Norovirus Epidemiology and Genetic Diversity in Leipzig, Germany during 2013–2017
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. RNA Extraction, Detection, Sequencing and Typing
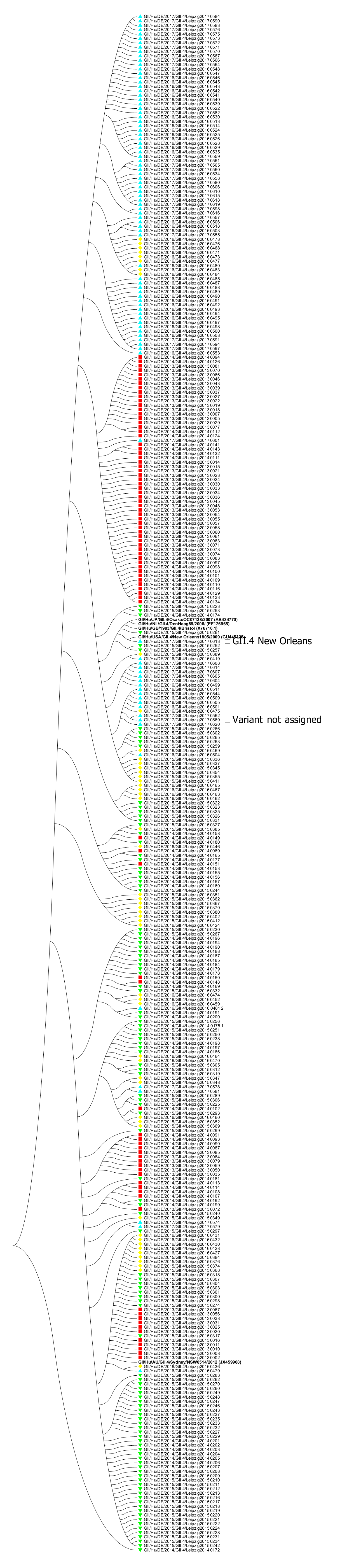
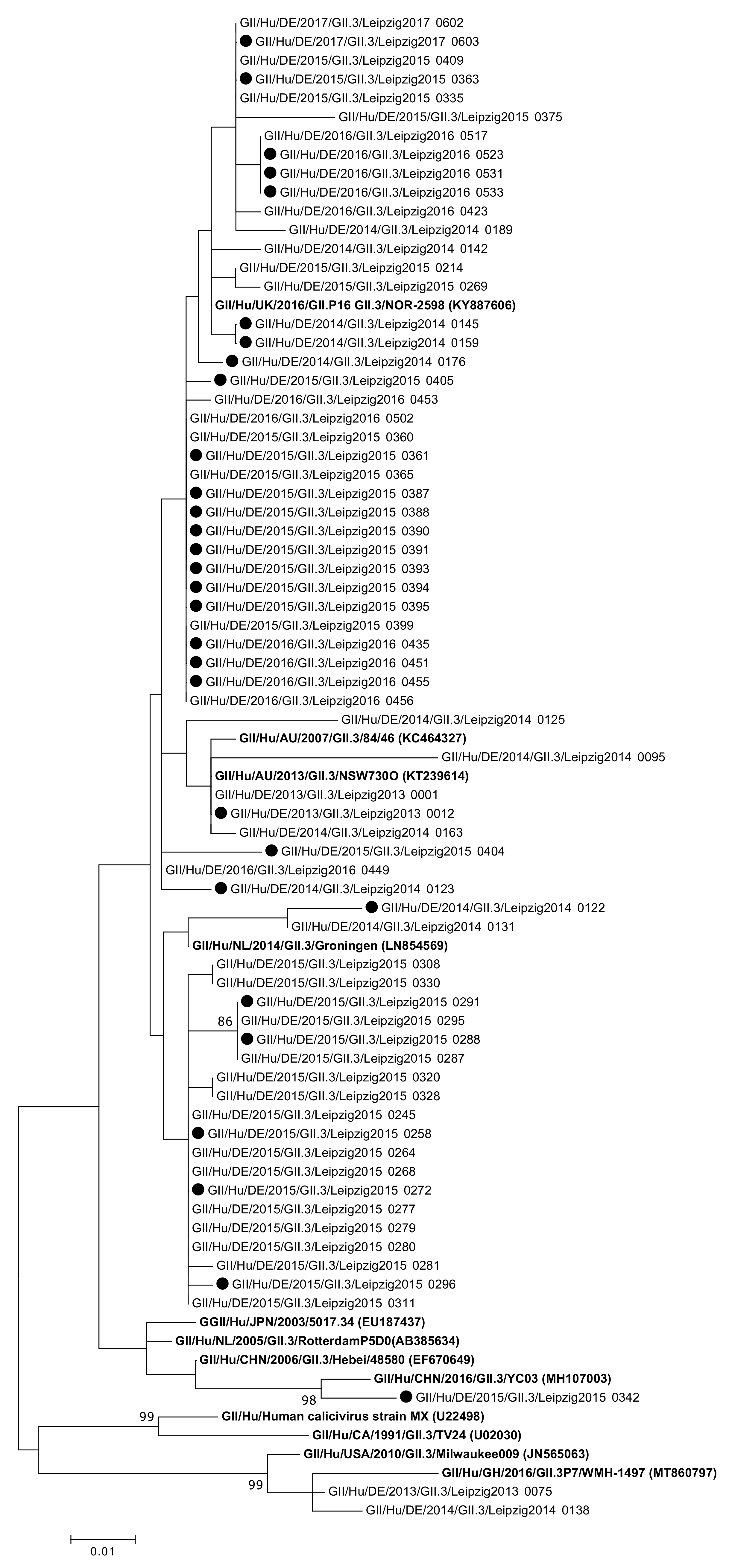
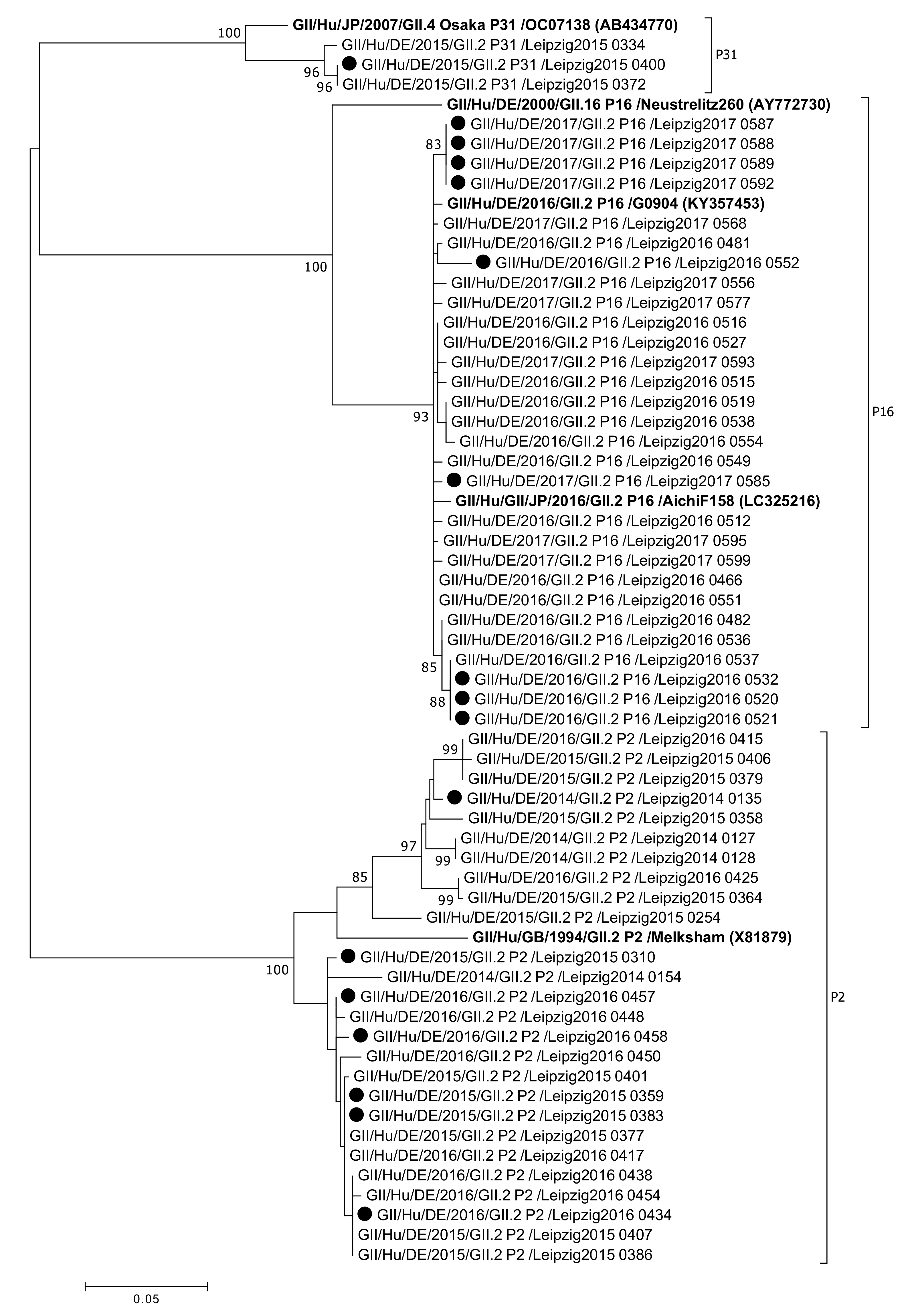
2.3. Analysis of Sequences and Phylogeny
2.4. Classification of Nosocomial and Community-Acquired Infections
2.5. Statistical Analysis
2.6. Ethical Clearance
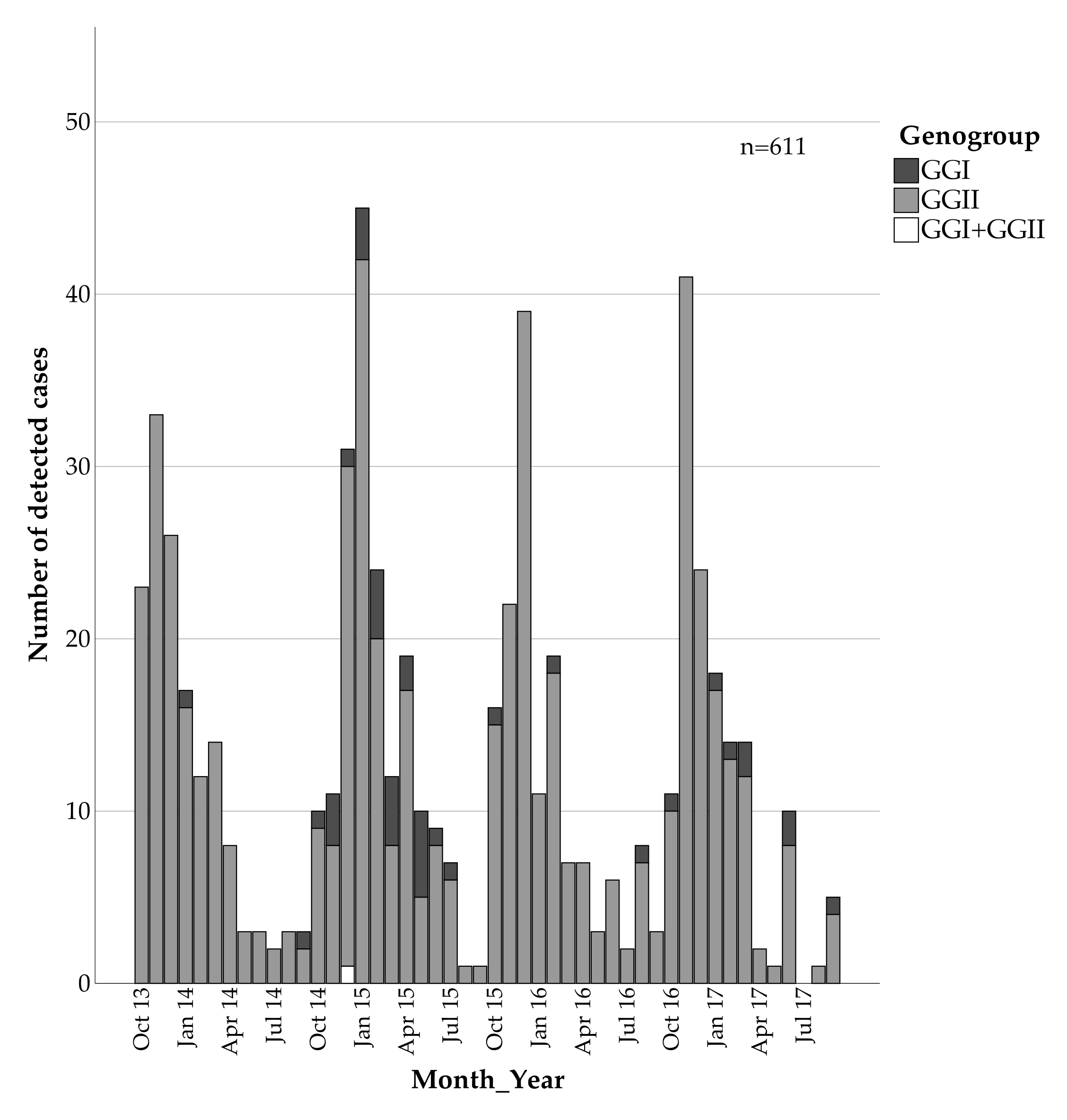
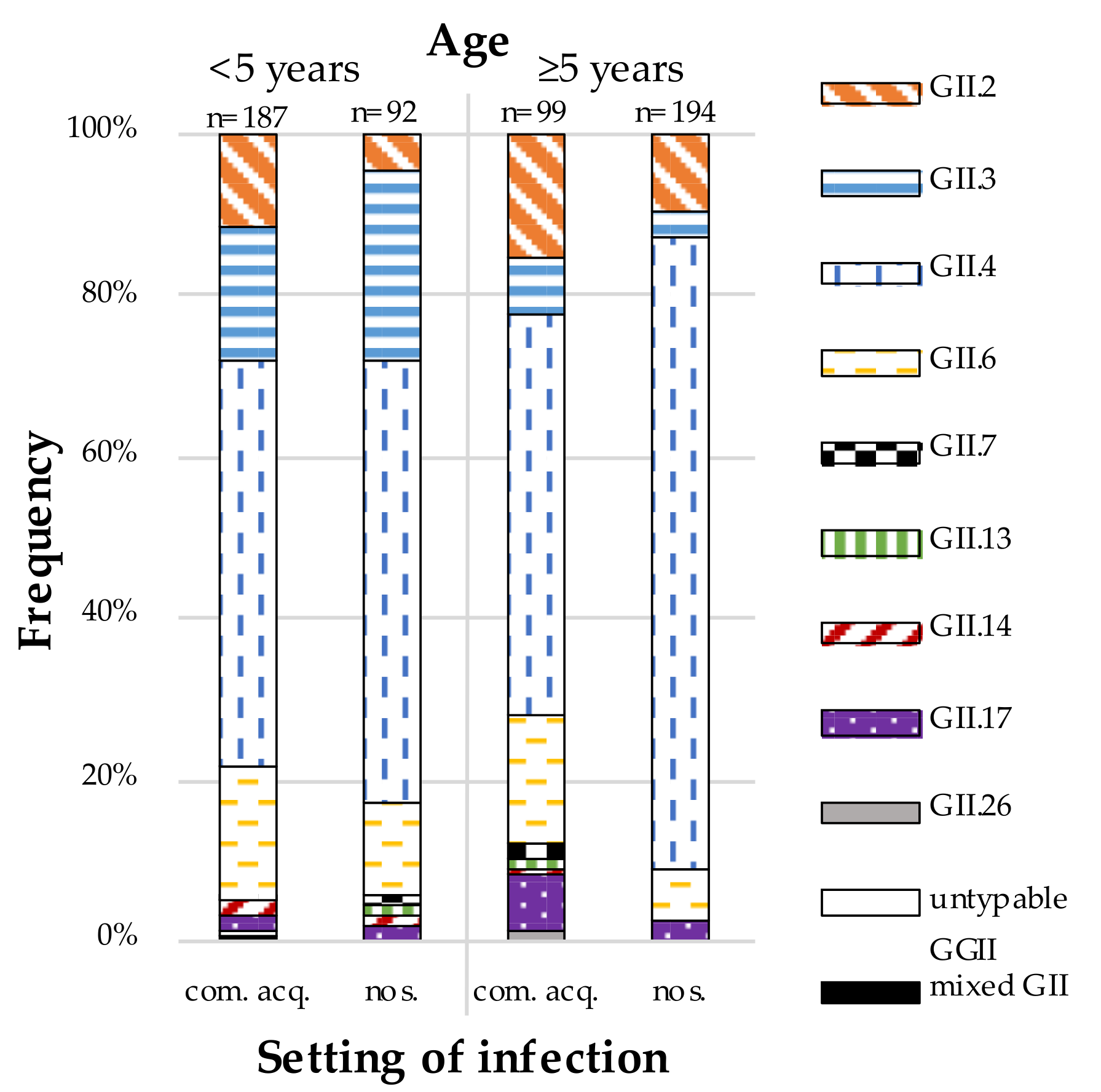
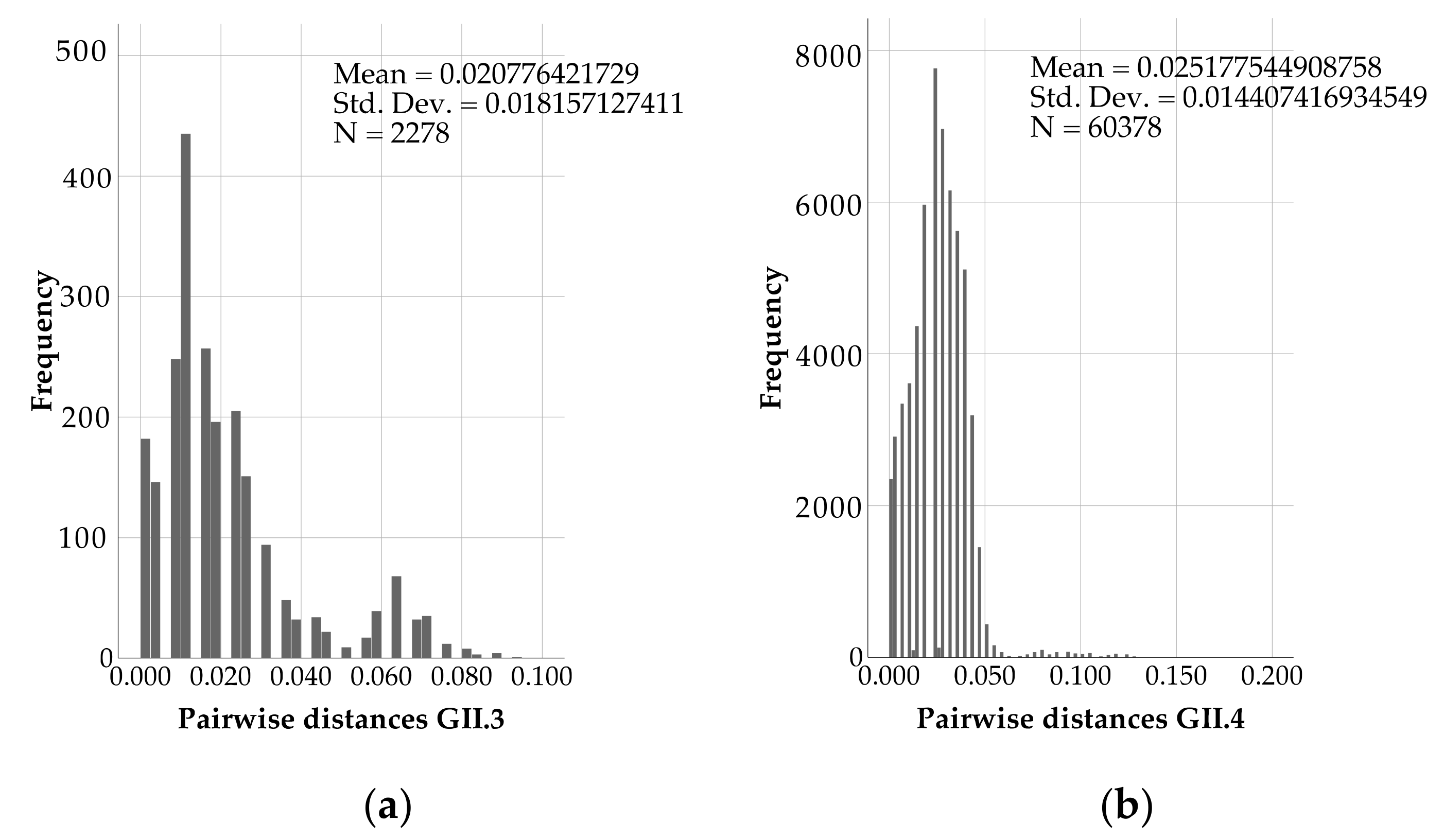
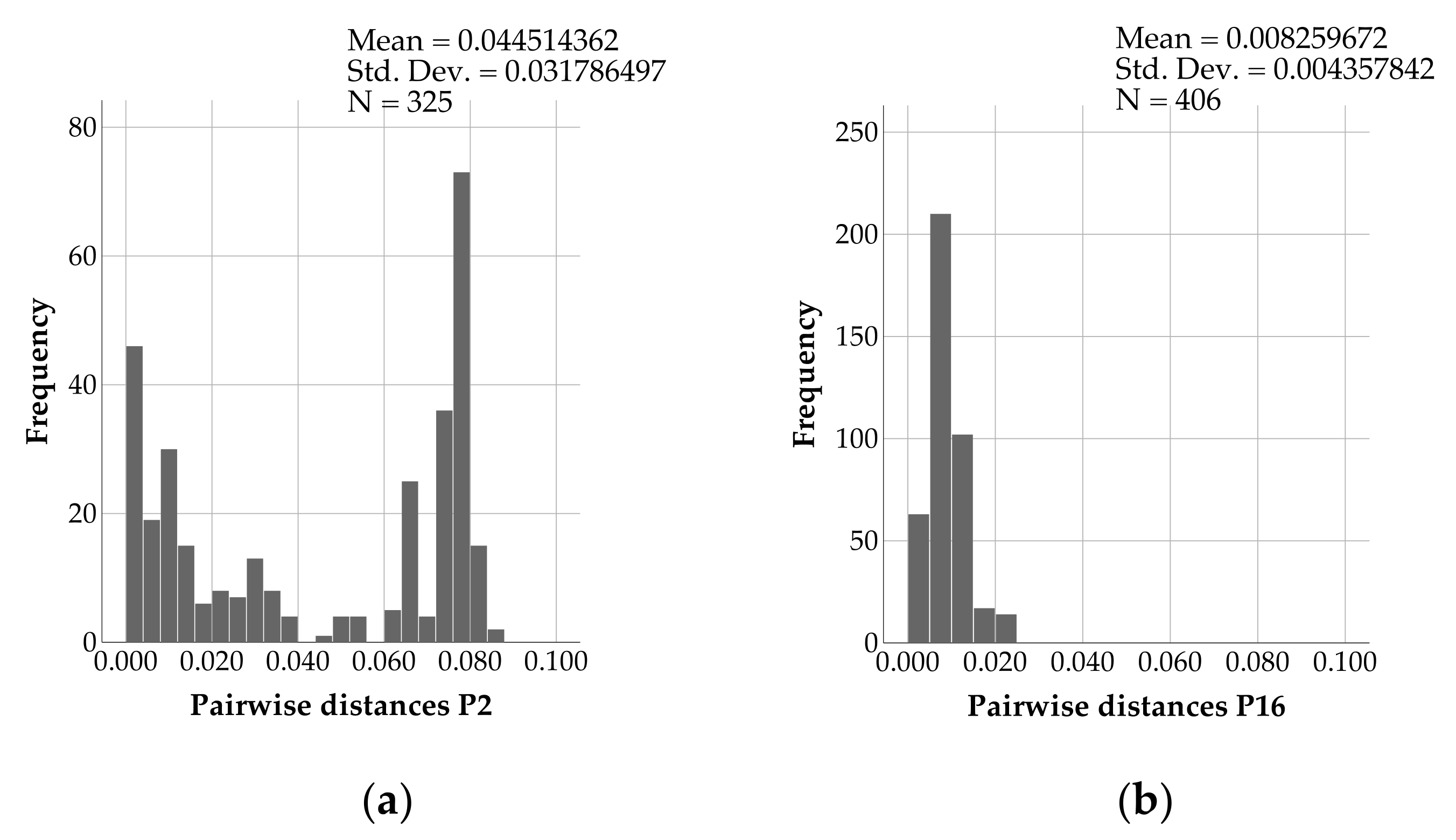
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ahmed, S.M.; Hall, A.J.; Robinson, A.E.; Verhoef, L.; Premkumar, P.; Parashar, U.D.; Koopmans, M.; Lopman, B.A. Global Prevalence of Norovirus in Cases of Gastroenteritis: A Systematic Review and Meta-Analysis. Lancet Infect. Dis. 2014, 14, 725–730. [Google Scholar] [CrossRef] [Green Version]
- Patel, M.M.; Widdowson, M.-A.; Glass, R.I.; Akazawa, K.; Vinjé, J.; Parashar, U.D. Systematic Literature Review of Role of Noroviruses in Sporadic Gastroenteritis. Emerg. Infect. Dis. 2008, 14, 1224–1231. [Google Scholar] [CrossRef] [PubMed]
- Koo, H.L.; Neill, F.H.; Estes, M.K.; Munoz, F.M.; Cameron, A.; DuPont, H.L.; Atmar, R.L. Noroviruses: The Most Common Pediatric Viral Enteric Pathogen at a Large University Hospital After Introduction of Rotavirus Vaccination. J. Pediatr. Infect. Dis. Soc. 2013, 2, 57–60. [Google Scholar] [CrossRef] [Green Version]
- Payne, D.C.; Vinjé, J.; Szilagyi, P.G.; Edwards, K.M.; Staat, M.A.; Weinberg, G.A.; Hall, C.B.; Chappell, J.; Bernstein, D.I.; Curns, A.T.; et al. Norovirus and Medically Attended Gastroenteritis in U.S. Children. N. Engl. J. Med. 2013, 368, 1121–1130. [Google Scholar] [CrossRef] [Green Version]
- Hemming, M.; Räsänen, S.; Huhti, L.; Paloniemi, M.; Salminen, M.; Vesikari, T. Major Reduction of Rotavirus, but Not Norovirus, Gastroenteritis in Children Seen in Hospital after the Introduction of RotaTeq Vaccine into the National Immunization Programme in Finland. Eur. J. Pediatr. 2013, 172, 739–746. [Google Scholar] [CrossRef]
- Ahmed, S.M.; Lopman, B.A.; Levy, K. A Systematic Review and Meta-Analysis of the Global Seasonality of Norovirus. PLoS ONE 2013, 8, e75922. [Google Scholar] [CrossRef]
- Hardstaff, J.L.; Clough, H.E.; Lutje, V.; McIntyre, K.M.; Harris, J.P.; Garner, P.; O’Brien, S.J. Foodborne and Food-Handler Norovirus Outbreaks: A Systematic Review. Foodborne Pathog. Dis. 2018, 15, 589–597. [Google Scholar] [CrossRef] [Green Version]
- Vega, E.; Barclay, L.; Gregoricus, N.; Shirley, S.H.; Lee, D.; Vinje, J. Genotypic and Epidemiologic Trends of Norovirus Outbreaks in the United States, 2009 to 2013. J. Clin. Microbiol. 2014, 52, 147–155. [Google Scholar] [CrossRef] [Green Version]
- Kroneman, A.; Harris, J.; Vennema, H.; Duizer, E.; van Duynhoven, Y.; Gray, J.; Iturriza, M.; Bottiger, B.; Falkenhorst, G.; Johnsen, C.; et al. Data Quality of 5 Years of Central Norovirus Outbreak Reporting in the European Network for Food-Borne Viruses. J. Public Health 2008, 30, 82–90. [Google Scholar] [CrossRef] [PubMed]
- Dolin, R.; Blacklow, N.R.; DuPont, H.; Formal, S.; Buscho, R.F.; Kasel, J.A.; Chames, R.P.; Hornick, R.; Chanock, R.M. Transmission of Acute Infectious Nonbacterial Gastroenteritis to Volunteers by Oral Administration of Stool Filtrates. J. Infect. Dis. 1971, 123, 307–312. [Google Scholar] [CrossRef] [PubMed]
- Robilotti, E.; Deresinski, S.; Pinsky, B.A. Norovirus. Clin. Microbiol. Rev. 2015, 28, 134–164. [Google Scholar] [CrossRef] [Green Version]
- Lopman, B.A.; Reacher, M.H.; Vipond, I.B.; Sarangi, J.; Brown, D.W.G. Clinical Manifestation of Norovirus Gastroenteritis in Health Care Settings. Clin. Infect. Dis. 2004, 39, 318–324. [Google Scholar] [CrossRef] [Green Version]
- Murata, T.; Katsushima, N.; Mizuta, K.; Muraki, Y.; Hongo, S.; Matsuzaki, Y. Prolonged Norovirus Shedding in Infants ≤6 Months of Age with Gastroenteritis. Pediatr. Infect. Dis. J. 2007, 26, 46–49. [Google Scholar] [CrossRef] [PubMed]
- Bok, K.; Green, K.Y. Norovirus Gastroenteritis in Immunocompromised Patients. N. Engl. J. Med. 2012, 367, 2126–2132. [Google Scholar] [CrossRef] [Green Version]
- Jiang, X.; Wang, M.; Wang, K.; Estes, M.K. Sequence and Genomic Organization of Norwalk Virus. Virology 1993, 195, 51–61. [Google Scholar] [CrossRef]
- Thorne, L.G.; Goodfellow, I.G. Norovirus Gene Expression and Replication. J. Gen. Virol. 2014, 95, 278–291. [Google Scholar] [CrossRef]
- Chhabra, P.; de Graaf, M.; Parra, G.I.; Chan, M.C.-W.; Green, K.; Martella, V.; Wang, Q.; White, P.A.; Katayama, K.; Vennema, H.; et al. Updated Classification of Norovirus Genogroups and Genotypes. J. Gen. Virol. 2019, 100, 1393–1406. [Google Scholar] [CrossRef] [PubMed]
- Kageyama, T.; Kojima, S.; Shinohara, M.; Uchida, K.; Fukushi, S.; Hoshino, F.B.; Takeda, N.; Katayama, K. Broadly Reactive and Highly Sensitive Assay for Norwalk-Like Viruses Based on Real-Time Quantitative Reverse Transcription-PCR. J. Clin. Microbiol. 2003, 41, 1548–1557. [Google Scholar] [CrossRef] [Green Version]
- Hallowell, B.D.; Parashar, U.D.; Hall, A.J. Epidemiologic Challenges in Norovirus Vaccine Development. Hum. Vaccines Immunother. 2019, 15, 1279–1283. [Google Scholar] [CrossRef] [PubMed]
- Sherwood, J.; Mendelman, P.M.; Lloyd, E.; Liu, M.; Boslego, J.; Borkowski, A.; Jackson, A.; Faix, D. Efficacy of an Intramuscular Bivalent Norovirus GI.1/GII.4 Virus-like Particle Vaccine Candidate in Healthy US Adults. Vaccine 2020, 38, 6442–6449. [Google Scholar] [CrossRef] [PubMed]
- Kim, L.; Liebowitz, D.; Lin, K.; Kasparek, K.; Pasetti, M.F.; Garg, S.J.; Gottlieb, K.; Trager, G.; Tucker, S.N. Safety and Immunogenicity of an Oral Tablet Norovirus Vaccine, a Phase I Randomized, Placebo-Controlled Trial. JCI Insight 2018, 3, e121077. [Google Scholar] [CrossRef]
- Esposito, S.; Principi, N. Norovirus Vaccine: Priorities for Future Research and Development. Front. Immunol. 2020, 11, 1383. [Google Scholar] [CrossRef]
- Bok, K.; Abente, E.J.; Realpe-Quintero, M.; Mitra, T.; Sosnovtsev, S.V.; Kapikian, A.Z.; Green, K.Y. Evolutionary Dynamics of GII.4 Noroviruses over a 34-Year Period. J. Virol. 2009, 83, 11890–11901. [Google Scholar] [CrossRef] [Green Version]
- Bull, R.A.; Eden, J.-S.; Rawlinson, W.D.; White, P.A. Rapid Evolution of Pandemic Noroviruses of the GII.4 Lineage. PLoS Pathog. 2010, 6, e1000831. [Google Scholar] [CrossRef]
- Mattison, C.P.; Cardemil, C.V.; Hall, A.J. Progress on Norovirus Vaccine Research: Public Health Considerations and Future Directions. Expert Rev. Vaccines 2018, 17, 773–784. [Google Scholar] [CrossRef] [PubMed]
- Mallory, M.; Lindesmith, L.; Graham, R.; Baric, R. GII.4 Human Norovirus: Surveying the Antigenic Landscape. Viruses 2019, 11, 177. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vinjé, J. Advances in Laboratory Methods for Detection and Typing of Norovirus. J. Clin. Microbiol. 2015, 53, 373–381. [Google Scholar] [CrossRef] [Green Version]
- Vinjé, J.; Hamidjaja, R.A.; Sobsey, M.D. Development and Application of a Capsid VP1 (Region D) Based Reverse Transcription PCR Assay for Genotyping of Genogroup I and II Noroviruses. J. Virol. Methods 2004, 116, 109–117. [Google Scholar] [CrossRef]
- Stals, A.; Mathijs, E.; Baert, L.; Botteldoorn, N.; Denayer, S.; Mauroy, A.; Scipioni, A.; Daube, G.; Dierick, K.; Herman, L.; et al. Molecular Detection and Genotyping of Noroviruses. Food Environ. Virol. 2012, 4, 153–167. [Google Scholar] [CrossRef]
- Hoehne, M.; Schreier, E. Detection of Norovirus Genogroup I and II by Multiplex Real-Time RT- PCR Using a 3’-Minor Groove Binder-DNA Probe. BMC Infect. Dis. 2006, 6, 69. [Google Scholar] [CrossRef] [Green Version]
- Bernard, H.; Höhne, M.; Niendorf, S.; Altmann, D.; Stark, K. Epidemiology of Norovirus Gastroenteritis in Germany 2001–2009: Eight Seasons of Routine Surveillance. Epidemiol. Infect. 2014, 142, 63–74. [Google Scholar] [CrossRef] [Green Version]
- Kojima, S.; Kageyama, T.; Fukushi, S.; Hoshino, F.B.; Shinohara, M.; Uchida, K.; Natori, K.; Takeda, N.; Katayama, K. Genogroup-Specific PCR Primers for Detection of Norwalk-like Viruses. J. Virol. Methods 2002, 100, 107–114. [Google Scholar] [CrossRef]
- Trujillo, A.A.; McCaustland, K.A.; Zheng, D.-P.; Hadley, L.A.; Vaughn, G.; Adams, S.M.; Ando, T.; Glass, R.I.; Monroe, S.S. Use of TaqMan Real-Time Reverse Transcription-PCR for Rapid Detection, Quantification, and Typing of Norovirus. J. Clin. Microbiol. 2006, 44, 1405–1412. [Google Scholar] [CrossRef] [Green Version]
- Won, Y.-J.; Park, J.-W.; Han, S.; Cho, H.-G.; Kang, L.-H.; Lee, S.-G.; Ryu, S.-R.; Paik, S.-Y. Full-Genomic Analysis of a Human Norovirus Recombinant GII.12/13 Novel Strain Isolated from South Korea. PLoS ONE 2013, 8, e85063. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lu, J.; Fang, L.; Sun, L.; Zeng, H.; Li, Y.; Zheng, H.; Wu, S.; Yang, F.; Song, T.; Lin, J.; et al. Association of GII.P16-GII.2 Recombinant Norovirus Strain with Increased Norovirus Outbreaks, Guangdong, China, 2016. Emerg. Infect. Dis. 2017, 23, 1188–1190. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, J.-S.; Lee, S.-G.; Jin, J.-Y.; Cho, H.-G.; Jheong, W.-H.; Paik, S.-Y. Complete Nucleotide Sequence Analysis of the Norovirus GII.4 Sydney Variant in South Korea. BioMed Res. Int. 2015, 2015, 374637. [Google Scholar] [CrossRef] [PubMed]
- Kroneman, A.; Vennema, H.; Deforche, K.; Avoort, H.v.d.; Peñaranda, S.; Oberste, M.S.; Vinjé, J.; Koopmans, M. An Automated Genotyping Tool for Enteroviruses and Noroviruses. J. Clin. Virol. 2011, 51, 121–125. [Google Scholar] [CrossRef]
- Franck, K.T.; Nielsen, R.T.; Holzknecht, B.J.; Ersbøll, A.K.; Fischer, T.K.; Böttiger, B. Norovirus Genotypes in Hospital Settings: Differences Between Nosocomial and Community-Acquired Infections. J. Infect. Dis. 2015, 212, 881–888. [Google Scholar] [CrossRef] [Green Version]
- Smith, P.W.; Bennett, G.; Bradley, S.; Drinka, P.; Lautenbach, E.; Marx, J.; Mody, L.; Nicolle, L.; Stevenson, K. Shea/Apic Guideline: Infection Prevention and Control In The Long-Term Care Facility. Infect. Control Hosp. Epidemiol. 2008, 29, 785–814. [Google Scholar] [CrossRef] [Green Version]
- Niendorf, S.; Jacobsen, S.; Faber, M.; Eis-Hübinger, A.M.; Hofmann, J.; Zimmermann, O.; Höhne, M.; Bock, C.T. Steep Rise in Norovirus Cases and Emergence of a New Recombinant Strain GII.P16-GII.2, Germany, Winter 2016. Eurosurveillance 2017, 22, 30447. [Google Scholar] [CrossRef]
- Franck, K.T.; Fonager, J.; Ersbøll, A.K.; Böttiger, B. Norovirus Epidemiology in Community and Health Care Settings and Association with Patient Age, Denmark. Emerg. Infect. Dis. 2014, 20, 1123–1131. [Google Scholar] [CrossRef] [Green Version]
- Suzuki, Y.; Doan, Y.H.; Kimura, H.; Shinomiya, H.; Shirabe, K.; Katayama, K. Predicting Genotype Compositions in Norovirus Seasons in Japan: NOROCAST: Norovirus Forecasting System. Microbiol. Immunol. 2016, 60, 418–426. [Google Scholar] [CrossRef] [Green Version]
- Phan, T.G.; Kaneshi, K.; Ueda, Y.; Nakaya, S.; Nishimura, S.; Yamamoto, A.; Sugita, K.; Takanashi, S.; Okitsu, S.; Ushijima, H. Genetic Heterogeneity, Evolution, and Recombination in Noroviruses. J. Med. Virol. 2007, 79, 1388–1400. [Google Scholar] [CrossRef]
- Monica, B.; Ramani, S.; Banerjee, I.; Primrose, B.; Iturriza-Gomara, M.; Gallimore, C.I.; Brown, D.W.; Moses, P.D.; Gray, J.J.; Kang, G. Human Caliciviruses in Symptomatic and Asymptomatic Infections in Children in Vellore, South India. J. Med. Virol. 2007, 79, 544–551. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Beersma, M.F.C.; Schutten, M.; Vennema, H.; Hartwig, N.G.; Mes, T.H.M.; Osterhaus, A.D.M.E.; van Doornum, G.J.J.; Koopmans, M. Norovirus in a Dutch Tertiary Care Hospital (2002–2007): Frequent Nosocomial Transmission and Dominance of GIIb Strains in Young Children. J. Hosp. Infect. 2009, 71, 199–205. [Google Scholar] [CrossRef] [PubMed]
- Boon, D.; Mahar, J.E.; Abente, E.J.; Kirkwood, C.D.; Purcell, R.H.; Kapikian, A.Z.; Green, K.Y.; Bok, K. Comparative Evolution of GII.3 and GII.4 Norovirus over a 31-Year Period. J. Virol. 2011, 85, 8656–8666. [Google Scholar] [CrossRef] [Green Version]
- Cannon, J.L.; Bonifacio, J.; Bucardo, F.; Buesa, J.; Bruggink, L.; Chan, M.C.-W.; Fumian, T.M.; Giri, S.; Gonzalez, M.D.; Hewitt, J.; et al. Global Trends in Norovirus Genotype Distribution among Children with Acute Gastroenteritis. Emerg. Infect. Dis. 2021, 27, 1438–1445. [Google Scholar] [CrossRef] [PubMed]
- Mahar, J.E.; Donker, N.C.; Bok, K.; Talbo, G.H.; Green, K.Y.; Kirkwood, C.D. Identification and Characterization of Antibody-Binding Epitopes on the Norovirus GII.3 Capsid. J. Virol. 2014, 88, 1942–1952. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hall, A.J.; Lopman, B.A.; Payne, D.C.; Patel, M.M.; Gastañaduy, P.A.; Vinjé, J.; Parashar, U.D. Norovirus Disease in the United States. Emerg. Infect. Dis. 2013, 19, 1198–1205. [Google Scholar] [CrossRef]
- Shah, M.P.; Hall, A.J. Norovirus Illnesses in Children and Adolescents. Infect. Dis. Clin. N. Am. 2018, 32, 103–118. [Google Scholar] [CrossRef]
- Spackova, M.; Altmann, D.; Eckmanns, T.; Koch, J.; Krause, G. High Level of Gastrointestinal Nosocomial Infections in the German Surveillance System, 2002–2008. Infect. Control Hosp. Epidemiol. 2010, 31, 1273–1278. [Google Scholar] [CrossRef] [PubMed]
- Verhoef, L.; Vennema, H.; van Pelt, W.; Lees, D.; Boshuizen, H.; Henshilwood, K.; Koopmans, M.; on behalf of the Food-Borne Viruses in Europe Network. Use of Norovirus Genotype Profiles to Differentiate Origins of Foodborne Outbreaks. Emerg. Infect. Dis. 2010, 16, 617–624. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Prevention of Hospital-Acquired Infections: A Practical Guide; Ducel, G., Fabry, J., Nicolle, L., Eds.; World Health Organization: Geneva, Switzerland, 2002. [Google Scholar]
- Cotten, M.; Petrova, V.; Phan, M.V.T.; Rabaa, M.A.; Watson, S.J.; Ong, S.H.; Kellam, P.; Baker, S. Deep Sequencing of Norovirus Genomes Defines Evolutionary Patterns in an Urban Tropical Setting. J. Virol. 2014, 88, 11056–11069. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Characteristics | Norovirus Positivity | OR (95%CI) | p | |
|---|---|---|---|---|
| Yes | No | |||
| Sex | 0.909 (0.770, 1.073) | >0.2 | ||
| Male | 333 | 3596 | ||
| Female | 278 | 3302 | ||
| Season | NA | >0.2 | ||
| 2013/2014 | 147 | 1722 | ||
| 2014/2015 | 180 | 1780 | ||
| 2015/2016 | 143 | 1660 | ||
| 2016/2017 | 141 | 1736 | ||
| Age | 2.847 (2.407, 3.367) | <0.001 | ||
| <5 years | 294 | 1695 | ||
| ≥5 years | 317 | 5203 | ||
| Time of infection | 3.826 (3.110; 4.705) | <0.001 | ||
| October to March | 494 | 3619 | ||
| April to September | 117 | 3279 | ||
| Characteristics | Setting of Infection | OR (95%CI) | p | |
|---|---|---|---|---|
| Community Acquired | Nosocomial | |||
| Genotype | NA | <0.001 | ||
| GI.1 | 1 | 0 | ||
| GI.2 | 5 | 2 | ||
| GI.3 | 17 | 5 | ||
| GI.4 | 1 | 0 | ||
| GI.5 | 2 | 0 | ||
| GI.6 | 3 | 2 | ||
| GII.2 | 36 | 22 | ||
| GII.3 | 38 | 29 | ||
| GII.4 | 144 | 202 | ||
| GII.6 | 46 | 23 | ||
| GII.7 | 3 | 1 | ||
| GII.13 | 1 | 1 | ||
| GII.14 | 4 | 1 | ||
| GII.17 | 11 | 7 | ||
| GII.26 | 1 | 0 | ||
| GI.3 and GII.4 | 1 | 0 | ||
| GII.2 and GII.4 | 1 | 0 | ||
| Genogroup | 3.234 (1.504, 6.953) | 0.002 | ||
| GGI | 29 | 9 | ||
| GGII | 285 | 286 | ||
| Age | 0.268 (0.191, 0.374) | <0.001 | ||
| <5 years | 201 | 94 | ||
| ≥5 years | 115 | 201 | ||
| GII.3 | 0.795 (0.476, 1.326) | >0.4 | ||
| Yes | 38 | 29 | ||
| No | 277 | 266 | ||
| GII.4 | 2.579 (1.853, 3.591) | <0.001 | ||
| Yes | 144 | 202 | ||
| No | 171 | 93 | ||
| GII.6 | 2.022 (1.193, 3.429) | 0.01 | ||
| Yes | 46 | 23 | ||
| No | 269 | 272 | ||
| Norovirus Genotype | 2013/2014 | 2014/2015 | 2015/2016 | 2016/2017 | Σ |
|---|---|---|---|---|---|
| GII.2 | 1 | 1 | 14 | 9 | 25 |
| GII.3 | 9 | 22 | 19 | 3 | 53 |
| GII.4 | 53 | 30 | 34 | 28 | 145 |
| GII.6 | 24 | 6 | 9 | 2 | 41 |
| GII.17 | 1 | 0 | 5 | 0 | 6 |
| Other GGII | 0 | 0 | 6 | 2 | 8 |
| Untypable GGII | 0 | 1 | 0 | 0 | 1 |
| Σ | 88 | 60 | 87 | 44 | 279 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ennuschat, N.; Härtel, S.; Pietsch, C.; Liebert, U.G. Norovirus Epidemiology and Genetic Diversity in Leipzig, Germany during 2013–2017. Viruses 2021, 13, 1961. https://doi.org/10.3390/v13101961
Ennuschat N, Härtel S, Pietsch C, Liebert UG. Norovirus Epidemiology and Genetic Diversity in Leipzig, Germany during 2013–2017. Viruses. 2021; 13(10):1961. https://doi.org/10.3390/v13101961
Chicago/Turabian StyleEnnuschat, Nora, Sabine Härtel, Corinna Pietsch, and Uwe G. Liebert. 2021. "Norovirus Epidemiology and Genetic Diversity in Leipzig, Germany during 2013–2017" Viruses 13, no. 10: 1961. https://doi.org/10.3390/v13101961
APA StyleEnnuschat, N., Härtel, S., Pietsch, C., & Liebert, U. G. (2021). Norovirus Epidemiology and Genetic Diversity in Leipzig, Germany during 2013–2017. Viruses, 13(10), 1961. https://doi.org/10.3390/v13101961






