The Current Evidence Regarding COVID-19 and Pregnancy: Where Are We Now and Where Should We Head to Next?
Abstract
:1. Introduction
2. Materials and Methods
3. Complications of COVID-19 Reported in Pregnancy
4. Challenges during Delivery of Pregnant Patients with COVID-19
5. Neonates’ Health Status
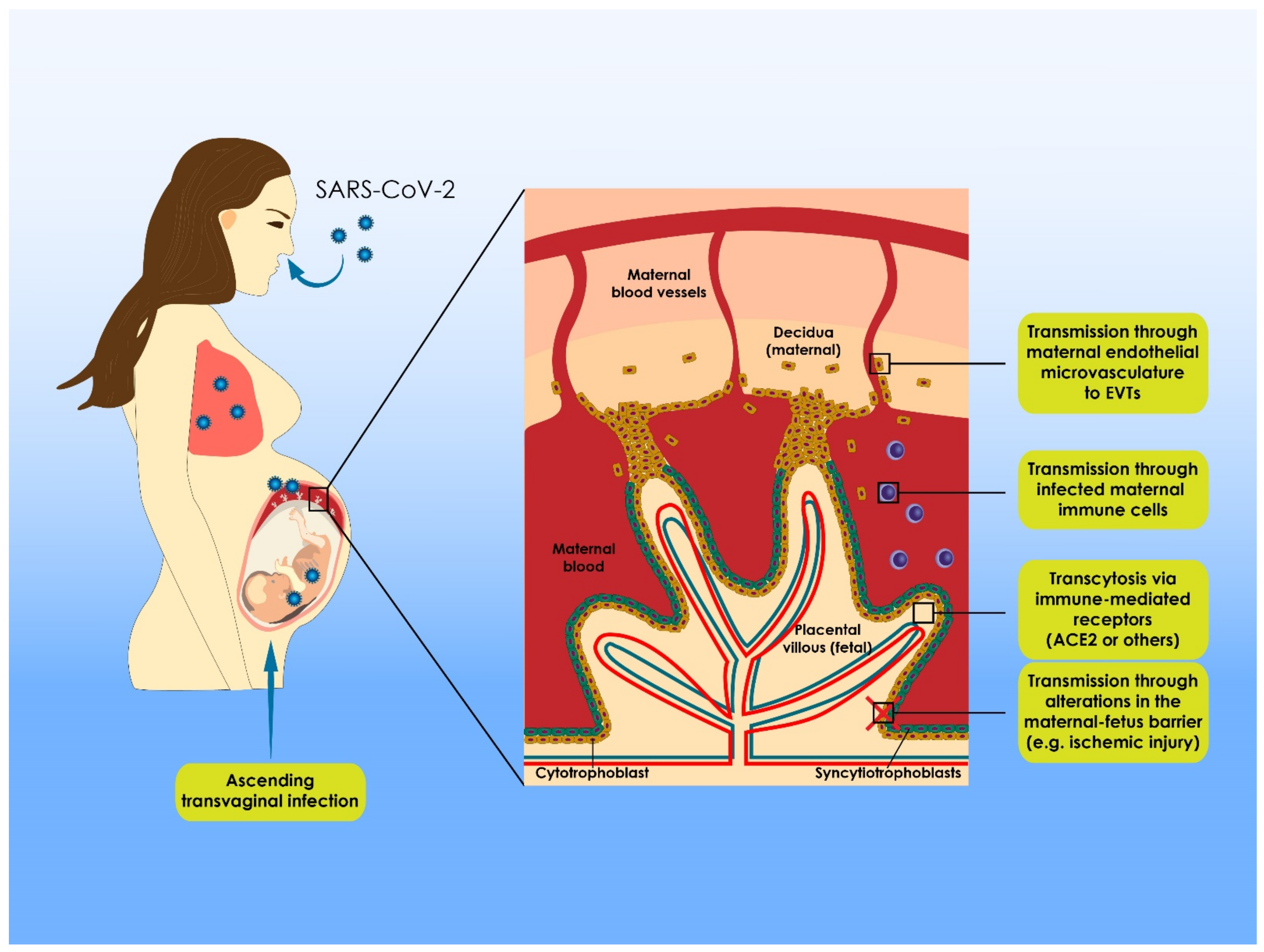
6. Delineating the Phenomenon of Vertical Transmission
7. Risks Entailed in Breastfeeding
8. Vaccination Debate
9. Discussion
10. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zeng, H.; Xu, C.; Fan, J.; Tang, Y.; Deng, Q.; Zhang, W.; Long, X. Antibodies in Infants Born to Mothers with COVID-19 Pneumonia. JAMA 2020, 323, 1848–1849. [Google Scholar] [CrossRef] [PubMed]
- Anifandis, G.; Tempest, H.G.; Oliva, R.; Swanson, G.M.; Simopoulou, M.; Easley, C.A.; Primig, M.; Messini, C.I.; Turek, P.J.; Sutovsky, P.; et al. COVID-19 and human reproduction: A pandemic that packs a serious punch. Syst. Biol. Reprod. Med. 2021, 67, 3–23. [Google Scholar] [CrossRef]
- Anifandis, G.; Messini, C.I.; Simopoulou, M.; Sveronis, G.; Garas, A.; Daponte, A.; Messinis, I.E. SARS-CoV-2 vs. human gametes, embryos and cryopreservation. Syst. Biol. Reprod. Med. 2021, 67, 260–269. [Google Scholar] [CrossRef] [PubMed]
- Narang, K.; Enninga, E.A.L.; Gunaratne, M.D.S.K.; Ibirogba, E.R.; Trad, A.T.A.; Elrefaei, A.; Theiler, R.N.; Ruano, R.; Szymanski, L.M.; Chakraborty, R.; et al. SARS-CoV-2 Infection and COVID-19 during Pregnancy: A Multidisciplinary Review. Mayo Clin. Proc. 2020, 95, 1750–1765. [Google Scholar] [CrossRef] [PubMed]
- Yu, N.; Li, W.; Kang, Q.; Zeng, W.; Feng, L.; Wu, J. No SARS-CoV-2 detected in amniotic fluid in mid-pregnancy. Lancet Infect. Dis. 2020, 20, 1364. [Google Scholar] [CrossRef]
- Tang, J.-Y.; Song, W.-Q.; Xu, H.; Wang, N. No evidence for vertical transmission of SARS-CoV-2 in two neonates with mothers infected in the second trimester. Infect. Dis. Lond. Engl. 2020, 52, 913–916. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Cheng, H.; Wang, J.; Ding, L.; Zhou, Z.; Liu, S.; Chang, L.; Rong, Z. Clinical Analysis of Neonates Born to Mothers with or without COVID-19: A Retrospective Analysis of 48 Cases from Two Neonatal Intensive Care Units in Hubei Province. Am. J. Perinatol. 2020, 37, 1317–1323. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, Z.; Xiong, G. Clinical characteristics and laboratory results of pregnant women with COVID-19 in Wuhan, China. Int. J. Gynaecol. Obstet. Off. Organ Int. Fed. Gynaecol. Obstet. 2020, 150, 312–317. [Google Scholar] [CrossRef]
- Breslin, N.; Baptiste, C.; Gyamfi-Bannerman, C.; Miller, R.; Martinez, R.; Bernstein, K.; Ring, L.; Landau, R.; Purisch, S.; Friedman, A.M.; et al. Coronavirus disease 2019 infection among asymptomatic and symptomatic pregnant women: Two weeks of confirmed presentations to an affiliated pair of New York City hospitals. Am. J. Obstet. Gynecol. MFM 2020, 2, 100118. [Google Scholar] [CrossRef]
- Narang, K.; Szymanski, L.M.; Kane, S.V.; Rose, C.H. Acute Pancreatitis in a Pregnant Patient with Coronavirus Disease 2019 (COVID-19). Obstet. Gynecol. 2021, 137, 431–433. [Google Scholar] [CrossRef]
- Sukhikh, G.; Petrova, U.; Prikhodko, A.; Starodubtseva, N.; Chingin, K.; Chen, H.; Bugrova, A.; Kononikhin, A.; Bourmenskaya, O.; Brzhozovskiy, A.; et al. Vertical Transmission of SARS-CoV-2 in Second Trimester Associated with Severe Neonatal Pathology. Viruses 2021, 13, 447. [Google Scholar] [CrossRef] [PubMed]
- Farhadi, R.; Mehrpisheh, S.; Ghaffari, V.; Haghshenas, M.; Ebadi, A. Clinical course, radiological findings and late outcome in preterm infant with suspected vertical transmission born to a mother with severe COVID-19 pneumonia: A case report. J. Med. Case Rep. 2021, 15, 213. [Google Scholar] [CrossRef]
- Garcia, J.J.; Turalde, C.W.; Bagnas, M.A.; Anlacan, V.M. Intravenous immunoglobulin in COVID-19 associated Guillain-Barré syndrome in pregnancy. BMJ Case Rep. 2021, 14, e242365. [Google Scholar] [CrossRef] [PubMed]
- Metz, T.D.; Clifton, R.G.; Hughes, B.L.; Sandoval, G.; Saade, G.R.; Grobman, W.A.; Manuck, T.A.; Miodovnik, M.; Sowles, A.; Clark, K.; et al. Disease Severity and Perinatal Outcomes of Pregnant Patients with Coronavirus Disease 2019 (COVID-19). Obstet. Gynecol. 2021, 137, 571–580. [Google Scholar] [CrossRef]
- Knight, M.; Bunch, K.; Vousden, N.; Morris, E.; Simpson, N.; Gale, C.; O’Brien, P.; Quigley, M.; Brocklehurst, P.; Kurinczuk, J.J.; et al. Characteristics and outcomes of pregnant women admitted to hospital with confirmed SARS-CoV-2 infection in UK: National population based cohort study. BMJ 2020, 369, m2107. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Li, Q.; Zheng, D.; Jiang, H.; Wei, Y.; Zou, L.; Feng, L.; Xiong, G.; Sun, G.; Wang, H.; et al. Clinical Characteristics of Pregnant Women with COVID-19 in Wuhan, China. N. Engl. J. Med. 2020, 382, e100. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Liu, C.; Dong, L.; Zhang, C.; Chen, Y.; Liu, J.; Zhang, C.; Duan, C.; Zhang, H.; Mol, B.W.; et al. Coronavirus disease 2019 among pregnant Chinese women: Case series data on the safety of vaginal birth and breastfeeding. BJOG Int. J. Obstet. Gynaecol. 2020, 127, 1109–1115. [Google Scholar] [CrossRef]
- Smithgall, M.C.; Liu-Jarin, X.; Hamele-Bena, D.; Cimic, A.; Mourad, M.; Debelenko, L.; Chen, X. Third-trimester placentas of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2)-positive women: Histomorphology, including viral immunohistochemistry and in-situ hybridization. Histopathology 2020, 77, 994–999. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Han, L.; Peng, M.; Lv, Y.; Ouyang, Y.; Liu, K.; Yue, L.; Li, Q.; Sun, G.; Chen, L.; et al. Maternal and Neonatal Outcomes of Pregnant Women with Coronavirus Disease 2019 (COVID-19) Pneumonia: A Case-Control Study. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 2020, 71, 2035–2041. [Google Scholar] [CrossRef] [Green Version]
- Hosier, H.; Farhadian, S.F.; Morotti, R.A.; Deshmukh, U.; Lu-Culligan, A.; Campbell, K.H.; Yasumoto, Y.; Vogels, C.B.; Casanovas-Massana, A.; Vijayakumar, P.; et al. SARS-CoV-2 infection of the placenta. J. Clin. Investig. 2020, 130, 4947–4953. [Google Scholar] [CrossRef]
- Salvatore, C.M.; Han, J.-Y.; Acker, K.P.; Tiwari, P.; Jin, J.; Brandler, M.; Cangemi, C.; Gordon, L.; Parow, A.; DiPace, J.; et al. Neonatal management and outcomes during the COVID-19 pandemic: An observation cohort study. Lancet Child Adolesc. Health 2020, 4, 721–727. [Google Scholar] [CrossRef]
- Afshar, Y.; Gaw, S.L.; Flaherman, V.J.; Chambers, B.D.; Krakow, D.; Berghella, V.; Shamshirsaz, A.A.; Boatin, A.A.; Aldrovandi, G.; Greiner, A.; et al. Clinical Presentation of Coronavirus Disease 2019 (COVID-19) in Pregnant and Recently Pregnant People. Obstet. Gynecol. 2020, 136, 1117–1125. [Google Scholar] [CrossRef]
- Dong, Y.; Chi, X.; Hai, H.; Sun, L.; Zhang, M.; Xie, W.-F.; Chen, W. Antibodies in the breast milk of a maternal woman with COVID-19. Emerg. Microbes Infect. 2020, 9, 1467–1469. [Google Scholar] [CrossRef]
- Majachani, N.; Francois, J.L.M.; Fernando, A.K.; Zuberi, J. A Case of a Newborn Baby Girl Infected with SARS-CoV-2 Due to Transplacental Viral Transmission. Am. J. Case Rep. 2020, 21, e925766. [Google Scholar] [CrossRef]
- Hinojosa-Velasco, A.; de Oca, P.V.B.-M.; García-Sosa, L.E.; Mendoza-Durán, J.G.; Pérez-Méndez, M.J.; Dávila-González, E.; Ramírez-Hernández, D.G.; García-Mena, J.; Zárate-Segura, P.; Reyes-Ruiz, J.M.; et al. A case report of newborn infant with severe COVID-19 in Mexico: Detection of SARS-CoV-2 in human breast milk and stool. Int. J. Infect. Dis. IJID Off. Publ. Int. Soc. Infect. Dis. 2020, 100, 21–24. [Google Scholar] [CrossRef] [PubMed]
- Pulinx, B.; Kieffer, D.; Michiels, I.; Petermans, S.; Strybol, D.; Delvaux, S.; Baldewijns, M.; Raymaekers, M.; Cartuyvels, R.; Maurissen, W. Vertical transmission of SARS-CoV-2 infection and preterm birth. Eur. J. Clin. Microbiol. Infect. Dis. 2020, 39, 2441–2445. [Google Scholar] [CrossRef] [PubMed]
- Sisman, J.; Jaleel, M.A.; Moreno, W.; Rajaram, V.; Collins, R.R.J.; Savani, R.C.; Rakheja, D.; Evans, A.S. Intrauterine Transmission of SARS-CoV-2 Infection in a Preterm Infant. Pediatr. Infect. Dis. J. 2020, 39, e265–e267. [Google Scholar] [CrossRef] [PubMed]
- Costa, S.; Posteraro, B.; Marchetti, S.; Tamburrini, E.; Carducci, B.; Lanzone, A.; Valentini, P.; Buonsenso, D.; Sanguinetti, M.; Vento, G.; et al. Excretion of SARS-CoV-2 in human breast milk. Clin. Microbiol. Infect. 2020, 26, 1430–1432. [Google Scholar] [CrossRef] [PubMed]
- Slayton-Milam, S.; Sheffels, S.; Chan, D.; Alkinj, B. Induction of Labor in an Intubated Patient with Coronavirus Disease 2019 (COVID-19). Obstet. Gynecol. 2020, 136, 962–964. [Google Scholar] [CrossRef]
- Alwardi, T.H.; Ramdas, V.; Al Yahmadi, M.; Al Aisari, S.; Bhandari, S.; Saif Al Hashami, H.; Al Jabri, A.; Manikoth, P.; Malviya, M. Is Vertical Transmission of SARS-CoV-2 Infection Possible in Preterm Triplet Pregnancy? A Case Series. Pediatr. Infect. Dis. J. 2020, 39, e456–e458. [Google Scholar] [CrossRef]
- Marzollo, R.; Aversa, S.; Prefumo, F.; Saccani, B.; Perez, C.R.; Sartori, E.; Motta, M. Possible Coronavirus Disease 2019 Pandemic and Pregnancy: Vertical Transmission Is Not Excluded. Pediatr. Infect. Dis. J. 2020, 39, e261–e262. [Google Scholar] [CrossRef]
- Pierce-Williams, R.A.M.; Burd, J.; Felder, L.; Khoury, R.; Bernstein, P.S.; Avila, K.; Penfield, C.A.; Roman, A.S.; DeBolt, C.A.; Stone, J.L.; et al. Clinical course of severe and critical coronavirus disease 2019 in hospitalized pregnancies: A United States cohort study. Am. J. Obstet. Gynecol. MFM 2020, 2, 100134. [Google Scholar] [CrossRef] [PubMed]
- Baud, D.; Greub, G.; Favre, G.; Gengler, C.; Jaton, K.; Dubruc, E.; Pomar, L. Second-Trimester Miscarriage in a Pregnant Woman with SARS-CoV-2 Infection. JAMA 2020, 323, 2198–2200. [Google Scholar] [CrossRef]
- Sentilhes, L.; De Marcillac, F.; Jouffrieau, C.; Kuhn, P.; Thuet, V.; Hansmann, Y.; Ruch, Y.; Fafi-Kremer, S.; Deruelle, P. Coronavirus disease 2019 in pregnancy was associated with maternal morbidity and preterm birth. Am. J. Obstet. Gynecol. 2020, 223, 914.e1–914.e15. [Google Scholar] [CrossRef]
- Vivanti, A.J.; Mattern, J.; Vauloup-Fellous, C.; Jani, J.; Rigonnot, L.; El Hachem, L.; Le Gouez, A.; Desconclois, C.; Ben M’Barek, I.; Sibiude, J.; et al. Retrospective Description of Pregnant Women Infected with Severe Acute Respiratory Syndrome Coronavirus 2, France. Emerg. Infect. Dis. 2020, 26, 2069–2076. [Google Scholar] [CrossRef]
- Chen, H.; Guo, J.; Wang, C.; Luo, F.; Yu, X.; Zhang, W.; Li, J.; Zhao, D.; Xu, D.; Gong, Q.; et al. Clinical characteristics and intrauterine vertical transmission potential of COVID-19 infection in nine pregnant women: A retrospective review of medical records. Lancet 2020, 395, 809–815. [Google Scholar] [CrossRef] [Green Version]
- Yu, N.; Li, W.; Kang, Q.; Xiong, Z.; Wang, S.; Lin, X.; Liu, Y.; Xiao, J.; Liu, H.; Deng, D.; et al. Clinical features and obstetric and neonatal outcomes of pregnant patients with COVID-19 in Wuhan, China: A retrospective, single-centre, descriptive study. Lancet Infect. Dis. 2020, 20, 559–564. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Zhao, R.; Zheng, S.; Chen, X.; Wang, J.; Sheng, X.; Zhou, J.; Cai, H.; Fang, Q.; Yu, F.; et al. Lack of Vertical Transmission of Severe Acute Respiratory Syndrome Coronavirus 2, China. Emerg. Infect. Dis. 2020, 26, 1335–1336. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Wang, J.; Li, W.; Zhou, Z.; Liu, S.; Rong, Z. Clinical characteristics of 19 neonates born to mothers with COVID-19. Front. Med. 2020, 193–198. [Google Scholar] [CrossRef] [Green Version]
- Khan, S.; Jun, L.; Siddique, R.; Li, Y.; Han, G.; Xue, M.; Nabi, G.; Liu, J. Association of COVID-19 with pregnancy outcomes in health-care workers and general women. Clin. Microbiol. Infect. Off. Publ. Eur. Soc. Clin. Microbiol. Infect. Dis. 2020, 26, 788–790. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.; Peng, L.; Siddique, R.; Nabi, G.; Xue, M.; Liu, J.; Han, G. Impact of COVID-19 infection on pregnancy outcomes and the risk of maternal-to-neonatal intrapartum transmission of COVID-19 during natural birth. Infect. Control Hosp. Epidemiol. 2020, 748–750. [Google Scholar] [CrossRef] [Green Version]
- Lang, G.; Zhao, H. Can SARS-CoV-2-infected women breastfeed after viral clearance? J. Zhejiang Univ. Sci. B 2020, 405–407. [Google Scholar] [CrossRef]
- Lyra, J.; Valente, R.; Rosário, M.; Guimarães, M. Cesarean Section in a Pregnant Woman with COVID-19: First Case in Portugal. Acta Med. Port. 2020, 33, 429–431. [Google Scholar] [CrossRef]
- Martinelli, I.; Ferrazzi, E.; Ciavarella, A.; Erra, R.; Iurlaro, E.; Ossola, M.; Lombardi, A.; Blasi, F.; Mosca, F.; Peyvandi, F. Pulmonary embolism in a young pregnant woman with COVID-19. Thromb. Res. 2020, 191, 36–37. [Google Scholar] [CrossRef]
- Dong, L.; Tian, J.; He, S.; Zhu, C.; Wang, J.; Liu, C.; Yang, J. Possible Vertical Transmission of SARS-CoV-2 from an Infected Mother to Her Newborn. JAMA 2020, 323, 1846–1848. [Google Scholar] [CrossRef] [Green Version]
- Yang, P.; Wang, X.; Liu, P.; Wei, C.; He, B.; Zheng, J.; Zhao, D. Clinical characteristics and risk assessment of newborns born to mothers with COVID-19. J. Clin. Virol. Off. Publ. Pan Am. Soc. Clin. Virol. 2020, 127, 104356. [Google Scholar] [CrossRef]
- Chen, Y.; Peng, H.; Wang, L.; Zhao, Y.; Zeng, L.; Gao, H.; Liu, Y. Infants Born to Mothers with a New Coronavirus (COVID-19). Front. Pediatr. 2020, 8, 104. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhou, Z.; Zhang, J.; Zhu, F.; Tang, Y.; Shen, X. A Case of 2019 Novel Coronavirus in a Pregnant Woman With Preterm Delivery. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 2020, 71, 844–846. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, H.; Wang, L.; Fang, C.; Peng, S.; Zhang, L.; Chang, G.; Xia, S.; Zhou, W. Clinical analysis of 10 neonates born to mothers with 2019-nCoV pneumonia. Transl. Pediatr. 2020, 9, 51–60. [Google Scholar] [CrossRef]
- Breslin, N.; Baptiste, C.; Miller, R.; Fuchs, K.; Goffman, D.; Gyamfi-Bannerman, C.; D’Alton, M. Coronavirus disease 2019 in pregnancy: Early lessons. Am. J. Obstet. Gynecol. MFM 2020, 2, 100111. [Google Scholar] [CrossRef] [PubMed]
- Vivanti, A.J.; Vauloup-Fellous, C.; Prevot, S.; Zupan, V.; Suffee, C.; Do Cao, J.; Benachi, A.; De Luca, D. Transplacental transmission of SARS-CoV-2 infection. Nat. Commun. 2020, 11, 3572. [Google Scholar] [CrossRef]
- Ferraiolo, A.; Barra, F.; Kratochwila, C.; Paudice, M.; Vellone, V.G.; Godano, E.; Varesano, S.; Noberasco, G.; Ferrero, S.; Arioni, C. Report of Positive Placental Swabs for SARS-CoV-2 in an Asymptomatic Pregnant Woman with COVID-19. Medicina 2020, 56, 306. [Google Scholar] [CrossRef]
- Alzamora, M.C.; Paredes, T.; Caceres, D.; Webb, C.M.; Valdez, L.M.; La Rosa, M. Severe COVID-19 during Pregnancy and Possible Vertical Transmission. Am. J. Perinatol. 2020, 37, 861–865. [Google Scholar] [CrossRef] [Green Version]
- Hantoushzadeh, S.; Shamshirsaz, A.A.; Aleyasin, A.; Seferovic, M.D.; Aski, S.K.; Arian, S.E.; Pooransari, P.; Ghotbizadeh, F.; Aalipour, S.; Soleimani, Z.; et al. Maternal death due to COVID-19. Am. J. Obstet. Gynecol. 2020, 223, 109.e1–109.e16. [Google Scholar] [CrossRef] [PubMed]
- Blitz, M.J.; Rochelson, B.; Minkoff, H.; Meirowitz, N.; Prasannan, L.; London, V.; Rafael, T.J.; Chakravarthy, S.; Bracero, L.A.; Wasden, S.W.; et al. Maternal mortality among women with coronavirus disease 2019 admitted to the intensive care unit. Am. J. Obstet. Gynecol. 2020, 223, 595–599.e5. [Google Scholar] [CrossRef] [PubMed]
- Badr, D.A.; Mattern, J.; Carlin, A.; Cordier, A.-G.; Maillart, E.; El Hachem, L.; El Kenz, H.; Andronikof, M.; De Bels, D.; Damoisel, C.; et al. Are clinical outcomes worse for pregnant women at ≥20 weeks’ gestation infected with coronavirus disease 2019? A multicenter case-control study with propensity score matching. Am. J. Obstet. Gynecol. 2020, 223, 764–768. [Google Scholar] [CrossRef] [PubMed]
- Nejadrahim, R.; Khademolhosseini, S.; Kavandi, H.; Hajizadeh, R. Severe acute respiratory syndrome coronavirus-2- or pregnancy-related cardiomyopathy, a differential to be considered in the current pandemic: A case report. J. Med. Case Rep. 2021, 15, 143. [Google Scholar] [CrossRef] [PubMed]
- Goswami, J.; MacArthur, T.A.; Sridharan, M.; Pruthi, R.K.; McBane, R.D.; Witzig, T.E.; Park, M.S. A Review of Pathophysiology, Clinical Features, and Management Options of COVID-19 Associated Coagulopathy. Shock 2020, 55, 700. [Google Scholar] [CrossRef] [PubMed]
- Mohammadi, S.; Abouzaripour, M.; Hesam Shariati, N.; Hesam Shariati, M.B. Ovarian vein thrombosis after coronavirus disease (COVID-19) infection in a pregnant woman: Case report. J. Thromb. Thrombolysis 2020, 50, 604–607. [Google Scholar] [CrossRef]
- Gunduz, Z.B. Venous sinus thrombosis during COVID-19 infection in pregnancy: A case report. Sao Paulo Med. J. Rev. Paul. Med. 2021, 139, 190–195. [Google Scholar] [CrossRef] [PubMed]
- Tekin, A.B.; Zanapalioglu, U.; Gulmez, S.; Akarsu, I.; Yassa, M.; Tug, N. Guillain Barre Syndrome following delivery in a pregnant woman infected with SARS-CoV-2. J. Clin. Neurosci. 2021, 86, 190–192. [Google Scholar] [CrossRef]
- Villar, J.; Ariff, S.; Gunier, R.B.; Thiruvengadam, R.; Rauch, S.; Kholin, A.; Roggero, P.; Prefumo, F.; do Vale, M.S.; Cardona-Perez, J.A.; et al. Maternal and Neonatal Morbidity and Mortality Among Pregnant Women with and without COVID-19 Infection: The INTERCOVID Multinational Cohort Study. JAMA Pediatr. 2021, 175, 817–826. [Google Scholar] [CrossRef]
- Carrasco, I.; Muñoz-Chapuli, M.; Vigil-Vázquez, S.; Aguilera-Alonso, D.; Hernández, C.; Sánchez-Sánchez, C.; Oliver, C.; Riaza, M.; Pareja, M.; Sanz, O.; et al. SARS-CoV-2 infection in pregnant women and newborns in a Spanish cohort (GESNEO-COVID) during the first wave. BMC Pregnancy Childbirth 2021, 21, 326. [Google Scholar] [CrossRef] [PubMed]
- Oncel, M.Y.; Akın, I.M.; Kanburoglu, M.K.; Tayman, C.; Coskun, S.; Narter, F.; Er, I.; Oncan, T.G.; Memisoglu, A.; Cetinkaya, M.; et al. A multicenter study on epidemiological and clinical characteristics of 125 newborns born to women infected with COVID-19 by Turkish Neonatal Society. Eur. J. Pediatr. 2021, 180, 733–742. [Google Scholar] [CrossRef] [PubMed]
- Taghavi, S.-A.; Heidari, S.; Jahanfar, S.; Amirjani, S.; Aji-Ramkani, A.; Azizi-Kutenaee, M.; Bazarganipour, F. Obstetric, maternal, and neonatal outcomes in COVID-19 compared to healthy pregnant women in Iran: A retrospective, case-control study. Middle East Fertil. Soc. J. 2021, 26, 17. [Google Scholar] [CrossRef] [PubMed]
- Singh, V.; Choudhary, A.; Datta, M.R.; Ray, A. Maternal and Neonatal Outcomes of COVID-19 in Pregnancy: A Single-Centre Observational Study. Cureus 2021, 13, e13184. [Google Scholar] [CrossRef]
- Le Gouez, A.; Vivanti, A.J.; Benhamou, D.; Desconclois, C.; Mercier, F.J. Thrombocytopenia in pregnant patients with mild COVID-19. Int. J. Obstet. Anesth. 2020, 44, 13–15. [Google Scholar] [CrossRef] [PubMed]
- Omar, S.; Motawea, A.M.; Yasin, R. High-resolution CT features of COVID-19 pneumonia in confirmed cases. Egypt. J. Radiol. Nucl. Med. 2020, 51, 121. [Google Scholar] [CrossRef]
- Waratani, M.; Ito, F.; Tanaka, Y.; Mabuchi, A.; Mori, T.; Kitawaki, J. Severe coronavirus disease pneumonia in a pregnant woman at 25 weeks’ gestation: A case report. J. Obstet. Gynaecol. Res. 2021, 47, 1583–1588. [Google Scholar] [CrossRef]
- Coronavirus Disease (COVID-19): Pregnancy and Childbirth. Available online: https://www.who.int/news-room/q-a-detail/coronavirus-disease-COVID-19-pregnancy-and-childbirth (accessed on 24 January 2021).
- Yang, R.; Mei, H.; Zheng, T.; Fu, Q.; Zhang, Y.; Buka, S.; Yao, X.; Tang, Z.; Zhang, X.; Qiu, L.; et al. Pregnant women with COVID-19 and risk of adverse birth outcomes and maternal-fetal vertical transmission: A population-based cohort study in Wuhan, China. BMC Med. 2020, 18, 330. [Google Scholar] [CrossRef]
- McLaren, R.A.; London, V.; Atallah, F.; McCalla, S.; Haberman, S.; Fisher, N.; Stein, J.L.; Minkoff, H.L. Delivery for respiratory compromise among pregnant women with coronavirus disease 2019. Am. J. Obstet. Gynecol. 2020, 223, 451–453. [Google Scholar] [CrossRef]
- Sagheb, S.; Lamsehchi, A.; Jafary, M.; Atef-Yekta, R.; Sadeghi, K. Two seriously ill neonates born to mothers with COVID-19 pneumonia- a case report. Ital. J. Pediatr. 2020, 46, 137. [Google Scholar] [CrossRef]
- Peng, Z.; Wang, J.; Mo, Y.; Duan, W.; Xiang, G.; Yi, M.; Bao, L.; Shi, Y. Unlikely SARS-CoV-2 vertical transmission from mother to child: A case report. J. Infect. Public Health 2020, 13, 818–820. [Google Scholar] [CrossRef] [PubMed]
- Fenizia, C.; Biasin, M.; Cetin, I.; Vergani, P.; Mileto, D.; Spinillo, A.; Gismondo, M.R.; Perotti, F.; Callegari, C.; Mancon, A.; et al. Analysis of SARS-CoV-2 vertical transmission during pregnancy. Nat. Commun. 2020, 11, 5128. [Google Scholar] [CrossRef]
- Gude, N.M.; Roberts, C.T.; Kalionis, B.; King, R.G. Growth and function of the normal human placenta. Thromb. Res. 2004, 114, 397–407. [Google Scholar] [CrossRef] [PubMed]
- Komine-Aizawa, S.; Takada, K.; Hayakawa, S. Placental barrier against COVID-19. Placenta 2020, 99, 45–49. [Google Scholar] [CrossRef]
- Carbayo-Jiménez, T.; Carrasco-Colom, J.; Epalza, C.; Folgueira, D.; Pérez-Rivilla, A.; Barbero-Casado, P.; Blázquez-Gamero, D.; Galindo-Izquierdo, A.; Pallás-Alonso, C.; Moral-Pumarega, M.T. Severe Acute Respiratory Syndrome Coronavirus 2 Vertical Transmission from an Asymptomatic Mother. Pediatr. Infect. Dis. J. 2021, 40, e115–e117. [Google Scholar] [CrossRef]
- Shende, P.; Gaikwad, P.; Gandhewar, M.; Ukey, P.; Bhide, A.; Patel, V.; Bhagat, S.; Bhor, V.; Mahale, S.; Gajbhiye, R.; et al. Persistence of SARS-CoV-2 in the first trimester placenta leading to transplacental transmission and fetal demise from an asymptomatic mother. Hum. Reprod. 2021, 36, 899–906. [Google Scholar] [CrossRef]
- Penfield, C.A.; Brubaker, S.G.; Limaye, M.A.; Lighter, J.; Ratner, A.J.; Thomas, K.M.; Meyer, J.A.; Roman, A.S. Detection of severe acute respiratory syndrome coronavirus 2 in placental and fetal membrane samples. Am. J. Obstet. Gynecol. MFM 2020, 2, 100133. [Google Scholar] [CrossRef] [PubMed]
- Mok, T.; Contreras, D.; Chmait, R.H.; Goldstein, J.; Pluym, I.D.; Tabsh, K.; Aldrovandi, G.; Afshar, Y. Complicated Monochorionic–Diamniotic Twins in a Pregnant Woman with COVID-19 in the Second Trimester. Am. J. Perinatol. 2021, 38, 747–752. [Google Scholar] [CrossRef]
- Valdespino-Vázquez, M.Y.; Helguera-Repetto, C.A.; León-Juárez, M.; Villavicencio-Carrisoza, O.; Flores-Pliego, A.; Moreno-Verduzco, E.R.; Díaz-Pérez, D.L.; Villegas-Mota, I.; Carrasco-Ramírez, E.; López-Martínez, I.E.; et al. Fetal and placental infection with SARS-CoV-2 in early pregnancy. J. Med. Virol. 2021, 93, 4480–4487. [Google Scholar] [CrossRef] [PubMed]
- Trombetta, A.; Comar, M.; Tommasini, A.; Canton, M.; Campisciano, G.; Zanotta, N.; Cason, C.; Maso, G.; Risso, F.M. SARS-CoV-2 Infection and Inflammatory Response in a Twin Pregnancy. Int. J. Environ. Res. Public. Health 2021, 18, 3075. [Google Scholar] [CrossRef]
- Hecht, J.L.; Quade, B.; Deshpande, V.; Mino-Kenudson, M.; Ting, D.T.; Desai, N.; Dygulska, B.; Heyman, T.; Salafia, C.; Shen, D.; et al. SARS-CoV-2 can infect the placenta and is not associated with specific placental histopathology: A series of 19 placentas from COVID-19-positive mothers. Mod. Pathol. 2020, 33, 2092–2103. [Google Scholar] [CrossRef]
- Fouda, G.G.; Martinez, D.R.; Swamy, G.K.; Permar, S.R. The Impact of IgG transplacental transfer on early life immunity. ImmunoHorizons 2018, 2, 14–25. [Google Scholar] [CrossRef]
- Gao, J.; Hu, X.; Sun, X.; Luo, X.; Chen, L. Possible intrauterine SARS-CoV-2 infection: Positive nucleic acid testing results and consecutive positive SARS-CoV-2-specific antibody levels within 50 days after birth. Int. J. Infect. Dis. 2020, 99, 272–275. [Google Scholar] [CrossRef] [PubMed]
- Filimonovic, D.; Lackovic, M.; Filipovic, I.; Orlic, N.K.; Markovic, V.M.; Djukic, V.; Stevanovic, I.P.; Mihajlovic, S. Intrauterine transfusion in COVID-19 positive mother vertical transmission risk assessment. Eur. J. Obstet. Gynecol. Reprod. Biol. 2020, 252, 617–618. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Li, W.; Hu, X.; Wei, Y.; Wu, J.; Luo, X.; Chen, S.; Chen, L. Disappearance of SARS-CoV-2 Antibodies in Infants Born to Women with COVID-19, Wuhan, China. Emerg. Infect. Dis. 2020, 26, 2491–2494. [Google Scholar] [CrossRef]
- Niewiesk, S. Maternal Antibodies: Clinical Significance, Mechanism of Interference with Immune Responses, and Possible Vaccination Strategies. Front. Immunol. 2014, 5, 446. [Google Scholar] [CrossRef] [Green Version]
- Groß, R.; Conzelmann, C.; Müller, J.A.; Stenger, S.; Steinhart, K.; Kirchhoff, F.; Münch, J. Detection of SARS-CoV-2 in human breastmilk. Lancet 2020, 395, 1757–1758. [Google Scholar] [CrossRef]
- Yu, Y.; Li, Y.; Hu, Y.; Li, B.; Xu, J. Breastfed 13 month-old infant of a mother with COVID-19 pneumonia: A case report. Int. Breastfeed. J. 2020, 15, 68. [Google Scholar] [CrossRef]
- Hanson, L.A. Breastfeeding provides passive and likely long-lasting active immunity. Ann. Allergy Asthma Immunol. Off. Publ. Am. Coll. Allergy Asthma Immunol. 1998, 81, 523–533. [Google Scholar] [CrossRef]
- Lugli, L.; Bedetti, L.; Lucaccioni, L.; Gennari, W.; Leone, C.; Ancora, G.; Berardi, A. An Uninfected Preterm Newborn Inadvertently Fed SARS-CoV-2-Positive Breast Milk. Pediatrics 2020, 146, e2020004960. [Google Scholar] [CrossRef]
- Shlomai, N.O.; Kasirer, Y.; Strauss, T.; Smolkin, T.; Marom, R.; Shinwell, E.S.; Simmonds, A.; Golan, A.; Morag, I.; Waisman, D.; et al. Neonatal SARS-CoV-2 Infections in Breastfeeding Mothers. Pediatrics 2021, 147. [Google Scholar] [CrossRef]
- Rasmussen, S.A.; Watson, A.K.; Kennedy, E.D.; Broder, K.R.; Jamieson, D.J. Vaccines and pregnancy: Past, present, and future. Semin. Fetal. Neonatal Med. 2014, 19, 161–169. [Google Scholar] [CrossRef]
- Rasmussen, S.A.; Kelley, C.F.; Horton, J.P.; Jamieson, D.J. Coronavirus Disease 2019 (COVID-19) Vaccines and Pregnancy. Obstet. Gynecol. 2021, 137, 408–414. [Google Scholar] [CrossRef] [PubMed]
- Anand, P.; Stahel, V.P. Review the safety of COVID-19 mRNA vaccines: A review. Patient Saf. Surg. 2021, 15, 20. [Google Scholar] [CrossRef]
- Graham, J.M. Update on the gestational effects of maternal hyperthermia. Birth Defects Res. 2020, 112, 943–952. [Google Scholar] [CrossRef]
- Shimabukuro, T.T.; Kim, S.Y.; Myers, T.R.; Moro, P.L.; Oduyebo, T.; Panagiotakopoulos, L.; Marquez, P.L.; Olson, C.K.; Liu, R.; Chang, K.T.; et al. Preliminary Findings of mRNA COVID-19 Vaccine Safety in Pregnant Persons. N. Engl. J. Med. 2021, 384, 2273–2282. [Google Scholar] [CrossRef] [PubMed]
- Levy, A.T.; Singh, S.; Riley, L.E.; Prabhu, M. Acceptance of COVID-19 vaccination in pregnancy: A survey study. Am. J. Obstet. Gynecol. MFM 2021, 3, 100399. [Google Scholar] [CrossRef] [PubMed]
- Panahi, L.; Amiri, M.; Pouy, S. Risks of Novel Coronavirus Disease (COVID-19) in Pregnancy; a Narrative Review. Arch. Acad. Emerg. Med. 2020, 8, e34. [Google Scholar]
- Lancet, T. Science during COVID-19: Where do we go from here? Lancet 2020, 396, 1941. [Google Scholar] [CrossRef]
- Ferrari, R. Writing narrative style literature reviews. Med. Writ. 2015, 24, 230–235. [Google Scholar] [CrossRef]
- Male, V. Are COVID-19 vaccines safe in pregnancy? Nat. Rev. Immunol. 2021, 21, 200–201. [Google Scholar] [CrossRef] [PubMed]
- Simopoulou, M.; Sfakianoudis, K.; Giannelou, P.; Rapani, A.; Siristatidis, C.; Bakas, P.; Vlahos, N.; Pantos, K. Navigating assisted reproduction treatment in the time of COVID-19: Concerns and considerations. J. Assist. Reprod. Genet. 2020, 37, 2663–2668. [Google Scholar] [CrossRef] [PubMed]
| Study | No of Pregnant Women | Trimester/ Gestation | No of Asymptomatic Women | Fever | Cough | Dyspnea | Myalgia | Headache | Diarrhea | Other |
|---|---|---|---|---|---|---|---|---|---|---|
| [10] | 1 | 33 w | - | 0 | 1 | 1 | 1 | 0 | 0 | Nausea, vomiting, acute pancreatitis |
| [11] | 1 | 21 w | - | 1 | 1 | 0 | 0 | 0 | 0 | Anosmia, ageusia |
| [12] | 1 | 32 w | - | 1 | 1 | 0 | 1 | 0 | 0 | Anorexia, nausea |
| [13] | 1 | 20 w | - | 0 | 1 | 0 | 0 | 0 | 0 | Acroparaesthesia, bilateral lower extremity weakness, dysphonia, dysphagia, Guillain–Barré syndrome |
| [14] | 1219 | 37.7 w (median) | 579 | 214 | 414 | 230 | 232 | 188 | 63 | Nasal stiffness, chills, anosmia, fatigue, sore throat, nausea |
| [15] | 427 | 29–38 w | - | >250 | >200 | >150 | >50 | >50 | >20 | Vomiting, rhinorrhea, lethargy, sore throat |
| [16] | 118 | 3rd (75) | 6 | 84 | 81 | 8 | - | 7 | 8 | Chest tightness, fatigue |
| [17] | 13 | 1st (5) 2nd (3) 3rd (5) | - | 8 | 5 | 1 | 1 | - | 1 | - |
| [18] | 51 | 3rd | 26 | 27 | 31 | - | 14 | - | - | Fatigue |
| [19] | 16 | 3rd | - | 12 | 0 | 0 | - | - | - | - |
| [20] | 1 | 22 w | - | 1 | 1 | - | 1 | - | 1 | Vaginal bleeding, abdominal pain |
| [21] | 78 | 27–41 w | 20 | 24 | 29 | 8 | 11 | 7 | 5 | Anosmia, rhinorrhea |
| [22] | 594 (including 6 w postpartum Women) | 1st (77) 2nd (241) 3rd (196) Postpartum (76) | - | 71 | 119 | - | 71 | - | - | Sore throat |
| [23] | 1 | 38 w | - | - | 1 | - | - | - | - | Chest tightness |
| [6] | 2 | 24 w, 27 w | - | 2 | 0 | 1 | - | 0 | - | - |
| [7] | 15 | 37 w | - | 10 | 6 | - | - | - | 1 | - |
| [24] | 1 | 34 w | - | - | - | 1 | 1 | - | - | - |
| [25] | 1 | 38 w | - | 1 | 1 | - | - | 1 | 1 | Rhinorrhea, sore throat |
| [26] | 1 | 22 w | - | 1 | - | - | - | - | - | Rhinitis |
| [27] | 1 | 34 w | - | 1 | - | - | - | - | 1 | - |
| [28] | 2 | 34 w, 37 w | - | 1 | 1 | 1 | - | - | 1 | - |
| [8] | 30 | 30–40.9 w | 8 | 11 | 5 | - | - | - | - | Abdominal pain, haemoptysis, fatigue, poor appetite |
| [29] | 1 | 33 w | - | - | - | - | - | - | - | - |
| [30] | 1 | 32 w | - | 1 | - | - | - | - | - | Flu-like symptoms |
| [31] | 1 | 38 w | - | 1 | - | - | - | - | - | - |
| [32] | 64 | 29.9 ± 5.8 w | - | - | - | - | - | - | - | - |
| [33] | 1 | 19 w | - | 1 | 1 | - | 1 | - | 1 | Sore throat, fatigue |
| [34] | 38 | 29.3 ± 8.5 | - | 10 | 25 | 13 | - | - | 7 | Sore throat, fatigue, anosmia |
| [35] | 100 | 31.3 w (median) | - | 62 | 80 | 30 | 26 | - | 10 | Anosmia, sore throat |
| [36] | 9 | 36–39 w | - | 7 | 4 | 1 | 3 | - | 1 | Sore throat, malaise |
| [37] | 7 | 37–41 w | - | 6 | 1 | 1 | - | - | 1 | - |
| [38] | 1 | 35 w | - | - | 1 | - | - | - | - | - |
| [39] | 19 | 35–41 w | - | 11 | 5 | 5 | - | - | 2 | - |
| [40] | 17 | 35–41 w | - | 3 | 6 | 2 | - | - | 3 | Nasal congestion, sputum production |
| [36] | 1 | 35 w | - | 1 | - | 1 | - | - | - | Fatigue |
| [41] | 3 | 34–38 w | - | 2 | 3 | 1 | - | - | - | - |
| [42] | 1 | 35 w | - | - | 1 | - | - | - | - | - |
| [43] | 1 | 39 w | - | - | 1 | - | - | - | - | - |
| [44] | 1 | 29 w | - | 1 | - | 1 | - | - | - | Rhinitis |
| [45] | 1 | 34 w | - | 1 | - | 1 | - | - | - | Nasal congestion |
| [46] | 7 | >36 w | - | 7/7 | 6/7 | - | - | - | 6/7 | - |
| [47] | 4 | 3rd | - | 3/4 | 2/4 | 2/4 | 2/4 | - | - | Fatigue |
| [48] | 1 | 30 w | - | 1/1 | - | - | - | - | - | - |
| [49] | 9 | - | 9/9 | 9/9 | - | - | - | 1 | - | |
| [50] | 7 | 28–37 w | 2/7 | 2/7 | 3/7 | - | 3/7 | 2/7 | - | Chest pain |
| [51] | 1 | 35 w | - | 1/1 | 1/1 | - | - | - | - | - |
| [52] | 1 | 38 w | 1 | - | - | - | - | - | - | - |
| [53] | 1 | 33 w | - | 1/1 | - | 1/1 | - | - | - | - |
| [54] | 9 | 24–36 w | - | 9/9 | 9/9 | 5/9 | 4/9 | - | - | - |
| Study | No of Pregnant Women | Completed Pregnancy | Vaginal Birth | C-Section | Preterm Delivery | Neonatal Adverse Outcomes | NICU Admission | Neonatal Death | Stillbirth | Miscarriage |
|---|---|---|---|---|---|---|---|---|---|---|
| [10] | 1 | 1 | 1 | 0 | 1 | 0 | 1 | 0 | 0 | 0 |
| [11] | 1 | 1 | 0 | 1 | 1 | SGA | 1 | 1 | 0 | 0 |
| [12] | 1 | 1 | 0 | 1 | 1 | Respiratory distress | 1 | 0 | 0 | 0 |
| [14] | 1219 | 1196 | - | 450 | 204 | - | 254 | 5 | - | - |
| [15] | 427 | 266 | 106 | 156 | 66 | Neonatal encephalopathy | 67 | 2 | 3 | 4 |
| [16] | 118 | 68 | 5 | 63 | 14 | - | - | 0 | 0 | - |
| [17] | 1 | 1 | - | 1 | - | - | - | - | - | - |
| [18] | 13 | 6 | 1 | 4 | 2 | Neonatal pneumonia | 0 | 0 | 0 | 1 |
| [19] | 51 | 51 | 26 | 25 | 10 | - | - | 0 | 0 | 0 |
| [20] | 16 | 16 | 2 | 14 | 3 | - | - | 0 | 0 | 0 |
| [21] | 1 | 0 | 0 | 0 | 0 | - | - | - | - | 1 |
| [75] | 31 | 31 | 25 | 6 | 1 | 2 infected neonates | 2 | 0 | 0 | 0 |
| [22] | 116 | 106 | 63 | 43 | 14 | Prolonged QT syndrome, mild respiratory distress, short bowel syndrome, tachycardia | 12 | 0 | 0 | 0 |
| [6] | 1 | 1 | 1 | - | - | - | 1: quarantine | - | - | - |
| [71] | 65 | 65 | 13 | 52 | 9 | Asphyxia, fever, diarrhea | - | - | - | - |
| [7] | 2 | 2 | 1 | 1 | 0 | Jaundice | 0 | 0 | 0 | 0 |
| [24] | 15 | 15 | 1 | 14 | 1 | NRDS | 15: quarantine | 0 | 0 | 0 |
| [25] | 1 | 1 | - | 1 | 1 | - | 1 | 0 | 0 | 0 |
| [26] | 1 | 1 | - | 1 | - | Severe COVID-19 | 1 | 0 | 0 | 0 |
| [27] | 1 | 1 | 1 | - | 2 | - | - | - | - | 2 (twins) |
| [28] | 1 | 1 | 1 | - | 1 | Jaundice, fever, respiratory distress hypoxia | 1 | 0 | 0 | 0 |
| [8] | 2 | 2 | - | 2 | 1 | - | - | - | - | - |
| [29] | 30 | 30 | 7 | 23 | 5 | - | - | - | - | - |
| [30] | 1 | 1 | 1 | - | 1 | Intubation | 1 | - | - | - |
| [31] | 1 | 1(triplets) | - | 3 | 3 | 1: NCPAP | 3 | 0 | 0 | 0 |
| [32] | 1 | 1 | 1 | - | - | Abdominal distension, respiratory acidosis, intubation | 1 | 0 | 0 | 0 |
| [33] | 64 | 32 | 8 | 24 | 29 | 2: IUGR | 21 | 0 | 0 | 0 |
| [34] | 1 | 1 | 1 | - | - | - | - | - | - | 1 |
| [35] | 38 | 17 | 10 | 7 | 10 | 3: intubated | 3 | 0 | 0 | 1 |
| [36] | 100 | 33 | 17 | 16 | 20 | 6: intubated | 10 | 1 | 0 | 0 |
| [36] | 9 | 9 | 0 | 9 | 4 | - | 0 | 0 | 0 | 0 |
| [37] | 7 | 7 | 0 | 7 | 0 | 1: mild pulmonary infection | 0 | 0 | 0 | 0 |
| [38] | 1 | 1 | - | 1 | 1 | - | 0 | 0 | 0 | 0 |
| [39] | 19 | 19 | 1 | 18 | 0 | - | 19: isolation | 0 | 0 | 0 |
| [40] | 17 | 17 | 0 | 17 | 3 | 5: neonatal pneumonia | 0 | 0 | 0 | |
| [36] | 1 | 1 | - | 1 | 1 | Tachypnea, moaning, periodic breath | 1 | 0 | 0 | 0 |
| [41] | 3 | 3 | 3 | 0 | 1 | - | 0 | 0 | 0 | 0 |
| [42] | 1 | 1 | 0 | 1 | 1 | - | 0 | 0 | 0 | 0 |
| [43] | 1 | 1 | 0 | 1 | 0 | - | 0 | 0 | 0 | 0 |
| [44] | 1 | 1 | 0 | 1 | 1 | - | 1 | 0 | 0 | 0 |
| [45] | 1 | 1 | 0 | 1 | 0 | - | 1: quarantine | 0 | 0 | 0 |
| [46] | 7 | 7 | 0 | 7 | 4 | Respiratory distress | 5 | 0 | 0 | 0 |
| [47] | 4 | 4 | 1 | 3 | 0 | TTN, rash | 2 | 0 | 0 | 0 |
| [48] | 1 | 1 | 0 | 1 | 1 | - | 1: isolation | 0 | 0 | 0 |
| [49] | 9 | 9 | 2 | 7 | 6 | Dyspnea, fever, vomit, NRDS, pneumothorax, thrombopenia | 1 | 0 | 0 | |
| [51] | 1 | 1 | 0 | 1 | 1 | Intubation, neurological symptoms | 1 | 0 | 0 | 0 |
| [52] | 1 | 1 | 0 | 1 | 0 | - | 0 | 0 | 0 | 0 |
| [53] | 1 | 1 | 0 | 1 | 1 | Intubation | 1 | 0 | 0 | 0 |
| [54] | 9 | 9 | 1 | 8 | Intubation, pneumonia | 2 | 4 | - |
| Study | Neonatal Throat Swab (+) | Amniotic Fluid (+) | Vaginal Secretions (+) | Placenta (+) | Breastmilk Viral RNA (+) | IgM (+) | IgG (+) | Cord Blood Viral RNA (+) | IgM (+) | IgG (+) | Neonatal Serum IgM (+) | IgG (+) | Other (+) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| [83] | 0/1 | - | - | - | - | - | - | - | - | - | - | 1/1 | |
| [11] | - | - | - | 1/1 | - | - | - | - | - | - | - | - | Umbilical cord |
| [12] | 1/1 | 1/1 | - | - | - | - | - | - | - | - | - | - | |
| [81] | - | 0/1 | - | 0/1 | - | - | - | - | - | - | - | - | |
| [78] | 1/1 | - | - | - | - | - | - | - | - | - | 1/1 | 1/1 | |
| [79] | - | 1/1 | - | 1/1 | - | - | - | - | - | - | - | - | Fetal membranes |
| [82] | - | - | - | 2/2 | - | - | - | - | - | - | - | - | Fetal lungs and kidneys |
| [15] | 12/240 | - | - | - | - | - | - | - | - | - | - | - | |
| [16] | 0/8 | - | - | - | 0/3 | - | - | - | - | - | - | - | |
| [17] | - | 0/1 | - | 0/1 | 0/1 | 1/1 | 1/1 | 0/1 | 1/1 | 1/1 | - | - | |
| [18] | 0/5 | 0/9 | 0/13 | 0/9 | 1/3 | - | - | - | - | - | 1/5 | 1/5 | |
| [20] | 0/3 | - | - | - | - | - | - | - | - | - | - | - | |
| [21] | - | - | - | 1/1 | - | - | - | - | - | - | - | - | Umbilical cord |
| [75] | 2/31 | 0/3 | 1/30 | 2/31 | 1/11 | 1/10 | 0/10 | 1/30 | 1/30 | 12/30 | - | - | |
| [22] | 0/120 | - | - | - | - | - | - | - | - | - | - | - | |
| [6] | 0/1 | - | 0/1 | - | 0/1 | 0/1 | 1/1 | - | - | - | 0/1 | 1/1 | |
| [71] | 0/38 | - | - | - | - | - | - | - | - | - | - | - | |
| [7] | 0/2 | - | - | - | - | - | - | - | - | - | 0/2 | 2/2 | |
| [24] | 0/15 | 0/15 | - | 0/15 | - | - | - | - | - | - | - | - | |
| [25] | 1/1 | - | - | - | - | - | - | - | - | - | - | - | |
| [26] | 1/1 | - | - | - | 1/1 | - | - | - | - | - | - | - | Infant’s and mother’s stool sample |
| [27] | - | 2/2 | - | 2/2 | - | - | - | - | - | - | - | - | Maternal Blood sample |
| [28] | 1/1 | - | - | - | - | - | - | - | - | - | - | - | |
| [8] | 0/2 | 0/2 | - | 1/2 | 1/2 | - | - | 1/2 | - | - | - | - | |
| [29] | 0/30 | - | - | - | - | - | - | - | - | - | - | - | |
| [30] | 0/1 | 0/1 | - | 0/1 | 0/1 | - | - | - | - | - | - | - | |
| [31] | 3/3 | - | - | - | - | - | - | - | - | - | - | - | |
| [32] | 1/1 | - | - | - | - | - | - | - | - | - | - | - | Tracheal aspiration, anal swab |
| [33] | 1/33 | - | - | - | - | - | - | - | - | - | - | - | |
| [34] | - | 0/1 | 0/1 | 1/1 | - | - | - | - | - | - | - | - | |
| [36] | 1/36 | - | - | - | - | - | - | - | - | - | - | - | |
| [36] | 0/6 | 0/6 | - | - | 0/6 | - | - | 0/6 | - | - | - | - | |
| [37] | 1/3 | - | - | 0/1 | - | - | - | 0/1 | - | - | - | - | |
| [38] | 0/1 | 0/1 | - | 0/1 | 0/1 | - | - | 0/1 | - | - | - | - | |
| [39] | 0/19 | 0/19 | - | - | 0/10 | - | - | 0/19 | - | - | - | - | |
| [40] | 2/17 | - | - | - | - | - | - | - | - | - | - | - | |
| [36] | 0/1 | 0/1 | 0/1 | 0/1 | 0/1 | - | - | 0/1 | - | - | - | - | |
| [41] | 0/3 | - | - | - | - | - | - | - | - | - | - | - | |
| [42] | 0/1 | 0/1 | - | 0/1 | 0/1 | - | - | 0/1 | - | - | - | - | |
| [43] | 0/1 | - | - | - | - | - | - | - | - | - | - | - | |
| [45] | 0/1 | - | 0/1 | - | 0/1 | - | - | - | - | - | 1/1 | 1/1 | |
| [46] | 0/6 | 0/5 | - | - | - | - | - | 0/5 | - | - | - | - | |
| [47] | 0/3 | - | - | - | - | - | - | - | - | - | - | - | |
| [48] | 0/1 | 0/1 | - | 0/1 | - | - | - | 0/1 | - | - | - | - | |
| [49] | 0/9 | - | - | - | - | - | - | - | - | - | - | - | |
| [51] | 1/1 | 1/1 | 1/1 | 1/1 | - | - | - | - | - | - | - | - | Rectal swab, neonatal blood |
| [52] | 0/1 | - | - | 1/1 | - | - | - | - | - | - | 0/1 | 0/1 | |
| [53] | 1/1 | - | - | - | - | - | - | - | - | - | 0/1 | 0/1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kalampokas, T.; Rapani, A.; Papageorgiou, M.; Grigoriadis, S.; Maziotis, E.; Anifandis, G.; Triantafyllidou, O.; Tzanakaki, D.; Neofytou, S.; Bakas, P.; et al. The Current Evidence Regarding COVID-19 and Pregnancy: Where Are We Now and Where Should We Head to Next? Viruses 2021, 13, 2000. https://doi.org/10.3390/v13102000
Kalampokas T, Rapani A, Papageorgiou M, Grigoriadis S, Maziotis E, Anifandis G, Triantafyllidou O, Tzanakaki D, Neofytou S, Bakas P, et al. The Current Evidence Regarding COVID-19 and Pregnancy: Where Are We Now and Where Should We Head to Next? Viruses. 2021; 13(10):2000. https://doi.org/10.3390/v13102000
Chicago/Turabian StyleKalampokas, Theodoros, Anna Rapani, Maria Papageorgiou, Sokratis Grigoriadis, Evangelos Maziotis, George Anifandis, Olga Triantafyllidou, Despoina Tzanakaki, Spyridoula Neofytou, Panagiotis Bakas, and et al. 2021. "The Current Evidence Regarding COVID-19 and Pregnancy: Where Are We Now and Where Should We Head to Next?" Viruses 13, no. 10: 2000. https://doi.org/10.3390/v13102000
APA StyleKalampokas, T., Rapani, A., Papageorgiou, M., Grigoriadis, S., Maziotis, E., Anifandis, G., Triantafyllidou, O., Tzanakaki, D., Neofytou, S., Bakas, P., Simopoulou, M., & Vlahos, N. (2021). The Current Evidence Regarding COVID-19 and Pregnancy: Where Are We Now and Where Should We Head to Next? Viruses, 13(10), 2000. https://doi.org/10.3390/v13102000










