviral agent suppressing hepatitis C virus in human hepatocytes. Hepatology 2011, 54, 1570–1579. [Google Scholar] [CrossRef] [PubMed]
- Gutierrez, J.A. Vitamin D Metabolites Inhibit Hepatitis C Virus and Modulate Cellular Gene Expression. J. Virol. Antivir. Res. 2014, 3. [Google Scholar] [CrossRef] [PubMed]
- Esmat, G.; El-Raziky, M.; Elsharkawy, A.; Sabry, D.; Hassany, M.; Ahmed, A.; Assem, N.; El Kassas, M.; Doss, W. Impact of Vitamin D Supplementation on Sustained Virological Response in Chronic Hepatitis C Genotype 4 Patients Treated by Pegylated Interferon/Ribavirin. J. Interferon Cytokine Res. 2015, 35, 49–54. [Google Scholar] [CrossRef]
- Petta, S.; Ferraro, D.; Cammà, C.; Cabibi, D.; Di Cristina, A.; Di Marco, V.; Di Stefano, R.; Grimaudo, S.; Mazzola, A.; Levrero, M.; et al. Vitamin D levels and IL28B polymorphisms are related to rapid virological response to standard of care in genotype 1 chronic hepatitis C. Antivir. Ther. 2012, 17, 823–831. [Google Scholar] [CrossRef] [PubMed]
- Nimer, A.; Mouch, A. Vitamin D improves viral response in hepatitis C genotype 2-3 naïve patients. World J. Gastroenterol. WJG 2012, 18, 800. [Google Scholar] [CrossRef]
- Petta, S.; Cammà, C.; Scazzone, C.; Tripodo, C.; Di Marco, V.; Bono, A.; Craxí, A. Low vitamin D serum level is related to severe fibrosis and low responsiveness to interferon-based therapy in genotype 1 chronic hepatitis C. Hepatology 2010, 51, 1158–1167. [Google Scholar] [CrossRef]
- Arima, K.; Sato, K.; Tanaka, G.; Kanaji, S.; Terada, T.; Honjo, E.; Kuroki, R.; Matsuo, Y.; Izuhara, K. Characterization of the Interaction between Interleukin-13 and Interleukin-13 Receptors. J. Biol. Chem. 2005, 280, 24915–24922. [Google Scholar] [CrossRef]
- Brightling, C.E.; Saha, S.; Hollins, F. Interleukin-13: Prospects for new treatments. Clin. Exp. Allergy 2009, 40, 42–49. [Google Scholar] [CrossRef]
- Opal, S.M.; DePalo, V.A. Anti-inflammatory cytokines. Chest 2000, 117, 1162–1172. [Google Scholar] [CrossRef] [PubMed]
- Wynn, T.A. IL-13 effector functions. Annu. Rev. Immunol. 2003, 21, 425–456. [Google Scholar] [CrossRef]
- Deng, Y.; Xie, M.; Xie, L.; Wang, J.; Li, T.; He, Y.; Li, R.; Li, S.; Qin, X. Association between Polymorphism of the Interleukin-13 Gene and Susceptibility to Hepatocellular Carcinoma in the Chinese Population. PLoS ONE 2015, 10, e0116682. [Google Scholar] [CrossRef]
- Seyfizadeh, N.; Seyfizadeh, N.; Gharibi, T.; Babaloo, Z. Interleukin-13 as an important cytokine: A review on its roles in some human diseases. Acta Microbiol. Immunol. Hung. 2015, 62, 341–378. [Google Scholar] [CrossRef][Green Version]
- Weng, H.-L.; Liu, Y.; Chen, J.-L.; Huang, T.; Xu, L.-J.; Godoy, P.; Hu, J.-H.; Zhou, C.; Stickel, F.; Marx, A.; et al. The etiology of liver damage imparts cytokines transforming growth factor β1 or interleukin-13 as driving forces in fibrogenesis. Hepatology 2009, 50, 230–243. [Google Scholar] [CrossRef] [PubMed]
- Formentini, A.; Braun, P.; Fricke, H.; Link, K.-H.; Henne-Bruns, D.; Kornmann, M. Expression of interleukin-4 and interleukin-13 and their receptors in colorectal cancer. Int. J. Color. Dis. 2012, 27, 1369–1376. [Google Scholar] [CrossRef] [PubMed]
- Zhu, V.F.; Yang, J.; LeBrun, D.; Li, M. Understanding the role of cytokines in Glioblastoma Multiforme pathogenesis. Cancer Lett. 2012, 316, 139–150. [Google Scholar] [CrossRef] [PubMed]
- Salama, A.; El-Ragehy, N.; Ali, A.; Elmahdy, E. Role of interleukin-33 in patients with chronic hepatitis c in menoufia university hospitals, Egypt. Menoufia Med. J. 2017, 30, 249–254. [Google Scholar]
- Jílková, Z.M.; Seigneurin, A.; Coppard, C.; Ouaguia, L.; Aspord, C.; Marche, P.N.; Leroy, V.; Decaens, T. Circulating IL-13 Is Associated with De Novo Development of HCC in HCV-Infected Patients Responding to Direct-Acting Antivirals. Cancers 2020, 12, 3820. [Google Scholar] [CrossRef]
- Debes, J.D.; van Tilborg, M.; Groothuismink, Z.M.; Hansen, B.E.; Wiesch, J.S.Z.; von Felden, J.; de Knegt, R.J.; Boonstra, A. Levels of Cytokines in Serum Associate with Development of Hepatocellular Carcinoma in Patients with HCV Infection Treated with Direct-Acting Antivirals. Gastroenterology 2018, 154, 515–517. [Google Scholar] [CrossRef]
- Sodroski, C.; Lowey, B.; Hertz, L.; Liang, T.J.; Li, Q. MicroRNA-135a Modulates Hepatitis C Virus Genome Replication through Downregulation of Host Antiviral Factors. Virol. Sin. 2019, 34, 197–210. [Google Scholar] [CrossRef]
- Perz, J.F.; Armstrong, G.L.; Farrington, L.A.; Hutin, Y.J.; Bell, B.P. The contributions of hepatitis B virus and hepatitis C virus infections to cirrhosis and primary liver cancer worldwide. J. Hepatol. 2006, 45, 529–538. [Google Scholar] [CrossRef]
- Broutier, L.; Mastrogiovanni, G.; Verstegen, M.M.A.; Francies, H.E.; Gavarró, L.M.; Bradshaw, C.R.; Allen, G.E.; Arnes-Benito, R.; Sidorova, O.; Gaspersz, M.P.; et al. Human primary liver cancer–derived organoid cultures for disease modeling and drug screening. Nat. Med. 2017, 23, 1424–1435. [Google Scholar] [CrossRef]
- European Association for the Study of the Liver. EASL-EORTC clinical practice guidelines: Management of hepatocellular carcinoma. J. Hepatol. 2012, 56, 908–943. [Google Scholar] [CrossRef]
- Reig, M.; Mariño, Z.; Perelló, C.; Iñarrairaegui, M.; Ribeiro, A.; Lens, S.; Bruix, J. Unexpected high rate of early tumor recurrence in patients with HCV-related HCC undergoing interferon-free therapy. J. Hepatol. 2016, 65, 719–726. [Google Scholar] [CrossRef]
- Conti, F.; Buonfiglioli, F.; Scuteri, A.; Crespi, C.; Bolondi, L.; Caraceni, P.; Brillanti, S. Early occurrence and recurrence of hepatocellular carcinoma in HCV-related cirrhosis treated with direct-acting antivirals. J. Hepatol. 2016, 65, 727–733. [Google Scholar] [CrossRef] [PubMed]
- Baack, B.; Smith, B.D.; Yartel, A.; Pitasi, M.; Falck-Ytter, Y. Eradication of hepatitis C virus infection and the development of hepatocellular carcinoma: A meta-analysis of observational studies. Ann. Intern. Med. 2013, 158, 329–337. [Google Scholar]
- Nault, J.-C.; Colombo, M. Hepatocellular carcinoma and direct acting antiviral treatments: Controversy after the revolution. J. Hepatol. 2016, 65, 663–665. [Google Scholar] [CrossRef] [PubMed]
- Kozbial, K.; Moser, S.; Schwarzer, R.; Laferl, H.; Al-Zoairy, R.; Stauber, R.; Ferenci, P. Unexpected high incidence of hepatocellular carcinoma in cirrhotic patients with sustained virologic response following interferon-free direct-acting antiviral treatment. J. Hepatol. 2016, 65, 856–858. [Google Scholar] [CrossRef]
- Rinaldi, L.; Di Francia, R.; Coppola, N.; Guerrera, B.; Imparato, M.; Monari, C.; Adinolfi, L.E. Hepatocellular carcinoma in HCV cirrhosis after viral clearance with direct acting antiviral therapy: Preliminary evidence and possible meanings. Wcrj 2016, 3, e748. [Google Scholar]
- Faillaci, F.; Marzi, L.; Critelli, R.; Milosa, F.; Schepis, F.; Turola, E.; Villa, E. Liver A ngiopoietin-2 Is a Key Predictor of De Novo or Recurrent Hepatocellular Cancer after Hepatitis C Virus Direct-Acting Antivirals. Hepatology 2018, 68, 1010–1024. [Google Scholar] [CrossRef]
- Waked, I.; Esmat, G.; Elsharkawy, A.; El-Serafy, M.; Abdel-Razek, W.; Ghalab, R.; Elshishiney, G.; Salah, A.; Megid, S.A.; Kabil, K.; et al. Screening and Treatment Program to Eliminate Hepatitis C in Egypt. N. Engl. J. Med. 2020, 382, 1166–1174. [Google Scholar] [CrossRef]
- Foster, G.R.; Afdhal, N.; Roberts, S.K.; Bräu, N.; Gane, E.J.; Pianko, S.; Lawitz, E.; Thompson, A.; Shiffman, M.L.; Cooper, C.; et al. Sofosbuvir and Velpatasvir for HCV Genotype 2 and 3 Infection. N. Engl. J. Med. 2015, 373, 2608–2617. [Google Scholar] [CrossRef]
- European Association for the Study of the Liver. EASL recommendations on treatment of hepatitis C 2016. J. Hepatol. 2017, 66, 153–194. [Google Scholar] [CrossRef]
- Poordad, F.; Schiff, E.R.; Vierling, J.M.; Landis, C.; Fontana, R.J.; Yang, R.; McPhee, F.; Hughes, E.A.; Noviello, S.; Swenson, E.S. Daclatasvir with sofosbuvir and ribavirin for hepatitis C virus infection with advanced cirrhosis or post-liver transplantation recurrence. Hepatology 2016, 63, 1493–1505. [Google Scholar] [CrossRef]
- Saadoun, D.; Pol, S.; Ferfar, Y.; Alric, L.; Hezode, C.; Ahmed, S.N.S.; Martin, L.D.S.; Comarmond, C.; Bouyer, A.S.; Musset, L.; et al. Efficacy and Safety of Sofosbuvir Plus Daclatasvir for Treatment of HCV-Associated Cryoglobulinemia Vasculitis. Gastroenterology 2017, 153, 49–52. [Google Scholar] [CrossRef]
- Ahmed, O.A.; Safwat, E.; Khalifa, M.O.; Elshafie, A.I.; Fouad, M.H.A.; Salama, M.M.; Abd-Elsalam, S. Sofosbuvir plus daclatasvir in treatment of chronic hepatitis C genotype 4 infection in a cohort of Egyptian patients: An experiment the size of Egyptian village. Int. J. Hepatol. 2018, 2018, 9616234. [Google Scholar] [CrossRef]
- Aly, O.A.; Yousry, W.A.; Teama, N.M.; Shona, E.M.; ElGhandour, A.M. Sofosbuvir and daclatasvir are safe and effective in treatment of recurrent hepatitis C virus in Egyptian patients underwent living donor liver transplantation. Egypt. Liver J. 2020, 10, 1–6. [Google Scholar] [CrossRef]
- Yoshida, E.M.; Sulkowski, M.S.; Gane, E.J., Jr.; Herring, R.W.; Ratziu, V.; Ding, X.; Wang, J.; Chuang, S.; Ma, J.; McNally, J.; et al. Concordance of sustained virological response 4, 12, and 24 weeks post-treatment with sofosbuvir-containing regimens for hepatitis C virus. Hepatology 2014, 61, 41–45. [Google Scholar] [CrossRef]
- Elsabaawy, M.M.; Gameel, K.; Eldemerdash, H.; Zakareia, T.; Eltahawy, M.; Albert, M.; Eljaky, A. Sustained virological response 12 versus sustained virological response 24 as evaluation endpoints in chronic hepatitis C virus Egyptian patients treated with sofosbuvir-based regimens. Egypt. J. Intern. Med. 2019, 31, 495–501. [Google Scholar]
- European Association for the Study of the Liver. EASL Clinical Practice Guidelines: Management of hepatocellular carcinoma. J. Hepatol. 2018, 69, 182–236. [Google Scholar] [CrossRef]
- Zou, Y.; Jing, C.; Liu, L.; Wang, T. Serum microRNA-135a as a diagnostic biomarker in non-small cell lung cancer. Medicine 2019, 98, e17814. [Google Scholar] [CrossRef] [PubMed]
- Karoney, M.J.; Siika, A.M. Hepatitis C virus (HCV) infection in Africa: A review. Pan Afr. Med. J. 2013, 14. [Google Scholar] [CrossRef] [PubMed]
- Kandeel, A.; Genedy, M.; El-Refai, S.; Funk, A.L.; Fontanet, A.; Talaat, M. The prevalence of hepatitis C virus infection in Egypt 2015: Implications for future policy on prevention and treatment. Liver Int. 2017, 37, 45–53. [Google Scholar] [CrossRef]
- Zeuzem, S.; Hultcrantz, R.; Bourliere, M.; Goeser, T.; Marcellin, P.; Sanchez-Tapias, J.; Albrecht, J. Peginterferon alfa-2b plus ribavirin for treatment of chronic hepatitis C in previously untreated patients infected with HCV genotypes 2 or 3. J. Hepatol. 2004, 40, 993–999. [Google Scholar] [CrossRef] [PubMed]
- Lawitz, E.; Mangia, A.; Wyles, D.; Rodriguez-Torres, M.; Hassanein, T.; Gordon, S.C.; Schultz, M.; Davis, M.; Kayali, Z.; Reddy, K.R.; et al. Sofosbuvir for Previously Untreated Chronic Hepatitis C Infection. N. Engl. J. Med. 2013, 368, 1878–1887. [Google Scholar] [CrossRef]
- Sulkowski, M.S.; Gardiner, D.F.; Rodriguez-Torres, M.; Reddy, K.R.; Hassanein, T.; Jacobson, I.; Lawitz, E.; Lok, A.S.; Hinestrosa, F.; Thuluvath, P.J.; et al. Daclatasvir plus Sofosbuvir for Previously Treated or Untreated Chronic HCV Infection. N. Engl. J. Med. 2014, 370, 211–221. [Google Scholar] [CrossRef] [PubMed]
- Lawitz, E.; Poordad, F.F.; Pang, P.S.; Hyland, R.H.; Ding, X.; Mo, H.; Symonds, W.T.; McHutchison, J.G.; E Membreno, F. Sofosbuvir and ledipasvir fixed-dose combination with and without ribavirin in treatment-naive and previously treated patients with genotype 1 hepatitis C virus infection (LONESTAR): An open-label, randomised, phase 2 trial. Lancet 2014, 383, 515–523. [Google Scholar] [CrossRef]
- Paolucci, S.; Fiorina, L.; Mariani, B.; Landini, V.; Gulminetti, R.; Novati, S.; Maserati, R.; Barbarini, G.; Bruno, R.; Baldanti, F. Development and persistence of DAA resistance associated mutations in patients failing HCV treatment. J. Clin. Virol. 2015, 72, 114–118. [Google Scholar] [CrossRef] [PubMed]
- Paolucci, S.; Premoli, M.; Novati, S.; Gulminetti, R.; Maserati, R.; Barbarini, G.; Sacchi, P.; Piralla, A.; Sassera, D.; De Marco, L.; et al. Baseline and Breakthrough Resistance Mutations in HCV Patients Failing DAAs. Sci. Rep. 2017, 7, 16017. [Google Scholar] [CrossRef] [PubMed]
- Reddy, K.R.; Lim, J.K.; Kuo, A.; Di Bisceglie, A.M.; Galati, J.S.; Morelli, G.; Everson, G.T.; Kwo, P.Y.; Brown, R.S.; Sulkowski, M.S.; et al. All-oral direct-acting antiviral therapy in HCV-advanced liver disease is effective in real-world practice: Observations through HCV-TARGET database. Aliment. Pharmacol. Ther. 2016, 45, 115–126. [Google Scholar] [CrossRef]
- Abo-Amer, Y.E.E.; Badawi, R.; El-Abgeegy, M.; Elsergany, H.F.; Mohamed, A.A.; Mostafa, S.M.; Abd-Elsalam, S. Quadruple Therapy Offers High SVR Rates in Patients with HCV Genotype 4 with Previous Treatment Failure. Adv. Virol. 2020, 2020, 9075905. [Google Scholar] [CrossRef]
- Llovet, J.M.; Peña, C.E.; Lathia, C.D.; Shan, M.; Meinhardt, G.; Bruix, J. Plasma Biomarkers as Predictors of Outcome in Patients with Advanced Hepatocellular Carcinoma. Clin. Cancer Res. 2012, 18, 2290–2300. [Google Scholar] [CrossRef] [PubMed]
- Ferrín, G.; Aguilar-Melero, P.; Rodriguez-Peralvarez, M.; Montero-Álvarez, J.L.; De La Mata, M. Biomarkers for hepatocellular carcinoma: Diagnostic and therapeutic utility. Hepatic Med. Évid. Res. 2015, 7, 1–10. [Google Scholar] [CrossRef][Green Version]
- Fontana, R.J.; Kronfol, Z.; Lindsay, K.L.; Bieliauskas, L.A.; Padmanabhan, L.; Back-Madruga, C.; Lok, A.S.; Stoddard, A.M.; HALT-C Trial Group. Changes in mood states and biomarkers during peginterferon and ribavirin treatment of chronic hepatitis C. Am. J. Gastroenterol. 2008, 103, 2766–2775. [Google Scholar] [CrossRef]
- Guzmán-Fulgencio, M.; Jiménez, J.L.; Berenguer, J.; Fernández-Rodríguez, A.; López, J.C.; Cosín, J.; Resino, S. Plasma IL-6 and IL-9 predict the failure of interferon-α plus ribavirin therapy in HIV/HCV-coinfected patients. J. Antimicrob. Chemother. 2012, 67, 1238–1245. [Google Scholar] [CrossRef]
- Hammad, L.N.; Abdelraouf, S.M.; Hassanein, F.S.; Mohamed, W.A.; Schaalan, M.F. Circulating IL-6, IL-17 and vitamin D in hepatocellular carcinoma: Potential biomarkers for a more favorable prognosis? J. Immunotoxicol. 2013, 10, 380–386. [Google Scholar] [CrossRef] [PubMed]
- Raza, A.; Mittal, S.; Sood, G.K. Sood, Interferon-associated retinopathy during the treatment of chronic hepatitis C: A systematic review. J. Viral Hepat. 2013, 20, 593–599. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.-F.; Dai, C.-Y.; Hwang, S.-J.; Ho, C.-K.; Hsiao, P.-J.; Hsieh, M.-Y.; Lee, L.-P.; Lin, Z.-Y.; Chen, S.-C.; Hsieh, M.-Y.; et al. Hepatitis C Viremia Increases the Association With Type 2 Diabetes Mellitus in a Hepatitis B and C Endemic Area: An Epidemiological Link With Virological Implication. Am. J. Gastroenterol. 2007, 102, 1237–1243. [Google Scholar] [CrossRef]
- Imazeki, F.; Yokosuka, O.; Fukai, K.; Kanda, T.; Kojima, H.; Saisho, H. Prevalence of diabetes mellitus and insulin resistance in patients with chronic hepatitis C: Comparison with hepatitis B virus-infected and hepatitis C virus-cleared patients. Liver Int. 2008, 28, 355–362. [Google Scholar] [CrossRef] [PubMed]
- Panetta, J.D.; Gilani, N. Interferon-induced retinopathy and its risk in patients with diabetes and hypertension undergoing treatment for chronic hepatitis C virus infection. Aliment. Pharmacol. Ther. 2009, 30, 597–602. [Google Scholar] [CrossRef]
- Fontana, R.J.; Sanyal, A.J.; Mehta, S.; Doherty, M.C.; Neuschwander-Tetri, B.A.; Everson, G.T.; Kahn, J.A.; Malet, P.F.; Sheikh, M.Y.; Chung, R.T.; et al. Portal Hypertensive Gastropathy in Chronic Hepatitis C Patients with Bridging Fibrosis and Compensated Cirrhosis: Results from the HALT-C Trial. Am. J. Gastroenterol. 2006, 101, 983–992. [Google Scholar] [CrossRef]
- Ridruejo, E.; Pinero, F.; Mendizabal, M.; Cheinquer, H.; Wolff, F.H.; Anders, M.; Latin American Liver Research Educational and Awareness Network (LALREAN). Decompensated cirrhosis and liver transplantation negatively impact in DAA treatment response: Real-world experience from HCV-LALREAN cohort. J. Med. Virol. 2020, 92, 3545–3555. [Google Scholar] [CrossRef]
- Osburn, W.O.; Levine, J.S.; Chattergoon, M.A.; Thomas, D.L.; Cox, A.L. Anti-inflammatory cytokines, pro-fibrogenic chemokines and persistence of acute HCV infection. J. Viral Hepat. 2013, 20, 404–413. [Google Scholar] [CrossRef]
- Liu, Y.; Meyer, C.; Müller, A.; Herweck, F.; Liebe, R.; Müllenbach, R.; Mertens, P.R.; Dooley, S.; Weng, H.-L. IL-13 Induces Connective Tissue Growth Factor in Rat Hepatic Stellate Cells via TGF-β–Independent Smad Signaling. J. Immunol. 2011, 187, 2814–2823. [Google Scholar] [CrossRef]
- Fierro, N.A.; González-Aldaco, K.; Torres-Valadez, R.; Trujillo-Trujillo, M.E.; Roman, S.; Trujillo-Ochoa, J.L. Spontaneous hepatitis C viral clearance and hepatitis C chronic infection are associated with distinct cytokine profiles in Mexican patients. Memórias Inst. Oswaldo Cruz 2015, 110, 267–271. [Google Scholar] [CrossRef][Green Version]
- Pascut, D.; Pratama, M.Y.; Tiribelli, C. HCC occurrence after DAA treatments: Molecular tools to assess the post-treatment risk and surveillance. Hepatic Oncol. 2020, 7. [Google Scholar] [CrossRef] [PubMed]
- Gao, Q.J.; Liu, D.W.; Zhang, S.Y.; Jia, M.; Wang, L.M.; Wu, L.H.; Tong, L.X. Polymorphisms of some cytokines and chronic hepatitis B and C virus infection. World J. Gastroenterol. WJG 2009, 15, 5610. [Google Scholar] [CrossRef]
- McFarlane, E.; Carter, K.C.; McKenzie, A.N.; Kaye, P.M.; Brombacher, F.; Alexander, J. Endogenous IL-13 Plays a Crucial Role in Liver Granuloma Maturation During Leishmania donovani Infection, Independent of IL-4Rα–Responsive Macrophages and Neutrophils. J. Infect. Dis. 2011, 204, 36–43. [Google Scholar] [CrossRef]
- Allah, E.S.H.A.; Hussein, S.; Hafez, R.; El-Amin, H.A. Do Sofosbuvir and Daclatasvir Affect Vitamin D and Iron Status in Chronic Hepatitis C Virus Patients? Role of Hepcidin. Bull. Egypt. Soc. Physiol. Sci. 2020, 41, 15–27. [Google Scholar] [CrossRef]
- Gayam, V.; Mandal, A.K.; Khalid, M.; Mukhtar, O.; Gill, A.; Garlapati, P.; Tiongson, B.; Sherigar, J.; Mansour, M.; Mohanty, S. Association Between Vitamin D Levels and Treatment Response to Direct-Acting Antivirals in Chronic Hepatitis C: A Real-World Study. Gastroenterol. Res. 2018, 11, 309–316. [Google Scholar] [CrossRef] [PubMed]
- Loftfield, E.; O’Brien, T.R.; Pfeiffer, R.M.; Howell, C.D.; Horst, R.; Prokunina-Olsson, L.; Weinstein, S.J.; Albanes, D.; Morgan, T.R.; Freedman, N.D. Vitamin D Status and Virologic Response to HCV Therapy in the HALT-C and VIRAHEP-C Trials. PLoS ONE 2016, 11, e0166036. [Google Scholar] [CrossRef]
- Chen, H.-W.; Lin, H.-H.; Shih, Y.-L.; Hsieh, T.-Y.; Lin, J.-C. Vitamin D in Patients with Chronic Hepatitis C Virus Infection Receiving the Direct Antiviral Agents. OBM Hepatol. Gastroenterol. 2020, 4, 1–4. [Google Scholar] [CrossRef]
- Backstedt, D. 25-Vitamin D levels in chronic hepatitis C infection: Association with cirrhosis and sustained virologic response. Ann. Gastroenterol. 2017, 30, 344–348. [Google Scholar] [CrossRef]
- van der Meer, A.J.; & Berenguer, M. Reversion of disease manifestations after HCV eradication. J. Hepatol. 2016, 65, S95–S108. [Google Scholar] [CrossRef]
- Huang, K.-T.; Kuo, I.-Y.; Tsai, M.-C.; Wu, C.-H.; Hsu, L.-W.; Chen, L.-Y.; Kung, C.-P.; Cheng, Y.-F.; Goto, S.; Chou, Y.-W.; et al. Factor VII-Induced MicroRNA-135a Inhibits Autophagy and Is Associated with Poor Prognosis in Hepatocellular Carcinoma. Mol. Ther. Nucleic Acids 2017, 9, 274–283. [Google Scholar] [CrossRef]
- von Felden, J.; Heim, D.; Schulze, K.; Krech, T.; Ewald, F.; Nashan, B.; Wege, H. High expression of micro RNA-135A in hepatocellular carcinoma is associated with recurrence within 12 months after resection. BMC Cancer 2017, 17, 1–8. [Google Scholar] [CrossRef][Green Version]
- Liu, S.; Guo, W.; Shi, J.; Li, N.; Yu, X.; Xue, J.; Liu, S. MicroRNA-135a contributes to the development of portal vein tumor thrombus by promoting metastasis in hepatocellular carcinoma. J. Hepatol. 2012, 56, 389–396. [Google Scholar] [CrossRef]
- Kitson, M.T.; Roberts, S.K. D-livering the message: The importance of vitamin D status in chronic liver disease. J. Hepatol. 2012, 57, 897–909. [Google Scholar] [CrossRef]
- Rode, A.; Fourlanos, S.; Nicoll, A. Oral vitamin D replacement is effective in chronic liver disease. Gastroentérol. Clin. Biol. 2010, 34, 618–620. [Google Scholar] [CrossRef] [PubMed]
- Lange, C.M.; Bojunga, J.; Ramos-Lopez, E.; von Wagner, M.; Hassler, A.; Vermehren, J.; Herrmann, E.; Badenhoop, K.; Zeuzem, S.; Sarrazin, C. Vitamin D deficiency and a CYP27B1-1260 promoter polymorphism are associated with chronic hepatitis C and poor response to interferon-alfa based therapy. J. Hepatol. 2011, 54, 887–893. [Google Scholar] [CrossRef]
- Abu-Mouch, S. Vitamin D supplementation improves sustained virologic response in chronic hepatitis C (genotype 1)-naïve patients. World J. Gastroenterol. 2011, 17, 5184. [Google Scholar] [CrossRef] [PubMed]
- Finkelmeier, F.; Kronenberger, B.; Köberle, V.; Bojunga, J.; Zeuzem, S.; Trojan, J.; Waidmann, O. Severe 25-hydroxyvitamin D deficiency identifies a poor prognosis in patients with hepatocellular carcinoma–a prospective cohort study. Aliment. Pharmacol. Ther. 2014, 39, 1204–1212. [Google Scholar] [CrossRef]
- Fahey, S.; Dempsey, E.; Long, A. The role of chemokines in acute and chronic hepatitis C infection. Cell. Mol. Immunol. 2013, 11, 25–40. [Google Scholar] [CrossRef]
- Ponziani, F.R.; Bhoori, S.; Castelli, C.; Putignani, L.; Rivoltini, L.; Del Chierico, F.; Sanguinetti, M.; Morelli, D.; Sterbini, F.P.; Petito, V.; et al. Hepatocellular Carcinoma Is Associated With Gut Microbiota Profile and Inflammation in Nonalcoholic Fatty Liver Disease. Hepatology 2019, 69, 107–120. [Google Scholar] [CrossRef] [PubMed]
- Bonecchi, R. Chemokines and chemokine receptors: An overview. Front. Biosci. 2009, 14, 540–551. [Google Scholar] [CrossRef] [PubMed]
- Noh, R.; Lee, D.H.; Kwon, B.W.; Kim, Y.H.; Kim, S.B.; Song, I.H. Clinical Impact of Viral Load on the Development of Hepatocellular Carcinoma and Liver-Related Mortality in Patients with Hepatitis C Virus Infection. Gastroenterol. Res. Pract. 2016, 2016, 7476231. [Google Scholar] [CrossRef] [PubMed]
- Ioannou, G.N.; Green, P.K.; Berry, K. HCV eradication induced by direct-acting antiviral agents reduces the risk of hepatocellular carcinoma. J. Hepatol. 2018, 68, 25–32. [Google Scholar] [CrossRef] [PubMed]
- Prenner, S.B.; VanWagner, L.B.; Flamm, S.L.; Salem, R.; Lewandowski, R.J.; Kulik, L. Hepatocellular carcinoma decreases the chance of successful hepatitis C virus therapy with direct-acting antivirals. J. Hepatol. 2017, 66, 1173–1181. [Google Scholar] [CrossRef] [PubMed]
- Romano, A.; Angeli, P.; Piovesan, S.; Noventa, F.; Anastassopoulos, G.; Chemello, L.; Alberti, A. Newly diagnosed hepatocellular carcinoma in patients with advanced hepatitis C treated with DAAs: A prospective population study. J. Hepatol. 2018, 69, 345–352. [Google Scholar] [CrossRef]
- Innes, H.; Barclay, S.T.; Hayes, P.C.; Fraser, A.; Dillon, J.; Stanley, A.; Bathgate, A.; McDonald, S.A.; Goldberg, D.; Valerio, H.; et al. The risk of hepatocellular carcinoma in cirrhotic patients with hepatitis C and sustained viral response: Role of the treatment regimen. J. Hepatol. 2018, 68, 646–654. [Google Scholar] [CrossRef]
- Calvaruso, V.; Cabibbo, G.; Cacciola, I.; Petta, S.; Madonia, S.; Bellia, A.; Tinè, F.; Distefano, M.; Licata, A.; Giannitrapani, L.; et al. Incidence of Hepatocellular Carcinoma in Patients with HCV-Associated Cirrhosis Treated with Direct-Acting Antiviral Agents. Gastroenterology 2018, 155, 411–421. [Google Scholar] [CrossRef]
- Kanwal, F.; Kramer, J.; Asch, S.M.; Chayanupatkul, M.; Cao, Y.; El-Serag, H.B. Risk of Hepatocellular Cancer in HCV Patients Treated with Direct-Acting Antiviral Agents. Gastroenterology 2017, 153, 996–1005. [Google Scholar] [CrossRef]
| Responders (n = 880) | Non Responders (n = 70) | Controls (n = 50) | p Value Comparison 3 Groups | p Value Comparison Responders vs. Non Responders | |
|---|---|---|---|---|---|
| Age | 47.83 ± 9.78 (30–70) | 50.14 ± 9.5 (32–71) | 49.62 ± 9.024 (33–69) | 0.082 | 0.0549 |
| Sex M/F | 418/462 (47.5/52.5) | 33/37 (47.1/52.9) | 30/20 (60/40) | 0.224 | 0.99 |
| Smoking | 286 (32.5) | 20 (28.6) | 0 | <0.001 | 0.51 |
| Comorbidities | 528 (60) | 57 (81.4) | 0 | <0.001 | <0.001 |
| Severity of liver disease Non-cirrhotic Cirrhotic | 748 (85) 132 (15) | 26 (37.1) 44 (62.9) | 0 0 | <0.001 | <0.001 |
| HCC incidence | 0 | 25 (35.7) | 0 | <0.001 | <0.001 |
| IL-13 (pg/mL) | 102 ± 20.6 (24–187) | 308.9 ± 113.4 (136–542) | 75.2 ± 4.8 (58–81) | <0.001 | <0.001 |
| miRNA-135a (fold change) | 2.1 ± 0.9 (0.4–4.6) | 4.6 ± 0.7 (2.7–6.7) | 1.2 ± 0.25 (0.7–1.6) | <0.001 | <0.001 |
| VIT D (ng/mL) | 32.2 (2.5–71) | 12.65 (2–45) | 44.1 (30–70) | <0.001 | <0.001 |
| HCV-RNA (IU/mL) | 4.7 × 105 (0.8 × 103–6 × 108) | 9.6 × 106 (1.2 × 104–1.26 × 108) | - | - | <0.001 |
| Non Responders without HCC (n = 45) | Non Responders with HCC (n = 25) | p Value | |
|---|---|---|---|
| Age (years) | 49.71 ± 9.37 (32–71) | 50.92 ± 9.798 (34–69) | 0.49 |
| Sex M/F | 23/22 (51.1/48.9) | 10/15(40/60) | 0.45 |
| Smoking | 12 (26.7 %) | 8 (32 %) | 0.78 |
| Comorbidities | 34 (75.6 %) | 23 (92 %) | 0.116 |
| IL-13 (pg/mL) | 262.8 ± 106.3 (136–532) | 391.8 ± 71.5 (267–542) | <0.001 |
| miRNA-135a (fold change) | 4.5 ± 0.8 (2.7–6.7) | 4.8 ± 0.5 (3.7–5.67) | 0.0426 |
| VIT D (ng/mL) | 14.7 (2–45) | 11.97 (2–41) | 0.13 |
| HCV-RNA (IU/mL) | 4.6 × 106 (1.21 × 104–4.2 × 107) | 1.8 × 107 (3.7 × 106–1.26 × 108) | 0.002 |
| AUC (95%CI) | SE | SP | +LR | |
|---|---|---|---|---|
| (a) For prediction of DAA treatment failure | ||||
| IL-13 (>135.9 pg/mL) | 0.999 (0.998–1) | 100 | 96.1 | 25.9 |
| miRNA135a (>3.562-fold change) | 0.978 (0.968–0.989) | 97.14 | 91.59 | 11.7 |
| Vit D (<21.13 ng/mL) | 0.851 (0.807–0.895) | 82.9 | 82.7 | 4.8 |
| (b) For prediction of HCC development | ||||
| IL-13 (>249 pg/mL) | 0.992 (0.987–0.997) | 100 | 98.4 | 61.7 |
| miRNA135a (>3.66 fold change) | 0.976 (0.964–0.989) | 100 | 88.96 | 9.06 |
| Vit D (<21.04 ng/mL) | 0.872 (0.809–0.935) | 92 | 79.9 | 4.6 |
| (c) For prediction of DAA treatment failure in non-cirrhotic patients | ||||
| IL-13 (>135.9 pg/mL) | 0.998 (0.995–1.000) | 100 | 96.9 | 32.5 |
| mRNA135a (>3.562-fold change) | 0.967 (0.950–0.983) | 96.2 | 91.7 | 11.6 |
| Vit D (<24.11 ng/mL) | 0.861 (0.780–0.942) | 84.6 | 83.7 | 5.2 |
| (d) For prediction of DAA treatment failure in cirrhotic patients | ||||
| Il-13 (>166.3 pg/mL) | 1 (0.979–1.000) | 100 | 100 | - |
| mRNA135a (>3.643 fold change) | 0.985 (0.971–1.000) | 97.73 | 92.42 | 12.9 |
| Vit D (<22.5 ng/mL) | 0.657 (0.573–0.742) | 86.4 | 50 | 1.7 |
| (e) For prediction of HCC development in cirrhotic patients | ||||
| Il-13 (>232.5 pg/mL) | 0.949 (0.9184–0.9798) | 100 | 90.1 | 10.1 |
| mRNA135a (>3.643 fold change) | 0.936 (0.900–0.972) | 100 | 81.46 | 5.4 |
| Vit D (<22.5 ng/mL) | 0.6575 (0.558–0.757) | 92 | 46.4 | 1.7 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ali, M.E.; Halby, H.M.; Ali, M.Y.; Hassan, E.A.; El-Mokhtar, M.A.; Sayed, I.M.; Thabet, M.M.; Fouad, M.; El-Ashmawy, A.M.; Mahran, Z.G. Role of Serum Vitamin D, Interleukin 13, and microRNA-135a in Hepatocellular Carcinoma and Treatment Failure in Egyptian HCV-Infected Patients Receiving Direct Antiviral Agents. Viruses 2021, 13, 2008. https://doi.org/10.3390/v13102008
Ali ME, Halby HM, Ali MY, Hassan EA, El-Mokhtar MA, Sayed IM, Thabet MM, Fouad M, El-Ashmawy AM, Mahran ZG. Role of Serum Vitamin D, Interleukin 13, and microRNA-135a in Hepatocellular Carcinoma and Treatment Failure in Egyptian HCV-Infected Patients Receiving Direct Antiviral Agents. Viruses. 2021; 13(10):2008. https://doi.org/10.3390/v13102008
Chicago/Turabian StyleAli, Mohamed E., Hamada M. Halby, Mamdouh Yones Ali, Elham Ahmed Hassan, Mohamed A. El-Mokhtar, Ibrahim M. Sayed, Marwa M. Thabet, Magdy Fouad, Ahmed M. El-Ashmawy, and Zainab Gaber Mahran. 2021. "Role of Serum Vitamin D, Interleukin 13, and microRNA-135a in Hepatocellular Carcinoma and Treatment Failure in Egyptian HCV-Infected Patients Receiving Direct Antiviral Agents" Viruses 13, no. 10: 2008. https://doi.org/10.3390/v13102008
APA StyleAli, M. E., Halby, H. M., Ali, M. Y., Hassan, E. A., El-Mokhtar, M. A., Sayed, I. M., Thabet, M. M., Fouad, M., El-Ashmawy, A. M., & Mahran, Z. G. (2021). Role of Serum Vitamin D, Interleukin 13, and microRNA-135a in Hepatocellular Carcinoma and Treatment Failure in Egyptian HCV-Infected Patients Receiving Direct Antiviral Agents. Viruses, 13(10), 2008. https://doi.org/10.3390/v13102008






